Abstract
A solid phase parallel synthesis using SynPhase™ technology was used to couple a series of 21 carboxylic with three different 4-(4-arylpiperazinyl)butanamines. The resulting library was evaluated as dopamine D3 receptor ligands giving rise to several compounds with affinities in the low nanomolar concentration range (9e and 9n with binding affinities at D3 receptors of 0.10 and 0.35 nM respectively).
Introduction
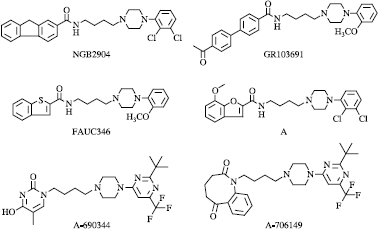
Since its discovery in 1990 [Citation1], the dopamine D3 receptor has been widely studied [Citation2]. Its localisation in the limbic area of the brain [Citation3] as well as the early investigations with agonists [Citation4] or antagonists [Citation5] led to the current situation that the therapeutic use of such compounds is mainly directed towards drug abuse. More recent studies have shown that potential therapeutic uses could be in other neurological and neuropsychiatric disorders [Citation6]. The aromatic amide BP 8975 () is the first representative of potent selective antagonists or partial agonists at this receptor. It has been used as prototype for further analogues by many groups.
Modification of the aromatic amide () has mainly focused on its replacement with other polyaromatics such as fluorene NGB2904 [Citation7] and biphenyl GR103691, [Citation8] or heteroaromatics like benzothiophene FAUC346 [Citation9] or benzofurane A [Citation10]. More recently, isosteric replacement of the aromatic amide with pyrimidone [Citation11] or incorporation into a benzodiazepinedione [Citation12] has been reported.
In the continuation of the previously reported modulations of BP897 [Citation13], we have performed some variations of both amide and aryl piperazine moieties using a combinatorial solid phase approach. Thus, three sub-series (8–10) of compounds were synthesized corresponding to variation of R1 substituent (). Concerning the amide part, three kinds of R2 substituents have been introduced: aryl (a–g), heteroaryl (h–j) and aryl connected through variable alkyl linkers (k–u).
Materials and methods
General
Syntheses were performed on SynPhase™ Lanterns (Code SPPS, D series, Linker BAL, 35 μmoles). 1H NMR Spectra were recorded on a Jeol GSX spectrometer at 270 MHz. High resolution mass spectra (HRMS) were recorded at the “Centre Régional de Mesures Physiques de l'Ouest” on a ZabSpec Tof Micromass spectrometer using a LSIMS (Cs+) or electrospray ionisation mode. Reagent-grade solvents were purchased from chemical suppliers and used directly without further purification unless otherwise specified. Thin-layer chromatography using CH2Cl2/CH3OH (95/5) as an eluent was performed on Merck silica gel 60 F254 (layer thickness: 0.22 mm) and assessed the purity of the compounds (>95%). The compounds were visualised using UV light, ninhydrin or iodine. The structure of compounds was checked using 1H NMR and HRMS.
Synthesis
Anchorage of amines to BAL lanterns
To a solution of a primary amine (1 M), NaBH3CN (0.063 g; 0.1 M) and AcOH (0.1 mL) in N,N-dimethylformamide (DMF) (10 mL) were added 21 SynPhase Lanterns 1. The solution was heated to 60°C for 16 h. The lanterns 2–4 were then washed with 3 × DMF and 3 × CH2Cl2 (general procedure) and dried under vacuum. One lantern of each amine pool was taken to form 21 identical pools that will react with acyl chlorides or carboxylic acids.
Formation of amide bond from acyl chlorides
To a pool of 3 lanterns 2–4 was added a solution of N-methylmorpholine (0.25 M) and R2–COCl (0.25 M) in dichloromethane (2 mL) for 1 h at room temperature. The lanterns 5a–7b were then washed according to the general procedure and dried under vacuum.
