Abstract
The antibacterial, antifungal, acute cytotoxicity, phytotoxicity and insecticidal profile of the crude extract and various fractions of Indigofera gerardiana have been studied. Six bacterial and fungal strains were used, of which Samonella typhi and Microsporum canis were the most susceptible strains with MICs 0.37 mg/mL and 0.09 mg/mL, respectively. The crude extract and the fractions showed low insecticidal activity against Sitophilus oryzae, Rhyzopertha dominica and Callosbruchus analis but no activity against Tribolium castaneum. The brine shrimp lethality assay showed absence of any measurable cytotoxicity of the crude extract and fractions, showing a good safety profile at a preliminary level. All the fractions except crude extract revealed profound and highly significant herbicidal activity against Lemna minor at the concentration of 1000 μg/mL. Indigofera gerardiana was shown by in-vitro assays to be a potential source for natural antifungal, antibacterial and herbicidal agents.
Introduction
The genius indigofera, comprises of 700 species. All of these herbs or shrubs, distributed throughout the tropical regions of the globe. In Pakistan, it is represented 24 spices (Nisar and Ali, 1997). The plant Indigofera gerardiana commonly known as Ghorega belongs to leguminoseae (Fabeaceae) [Citation1]. It is widely distributed in northern pars of Pakistan and finds various medicinal uses in the indigenous system of medicine. in northern areas of Pakistan, this plant is traditionally used for relieving abdominal and spastic pains and the infectious diseases especially the skin infections involving microorganisms. In literature considerable work has been done on various species of the genus Indigofera. Indigofera daleoides Benth is used traditionally used for the treatment of diarrhea, was found to be active against various pathogenic bacterial strains [Citation3]. Similarly Indigofera oblongifolia has showed its antimicrobial [Citation4], hepatoprotective [Citation5] and lipoxygenase inihibitory activity [Citation6]. Abubakar et al. has reported the snake-venom neutralizing activity of Indigofera pulchra [Citation7]. Antioxidant and free radical scavenging and anti-dyslipidemic activities of Indigofera tinctoria has been reported [Citation8,Citation9]. Indigofera emarginella. has shown in-vitro atimalarial activity against Plasmodium falciparum. Chakrabarti et al. have reported the antidiabetic activity of Indigofera mysorens [Citation11].
This study was aimed at investigating the antibacterial and antifungal activities of Indigofera gerardiana to explore the scientific basis for its folk use in infectious diseases caused by the microbial pathogens. Brine shrimp lethality assay was also performed to assess its safety. Additionally, we have attempted to explore the insight of Indigofera gerardiana for its insecticidal and phytotoxicity potential to be developed as natural insecticides and herbicides.
Experimental
Plant material
Indegofera gerardiana was collected as a whole plant from upper Dir, NWFP (Pakistan) during the month of March–April 2005. The plant was identified by Prof Dr Jahandar Shah, plant taxonomist and Vice Chancellor, University of Malakand, Chakdara.
Extraction
The plant sample with an appropriate quantity obtained after the preliminary necessary preparations such as drying under shadow for three weeks then chopped into small pieces and pulverized into a fine powder. The powdered plant material (20 kg) was soaked in distilled methanol with occasional stirring at room temperature. After 15 days, the methanol soluble materials were filtered off. The process was repeated 3 times and the filtrate was concentrated in vacuo at 40°C to afford dark brown. The crude methanolic extract (40 g) was suspended in distilled water and successively extracted with hexane (11% w/w of crude extract), chloroform (31.9% w/w of crude extract), ethylacetate (21.6% w/w of crude extract), n-butanol (17.2% w/w of crude extract), and finally the aqueous (18.2% w/w of crude extract) fraction was obtained. Each organic extract was then evaporated to dryness. Stock extracts solutions were prepared at 200 mg/mL in distilled water and stored at ambient temperature for further use.
Fungal and bacterial strains
Tests were performed on six fungi and six bacteria reference strains. Bacterial strains were Escherchia coli ATCC 25922, Bacillus subtilis ATCC 6633, Shigella flexeneri (clinical isolate), Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853 and Salmonella typhi ATCC 19430. Fungal strains include Trichophyton longifusus (clinical isolate), Candida albicans ATCC 2091, Aspergillus flavus ATCC 32611, Microspoum canis ATCC 11622, Fusarium solani 11712 and Candida glaberata ATCC 90030. They were maintained on agar slant at 4°C. The strains were activated at 37°C for 24 h on nutrient agar (NA) or Sabouraud glucose agar (SGA) respectively for bacteria and fungi, prior to any screening.
