Abstract
Highly reactive radicals are implicated in many pathological conditions. The quest for radical scavengers or antioxidants, spans the previous decades. A new series of complexes of the type [Cu (dien) (2a-2tzn) Y2] and [Cu (dienXXY2) (2a-5mt)] and of the type [Cu (dptaS) Cl2] and [Cu (dptaS) Br2] (dptaS = 1, 3-propanediamine) or Schiff mono-base of dipropylenetriamine with 2-thiophene-carboxaldehyde, has been tested for anti-inflammatory and antioxidant activity. The tested compounds inhibit the carrageenin-induced rat paw edema (52.0–82.6%) and present important scavenging activity. Compound 6 is the most potent (82.6%) in the in vivo experiment. Lipophilicity-as RM values – has been determined. The results support that in general, adducts of the type [Cu (dienXXY2) (2a-5mt)] exhibit increased activity compared to the starting material of type [Cu (dienXXY2)]. An attempt to correlate the biological results with their structural characteristics and physicochemical parameters has been made
Introduction
In recent years, considerable interest has been shown in the chemistry of transition metal complexes of Schiff bases [Citation1–3]. Specifically the linear or cyclic Schiff bases have attracted much attention since most of their compounds prepared to date, exhibit noteworthy bioactivity, which is due to the participation of the specific metal atom and ligands. Their use as analgesic, anti-inflammatory, antibiotic, antimicrobial [Citation4] and especially as anticancer [Citation5] agents is well known. Recently, a new series of Schiff mono/ dibase coordination compounds with 2-amino-5-methyl-thiazole (2a-5mt), with promising anticancer and antibacterial activities, has been reported [Citation6,7].
Highly reactive hydroxyl radicals are implicated in many pathological conditions, including cancer, diabetes, Alzheimer's and Parkinson's diseases, heart diseases, and aging [Citation8]. Harmful hydroxyl radicals attack any component in living organisms, including proteins, DNA, and lipids. These deleterious species are generated from the less damaging reactive oxygen species—superoxide radical anion and hydrogen peroxide—in a Fenton reaction. The quest for Fenton inhibitors, either radical scavengers or metal-ion chelators, spans the last three decades. Nowadays, antioxidants that exhibit 1, 1-diphenyl-picrylhydrazyl (DPPH) radical scavenging activity are increasingly receiving attention. They have been reported to have interesting anticancer, anti-aging and anti-inflammatory activities. Consequently, compounds with antioxidant properties could be expected to offer protection against rheumatoid arthritis and inflammation and to lead to potentially effective drugs. In fact, many non- steroidal anti-inflammatory drugs have been reported to act either as inhibitors of free radical production or as radical scavengers.
Copper complexes of several ligands have been prepared and evaluated for anti-inflammatory [Citation9–18] activity. Other compounds known for their anti-inflammatory properties are the S, N-heterocyclic ligands, e.g. thiazoline and its derivatives [Citation12–13]. The bioactivity of S, N- thiazoles is largely due to their structural similarities with the imidazolyl entities of proteins [Citation19], as well as biological features [Citation20–21] Their participation in the formation of compounds can modify [Citation20–29] the bioactive and pharmaceutical characteristics of the adducts. Among the thiazoles employed to date, the 2-amino-5-methylthiazole is most appropriate for the enhancement of the bioactivity of already synthesized compounds. This unique characteristic of the 2a-5mt molecule could be attributed to its electronic distribution resulting from the positive inductive ( + I) effect of the lipophilic methyl, the positive resonance ( + R) effect of the hydrophilic amino group and the negative resonance ( − R) effect of the thiazole ring. Furthermore, the position of the methyl group on the fifth carbon atom is essential due to steric or other reasons, since the 2a-4mt molecule where the methyl group is located on the fourth C atom, does not exhibit any activity.
