Abstract
New 1-[2-azido-2-(2,4-dichlorophenyl)ethyl]-1H/-imidazole were synthesized by nucleophilic substitution of various tertiary alcohols with azide anion in presence of boron trifluoride-diethyl etherate. Their antifungal activity was evaluated against Candida albicans, Candida glabrata, Aspergillus fumigatus and an azole-resistant petite mutant of C. glabrata. Preliminary SAR results are discussed.
Introduction
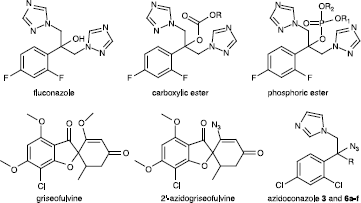
In these past two or three decades, fungal infections have increased in frequency among severaly immunocompromised hosts suffering from cancer or aids and in organ transplant cases. [Citation1,Citation2] On account of nephrotoxicity of amphotericin B [Citation3] and despite the recent discovery of echinocandins [Citation4], azoles which act by inhibiting cytochrome P450 14α-demethylase and present a low toxicity and a good distribution profile are usually preferred as first-line therapy [Citation5]. However, the extensive use of azole compounds has given rise to the emergence of azole-resistant isolates in pathogenic yeasts which are often associated to an increased activity of the efflux pump [Citation6]. Despite recent developements, there is still a need for a genuinely broad-spectrum and low toxicity antifungal azole agents capable to overcome this increased efflux [Citation7,Citation8] Considering this need, we recently reported the synthesis of new 2-aryl-1-azolyl-3-thienylbutan-2-ols [Citation9], isoxazoles and isoxazolines [Citation10] which kept a high activity against a Candida glabrata azole-resistant strain which overexpressed genes coding for the efflux pump proteins. [Citation6] These studies pointed out the utility of a five-membered heteroaromatic ring in the conazole side-chain for the escaping of the efflux pumps. Although modifications of the side-chain is very studied, some 2-position pharmacomodulations is less current. The most used conazole (fluconazole) was only modified by esterification between the 2-hydroxyl group and some carboxylic or phosphoric acids to form prodrugs with good solubility [Citation11,Citation12]. However, these prodrugs are less active than fluconazole and their hydrolysis capacity is weak. Therefore, it's very interesting to develop some new conazole with a pharmacomodulation in place of the 2-hydroxyl group. In this aim, we had worked on the subtitution of the 2-hydroxyl group by an azide anion. A such modification has ever encountered on griseofulvine with the substitution of the 2′-methoxy group while preserving the same antifungal activities against dermatophytes [Citation13]. Although the azide group is recognized for his great toxicity, the used of azidoconazoles could be justified for extremely infectious diseases caused by resistant strains. In this study, we have considered the antifungal potential of azide derivatives. So we would like to report herein the synthesis of new azide in the conazole series and an evaluation of their antifungal activity against an azole-resistant petite mutant of C. glabrata and its parent wild-type strain, but also on two other human pathogenic fungi, i.e. C. albicans and Aspergillus fumigatus.
Materials and methods
Chemistry
Instrumentation
Synthesis of 3 [Citation14], 5a [Citation15], 5c [Citation16], 5d [Citation17], 5e [Citation18] and 5f [Citation19] were performed as previously described. Alcohol 1 [Citation19] is commercially available from Acros Organics and ketone 4 [Citation20] from Ugarit Chimie. Si gel 60 (Macherey-Nagel, 230-400 mesh) was used for column chromatography and precoated Si gel plates (Macherey-Nagel, SIL G/UV254, 0.25 mm) were used for preparative TLC. Infrared (IR) spectra were determined on a BRUKER FT IR Vector 22 using KBr discs for solids or neat liquid films for liquids. NMR spectra were recorded in CDCl3 solution on a BRUKER AVANCE DRX 500 or a JEOL GSX 270 WB spectrometers.