Hole diffusion method
The antimicrobial tests were carried out by the hole diffusion method as described by Berghe and Vlietinck (1991) [Citation12] using a cell suspension of about 1.5 × 10− 6 CFU/mL obtained following Mac farland turbidity standard No. 0.5. The concentration of the suspension was standardised by adjusting the optical density to 0.1 at 600 nm wavelength (SHIMADZU UV-vis spectrophotometer) [Citation13]. Holes of 6 mm diameter were then made on the MHA plate (8 mm thick) and filled with 150 μl of methanolic extract, fractions or standard drug(s) in DMSO. The inoculated plates were incubated at 37°C for 24 h. Antimicrobial activity was evaluated by measuring the diameter of the zone of growth inhibition around the hole. The assay was repeated three times and the mean diameter was recorded. Imepenem, miconazole and amphotericin B were used as standard antibiotics for comparison with extracts and fractions.
MIC determination by macrodilution method
Extracts (10 mg/mL) were dissolved in DMSO and serially diluted with sterile water in microplates in a laminar flow cabinet [Citation14]. The same volume of an actively growing culture of the test bacteria and fungi was added to the different wells and cultures were grown overnight in 100% relative humidity at 37°C. The next morning tetrazolium violet was added to all the wells. Growth was indicated by a violet color of the culture. The lowest concentration of the test solution that led to an inhibition of growth was taken as the MIC. The negative control DMSO had no influence on the growth at the highest concentration used. Impenem, amphotericin B and miconazole were used as controls for comparison.
Cytotoxicity assay
Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22 × 32 cm), filled with artificial seawater which was prepared with commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment that got darkened while the minor compartment was opened to ordinary light. Two days later, nauplii were collected by a pipette from the lighted side. A sample of the test compound was prepared by dissolving 20 mg of each compound in DMF (2 mL). Three variable portions of this stock solution i.e., 500, 50 and 5 mg/mL were transferred to 9 vials (three for each dilution were used for each test sample and LD50 is the mean of three values) and one vial containing DMF (2 mL) was kept as control. The solvent was allowed to evaporate overnight. After two days, when the shrimp larvae were ready, 1 mL of seawater and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with seawater to 5 mL per vial. After 24 h, the number of survivors was counted by following the standard procedure [Citation15,Citation16]. Data were analyzed by Finney computer program to determine the LD50 values [Citation17].
Insecticidal activity
The insecticidal activity of the test compounds was determined by direct contact application using filter paper [Citation18]. 3 mL of each extract/fraction (1 mg/mL) was applied to filter papers (90 mm diameter). After drying, each filter paper was placed in the separate petri dish along with 10 adults of each Tribolium castaneum, Sitophilus oryzae, Trogoderma granarium, Callosobruchus analis and Rhyzopartha dominica. Permethrin (235.71 μg/cm2) was used as a reference insecticide. All these insects were kept without food for 24 h after which mortality count was done.
Phytotoxicity assay
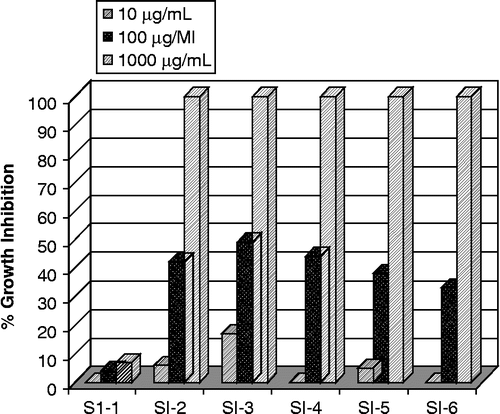
Phytotoxic activity of the crude extract was tested against the Lemna minor L. [Citation19]. Three flasks were inoculated with sufficient stock solution (20 mg/mL) to give a final concentration of 500, 50 and 5 μg/mL respectively. To each flask, 20 mL medium and 10 plants each containing a rosette of three fronds, was added. Paraquat was used as reference growth inhibitor. All flasks were incubated in the growth cabinet for seven days after which the growth regulation in percentage was calculated with reference to the negative control. IC50 was calculated with a Finney computer program.