Continuing our work [Citation6,7] on the biological activity of Schiff mono/dibase compounds with S,N-heterocyclic adducts we report here the anti-inflammatory and antioxidant activities of a new series of Schiff mono/ dibase coordination compounds with 2-amino-5-methyl-thiazole (2a-5mt) [Citation6]. (). Non-Steroidal anti-inflammatory Drugs (NSAIDs) have a broad spectrum and it has been suggested that the variations in both efficacy and their tolerability are partly due to differences in their physicochemical properties, which determine their distribution in the body and their ability to penetrate and enter the interior of membranes [Citation30,31]. Thus, partition coefficients such as RM values are performed [Citation32,33]
Figure 1. Structures of the tested complexes [Citation6,7,34]
![Figure 1. Structures of the tested complexes [Citation6,7,34]](/cms/asset/b5bad3a8-ad5b-423f-813d-c5ba208cb218/ienz_a_284259_f0001_b.gif)
Materials and methods
Materials
All the chemicals used were of analytical grade and commercially available by Merck. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), nordihydroguairetic acid (NDGA) are purchased from the Aldrich Chemical Co. Milwaukee, WI, (USA). Soybean Lipoxygenase, linoleic acid sodium salt, NADH, Nitrotetrazolium Blue (NBT) and indomethacin were obtained from Sigma Chemical, Co. (St. Louis, MO, USA) and carrageenin, type K, was commercially available. For the in vivo experiments, male and female Fischer-344 rats (180–240 g) were used. N-Methylphenazonium-methyl sulfate (PMS) was purchased by Fluka.
Synthesis, analytical data and structural information of the tested coordination compounds are given in references [Citation6,7,34]
Physical measurements
Determination of lipophilicity as RM values: Reversed phase TLC (RPTLC) was performed on silica gel plates impregnated with 55% (v/v) liquid paraffin in light petroleum ether. For the RM determination glass plates 20 × 20 cm Kieselgel 60F254 (Merck 5715) were used.
The mobile phase was a methanol/water mixture (75/25, v/v) containing 4% aqueous ammonia (27%) [Citation34]. The plates were developed in closed chromatography tanks saturated with the mobile phase at 24°C. Spots were detected under UV light or by iodine vapours. RM values were determined from the corresponding Rf values (from 10 individual measurements) using the equation RM = log [(1/Rf) − 1]. ()
Table I. Lipophilicity values: Experimentally determined RM values of the Cu (II) complexes.
Biological assays
In vitro assays
In the in vitro assays each experiment was performed at least in triplicate and the standard deviation of absorbance was less than 10% of the mean. For the in vitro tests a Lambda 20 (Perkin–Elmer) UV–Vis double beam spectrophotometer was used.
Determination of the reducing activity of the stable radical 1,1-diphenyl-picrylhydrazyl (DPPH)[Citation32,Citation33]
Various concentrations (0.1–0.2 mM) of the test compound in DMSO were added to an ethanolic solution of DPPH radical (0.1 mM). The mixture was shaken vigorously and allowed to stand for 20 min or 60 min; absorbance at 517 nm was determined spectrophotometrically and the percentage of activity was calculated. All tests were undertaken on three replicates and the results were averaged. ()
Table II. Antioxidant and antiinflammatory activities of Cu(II) complexes.
Competition of the tested compounds with DMSO for hydroxyl radicals [Citation32,33]
The hydroxyl radicals generated by the Fe3+/ascorbic acid system, were detected by the determination of formaldehyde produced from the oxidation of DMSO. The reaction mixture contained EDTA (0.1 mM), Fe3 + (167 lM), DMSO (33 mM) in phosphate buffer (50 mM, pH 7.4), the tested compounds (concentration 0.1 mM) and ascorbic acid (10 mM). After 30 min of incubation (37°C) the reaction was stopped with CCl3COOH (17% w/v). ()
Soybean lipoxygenase inhibition study in vitro [Citation32,33]
The tested compounds dissolved in DMSO were incubated at room temperature with sodium linoleate (0.1 ml) and 0.2 ml of enzyme solution (1/9x10− 4 w/v in saline). The conversion of sodium linoleate to 13-hydroperoxylinoleic acid at 234 nm was recorded and compared with the appropriate standard inhibitor. ()
Non-enzymatic assay of superoxide radicals measurement of superoxide radical scavenging activity [Citation32,33]
The superoxide producing system was set-up by mixing phenazine methylsulfate (PMS), NADH and air – oxygen. The production of superoxide was estimated by the nitroblue tetrazolium method. The reaction mixture containing compounds, 3μM PMS, 78μM NADH, and 25μM NBT in 19μM phosphate buffer pH 7.4 was incubated for 2 min at room temperature and the absorption measured at 560 nm against a blank containing PMS. The tested compounds were preincubated for 2 min before adding NADH. ()
