Abstract
New hydrazide derivatives of imidazo[1,2-a]pyridine have been synthesized and evaluated for anticandidal activity. The reaction of imidazo[1,2-a]pyridine-2-carboxylic acid hydrazides with various benzaldehydes gave N-(benzylidene)imidazo[1,2-a]pyridine-2-carboxylic acid hydrazide derivatives. Their anticandidal activities against Candida albicans and Candida glabrata (isolates obtained from Osmangazi University, Faculty of Medicine, Eskisehir, Turkey), Candida albicans (ATCC 90028), Candida utilis (NRLL Y-900), Candida tropicalis (NRLL Y-12968), Candida krusei (NRLL Y-7179), Candida zeylanoides (NRLL Y-1774), and Candida parapsilosis (NRLL Y-12696) were investigated.
Introduction
Invasive fungal infections are becoming increasingly implicated as a cause of crucial and fatal diseases. This is especially the case in immunocompromised patients, who are having a tendency to infections caused by opportunistic fungal pathogens that are normally kept in check by a functioning immune system [Citation1–3]. Common use of antifungal therapies for curative, pre-emptive or prophylactic intentions has been developed to overcome the threat of Candida colonisation and infection, but has also led to the development of resistance to the currently available antifungals. Intrinsic (Candida krusei, Candida glabatra) or acquired (Candida albicans) resistances to azole antifungals have been observed with an increasing occurrence. This situation highlights the need for advent of new and effective antimycotic agents. [Citation4–8].
As known, not only biochemical similarity of the human cell and fungi forms is a handicap for selective activity, but also the easily gained resistance is the main problem encountered in developing safe and efficient antifungals. While the incidence of systemic fungal infections has been increasing, the choice of suitable antifungal agents remains relatively limited although the advent of the new echinocandin class is a welcome development as is the expansion of members of the azole antifungals [Citation9–11]. The latter type of compounds exhibits activity by interfering with ergosterol biosynthesis through the inhibition of lanosterol 14α-demethylation catalysed by cytochrome P-450 [Citation12]. Imidazoles, the first group to be developed in azole antifungals also inhibit the accumulation of methylated sterols disrupts the organization of the lipidic bilayer of membranes. Furthermore, some imidazole drugs, at high concentrations, could exert direct inhibitory effects on membranes, without interference with sterols and sterol esters [Citation13–14]. The literature survey reveals that, although there are many examples of imidazole ring, there are not many study on imidazo[1,2-a]pyridine ring as an imidazole fused six-membered ring system.
On the other hand, it is well known that hydrazide-hydrazone group plays an important role for antimicrobial activity [Citation15–17].
In the design of new drugs, the development of hybrid molecules through the combination of different pharmacophores in one frame may lead to compounds with interesting biological profiles.
In the present study, prompted by these observations, the synthesis and anticandidal screening of new hydrazide-hydrazone derivatives of imidazopyridines as hybrid molecules including different pharmacophores were aimed.
Experimental
Chemistry
All melting points (m.p.) were determined in open capillaries on a Gallenkamp apparatus and are uncorrected. The purity of the compounds was routinely checked by thin layer chromatography (TLC) using silica gel 60G (Merck). Spectroscopic data were recorded by the following instruments. IR: Shimadzu IR-435 spectrophotometer; 1H-NMR: Bruker 250 MHz spectrometer, MS-FAB: VG Quattro Mass spectrometer. Elemental analyses were recorded on Perkin Emler EAL 240 spectrometer.
General procedure for synthesis of the compounds
3-Nitro-5-acetamido-6-methylimidazo[1,2-a]pyridine-2-carboxylic acid hydrazides (1)
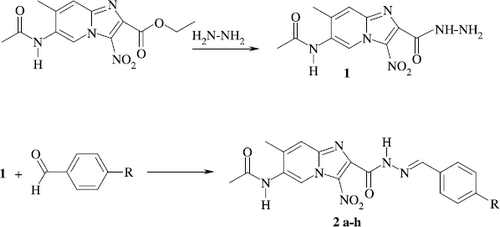
These compounds were prepared according to the previously reported method, by reacting ethyl 3-nitro-5-acetamido-6-methylimidazo[1,2-a]pyridine-2-carboxylates with hydrazine hydrate [Citation18,19] (Scheme ).
N-(benzylidene)-3-Nitro-5-acetamido-6-methylimidazo[1,2-a]pyridine-2-carboxylic acid hydrazide derivatives (2a-h)
Equimolar quantities of acid hydrazides (30 mmol) and appropriate benzaldehydes in 25 ml of absolute ethanol were refluxed for 3–5 h. The resulting solid was filtered and recrystallized from ethanol ().