Results and discussion
Antibacterial activity
Crude extract and the fractions showed zone of inhibition in mm against various bacterial strains (). Crude methanolic extract of Indigofera gerardiana. (SI-1), n-hexane fraction (SI-2), butanol fraction (SI-5) and the aqueous fraction (SI-6) showed good MICs and inhibited S. aureus, S. flexeneri, P. aeruginosa, E. coli and S. typhi ( and ). The fraction SI-2 remained the most active fraction (MIC = 0.37, 0.41 and 0.62 mg/mL for S. typhi, S. aureus and E. coli respectively).
Table I. Antibacterial activity of crude extract and the fractions of Indigofera gerardiana represented as zones of inhibition of bacterial growth (in mm). 0.2 mL of the test samples was 10 mg/mL, while for imipenum it was 10 μg/disc.
Table II. Antibacterial activity of crude extract and the fractions of Indigofera gerardiana represented as Minimum Inhibitory Concentration (MIC, μg/mL).
Antifungal activity
The Indegofera gerardiana crude extract (SI-1), n-hexane (SI-2), chloroform (SI-3) and aqueous fraction exhibited the most interesting inhibitory activities against T. longifusus, C. albicans, A. flavus, M. canis and F. solani. Crude extract and its fractions exhibited zone of inhibition (in mm) against these strains (). M. canis and F. solani were the highly susceptible strains. The species M. canis was inhibited remarkably with MIC ranging from 90 μg/mL to 290 μg/mL, followed by the F. solani with MIC ranging from 150 μg/mL to 810 μg/mL, C. albicans (MIC ranging from 170 μg/mL to 5000 μg/mL), A. flavus (MIC from 180 μg/mL to 5000 μg/mL) and T. longifusus (MIC in the range from 190 μg/mL to >10000 μg/mL). However, all the fractions as well as crude extract showed negligible activity against Candida glaberata ().
Table III. Antifungal activity of crude extract and the fractions of Indigofera gerardiana represented as % inhibition of fungal growth. 0.2 mL of the test samples was 20 mg/mL, while for miconazole and amphotericin B it was 10 μg/disc and 5 μg/disc, respectively.
Table IV. Antifungal activity of crude extract and the fractions of Indigofera gerardiana represented as Minimum Inhibitory Concentration (MIC, μg/mL).
Insecticidal activity
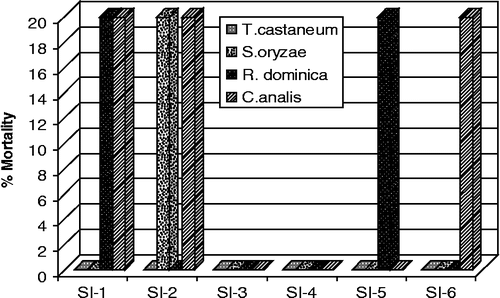
Four insects (T. castaneum, S. oryzae, C. analis and R. dominica) were used in the experiment () for contact-mortality model of insecticidal bioassay. Only three insects S. oryzae, R. dominica and C. analis (pulse beetle) were found weakly susceptible to the tested extract and fractions. Concentration used in the experiment for each extract/fractions was 1 mg/mL. Insect pests cause damage to stored grain and processed products by reducing dry weight and nutritional value [Citation20]. Around the world, residual chemical insecticides and fumigation are currently the methods of choice for the control of stored-product insects [Citation21]. The results reveals that crude extract, the subsequent organic (only n-hexane, n-butanol) and aqueous fractions possess low insecticidal activities.
Figure 1. Insecticidal activity of the crude extract and fractions of Indigofera gerardiana at the concentration of 1 mg/mL. SI-1 = Crude extract; SI-2 = n-hexane fraction; SI-3 = Chloroform fraction; SI-4 = Ethylacetate fraction; SI-5 = Butanol fraction and SI-6 = Aqueous fraction. T. castaneum = Tribolium castaneum; S. oryzae = Sitophilus oryzae; R. dominica = Rhyzopartha dominica; C. analis = Callosbruchus analis.
Cytotoxicity assay
Acute cytotoxicity of the crude extract and its fractions was found to be absent at the tested concentrations (10, 100 and 1000 μg/mL) in the brine shrimp lethality assay. This study is useful in evaluating the toxicity of the two metal complexes of diclofenac, which declared them safe for use as antifungal and antibacterial agents.