Formation of amide bond from carboxylic acids
To a pool of 3 lanterns 2–4 was added a solution of diisopropyl carbodiimide (DIC, 0.2 M), hydroxybenzotriazole (HOBt, 0.2 M) and R2–COOH (0.2 M) in DMF (2 mL) for 16 h at room temperature. The lanterns 5c-7u were then washed according to the general procedure and dried under vacuum.
Cleavage
The lanterns 5a-7u were then separated in a 96-well plate and 0.5 mL of trifluoroacetic acid in CH2Cl2 (20:80) was added. After 1 h at room temperature, the lanterns were removed and the cleavage solutions were evaporated under vacuum to yield the compounds 8a-10u (average yield: 48%).
N-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butyl}benzamide, trifluoroacetate salt (8a)
1H NMR (CD3OD) δ (ppm): 1.77 (qt, J = 7.0 Hz, J = 6.7 Hz, 2H), 1.90 (m, 2H), 3.16 (t, J = 12.0 Hz, 2H), 3.34 (m, 6H), 3.49 (t, J = 6.9 Hz, 2H), 3.53 (d, J = 13.0 Hz, 2H), 3.71 (d, J = 12.0 Hz, 2H), 7.17 (dd, J = 2.2, J = 7.3, 1H), 7.32 (m, 2H), 7.48 (t, J = 7.5 Hz, 2H), 7.56 (t, J = 7.5 Hz, 1H), 7.85 (m, 2H). Rf (CH2Cl2/CH3OH: 95/5) = 0.30.
N-{4-[4-(3-(Trifluoromethyl)phenyl)piperazin-1-yl]butyl}-5-phenylpentanamide, trifluoroacetate salt (9r)
1H NMR (DMSO-d6) δ (ppm):1.61 (m, 2H), 1.66 (q, J = 3.5 Hz, 4H), 1.80 (m, J = 3.6 Hz, J = 7.31 Hz, 2H), 2.25 (t, J = 6.8 Hz, 2H), 2.64 (t, J = 6.8 Hz, 2H), 3.13 (m, 2H), 3.24 (m, 6H), 3.33 (t, J = 1.6 Hz, 2H), 3.66 (m, 2H), 3.91 (m, 2H), 7.21 (m, 8H), 7.49 (t, J = 8.3 Hz, 1H). Rf (CH2Cl2/CH3OH: 95/5) = 0.25.
N-{4-[4-(2-Fluorophenyl)-piperazin-1-yl]-butyl}-5,6,7,8-tetrahydronaphthyl-2-amide, trifluoroacetate salt (10d)
1H NMR (DMSO-d6) δ (ppm): 1.73 (qt, J = 7.0 Hz, J = 7.3 Hz, 2H), 1.86 (m, 6H), 2.82 (d, J = 3.2 Hz, 4H), 3.14 (t, J = 12.2 Hz, 2H), 3.31 (m, 6H), 3.47 (t, J = 6.7 Hz, 2H), 3.62 (d, J = 13.3 Hz, 2H), 3.69 (d, J = 12.1 Hz, 2H), 7.12 (m, 5H), 7.54 (m, 2H). Rf (CH2Cl2/CH3OH: 95/5) = 0.38.
9b
HRMS [M + H]+. calcd for C22H25F4N3O: 424.2012; found: 424.2012. 8h: HRMS [M + H]+. calcd for C20H23BrCl2N4O: 485.0510; found: 485.0510. 9l: HRMS [M + H]+ calcd for C36H38F3N3O3: 618.2943; found: 618.2941. 8c: HRMS [M + H]+. calcd for C22H27Cl2N3O3S: 484.1228; found: 484.1234. 10g: HRMS [M + H]+. calcd for C28H32FN3O2: 462.2556; found: 462.2550. 9r: HRMS [M + H]+. calcd for C25H34FN3O: 412.2764; found: 412.2762.