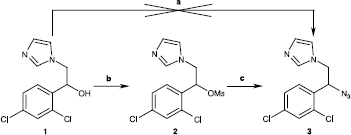
Methanesulfonic acid 1-(2,4-dichlorophenyl)-2-imidazol-1-yl-ethyl ester (2)
At 0°C, methanesulfonyl chloride (1.1 mmol) was added to a solution of alcohol 1 (1.0 mmol) and triethylamine (1.1 mmol) in dichloromethane. After 2 h, the reaction was warmed to room temperature and water (20 mL) was then added. The organic phase was extracted with dichloromethane (2 × 20 mL), dried over Na2SO4, filtered and evaporated under reduced pressure. The crude product was purified by flash chromatography on SiO2 with dichloromethane/ethanol (95/5) as eluent to give 2 (0,96 mmol; 96%). 1H-NMR (270 MHz; CDCl3): 2.71 (s, 3H, CH3), 4.24 (dd, 1H, J = 7.5 and 15.2 Hz, CH2a), 6.44 (dd, 1H, J = 3.0 and 15.2 Hz, CH2b), 6.07 (dd, 1H, J = 3.0 and 7.5 Hz, CH), 6.95 (s, 1Harom, H-2 imidazolyl), 7.09 (s, 1Harom, H-3 imidazolyl), 7.31 (m, 2Harom, H-5′ and H-6′), 7.43 (s, 1Harom, H-5 imidazolyl), 7.45 (m, 1Harom, H-3′); 13C-NMR (270 MHz; CDCl3): 38.1 (CH3), 50.4 (CH2), 77.8 (CH), 127.9 (CHarom), 128,1 (CHarom), 128.3 (CHarom), 129.3 (CHarom), 129.6 (CHarom), 129.8 (CHarom), 131.2 (Cq/arom), 131.8 (Cq/arom), 136.0 (Cq/arom); HRMS (MeOH/H2O, ESI+) m/z: 335,0033 (required: 335,0024).
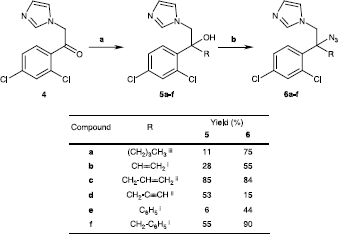
2-(2,4-Dichlorophenyl)-1H-imidazol-1-yl-but-3-en-2-ol (5b)
A solution of vinylmagnesium bromide (1 M in THF; 2.0 mmol) was dropped into a solution of ketone 4 (1.0 mmol) in freshly distilled THF (10 mL) and refluxed. The reaction was then quenched with water (20 mL) and THF was removed under reduced pressure. The organic phase was extracted with dichloromethane (2 × 20 mL), washed with a 10% aqueous solution of NaHCO3, dried over Na2SO4, filtered and concentrated under reduced pressure. The crude product was purified by flash chromatography on SiO2 with dichloromethane/ethanol (97/3) as eluent to give 5b (0,28 mmol; 28%). 1H-NMR (270 MHz; CDCl3): 4.37 (d, 1H, J = 14.1 Hz, CH2a), 4.70 (d, 1H, J = 14.1 Hz, CH2b), 5.26 (d, 1Hvinyl, J = 10.8 Hz, CH2a), 5.30 (d, 1Hvinyl, J = 17.3 Hz, CH2b), 6.46 (dd, 1Hvinyl, J = 10,8 and 17,3 Hz, CH), 6.74 (m, 2Harom, H-2 imidazolyl and H-3 imidazolyl), 7.13 (dd, 1Harom, J = 2.1 and 8.7 Hz, H-5′), 7.33 (s, 1Harom, H-5 imidazolyl), 7.35 (d, 1Harom, J = 2.1 Hz, H-3′), 7.63 (d, 1Harom, J = 8.7 Hz, H-6′); 13C-NMR (270 MHz; CDCl3): 53.9 (CH2), 76.1 (Cq), 117.1 (CH2/vinyl), 120.4 (CHarom), 127.2 (CHarom), 127.3 (CHarom), 129.9 (CHarom), 130.4 (CHarom), 131.1 (Cq/arom), 134.1 (Cq/arom), 137.9 (CHarom), 138.3 (Cq/arom), 138.5 (CHvinyl).