Phytotoxicity assay
The results of phytotoxicity assay of the test compounds are shown in . All the fractions except crude extract have exhibited highly significant phytotoxicity in the highest tested concentration (1000 μg/mL) with 100% inhibition of growth of L. minor. They also exhibited moderate inhibitory activities at concentration of 100 μg/mL but showed low herbicidal activities at 10 μg/mL concentration. There is a need to discover new herbicides since the number of herbicide-resistant weeds is increasing and conventional synthetic herbicides are becoming less and less effective against the resistant weed biotypes [Citation22] in addition to environmental and health related concerns. Therefore, new herbicides from natural sources are currently receiving more attention which could be appropriate and non-hazardous alternatives to the currently used synthetic agrochemicals as the natural products, generally, are effective, biodegradable and thus posing less threat to the environment. The results obtained from the current study indicated that Indigofera gerardiana might be useful as natural herbicides and could be a source of bioactive agrochemicals.
Conclusion
Increasing emergence of resistant bacterial and fungal strains requires timely and prompt measures in terms of developing new and effective antimicrobial agents Citation23–31. In this study we have made an effort to investigate new potential applications of Indigofera gerardiana. Antifungal and antibacterial activities of Indigofera gerardiana identified in the current study shows their potential for the treatment of bacterial and fungal infections in plants and animals caused by selected pathogenic strains. Insect pests are one of the main problem in storing food grains and other food materials as well as plants. Though insecticidal activities of the extract and fractions were found to be low against insects Sitophilus oryzae, Rhyzopertha dominica and Callosbruchus analis yet this study indicates that further screening and purification of the active compounds followed by structural modifications may lead to enhancement of their potency as well as their efficacy. However herbicidal activity was highly significant that requires the extract to be further screened and developed as a potential weedicide. The in-vitro cytotoxicity assay indicates their safety in terms of acute cytotoxicity though further studies should be conducted to assess their safety in animal models. In short, Indigofera gerardiana has a potential to be a source of valuable lead compounds against microbial infections as well as weeds.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- M Hamayun, A Khan, and MA Khan. (2003). Common medicinal folk recipes of DistrictBuner, NWFP, Pakistan [DB]. J Ethnobot Leaflets (http://www.siu.edu/∼/)
- E Nasir, and SI Ali. “Flora of West Pakistan”, Vol. 100, Rawalpindi: National Herbarium Agricultural Research Council; (1997). p 65–83.
- MC Mathabe, RV Nikolova, N Lall, and NZ Nyazema. (2006). Antibacterial activities of medicinal plants used for the treatment of diarrhoea in Limpopo Province, South Africa. J Ethnopharmacol 105:286–293.
- MU Dahot. (1999). Antibacterial and antifungal activity of small protein of Indigofera oblongifolia leaves. J Ethnopharmacol 64:277–282.
- M Shahjahan, G Vani, and CS Devi. (2005). Protective effect of Indigofera oblongifolia in CCl4-Induced Hepatotoxicity. J Med Food 8:261–265.
- A Sharif, E Ahmed, A Malik, N Riaz, N Afza, SA Nawaz, M Arshad, and MI Choudhary. (2005). Lipoxygenase inhibitory constituents from Indigofera oblongifolia. Arch Pharmacal Res 28:761–764.
- MS Abubakar, E Balogun, EM Abdurahman, AJ Nok, M Shok, and M Mohammed. (2006). Garba Ethnomedical treatment of poisonous snakebites: Plant extract neutralized Naja nigricollis Venom. Pharm Biol 44:343–348.
- D Prakash, S Suri, G Upadhyay, and BN Singh. (2007). Total phenol, antioxidant and free radical scavenging activities of some medicinal plants. Int J Food Sci Nutri 58:18–28.
- PJ Waako, E Katuura, P Smith, and P Folb. (2007). East African medicinal plants as a source of lead compounds for the development of new antimalarial drugs. Afri J Ecol 45:102–106.
- A Puri, T Khaliq, SM Rajendran, G Bhatia, R Chandra, and T Narender. (2007). Antidyslipidemic activity of Indigofera tinctoria. J Herb Pharmacother 7:59–64.
- R Chakrabarti, RKB Damarla, R Mullangi, VM Sharma, RK Vikramadithyan, and R Rajagopalan. (2006). Insulin sensitizing property of Indigofera mysorensis extract. J Ethnopharmacol 105:102–106.
- VA Berghe, and AJ Vlietinck. (1991). Screening methods for antibacterial and antiviral agents from higher plants. Method Plant Biochem 6:47–68.