Pharmacological characterisation
Affinity of compounds for the human D3 receptor was determined by [3H] spiperone binding CHO cells that had been tranfected by the cDNA coding for the human D3 receptor (hD3). [3H]Spiperone (0,5 at 2 nM) binding was performed in the presence of 2.5 to 5 μg of membrane proteins in a medium containing 120 mM of NaCl, 5 mM of KCl, and 50 mM of Tris HCl at pH 7.4; incubation for 60 min at room temperature was performed. Non-specific binding was estimated in the presence of 10 μM haloperidol. Non-transfected cells are devoid of any specific binding. The products were tested in duplicate experiments at five different concentrations: 0.1, 1, 10, 100 and 1 000 nM.
Results and discussion
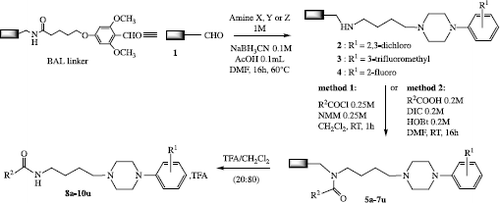
To minimize operations encountered in solution phase synthesis, we selected SynPhase™ Lanterns [Citation14] with BAL linker (Mimotopes Pty Ltd) that offer synthesis characteristics and functionality comparable to classical resins. Their easy and fast handling facilitates parallel synthesis of milligram scale individualised compounds (35 μmol per support) using the “split-pool” procedure [Citation15]. BAL linker allows fixation of primary amines through reductive amination then formation of amides that can easily be cleaved from the support.
The first step of our work on solid support (Scheme ) according to the split-pool technique consisted in a reductive amination to the BAL linker. Thus we formed three pools of 21 BAL lanterns 1 and put each one in a solution of the desired amine [Citation16,Citation17] (1 M) in DMF in the presence of NaBH3CN and a catalytic amount of acetic acid. The temperature was maintained at 60°C for 16 h in the separate solutions (10 mL) affording three pools (2–4).
The next step aimed at forming the amide bond from acyl chlorides or carboxylic acids. Thus 21 identical pools were formed with one lantern of each series 2–4. Two pools (a and b) were reacted for 1 h with acyl chlorides in the presence of N-methylmorpholine in dichloromethane. The other ones (c to u) were placed in DMF for 16 h in the presence of carboxylic acids and an equimolar mixture of DIC and HOBt, as usually performed in peptidic bond formation. In our synthesis, DIC that generates soluble diisopropylurea was prefered to dicyclohexylcarbodiimide (DCC).
For the final cleavage step, lanterns 5a-7u were distributed in a 96 well plate and a mixture of trifluoroacetic acid in dichloromethane was added, leading to 5 to 20 mg of the separate amides 8a-10u as their trifluoroacetate salts (average yield of 48%) after evaporation of the solvents. It is noteworthy that only amides could be separated from the support leading to very good purity according to TLC controls. 1H NMR Spectra of several compounds showed satisfactory results.
Binding affinities of 8a-10u at D3 receptors were then evaluated. Within the (2,3-dichlorophenyl)piperazine series (compounds 8a-u, ), all compounds except 8q display affinities in low nanomolar concentration range. This substitution generally gives better affinities than 3-trifluoromethyl (series 9) or 2-fluoro (series 10). The substitution has only little influence on the ranking order of potencies except for 8q, 9 h, 10q.
Table I. Binding affinities Ki [nM] of 8a-10u at D3 receptors using a stable transfected CHO cell line.
Benzamide substitution has been discussed previously [Citation13] with 2-methoxyphenylpiperazine derivatives: 2-substitution with methyl and benzoyl chains was found detrimental. In these series we found that fluoro substitution is well tolerated (8-10b) probably due to its small size or an internal hydrogen bond involving the amidic NH keeping the aromatic in a good orientation. Substitution in 3- or 4-position is well tolerated even with bulky substituents such as phenyl, benzoyl or benzyloxy 8e-10g. Heteroaromatics (pyridine 8h-10i, pyrazine 8-10j) show a slight decrease in affinity, probably due to their hydrophilic nature located too close to the amide. Note that 2-substitution with an hydroxyl (8-10i) is also well tolerated, probably due to an intermolecular hydrogen bond.