General procedure for compounds 6a-f
Boron trifluoride-diethyl etherate (1.97 mmol) was dropped at 0°C to a solution of sodium azide (1.54 mmol) and corresponding tertiary alcohol (0.16 mmol) in dried THF (10 mL). The mixture was stirred for 12 h, hydrolysed with water (1 mL) and concentrated. Then, dichloromethane (10 mL), water (10 mL) and a saturated aqueous solution of K2CO3 (2 mL) were added. The organic layer was extract with dichloromethane (2 × 10 mL), dried (Na2SO4) and evaporated under reduced pressure. The product was purified by flash chromatography on SiO2 with the adequat eluent.
1-(2-Azido-2-(2,4-dichlorophenyl)hexyl)-1H-imidazole (6a)
Eluent: dichloromethane/ethanol (95/5), 75%; IR νmax: 2100 (N3) cm− 1; 1H-NMR (270 MHz; CDCl3): 0.93 (t, 3H, J = 7.5 Hz, CH3), 1.33 (m, 2H, CH2), 1.66 (m, 2H, CH2), 2.74 (m, 2H, CH2), 5.17 (d, 1H, J = 15.8 Hz, CH2a), 5.32 (d, 1H, J = 15.8 Hz, CH2b), 6.36 (s, 1Harom, H-2 imidazolyl), 7.17 (dd, 1Harom, J = 1.9 and 8.5 Hz, H-5′), 7.29 (d, 1Harom, J = 1.9 Hz, H-3′), 7.51 (d, 1Harom, J = 8.5 Hz, H-6′), 8.15 (s, 1Harom, H-3 imidazolyl), 8.51 (s, 1Harom, H-5 imidazolyl); 13C-NMR (270 MHz; CDCl3): 13.8 (CH3), 22.3 (CH2), 31.5 (CH2), 35.9 (CH2), 55.2 (CH2), 74.5 (Cq), 121.3 (CHarom), 121.9 (CHarom), 127.7 (CHarom), 134.6 (Cq/arom), 135.4 (Cq/arom), 135.9 (Cq/arom), 136.7 (CHarom), 143.9 (CHarom), 160.6 (Cq/arom).
1-(2-Azido-2-(2,4-dichlorophenyl)but-3-enyl)-1H-imidazole (6b)
Eluent: dichloromethane/ethanol (96/4), 55%; IR νmax: 2099 (N3) cm− 1; 1H-NMR (270 MHz; CDCl3): 4.53 (d, 1H, J = 14.3 Hz, CH2a), 4.88 (d, 1H, J = 14.3 Hz, CH2b), 5.31 (d, 1Hvinyl, J = 17.3 Hz, CH2a′), 5.38 (d, 1Hvinyl, J = 10.7 Hz, CH2b′), 6.46 (dd, 1Hvinyl, J = 10.7 and 17.3 Hz, CH), 6.76 (s, 1Harom, H-2 imidazolyl), 7.00 (s, 1Harom, H-3 imidazolyl), 7.21 (dd, 1Harom, J = 2.1 and 8.5 Hz, H-5′), 7.41 (d, 1Harom, J = 2.1 Hz, H-3′), 7.54 (d, 1Harom, J = 8.5 Hz, H-6′), 8.06 (s, 1Harom, H-5 imidazolyl); 13C-NMR (270 MHz; CDCl3): 70.5 (CH2), 75.5 (Cq), 118.6 (CH2/vinyl), 121.7 (CHvinyl), 121.8 (CHarom), 127.7 (CHarom), 129.5 (CHarom), 130.8 (CHarom), 130.9 (CHarom), 135.1 (Cq/arom), 136.0 (Cq/arom), 136.3 (Cq/arom), 137.4 (CHarom).
1-(2-Azido-2-(2,4-dichlorophenyl)pent-4-enyl)-1H-imidazole (6c)
Eluent: dichloromethane/ethanol (96/4), 37%; IR νmax: 2097 (N3) cm− 1; 1H-NMR (270 MHz; CDCl3): 2.47 (dd, 1H, J = 8.3 and 13.9 Hz, CH2a), 3.46 (dd, 1H, J = 5.3 and 13.9 Hz, CH2b), 4.31 (d, 1H, J = 14.1 Hz, CH2a′), 4.91 (d, 1H, J = 14.1 Hz, CH2b′), 5.19 (m, 2Hvinyl, CH2a″ and CH2b″), 5.39 (m, 1Hvinyl, CH), 6.77 (s, 1Harom, H-2 imidazolyl), 7.05 (s, 1Harom, H-3 imidazolyl), 7.18 (dd, 1Harom, J = 2.6 and 8.9 Hz, H-5′), 7.41 (d, 1Harom, J = 2.6 Hz, H-3′), 7.44 (d, 1Harom, J = 8.9 Hz, H-6′), 8.04 (s, 1Harom, H-5 imidazolyl); 13C-NMR (270 MHz; CDCl3): 41.1 (CH2), 55.3 (CH2), 75.0 (Cq), 121.6 (CHvinyl), 121.9 (CHarom), 122.1 (CH2/vinyl), 127.7 (CHarom), 127.9 (CHarom), 135.1 (Cq/arom), 130.0 (CHarom), 130.2 (CHarom), 130.9 (CHarom), 135.1 (Cq/arom), 135.9 (Cq/arom).
