Abstract
Photometric microplate assay was performed for testing of paraoxon-inhibited acetylcholinesterase (AChE) using three reactivators for reactivation purposes: obidoxime, pralidoxime, and HI-6. 3-D graphs (percent of reactivation vs. concentration of reactivator and vs. time of reactivator effecting) were constructed for each reactivator to compare their efficacy. The best results were obtained using obidoxime where reactivation was near to 80%. Suitability of photometric microplates for following of reactivation procedures is discussed.
Introduction
Organophophates represent a wide group of toxic compounds including nerve agents prepared for military purposes (such as cyclosarin, sarin, soman, VX) and pesticides (such as chlorfevinphos, dichlorvos, paraoxon, and parathion). Presented compounds have similar toxicology pathway: they covalently modify serine in reaction centre of acetylcholinesterase (AChE) or butyrylcholinesterase (BChE). Structure of AChE was mapped and large similarities between different organisms were described [Citation1] so similar toxicology effect of organophosphates on evolutionary large group of animals could be expected. Intoxication with military nerve agents exerts fast effect; furthermore, chronic toxicity effect is less probable due to instability in environment. Pesticides are less toxic; however, pesticides residuums are stabile and especially present serious fulmination when water supplies are contaminated. Naturally occurred organisms are not able to metabolize pesticides nevertheless genetically modified organisms such as Pseudomonas putida were referred [Citation2]. Harmful effect of organophosphates on human body and diagnostic possibilities were extensively reviewed by Bajgar [Citation3].
Two basic ways can be chosen for treatment when someone is intoxicated. There could be employed suppression of rising cholinergic shock, typically atropine is used for this purposes. In another manner, treatment can be based on reactivation of inhibited AChE; large group of reactivators is convenient for these purposes [Citation4,5]. Typically used reactivators are monopyridinium and bispyridinium oximes such as pralidoxime (2-hydroxyiminomethyl-1-methylpyridinium chloride), obidoxime (1,3-bis(4-hydroxyiminomethylpyridinium)-2-oxa-propane dichloride), trimedoxime (1,3-bis(4-hydroxyiminomethylpyridinium)-propane dibromide), methoxime (1,1-bis(4-hydroxyiminomethylpyridinium)-methane dichloride), and HI-6 (1-(2-hydroxyiminomethylpyridinium)-3-(4-carbamoylpyridinium)-2-oxa-propane dichloride) [Citation6]. Nevertheless, reactivators could negatively influence organisms and slight toxicity effect was described [Citation7]. In another study, there was even described strong competition between one of reactivators: HI-6 and different origin cholinesterases [Citation8]. New reativators are object of extensive research [Citation9–17]; however, possibility to choose convenient reactivator for given organophosphate is quite difficult process.
The presented study is aimed at performance of photometric microplate assay for AChE reactivation after previous inhibition by representative pesticide: paraoxon. Maximization of contemporary obtained information is an expected advantage; 3-D graphs (percent of reactivation vs. concentration of reactivator and time preincubation) are constructed. Finally, optimal reactivation and its proper concentration is recommended.
Material and methods
Chemicals
Paraoxon (diethyl p-nitrophenyl phosphate) was obtained from Labor Dr. Ehrenstorfer-Schafers (Augsburg, Germany). Acetylthiocholine chloride (ATChCl), Ellman's reagent: 5,5′-ditiobis (2-nitrobenzoic acid) (DTBN) and human recombinant AChE in lyophilized form were purchased form Sigma-Aldrich (Prag, Czech Republic). Pralidoxime, obidoxime and HI-6 were synthesized at the Department of Toxicology of Faculty of Military Health Sciences in Hradec Kralove. Deionized water was prepared by Millipore system. All others chemicals were obtained in the standard purity.
Measuring protocol
96-well (8 × 12) microplates were purchased from Gama (Ceske Budejovice, Czech Republic). Total activity 0.01 nkat per well was injected and then added 10 μl of paraoxon 1 ppm or 10 μl of deionized water and let incubated for one hour. 1 ppm of paraoxon with given preincubation time caused demonstrably inhibition more than 95%. Then freshly prepared mixture consisting from 1 mM ATChCl and DTBN 0.4 mg/ml in phosphate buffered saline (PBS) was added in amount 40 μl per well. Finally, 10 μl of tested reactivator or deionized water was injected to well. Tested concentration range of reactivator was from 10− 8 to 10− 3 M. PBS was used for blank purposes. Absorbance was measured at 412 nm using microplate reader MRX (Dynatech Laboratories, Chantilly, VA, USA).