The presence of a spacer between aromatic and amide part of the molecules 8k-10u always seems unfavourable except for 8-10n whose structure is related to one of the previously described FAUC346 or A-706149.
In summary, we consider that solid phase parallel chemistry using SynPhase™ Lanterns was beneficial to our objective especially in terms of ease and purity of the final compounds. The obtaining of diverse compounds with nanomolar Ki values at human dopamine D3 receptors will allow selectivity and pharmacokinetic studies to find the best potential candidates for further development.
Acknowledgements
We gratefully acknowledge Philippe Jéhan and Marcel Morvan for technical assistance.
References
- P Sokoloff, B Giros, MP Martres, ML Bouthenet, and JC Schwartz. (1990). Nature 347:146–151.
- F Boeckler, and P Gmeiner. (2006). Pharmacol Therapeut 112:281–333.
- B Levant. (1997). Pharmacol Rev 49:231–252.
- SB Caine, and GF Koob. (1993). Science 260:1814–1816.
- M Pilla, S Perachon, F Sautel, F Garrido, A Mann, CG Wermuth, JC Schwartz, BJ Everitt, and P Sokoloff. (1999). Nature 400:371–375.
- RR Luedtke, and RH Mach. (2003). Curr Pharm Design 9:643–671.
- J Yuan, X Chen, R Brodbeck, R Primus, J Braun, JWF Wasley, A Thurkauf, and (a). (1998). Bioorg Med Chem Lett 8:2715–2718. (b) Robarge MJ, Husbands SM, Kieltyka A, Brodbeck R, Thurkauf A, Newman AH. J Med Chem 2001;44:3175-3186 (erratum 4056)
- PJ Murray, LA Harrison, MR Johnson, GM Robertson, DIC Scopes, DR Buli, SEA Graham, AG Hayes, GJ Kilpatrick, I Den Daas, C Large, MJ Sheehan, CM Stubbs, and MP Turpin. (1995). Bioorg Med Chem Lett 5:219–222.
- L Bettinetti, K Schlotter, H Hübner, and P Gmeiner. (2002). J Med Chem 45:4594–4597.
- M Leopoldo, F Berardi, NA Colabufo, P De Giorgio, E Lacivita, R Perrone, and V Tortorella. (2002). J Med Chem 45:5727–5735.
- H Geneste, G Backfisch, W Braje, J Delzer, A Haupt, CW Hutchins, LL King, A Kling, HJ Teschendorf, L Unger, W Wernet, and (a). (2006). Bioorg Med Chem Lett 16:490–494. (b) Geneste H, Amberg W, Backfisch G, Beyerbach A, Braje W, Delzer J, Haupt A, Hutchins CW, King LL, Sauer DR, Unger L, Wernet W. Bioorg Med Chem Lett 2006;16:1934-1937
- H Geneste, G Backfisch, W Braje, J Delzer, A Haupt, CW Hutchins, LL King, W Lubisch, G Steiner, HJ Teschendorf, L Unger, and W Wernet. (2006). Bioorg Med Chem Lett 16:658–662.
- P Heidler, V Zohrabi-Kalantari, T Calmels, M Capet, JC Schwartz, H Stark, and A Link. (2005). Bioorg Med Chem 13:2009–2014.
- JG Parsons, CS Sheehan, Z Wu, IW James, AM Bray, and For a recent review about SynPhase™ Lanterns, see:. (2003). Methods Enzymol 369:39–74.
- J Renault, M Lebranchu, A Lecat, and P Uriac. (2001). Tetrahedron Lett 42:6655–6658.
- J Yuan, X Chen, R Brodbeck, R Primus, J Braun, JWF Wasley, and A Thurkauf. (1998). Bioorg Med Chem Lett 8:2715–2718.
- M Capet, D Danvy, N Levoin, M Morvan, I Berrebi-Bertrand, T Calmels, P Robert, JC Schwartz, and JM Lecompte. (2006). US Pat Appl Publ US2006089364