1-(2-Azido-2-(2,4-dichlorophenyl)pent-4-ynyl)-1H-imidazole (6d)
Eluent: dichloromethane/ethanol (99/1), 15%; IR νmax: 2098 (N3) cm− 1; 1H-NMR (270 MHz; CDCl3): 2.19 (t, 1Halkyne, J = 2.5 Hz, CH), 3.09 (dd, 1H, J = 2.5 and 17.3 Hz, CH2a), 3.26 (dd, 1H, J = 2.5 and 17.3 Hz, CH2b), 4.55 (d, 1H, J = 14.5 Hz, CH2a′), 4.95 (d, 1H, J = 14.5 Hz, CH2b′), 6.76 (s, 1Harom, H-2 imidazolyl), 7.07 (s, 1Harom, H-3 imidazolyl), 7.23 (dd, 1Harom, J = 1.5 and 8.5 Hz, H-5′), 7.43 (d, 1Harom, J = 1.5 Hz, H-3′), 7.53 (d, 1Harom, J = 8.5 Hz, H-6′), 8.08 (s, 1Harom, H-5 imidazolyl); 13C-NMR (270 MHz; CDCl3): 28.5 (CH2), 54.3 (CH2), 74.5 (Cq), 74.6 (CHalkyne), 121.5 (CHarom), 122.2 (CHarom), 128.1 (CHarom), 129.6 (Cq/arom), 130.1 (CHarom), 130.4 (CHarom), 135.1 (Cq/arom), 136.1 (Cq/arom), 136.3 (CHarom).
1-(2-Azido-2-(2,4-dichlorophenyl)-2-phenylethyl)-1H-imidazole (6e)
Eluent: dichloromethane/ethanol (97/3), 44%; IR νmax: 2099 (N3) cm− 1; 1H-NMR (270 MHz; CDCl3): 4.84 (d, 1H, J = 13.2 Hz, CH2a), 5.23 (d, 1H, J = 13.2 Hz, CH2b), 6.60 (s, 1Harom, H-2 imidazolyl), 7.03 (s, 1Harom, H-3 imidazolyl), 7.16 (m, 2Harom, H-5′ and H-3′), 7.33 (m, 5Harom, H-2″, H-3″, H-4″, H-5″ and H-6″), 7.56 (d, 1Harom, J = 8.5 Hz, H-6′), 7.94 (s, 1Harom, H-5 imidazolyl); 13C-NMR (270 MHz; CDCl3): 55.7 (CH2), 76.7 (Cq), 121.5 (CHarom), 121.9 (CHarom), 125.6 (2CHarom), 127.5 (CHarom), 128.8 (CHarom), 129.9 (2CHarom), 129.2 (CHarom), 131.1 (CHarom), 132.1 (Cq/arom), 135.2 (Cq/arom), 136.1 (CHarom), 137.2 (Cq/arom), 141.0 (Cq/arom).
