Abstract
Many oral care products incorporate an antibacterial compound to prevent the formation of dental plaque which predisposes teeth to dental caries or periodontal disease [Citation]. Triclosan (TCN) is a commonly used antiplaque agent in toothpastes [CitationCitation]. Strategies to increase the delivery efficiency of antibacterials using formulation aids such as polyamidoamine (PAMAM) dendrimers are of interest.
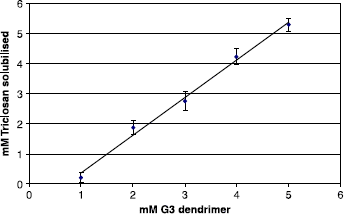
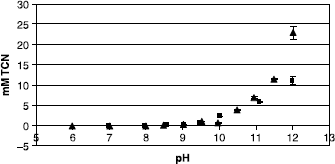
Solubilisation studies over the pH range 5-12 demonstrated an increase in the level of TCN solubilised with increasing dendrimer concentration (1 mM–5 mM). However, the dendrimer was unable to enhance TCN solubility at lower pH values and the solubilising effect observed was attributed to the ionization of TCN (pKa 8.14) resulting from dendrimer induced pH changes.
End group modification of G3 PAMAM dendrimer with phenylalanine in order to promote solubility through π–π stacking between TCN and the amino acid has been carried out. Phenylalanine:G3 PAMAM conjugates of different ratios (32:1, 21:1, 16:1) were synthesized. The fully conjugated dendrimer (32:1) had poor aqueous solubility, whereas the 21:1 and 16:1 dendrimer conjugates were water soluble. The 21:1 conjugate was tested for its ability to solubilise TCN, however, again there was no increase over control buffer solutions of the same pH. An alternative approach under investigation is to directly conjugate TCN to PAMAM dendrimers via a hydrolysable linkage.
| Abbreviation | ||
| PAMAM, | = | polyamidoamine |
| TCN, | = | Triclosan |
Introduction
All surfaces within the mouth, particularly the teeth, are covered by a microflora. The main component of the microflora is bacterial in origin, but viruses, fungi and protozoa are also present. Dental plaque is defined as “the diverse microbial community embedded in a matrix of host and bacterial polymers, growing on teeth as a biofilm” [Citation4]. In the absence of oral hygiene dental plaque can build up to a level which may predispose to dental caries or periodontal disease. These disease states involve a shift in the dominant species of bacteria within the plaque biofilm which may then predispose sites to disease. Therefore many dental products (mouthwash and toothpaste) incorporate an antimicrobial compound to help control plaque formation.
Triclosan (TCN) (1) () is a non-ionic, lipid-soluble bisphenol antibacterial which is widely used in oral care products. Triclosan has broad spectrum antimicrobial activity against many types of Gram-positive and Gram-negative non-sporulating bacteria, some fungi and yeasts [Citation2]. It has greater activity against Gram-negative anaerobic species (associated with gum disease states) and lesser activity against Gram-positive bacteria Citation5Citation6Citation7. Triclosan has been demonstrated to inhibit proteases of subgingival bacteria, reduce the uptake and metabolism of glucose by oral bacteria such as S. mutans and it is reported to reduce the integrity of bacterial membranes [Citation8].
For an antibacterial to be effective when delivered from an oral care product it should be efficiently retained and subsequently released at the site of interest. The use of mucoadhesive carrier systems is a strategy that may be used to enhance agent delivery and retention.
Dendrimers are good candidates for delivery systems as they can be modified by the addition of molecules to their surface groups [Citation9], for example, to enhance solubility. Active molecules can either be encapsulated within the dendrimer architecture [Citation10] or can be conjugated to surface groups [Citation9]. The surface groups can also be further modified to enable specific targeting of the dendrimer carrier [Citation11]. Polyamidoamine (PAMAM) dendrimers have been evaluated for a range of pharmaceutical applications [Citation12,Citation13]. In theory, these cationic dendrimers may have intrinsic mucoadhesive properties for use in the oral cavity, as mucin (which covers oral epithelia) is negatively charged [Citation14], thus creating an electrostatic attraction between mucus and dendrimer. An agent such as TCN, when encapsulated in the dendrimer architecture may then be slowly released into the oral cavity, potentially increasing efficacy. As a preliminary step in the assessment of dendrimers as potential carriers for antimicrobials, the solubilisation of TCN using various PAMAM dendrimers was investigated.