In vivo assays
Inhibition of the carrageenin-induced edema [Citation32,33]
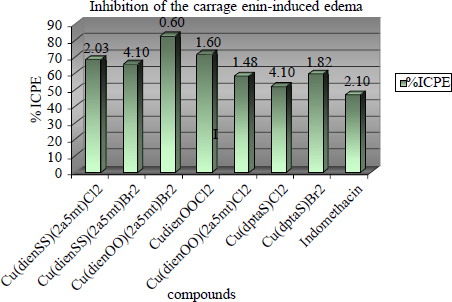
Edema was induced in the right hind paw of Fisher 344 rats (150–200 g) by the intradermal injection of 0.1 ml 2% carrageenin in water. Both sexes were used. Females pregnant were excluded. Each group was composed of 6–15 animals. The animals, which have been bred in our laboratory, were housed under standard conditions and received a diet of commercial food pellets and water ad libitum during the maintenance but they were entirely fasted during the experiment period. Our studies were in accordance with recognised guidelines on animal experimentation. The tested complexes 0.01 mmol/kg body weight, were diluted in water and they were given intraperitoneally simultaneously with the carrageenin injection. The rats were euthanized 3.5 h after carrageenin injection. The difference between the weight of the injected and uninjected paws was calculated for each animal. The change in paw weight was compared with that in control animals (treated with water) and expressed as a percent inhibition of the edema %ICPE values ( and ). Indomethacin was tested as a reference compound in 0.01 mmol//kg (47%).
%ICPE values are the mean from two different experiments with a standard error of the mean less than 10%.
Results and discussion
The reported compounds were tested for their antioxidant and anti-inflammatory activities.
The model of the scavenging of the stable DPPH radical is widely used to evaluate antioxidant activities in less time than other methods. DPPH is a stable free radical that can accept an electron or hydrogen radical and thus be converted into a stable, diamagnetic molecule. DPPH has an odd electron and so has a strong absorption band at 517 nm. When this electron becomes paired off, absorption decreases stoichiometrically with respect to the number of electrons taken up. Such a change in the absorbance produced in this reaction has been widely applied to test the capacity of numerous molecules to act as free radical scavengers. All compounds were tested for their interaction with the stable free radical DPPH. Butylated hydroxytoluene (BHT) and nordihydroguairetic acid (NDGA) were used as reference compounds. This interaction indicates their radical scavenging activity in an iron- free system and expresses their reducing activity. Compound 2, 4, 6, 7, 8 showed high interactions, whereas compounds 1, 3, 5, 9 and 10 were found to have very low activity. Compound 9 presents the highest activity at 0.1 mM. In general, no changes are observed among the interaction values, concerning time and concentration of the compounds (). Further investigations are in progress in order to attain a detailed study on their interaction with DPPH. The competition of complexes with dimethylsulfoxide (DMSO) for hydroxyl radicals, generated by the Fe3 + /ascorbic acid system expressed as the inhibition of formaldehyde production was used for the evaluation of their hydroxyl radical scavenging activity. Compounds 1, 2, 3, 5, 7–10 and 13 did not show any inhibition, whereas compounds 4, 6, 11 and 12 inhibited significantly high the DMSO oxidation in concentration 0.1 mM. ()
Non-enzymatic superoxide anion radicals were generated. The superoxide- producing system was set up by mixing phenazine methosulfate (PMS), nicotinamide-adenine-dinucleotide (NADH) and air–oxygen. The production of superoxide was estimated by the nitroblue tetrazolium method. Compound 1 did not show any result whereas compounds 2 and 7 present low activity. All the other compounds exhibit high scavenging activity (45.6–78.3% ). Taking under consideration the RM values, lipophilicity does not positively influence the scavenging activity. Considering the structures of these compounds and their reactivity against superoxide anion we can confirm that there is a relation between this activity and the electron -donor properties of the ligands. Several findings concerning the correlation of antioxidant activity and the structures of these complexes emerge from . a) For compounds 1–10 a factor that should be taken under consideration is the nature of the heterocyclic atom of the Schiff dibases, dien vs. dienSS, dienOO, since the activity of the substituted complexes differs depending on the presence of S or O, atom in the heterocyclic ring, thiophene, or furyl and (b) on the presence of 2a-5mt in the adducts. In general, the latter exhibit higher scavenging activity compared to the starting materials with some exceptions. (c) The observed differences in activity seem to depend partly on the nature of the counter anion, Cl or Br or NO3 since these can be regarded preferentially as leaving groups. The latter seems to be important for the monobases 11–13.