1-(2-Azido-2-(2,4-dichlorophenyl)-3-phenylpropyl)-1H-imidazole (6f)
Eluent: dichloromethane/ethanol (95/5), 75%; IR νmax: 2097 (N3) cm− 1; 1H-NMR (270 MHz; CDCl3): 3.05 (d, 1H, J = 13.7 Hz, CH2a), 3.94 (d, 1H, J = 13.7 Hz, CH2b), 4.44 (d, 1H, J = 14.3 Hz, CH2a′), 5.12 (d, 1H, J = 14.3 Hz, CH2b″), 6.77 (s, 1Harom, H-2 imidazolyl), 6.91 (m, 2Harom), 7.02 (m, 2Harom), 7.11 (m, 2Harom), 7.20 (m, 2Harom), 7.45 (d, 1Harom, J = 1.9 Hz, H-3′), 8.03 (s, 1Harom, H-5 imidazolyl); 13C-NMR (270 MHz; CDCl3): 42.1 (CH2), 55.4 (CH2), 76.1 (Cq), 121.5 (CHarom), 121.8 (CHarom), 127.7 (CHarom), 127.8 (CHarom), 128.4 (CHarom), 128.7 (Cq/arom), 128.8 (2CHarom), 130.0 (2CHarom), 130.6 (CHarom), 130.8 (CHarom), 133.1 (Cq/arom), 135.0 (Cq/arom), 135.8 (Cq/arom).
Antifungal activity
Test was performed following the guidelines of the approved reference method for yeasts [Citation21]. Antifungal activity was evaluated against an azole-susceptible strain of Candida glabrata designated 94.5579 and its derived azole-resistant petite mutant. MICs (Minimum Inhibitory Concentration) were determined using a microdilution assay in RPMI-1640 culture medium, inoculated with 0.5–2.5 × 103 cells/mL. The test was performed using sterile 96 flat shaped-well microtitre plates. Serial two-fold drug dilutions were made in DMSO. Dilutions of the compounds were dispensed at a volume of 5 μL per well, to obtain final concentrations ranging from 250 μg/mL to the concentration where no inhibition was seen. After 48 h at 37°C, the absorbance was measured at 630 nm and MICs90 were calculated at the minimum concentration required to inhibit at least 90% of the fungal growth compared to the drug-free control.
Results and discussion
Chemistry
Azidoconazoles 6a-f were prepared by a two-step synthesis from ketone 4. This last was reacted with various organometallic reagents to give alcohols 5a-f as intermediates. The key step in this straightfoward synthesis consisted in a nucleophilic substitution (SN1) of the 2-hydroxyl group of tertiary alcohol 5a-f with an azide anion in presence of a Lewis Acid, i.e. boron trifluoride-diethyl etherate [Citation22]. This azido-substitution was very fast and efficient for the majority of compounds 6a-f, excepted 6d for which reaction yield did not exceed 15%. Azidoconazole 3 was also prepared with a two-step synthesis from secondary alcohol 1. In this case, direct substitution of 2-hydroxyl group by an azide anion with Lewis Acid was unsuccessful. So, a more classical pathway was used with a mesylation of the secondary alcohol group followed by a nucleophilic substitution (SN2) in DMF with NaN3 [Citation14].
Antifungal activity
In our approach for the discovery of new antifungal derivatives against C. glabrata petite mutant, a first compound 3 was synthesized. Evaluation of its antifungal activity pointed out that the azidoconazole 3 was much more active against C. glabrata (MIC90 = 31 μg/mL) and C. glabrata petite mutant (MIC90 = 8 μg/mL) than its alcoholic analog 1 whose for MICs90 against C. glabrata and C. glabrata petite mutant were higher than 250 μg/mL. Morever, the pathogenic fungi A. Fumigatus was sensitive to azidoconazole 3 (MIC90 = 4 μg/mL). Starting from this promising result that clearly demonstrated the sensitivity of various pathogenic fungi to secondary azidoconazole, more tertiary azidoconazoles 6a-f were synthesized from conazoles 5a-f bearing a hydroxyl group like last generation antifungal azoles (i.e. fluconazole or voriconazole). Results obtained for all compounds 6a-f are heterogeneous. Among alcohols derivatives 5, compounds 5a and 5e are inactive against all tested strains. Substitution of the 2-hydroxyl group by an azide moiety lead to an increased activity. Azidoconazole 6e was much more active than its hydroxylated analogue 5e with MIC90 values ranging from 2 to 16 μg/mL. A weak