- ML Tereschuck, MVQ Riera, GR Castro, and LR Abdala. (1997). Antimicrobial activity of flavonoid from leaves of Tagetes minuta. J Ethnopharmacol 56:227–232.
- P Pithayanukul, J Tubprasert, and M Wuthi-Udomlert. (2007). In vitro antimicrobial activity of Zingiber cassumunar (Plai) oil and a 5% Plai oil gel. Phytother Res 21:164–169.
- JL McLaughlin, C-J Chang, and DL Smith. In: Atta-ur-Rahman, editor. Studies in natural products chemistry, “BenchTop” bioassays for the discovery of bioactive natural products: An update, structure and chemistry (part-B)., Vol. 9. The Netherlands: Elsevier Science Publishers B.V.; (1991). p 383.
- BN Meyer, NR Ferrigni, JE Putnam, LB Jacobsen, DE Nichols, and JL McLaughlin. (1982). Brine shrimp: A convenient general bioassay for active plant constituents. Planta Medica 45:31–34.
- DJ Finney. Probit analysis. 3rd ed.. Cambridge: Cambridge University Press; (1971).
- YJ Ahn, GH Kim, KY Cho. Bioassay system for insecticidal compounds. In: Proceedings of the third symposium on the biochemical methodology for the research and development of the bioactive substances, held at Seoul, Republic of Korea, 1995. p 495–506.
- JL McLaughlin, CJ Chang, and DL Smith. “Bench-Top” bioassays for the discovery of bioactive natural products an update. In: Atta-ur- Rahman, editor. Studies in natural products chemistry. Amsterdam: Elsevier Science Publishers; (1991).
- MA Mullen. (1992). Development of a pheromone trap for monitoring Tribolium castaneum. J Stored Prod Res 28:245–249.
- RN Sinha, and FL Watters. Insect pests of flour mills, grain elevators, and feed mills and their control. Ottawa, ON, Canada: Agriculture Canada, Publication 1776; (1985).
- PC Bhowmik, and Inderjit. (2003). Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot 22:661–671.
- MDF Pereira, R Chevrot, E Rosenfeld, V Thiery, and T Besson. (2007). Synthesis and evaluation of the antimicrobial activity of novel quinazolinones. J Enz Inhib Med Chem 22:577–583.
- ZH Chohan, A Scozzafava, and CT Supuran. (2002). Unsymmetrical 1,1′-disubstituted Ferrocenes: Synthesis of Co(ii), Cu(ii), Ni(ii) and Zn(ii) Chelates of Ferrocenyl -1-thiadiazolo-1′-tetrazole, -1-thiadiazolo-1′-triazole and -1-tetrazolo-1′-triazole with Antimicrobial Properties. J Enz Inhib Med Chem 7:261–266.
- I Kerimov, G Ayhan-Kilcigil, B Can-Eke, N Altanlar, and M Iscan. (2007). Synthesis, antifungal and antioxidant screening of some novel benzimidazole derivatives. J Enz Inhib Med Chem 22:696–701.
- RN Yadava, and L Tiwari. (2007). New antifungal flavone glycoside from Butea monosperma O. Kuntze. J Enz Inhib Med Chem 22:497–500.
- A Mastrolorenzo, A Scozzafava, and C Supuran. (2000). Antifungal activity of Ag(I) and Zn(Ii) complexes of Aminobenzolamide (5-Sulfanilylamido-1,3,4-Thiadiazole-2-Sulfonamide) derivatives. J Enz Inhib Med Chem 15:517–531.
- A Özdemir, G Turan-Zitouni, ZA Kaplancikli, and P Chevallet. (2007). Synthesis of some 4-arylidenamino-4H-1,2,4-triazole-3-thiols and their antituberculosis activity. J Enz Inhib Med Chem 22:511–516.
- B Murukan, and K Mohanan. (2007). Synthesis, characterization and antibacterial properties of some trivalent metal complexes with [(2-hydroxy-1-naphthaldehyde)-3-isatin]- bishydrazone. J Enz Inhib Med Chem 22:65–70.
- M Nisar, I Khan, SU Simjee, AH Gilani, Obaidullah, and H Perveen. (2008). Anticonvulsant, analgesic and antipyretic activities of Taxus wallichiana Zucc. J Ethnopharmacol 116:490–494.
- M Nisar, I Khan, A Bashir, A Ihsan, W Ahmad, and MI Choudhary. (2008). Antifungal and antibacterial activities of Taxus wallichiana Zucc. J Enz Inhib Med Chem 23:256–60.