Compounds 1–13 were further evaluated for the inhibition of soybean lipoxygenase (LO) by the UV absorbance- based enzyme assay [Citation33] (). Perusal of % inhibition values or IC50 values shows that compound 2 is the most active within the set, followed by compounds 6, 10 and 13.
Most of the LO inhibitors are antioxidants or free radical scavengers, since lipoxygenation occurs via a carbon-centered radical. Although lipophilicity is referred to [Citation35] as an important physicochemical property for LO inhibitors, all the above tested derivatives do not follow this concept with the exception of complex 2. Taking under consideration the RM values, as an expression of lipophilicity it seems that compound 2 with RM = − 0.332 is more potent compared to the rest molecules (). It seems that LO inhibition (log 1/IC50) in vitro is correlated with the superoxide anion scavenging activity log % PMS.
To assess the anti-inflammatory activity of the complexes, the rat carrageenin induced paw edema assay was employed as a model for acute inflammation. Indomethacin was included as a reference drug. The development of the edema induced by carrageenin has been described as a biphasic event. The first phase of the inflammatory response is mediated by histamine and serotonin. The second phase is mediated by kinins and presumably by prostaglandins. Since edemas of this type are highly sensitive to NSAIDs, carrageenin has been accepted as a useful agent for studying new NSAIDs. This model reliably predicts the anti-inflammatory efficacy of the NSAIDs during the second phase. It detects compounds that are anti-inflammatory agents, as a result of inhibition of prostaglandin amplification. Edema was induced in the right hind paw of Fisher 344 rats (150–200 g) by the intradermal injection of 0.1 ml 2% carrageenin in water. Complexes 2, 4, 6, 7, 8, 11 and 12 presenting the higher DPPH interaction values were tested (0.01 mmol/ml/kg body weight). They were dissolved in water and were given intraperitoneally simultaneously. Compounds 2, 4, 6, 7, and 12 were found to possess significant protection. The protection ranges from 52.0 to 82.6%. Compound 11 has the lowest effect (52.0%). Lipophilicity, as RM values, does not seem to affect the biological responses. From our results an attempt has been made to delineate the role of specific structural characteristics correlated with the anti-inflammatory activity. The main differences in terms of structural changes for compounds 1–10 are: a) the nature of the heterocyclic aldehyde, b) the nature of the anionic group Y2, c) the presence of a dien or dien's Schiff base adducts in the structure. The complexes derived from 2-furaldehyde (compounds 6, 7) seem to be more potent. The nature of Y = Cl / Br does not influence the in vivo results (compound 2 = 68.5%, compound 4 = 65.6%). However the presence of Y = Cl / Br in the case of the furyl complexes causes a significant change in carrageenin paw edema inhibition (compound 6 = 82.6%, compound 8 = 58.2%). The presence of Y = Cl is responsible for this decrease. Perusal of shows that adduct Cu (dienOO) 2a5mtBr2 is more potent than the corresponding adduct of the type Cu (dienSS) 2a5mtBr2. Not many changes are observed among the %ICPE protection values of the monobases Schiff complexes formed with the presence of 2-thiophene-carboxaldehyde (compounds 11, 12).
Attempts to correlate these expressions of activity with RM values in a linear or non-linear regression analysis, gave statistically non-significant correlations. Unfortunately the number of compounds is not enough to calculate a combination of all the effects. The in vitro/in vivo activities have not succeeded in providing a clear correlation among all the physicochemical parameters in a QSAR analysis.
Conclusion
Both antibacterial and antiproliferative studies [Citation6] as well as the anti-inflammatory and antioxidant results showed that adducts exhibit high bioactivity. This means that the insertion of 2a-5mt affects the aggregation of electronic, physicochemical and steric properties of the resulting compounds thus moderating their biological activity. The Cu (II) Schiff mono-bases coordination compounds also exhibit anti-inflammatory activities.
It is of interest that all the tested in vivo compounds possess higher protection compared to the reference drug indomethacin in equipotent dose. Taking also under consideration the in vivo results, compound 7 as a starting material emerges as new potential prototype whereas compound 6 is a very potent anti-inflammatory agent representing a new lead. The antiradical activity of the tested complexes supports, at least in part, the in vivo anti-inflammatory activity
To study the physiological relevance of our findings, we are currently investigating the most potent inhibitors identified here, in various biological systems. These results will be published in due course.