activity of 6a was observed on the C. albicans strain (MIC90 = 16 μg/mL). Activities of 5a and 6a pointed out the disfavorable effect of long saturated alkyl chain. However, introduction of unsaturated alkyl side chains (vinyl, allyl and propargyl) or aromatic ring seems to be favorable for the activity. Alcohols 5b-d and azides 6b-d are usually much more active against C. glabrata strains with MIC90 values ranging from 1 to 8 mg/mL. This observation is less significant when alcohol 5e and its azide analogue 6e are considered. In these cases, there is no increasing of activity against C. glabrata when hydroxyl group was replaced by an azide. Against C. glabrata petite mutant, a decrease of MIC90 showed that azidoconazole 6b, 6c, 6d and 6e are more active than their alcoholic analog 5b, 5c, 5d and 5e respectively and than frequently-used fluconazole and voriconazole. Also, one azidoconazole 6c shown an interesting slightly broad-spectrum activity against C. albicans (MIC90 = 8 μg/mL) and A. Fumigatus (MIC90 = 8 μg/mL). In conclusion, the antifungal profile of the tested compounds showed that C. glabrata petite mutant seems to be more sensitive than its parent to azidoconazoles 6a-f. Therefore, removing of the 2-hydroxyl group by an azido moiety using a nucleophilic substitution gave some azidoconazoles that could be specifically active against a conazole-resistant strain. Azido group seems to be an efficient parameter to consider in order to design new azoles with the aim to overcome the increased activity of the efflux pumps which is the major mechanism of the acquiered resistance to azoles in clinical isolates.
Table I. MIC90 for the tested compounds against several fungal strains.
Acknowledgements
We wish to express our thanks to “Région des Pays de la Loire” for financial support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- SK Fridkin, and WR Jarvis. (1996). Clin Microbiol Rev 9:499–511.
- SY Ablordeppey, P Fan, JH Ablordeppey, and L Mardenborough. (1999). Curr Med Chem 6:1151–1196.
- M Kleinberg. (2006). Int J Antimicrob Agents 27 (Suppl 1):12–16.
- MS Turner, RH Drew, and JR Perfect. (2006). Expert Opin Emerg Drugs 11:231–250.
- K Richardson, K Cooper, MS Marriott, MH Tarbit, PF Troke, and PJ Whittle. (1990). Rev Infect Dis 12 (Suppl 3):267–271.
- S Brun, T Bergès, P Poupard, C Vauzelle-Moreau, G Renier, D Chabasse, and JP Bouchara. (2004). Antimicrob Agents Chemother 481:1788–1796.
- A Chen, and JD Sobel. (2005). Expert Opin Emerg Drugs 10:21–33.
- S Sundriyal, RK Sharma, and R Jain. (2006). Curr Med Chem 13:1321–1335.
- F Chevreuil, A Landreau, D Séraphin, G Larcher, JP Bouchara, and P Richomme. (2006). J Enz Inhib Med Chem 21:293–303.
- F Chevreuil, A Landreau, D Séraphin, G Larcher, S Mallet, JP Bouchara, and P Richomme. (2007). J Enz Inhib Med Chem 22:563–569.
- N Nguyen-Hai, S Sardari, and M Selecky. (2004). Bioorgan Med Chem 12:6255–6269.
- A Bentley, M Butters, and SP Green. (2002). Organ Proc Res Develop 6:109–112.
- L Shapiro, and AK Ganguly. (1974). Chem Abstr 81:91336. US3816472
- A Stuetz, and H Egger. (1984). Chem Abstr 102:62244. DE3408127
- D Bawden, GE Gymer, and MS Marriott. (1983). Eur J Med Chem 18 (1):91–96.
- G Jeager, K Boeckmann, and KH Buechel. (1983). Chem Abstr 100:139121. DE3222191
- AM Jiyooji, and FC Haku. (1979). Chem Abstr 91:57005. JP54027563
- P Scharwaechter, K Gutsche, and W Kohlmann. (1977). Chem Abstr 88:89672. DE2623129
- EF Godefroi, J Heeres, J Van Custem, and PA Janssen. (1969). J Med Chem 12:784–791.
- ER Perez, A Loupy, M Liagre, AM De Guzzi Plepis, and PJ Cordeiro. (2003). Tetrahedron 59:865–870.
- National Commitee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing yeasts.Approved standard M27-A. National Commitee for Clinical Standards, Villanova, PA, (1997).
- GCB Harriman, J Shao, and JR Luly. (2000). Tetrahedron Lett 41:8853–8856.