Data processing
Following values were experimentally obtained: ΔA0 was absorbance provided by intact AChE (no paraoxon, no reactivator). The symbol ΔAi expresses absorbance for paraoxon inhibited AChE without reactivation. ΔAr is absorbance change provided by paraoxon inhibited AChE and consequently reactivated. The percentage of reactivation is expressed as follows:
Results
Three currently available reactivators were tested in photometric microplate system. Obidoxime is one of tested drugs. Potency of obidoxime for reactivation sarin likewise others nerve agents inhibited acetylcholinesterase was tested previously [Citation17,6]. Our presented testing proved quite good reactivation potency of obidoxime toward paraoxon inhibited AChE. Efficacy of obidoxime for paraoxon-inhibited AChE is summarized in .
Figure 1. Reactivation of paraoxon-inhibited AChE by obidoxime. Percent of reactivation vs. concentration of obidoxime and time from reactivation commencement is introduced.
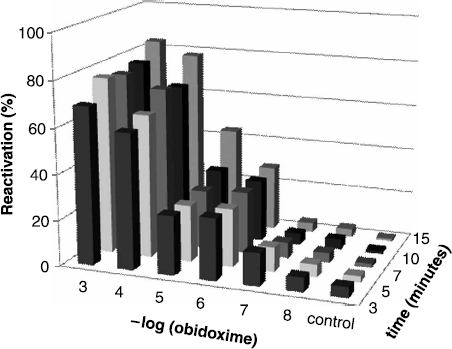
Obidoxime was capable to reactivate paraoxon inhibited AChE in concentration 10− 3 and 10− 4 M from the earlier time interval resulting in reactivation above 60% and longer incubation (more than 10 minutes) proved reactivation near to 80% (10− 3 M) or 75% (10− 4 M). A sharp decrease of reactivation is obvious in further obidoxime dilutions.
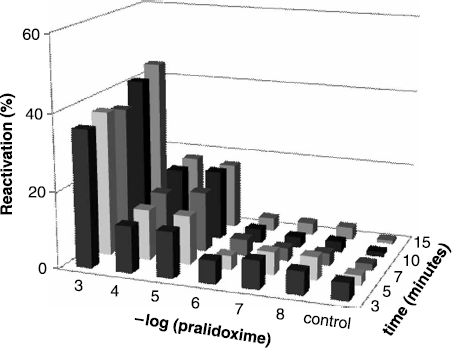
Pralidoxime was further tested reactivator. Applicability of pralidoxime for paraoxon inhibited AChE reactivation is not obvious as in the obidoxime case. Suitable concentration of pralidoxime is quite high: 10− 3 M. In this concentration the percent of reactivation was around 40%. However, this concentration is too high for physiological applicability. Reactivation efficacy for paraoxon-inhibited AChE is similar to that previously referred for cyclosarin-inhibited AChE [Citation13]. Reactivation results are shown in .
Although reactivation by pralidoxime is considered less suitable than that by obidoxime, we should consider fact that molecule of pralidoxime has only one oxime group in comparison with obidoxime. Also the position of this group is a very important feature which could dramatically affect the reactivation. For paraoxon-inhibited AChE oximes with oxime group in position-4 are prefered.
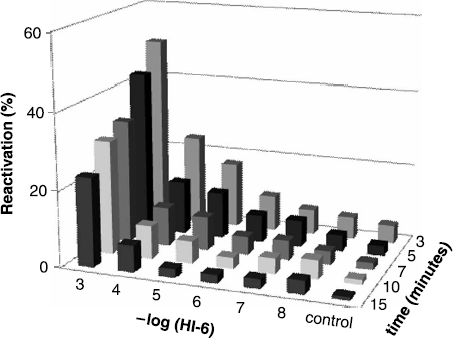
Complete summarizing of reactivation by HI-6 is expressed in . Reactivation potency of HI-6 is very low for paraoxon inhibited AChE in comparison with obidoxime and pralidoxime. Concentration 10− 3 M proved sufficient reactivation around 50% for short time interval; furthermore, competition between HI-6 and enzyme substrate and/or other inhibition mechanism caused decrease of reactivation percent in further time scale. Similar competition was observed in previous study [Citation8].
Discussion
In our In vitro experiments, we have tested reactivation potency of three oximes: obidoxime, pralidoxime, and HI-6. Proposed system based on photometric microplates was able to predict reactivation suitability for all tested reactivators and finally choose the most optimal one. The reactivator obidoxime was found as the most promising one. We did not found reference relevant to paraoxon-inhibited AChE, nevertheless, our system proved good correlation to mentioned works concerning to reactivation AChE after inhibition by nerve agents [Citation6,10,13,16] even in fact that our system is artificial one.