Acknowledgements
We would like to thank Dr C. Hansch and Biobyte Corp. 201 West 4th Str., Suite 204, Claremont CA California, 91711, USA for free access to the C-QSAR program and Associate Professor C. Bolos for the inspiration.
References
- K Nakajima, Y Ando, H Mano, and M Kojima. (1998). Photosubstitution reactivity, crystal structures, and electrochemistry of ruthenium(II) (III) complexes containing tetradentate (O2N2, S2N2, and P2N2) Schiff base ligands. Inorg Chim Acta 274 (2):184–191.
- A Golcu, M Tumer, H Demirelli, and RA Wheatley. (2005). Cd(II) and Cu(II) complexes of polydentate Schiff base ligands: Synthesis, characterization, properties and biological activity. Inorg Chim Acta 358 (6):1785–1797.
- ATh Chaviara, PC Christidis, A Papageorgiou, E Chrysogelou, DJ Hadjipavlou-Litina, and CA Bolos. (2005). In vivo anticancer, anti-inflammatory, and toxicity studies of mixed-ligand Cu(II) complexes of dien and its Schiff dibases with heterocyclic aldehydes and 2-amino-2-thiazoline. Crystal structure of [Cu(dien)(Br)(2a-2tzn)](Br)(H2O). J Inorg Biochem 99 (11):2102–2109.
- ME Hossain, MN Alam, J Begum, AM Akbar, M Nazimuddin, FE Smith, and RC Hynes. (1996). The preparation, characterization, crystal structure and biological activities of some copper(II) complexes of the 2-benzoylpyridine Schiff bases of S-methyl- and S-benzyldithiocarbazate. Inorg Chim Acta 249 (2):207–213.
- EM Hodnett, and PD Mooney. (1970). Antitumor activities of some Schiff bases. J Med Chem 13 (4): 786–786
- ATh Chaviara, PJ Cox, KH Repana, RM Papi, KT Papazisis, D Zambouli, AH Kortsaris, DA Kyriakidis, and CA Bolos. (2004). Copper(II) Schiff base coordination compounds of dien with heterocyclic aldehydes and 2-amino-5-methyl-thiazole: Synthesis, characterization, antiproliferative and antibacterial studies. Crystal structure of CudienOOCl2. J Inorg Biochem 98 (8):1271–1283.
- ATh Chaviara, PJ Cox, KH Repana, AA Pantazaki, KT Papazisis, AH Kortsaris, DA Kyriakidis, GSt Nikolov, and CA Bolos. (2005). The unexpected formation of biologically active Cu(II) Schiff mono-base complexes with 2-thiophene-carboxaldehyde and dipropylenetriamine: Crystal and molecular structure of CudptaSCl2. J Inorg Biochem 99 (2):467–476.
- DB Marks, AD Marks, CM Smith. Basic medical biochemistry: A clinical approach. Philadelphia: Lippincott Williams & Wilkins; (1996).
- CT Dillon, TW Hambley, BJ Kennedy, PA Lay, and JE Weder. (2004). Copper and zinc complexes as antiinflammatory drugs. Met Ions Biol Syst 41:253–277.
- RK Parashar, RC Sharma, and G Mohan. (1989). Biological activity of some Schiff bases and their metal complexes. Biol Trace Elem Res 23:145–150.
- S Okuyama, S Hashimoto, H Aihara, WM Willingham, and JR Sorenson. (1987). Copper complexes of non-steroidal antiinflammatory agents: Analgesic activity and possible opioid receptor activation. Agents Actions 21:130–144.
- DH Brown, WE Smith, JW Teape, and AJ Lewis. (1980). Antiinflammatory effects of some copper complexes. J Med Chem 23 (7):729–734.
- MH Shih, and FY Ke. (2004). Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg Med Chem 12 (17):4633–4643.
- K Singh, MS Barwa, and P Tyagi. (2005). Synthesis, characterization and biological studies of Co(II), Ni(II), Cu(II) and Zn(II) complexes with bidentate Schiff bases derived by heterocyclic ketone. Eur J Med Chem 41 (1):147–153.
- M Pellei, GG Lobbia, C Santini, R Spagna, M Camalli, D Fedeli, and G Falcioni. (2004). Synthesis, characterization and antioxidant activity of new copper(I) complexes of scorpionate and water soluble phosphane ligands. J Chem Soc Dalton Trans 17:2822–2828.