Table I. Some characteristics of the compounds.
2a: IR (KBr, cm−1): 3396 and 3145(NH), 1691 and 1679(C=O), 1520–1342(C=C and C=N)
1H-NMR (250 MHz) (DMSO-d6) δ (ppm): 2.25 (3H, s, CH3), 2.55 (3H, s, COCH3), 6.90-8.30 (7H, m, aromatic protons), 9.60 (1H, s, NH), 9.75 (1H, d [J = 6.20 Hz], CH), 12.25 (1H, br, N-NH). MS (FAB) [M + 1]: m/z 381. Anal. Calc. for C18H16N6O4: C, 56.84; H, 4.24; N, 22.09. Found: C, 56.85; H, 4.21; N, 22.12%.
2b: IR (KBr, cm−1): 3411 and 3162(NH), 1701 and 1682(C=O), 1541–1365(C=C and C=N)
1H-NMR (250 MHz) (DMSO-d6) δ (ppm): 2.20 (3H, s, CH3), 2.50 (3H, s, COCH3), 7.40-8.30 (6H, m, aromatic protons), 9.70 (1H, s, NH), 9.80 (1H, d [J = 6.23 Hz], CH), 12.30 (1H, br, N-NH). MS (FAB) [M + 1]: m/z 415. Anal. Calc. for C18H15ClN6O4: C, 52.12; H, 3.64; N, 20.26. Found: C, 52.15; H, 3.67; N, 20.28%.
2c: IR (KBr, cm−1): 3382 and 3143(NH), 1691 and 1668(C=O), 1504–1321(C=C and C=N)
1H-NMR (250 MHz) (DMSO-d6) δ (ppm): 2.25 (3H, s, CH3), 2.45 (3H, s, COCH3), 7.30-8.20 (6H, m, aromatic protons), 9.65 (1H, s, NH), 9.70 (1H, d [J = 5.91 Hz], CH), 12.10 (1H, br, N-NH). MS (FAB) [M + 2]: m/z 461. Anal. Calc. for C18H15BrN6O4: C, 47.08; H, 3.29; N, 18.30. Found: C, 47.11; H, 3.30; N, 18.27%.
2d: IR (KBr, cm−1): 3423 and 3151(NH), 1689 and 1672 (C=O), 1538–1311(C=C and C=N)
1H-NMR (250 MHz) (DMSO-d6) δ (ppm): 2.20 (3H, s, CH3), 2.45 (3H, s, COCH3), 7.35-8.25 (6H, m, aromatic protons), 9.70 (1H, s, NH), 9.75 (1H, d [J = 6.12 Hz], CH), 12.10 and 12.30 (1H, two s, N-NH). MS (FAB) [M + 1]: m/z 399. Anal. Calc. for C18H15FN6O4: C, 54.27; H, 3.80; N, 21.10. Found: C, 54.30; H, 3.77; N, 21.11%.
2e: IR (KBr, cm−1): 3497 and 3135(NH), 1715 and 1695(C=O), 1541–1381(C=C and C=N)
1H-NMR (250 MHz) (DMSO-d6) δ (ppm): 2.25 (3H, s, CH3), 2.40(3H, s, phenyl-CH3), 2.65 (3H, s, COCH3), 7.10-8.30 (6H, m, aromatic protons), 9.70 (1H, br s, NH), 9.80 (1H, d [J = 6.96 Hz], CH), 12.15 (1H, br, N-NH). MS (FAB) [M + 1]: m/z 395. Anal. Calc. for C19H18N6O4: C, 57.86; H, 4.60; N, 21.31. Found: C, 57.88; H, 4.62; N, 21.31%.
2f: IR (KBr, cm−1): 3405 and 3120(NH), 1695 and 1675(C=O), 1550–1385(C=C and C=N)
1H-NMR (250 MHz) (DMSO-d6) δ (ppm): 2.20 (3H, s, CH3), 2.60 (3H, s, COCH3), 3.75 and 3.90 (3H, two s, OCH3), 6.85-8.25 (6H, m, aromatic protons), 9.65 (1H, s, NH), 9.80 (1H, d [J = 5.51 Hz], CH), 12.00 and 12.20 (1H, two s, N-NH). MS (FAB) [M + 1]: m/z 411. Anal. Calc. for C19H18N6O5: C, 55.61; H, 4.42; N, 20.48. Found: C, 55.65; H, 4.43; N, 20.50%.