Proposed method brings intriguing simplification of proper reactivator selection. Paraoxon was used as representative pesticide but testing of other pesticides is expected in the future. We are able to examine fitness of tested reactivators group for given inhibitor within very short time. Furthermore, we are able to recognize the best concentration as well as time interval of the needed reactivation effect within one testing cycle. Displacement of laboratory animals from routinely used testing protocols is an advantage, too. Such very quick method could be used also as diagnostic tool for reactivability test in the case of intoxication by unknown organophosphorus inhibitor.
Acknowledgements
We would like to thank to the Ministry of Industry and Trade of the Czech Republic for the Project No.2A-1TP1/007
References
- J Wiesner, Z Kriz, K Kuca, D Jun, and J Koca. (2007). Acetylcholinesterases – the structural similarities and differences. J Enz Inhib Med Chem 22:417–424.
- MP Mattozzi, SK Tehara, T Hong, and JD Keasling. (2006). Mineralization of paraoxon and its use as a sole C and P source by a rationally designed catabolic pathway in Pseudomonas putida. Appl Environ Microb 72:6699–6706.
- J Bajgar. (2004). Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv Clin Chem 38:151–216.
- K Kuca, D Jun, and K Musilek. (2006). Structural requirements of acetylcholinesterase reactivators. Mini Rev Med Chem 6:269–277.
- J Bajgar, J Fusek, K Kuca, L Bartosova, and D Jun. (2007). Treatment of organophosphate intoxication using cholinesterase reactivators: Facts and fiction. Mini Rev Med Chem 7:461–466.
- K Kuca, D Jun, and J Bajgar. (2007). Currently used cholinesterase reactivators against nerve agent intoxication: Comparison of their effectivity in vitro. Drug Chem Toxicol 30:31–40.
- S Vesela, V Ondruska, K Kuca, and J Patocka. (2006). Test with Daphnia magna: A new approach to prescreen toxicity of newly synthesized acetylcholinesterase reactivotors. J Enz Inhib Med Chem 21:427–432.
- M Pohanka, D Jun, and K Kuca. (2007). Amperometric biosensor for evaluation of competitive cholinesterase inhibition by the reactivator HI-6. Anal Lett. 40:2351–2359.
- M Hrabinova, K Musilek, D Jun, and K Kuca. (2006). New group of xylene linker-containing acetylcholinesterase reactivators as antidotes against the nerve agent cyclosarin. J Enz Inhib Med Chem 21:512–519.
- K Kuca, and J Kassa. (2003). A comparison of the ability of a new bispyridinium oxime-1-(4-hydroxyiminomethylpyridinium)-4-(4-carbamoylpyridinium)butane dibromide and currently used oximes to reactivate nerve agent-inhibited rat brain acetylcholinesterase by in vitro methods. J Enz Inhib Med Chem 18:529–535.
- J Kassa, D Jun, and K Kuca. (2007). A comparison of reactivating efficacy of newly developed oximes (K074, K075) and currently available oximes (obidoxime, HI-6) in cyclosarin-and tabun- poisoned rats. J Enz Inhib Med Chem 22:297–300.
- K Musilek, O Holas, K Kuca, D Jun, V Dohnal, and M Dolezal. (2007). Synthesis of a novel series of non-symmetrical bispyridinium compounds bearing a xylene linker and evaluation of their reactivation activity against tabun and paraoxon-inhibited acetylcholinesterase. J Enz Inhib Med Chem 22:425–432.
- K Kuca, J Cabal, D Jun, J Bajgar, and M Hrabinova. (2006). Potency of new structurally different oximes to reactivate cyclosarin-inhibited human brain acetylcholinesterases. J Enz Inhib Med Chem 21:663–666.
- K Musilek, K Kuca, D Jun, V Dohnal, and M Dolezal. (2005). Synthesis of a novel series of bispyridinium compounds bearing a xylene linker and evaluation of their reactivation activity against chlorpyrifos-inhibited acetylcholinesterase. J Enz Inhib Med Chem 20:409–415.
- J Picha, K Kuca, M Kivala, M Kohout, J Cabal, and F Liska. (2005). A new group of monoquaternary reactivators of acetylcholinesterase inhibited by nerve agents. J Enz Inhib Med Chem 20:233–237.
- K Kuca, and J Patocka. (2004). Reactivation of cyclosarin-inhibited rat brain acetylcholinesterase by pyridinium-oximes. J Enz Inhib Med Chem 19:39–43.
- K Kuca, J Cabal, and J Kassa. (2005). In vitro reactivation of sarin-inhibited brain acetylcholinesterase from diferent species by various oximes. J Enz Inhib Med Chem 20:227–232.