- J Vanco, O Svajlenova, E Ramanska, J Muselik, and JJ Valentova. (2004). Antiradical activity of different copper(II) Schiff base complexes and their effect on alloxan-induced diabetes. J Trace Elem Med Biol 18 (2):155–161.
- C Santini, M Pellei, GG Lobbia, D Fedeli, and G Falcioni. (2003). Synthesis and characterization of new copper(I) complexes containing 4-(diphenylphosphane)benzoic acid and “scorpionate” ligands with “in vitro” superoxide scavenging activity. J Inorg Biochem 94 (4):348–354.
- M Gonsalez-Alvarez, G Alzuet, J Borras, LC Agudo, JM Montejo-Bernardo, and S Garcia-Granda. (2003). J Biol Inorg Chem 8:112–120.
- G Kornis. InAR Katritzky. editor>. 1, 3, 4-Thiadiazoles in Comprehensive Heterocyclic Chemistry., vol. 6 New York: Part 4B Pergamon Press; (1984). p545–578.
- P Comba. (1993). The relation between ligand structures, coordination stereochemistry, and electronic and thermodynamic properties. Coord Chem Rev 123 (1-2):1–48.
- TL Brown, and KJ Lee. (1993). Ligand steric properties. Coord Chem Rev 128 (1-2):89–116.
- CW Holzapfel, and GR Pettit. (1985). Antineoplastic agents. Par 108. Structural biochemistry. Part 23. Synthesis of the dolastatin thiazole amino acid component (gln)Thz. J Org Chem 50 (13):2323–2327.
- M Nakamura, T Shibata, K Nakane, T Nemoto, M Ojika, and K Yamada. (1995). Stereochemistry and total synthesis of dolastatin E. Tetrahedron Lett 36 (28):5059–5062.
- C Boden, and G Pattenden. (1994). Total synthesis of lissoclinamide 5, a cytotoxic cyclic peptide from the tunicate Lissoclinum patella. Tetrahedron Lett 35 (44):8271–8274.
- A Butler, and JV Walker. (1993). Marine Haloperoxidases. Chem Rev 93:1937–1944.
- JRJ Sorenson. Inflammatory Diseases and Copper.,vol. 2 Clifton: Humana Press; (1982).
- R Nagar, and G Mohan. (1991). Synthetic and pharmacological studies on some transition metal chelates involving N-pyrimidino benzamide-2-carboxylic acid as ligand. J Inorg Biochem 42:9–16.
- S Oga, SF Taniguchi, R Najjar, and AR Souza. (1991). Synthesis, characterization, and biological screening of a copper flurbiprofen complex with anti-inflammatory effects. J Inorg Biochem 41 (1):45–51.
- ZH Chohan, H Pervez, A Rauf, A Scozzafava, and CT Supuran. (2002). Antibacterial Co(II), Cu(II), Ni(II) and Zn(II) complexes of thiadiazole derived furanyl, thiophenyl and pyrrolyl Schiff bases. J Enz Inhib Med Chem 17 (2):117–122.
- RO Day, GG Graham, KM Williams, and PM Brooks. (1988). Variability in response to NSAIDs. Fact or fiction?. Drugs 36 (6):643–651.
- GA Ellis, and DR Blake. (1993). Why are non-steroidal anti-inflammatory drugs so variable in their efficacy? A description of ion trapping. Ann Rheum Dis 52 (3):241–243.
- E Pontiki, D Hadjipavlou-Litina, ATh Chaviara, and CA Bolos. (2006). Evaluation of anti-inflammatory and antioxidant activities of mixed-ligand Cu(II) complexes of dien and its Schiff dibases with heterocyclic aldehydes and 2-amino-2-thiazoline. Bioorg Med Chem Lett 16 (8):2234–2237.
- D Panagoulis, E Pontiki, E Skeva, C Raptopoulou, S Girousi, D Hadjipavlou-Litina, and C Dendrinou-Samara. (2007). Synthesis and pharmacochemical study of new Cu(II) complexes with thiophen-2-yl saturated and α,β-unsaturated substituted carboxylic acids. J Inorg Biochem 101 (4):623–634.
- Chaviara ATh. Thesis, Aristotles University,2006, Thessaloniki.
- Pontiki E, Hadjipavlou-Litina D. Lipoxygenase inhibitors: A comparative QSAR study review and evaluation of new QSARs, Med Res Rev, Epub ahead of print – 26 December 2006 in press.