2g: IR (KBr, cm−1): 3375 and 3131(NH), 1689 and 1670(C=O), 1511–1345(C=C and C=N)
1H-NMR (250 MHz) (DMSO-d6) δ (ppm): 2.20 (3H, s, CH3), 2.50 (3H, s, COCH3), 6.95-8.20 (6H, m, aromatic protons), 9.55 (1H, s, NH), 9.65 (1H, d [J = 5.81 Hz], CH), 12.10 (1H, br, N-NH). MS (FAB) [M + 1]: m/z 426. Anal. Calc. for C18H15N7O6: C, 50.83; H, 3.55; N, 23.05. Found: C, 50.80; H, 3.59; N, 23.06%.
2h: IR (KBr, cm−1): 3387 and 3158(NH), 1703 and 1688(C=O), 1582–1361(C=C and C=N)
1H-NMR (250 MHz) (DMSO-d6) δ (ppm): 2.25 (3H, s, CH3), 2.60 (3H, s, COCH3), 2.85-2.95 (6H, m, N(CH3)2), 7.05-8.35 (6H, m, aromatic protons), 9.60 (1H, s, NH), 9.70 (1H, d [J = 5.98 Hz], CH), 12.25 (1H, br, N-NH). MS (FAB) [M + 1]: m/z 424. Anal. Calc. for C20H21N7O4: C, 56.73; H, 5.00; N, 23.16. Found: C, 56.70; H, 4.97; N, 23.14%.
Microbiology
The activity of the compounds were first screened using an agar diffusion method for Candida albicans (clin isol.) and Candida tropicalis and all active compounds (inhibition zones >9–11 mm, at 2 mg/mL concentration) were further evaluated using the microdilution broth method to identify the minimum inhibitory concentrations (MIC) against all Candida sp. [Citation20]. Tested microorganism strains were: Candida albicans and Candida glabrata (isolates obtained from Osmangazi University, Faculty of Medicine, Eskisehir, Turkey), Candida albicans (ATCC 90028), Candida utilis (NRLL Y-900), Candida tropicalis (NRLL Y-12968), Candida krusei (NRLL Y-7179), Candida zeylanoides (NRLL Y-1774), and Candida parapsilosis (NRLL Y-12696).
Microorganisms
Microorganisms were stored in 15% glycerol containing micro-test tubes at − 86°C (strain numbers of microorganisms are given in ). All Candida strains were inoculated on Sabouraud Dextrose Agar (SDA) prior the experiments at 37°C. After sufficient growth Candida sp. were then transferred to Mueller Hinton Broth (MHB) for further incubation at the same conditions for another 24 h.
Table II. Antifungal activities of the compounds (mg/mL).
Anticandidal assay
Agar diffusion assay
1 mL of pregrown Candida suspension in MHB adjusted to McFarland No. 0.5 was inoculated to each Petri plate (9 cm and 20 mL MHA) under sterile conditions. With the aid of an sterile cork borer (8 mm) wells were formed in each Petri plate to accommodate the test samples (50 microlitre from a stock solution of 2 mg/mL DMSO) including the solvent and antifungal controls to incubate at 37°C for 24 h. Inhibition zones were visualized using a tetrazolium salt (TTC, Aldrich) where clear inhibition zones ≥ 9 mm were considered as active, for further testing.
Microdilution assay
The test compounds and the antimicrobial standards were first dissolved in dimethylsulfoxide (DMSO) which was used to prepare the stock solutions at an initial concentration of 2 mg/mL. Serial dilution series were prepared in 100 μL MHB with an equal amount of the test samples. The last row was filled only with water as growth control for the microorganisms. Overnight grown microorganism suspensions were first diluted in double strength MHB and standardized to 108 CFU/mL (using McFarland No: 0.5) under sterile conditions. Then each microorganisms suspension was pippetted into each well and incubated at 37°C for 24 h. Ketoconazole was used as a standard antifungal agent against Candida sp. Sterile distilled water and medium served as a positive growth control. The first well without turbidity was assigned as the minimum inhibitory concentration (MIC, in mg/mL). Average results of separately performed three experiments as given in .
Results and discussion
The structures of the obtained compounds were elucidated by spectral data. In the IR spectra, some significant stretching bands due N–H, C = O and C = N C = C were at about 3497–3120 cm− 1, 1715–1668 cm− 1 and 1582–1311 cm− 1 respectively.
The 1H-NMR spectra data were also consistent with the assigned structures. In the 250 MHz 1H-NMR spectrum of compounds, we observed paired peaks for each of the protons N = CH (9.60–9.80 ppm as doublets [ J = 5.51 − 6.96 Hz]), and N = NH (12.00–12.30 ppm as two singlets) corresponding to (E) and (Z) forms of the compounds. For each compound, the intensities of these paired peaks different from others, due to the variable amounts of (E) and (Z), which are usually unequal. The NH proton of acetamide was observed at 9.55–9.70 ppm as a singlet. All the other aromatic and aliphatic protons were observed at expected regions. The mass spectra (MS(FAB)) of compounds showed [M + 1] and [M + 2] peaks, in agreement with their molecular formula.
All compounds were evaluated for their antimicrobial properties. MIC's were recorded as the minimum concentration of compound, which inhibits the growth of tested microorganisms. Some compounds showed promising anticandidal activities, when compared with reference drug. When compared with Ketoconazole; the compound 2a, 2 g, 2 h showed similar antifungal activity against Candida glabrata (clinical isolate); also compounds 2b, 2f, 2 g, 2 h showed similar antifungal activities against Candida zeylanoides (NRLL Y-1774) ().
References
- MA Pfaller. (1998). The epidemiology of invasive mycoses-narrowing the gap. Clin Infect Dis 27:1148–1150.
- KA Marr, RA Carter, F Crippa, A Wald, and L Corey. (2002). Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis 34:909–917.
- S Hood, and DW Denning. (1996). Treatment of fungal infection in AIDS. J Antimicrob Chemoth 37:71–85.
- M Masia Canuto, and F Gutierrez Rodero. (2002). Antifungal drug resistance to azoles and polyenes. Lancet Infect Dis 2:550–563.
- CR Sims, L Ostrosky-Zeichner, and JH Rex. (2005). Invasive candidiasis in immunocompromised hospitalized patients. Arch Med Res 36:660–671.
- P Montravers, and K Jabbour. (2006). Managing the challenges of invasive fungal infections. Int J Antimicrob Agents 27:1–2.
- BD Alexander, and JR Perfect. (2000). Antifungal resistance trends towards the year 2000 implications for therapy and new approaches. Drugs 54:657–678.
- N Lebouvier, F Pagniez, M Duflos, P Le Pape, YG Min Na, L Bauta, and ML Borgnea. (2007). Synthesis and antifungal activities of new fluconazole analogues with azaheterocycle moiety. Bioorg Med Chem Lett 17:3686–3689.
- JR Rees, RW Pinner, and RA Hajjeh. (1993). The epidemiological features of invasive mycotic infections in the San Francisco Bay Area, 1992–1993: Results of population-based laboratory active surveillance. Clin Infect Dis 27:1138–1140.
- A Polak. (1999). The past, present and future of antimycotic combination therapy. Mycoses 42:355–370.
- R Musiol, J Jampilek, V Buchta, L Silva, H Niedbala, B Podeszwa, A Palka, K Majerz-Maniecka, B Oleksynd, and J Polanski. (2006). Antifungal properties of new series of quinoline derivatives. Bioorg Med Chem 14:3592–3598.
- DJ Sheehan, CA Hitchcock, and CM Sıbley. (1999). Current and emerging azole antifungal agents. Clin Microbiol Rev 12:40–79.
- H Yamaguchi. (1977). Antagonistic action of lipid components of membranes from Candida albicans and various other lipids on two imidazole antimycotics, clotrimazole and miconazole. Antimicrob Agents Chemother 12:16–25.
- H Yamaguchi. (1978). Protection by unsaturated lecithin against the imidazole antimycotics, clotrimazole and miconazole. Antimicrob Agents Chemother 13:423–426.
- P Vicini, F Zani, P Cozzini, and I Doytchinova. (2002). Hydrazones of 1,2-benzisothiazole hydrazides: Synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 37:553–564.
- KA Metwally, LM Abdel-Aziz, EM Lashine, MI Husseiny, and RH Badawy. (2006). Hydrazones of 2-aryl-quinoline-4-carboxylic acid hydrazides: Synthesis and preliminary evaluation as antimicrobial agents. Bioorg Med Chem 14:8675–8682.
- A Gürsoy, N Terzioğlu, and G Ötük. (1997). Synthesis of some new hydrazide-hydrazones, thiosemicarbazides and thiazolidinones as possible antimicrobials. Eur J Med Chem 32:753–757.
- HL Yale, K Losen, J Martins, M Holsing, MF Perry, and J Bernstein. (1953). Chemotherapy of experimental tuberculosis. VIII. The synthesis of acid hydrazides, their derivatives and related compound. J Am Chem Soc 75:1933–1942.
- L Bukowski, and M Janowiec. (1996). 1-Methyl-1H-2-imidazo[4,5-b]pyridinecarboxylic acid and of its derivatives with suspected antituberculotic activity. Pharmazie 51:27–30.
- NCCLS. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standardsecond ed.. (2002). NCCLS document M27-A2 [ISBN 1-56238-469-4].