Materials and methods
Chemicals were obtained from Sigma-Aldrich UK., Lancaster Synthesis Ltd and Fischer Scientific UK Ltd, unless otherwise stated. G0-3 PAMAM dendrimers were obtained from Dendritech, USA. Dioxane was dried by distillation over sodium metal. Deuterated solvents were obtained from Cambridge Isotope Laboratories Inc., Andover USA. Sephadex LH-20 was obtained from Sigma-Aldrich UK. Triclosan was a gift from Unilever Research & Development, Port Sunlight UK.
HPLC was performed on a Hewlett Packard Series II 1090 Liquid Chromatography system. A Hyperclone 5 μ ODS (C18) column 250 × 4.6 mm was obtained from Phenomenex UK. All HPLC solvents were HPLC grade. Water was distilled prior to use. U.V. spectroscopy was carried out on a Unicam UV 300 system.
NMR was performed on a Bruker Avance 300, with X-Win software. 1H and 13C spectra were measured at 300 MHz and 75.5 MHz, respectively, using d4-MeOD as the solvent. Chemical shifts for 1H and 13C NMR spectra were quoted in parts per million (δ) referenced to tetramethylsilane (TMS) at zero ppm. Multiplicities were indicated by s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), b (broad) or combinations thereof.
Dendrimer modification
2,5-Dioxopyrrolidin-1-yl 2-(tert-butyloxycarbonylamino)-3-phenylpropanoate (3)
Boc-protected-L-phenylalanine (2) (0.395 g, 1.49 mmol) was dissolved in anhydrous dioxane (10 mL) under an inert atmosphere. To this was added N-hydroxysuccinimide (0.326 g, 2.83 mmol) and N-(3-dimethylaminopropyl)-N′-ethyl-carbodiimide hydrochloride (EDC) (0.486 g, 2.54 mmol). The reaction mixture was stirred at room temperature for 12 h after which TLC (1:1 hexane-ethyl acetate) showed the reaction had reached completion. The solvent was evaporated in vacuo and the resulting oil dissolved in ethyl acetate (50 mL). The solution was washed with 0.5M HCl (1 × 50 mL), saturated NaHCO3 (2 × 50 mL) and saturated brine (1 × 50 mL). The organic layer was dried over MgSO4, filtered and reduced in vacuo to give (3) (0.364 g, 1.00 mmol) as a colourless solid (68% yield). 1H NMR: (300 MHz, d4-MeOD) δ ppm 1.36 (9H, bs, But), 2.83 (4H, bs, CH2CH2), 3.02 (1H, dd, Jgem 13.8 Hz JHH 9.6 Hz, PhCHAHB) 3.28-3.34 (1H, m, PhCHAHB), 4.73 (1H, dd, JHH 9.6 Hz JHH 4.8 Hz, PhCH2CH), 7.22-7.30 (5H, m, Ph).
G0 PAMAM tetra-tert-butyl 1-amino-1-oxo-3-phenylpropan-2-ylcarbamate (4)
2,5-Dioxopyrrolidin-1-yl 2-(tert-butyloxycarbonylamino)-3-phenylpropanoate (3) (0.364 g, 1.00 mmol) was dissolved in anhydrous dioxane (10 mL) under a nitrogen atmosphere. To the solution was added an anhydrous MeOH solution (8 mL) containing G0 PAMAM dendrimer (0.052 g, 0.101 mmol) and triethylamine (0.204 g, 2.016 mmol). The solution was stirred for 12 h and then the solvent evaporated in vacuo. The resultant oil was dissolved in a minimum amount of MeOH and purified on a Sephadex G10 column (0.01% acetic acid mobile phase). This yielded the fully conjugated G0:4 N-Boc-L-phenylalanine (4) (77.8 mg, 0.052 mmol, 51.5% yield). 1H NMR indicated approx 75% purity (from integration of PhCH2CH peaks of reacted and unreacted phenylalanine) with separation from the unreacted phenylalanine difficult due to the small difference in molecular weight between the compounds.
1H NMR: (300 MHz d4-MeOD) δ ppm 1.35 (36H, bs, But), 2.39-2.48 (8H, m, PAMAM-d), 2.72-2.79 (4H, m, PAMAM NCH2CH2N), 2.79-2.87 (4H, m, PhCHAHB) 2.87-2.96 (8H, m, PAMAM-c), 3.05-3.14 (4H m, PhCHAHB), 3.21-3.27 (16H, m, PAMAM-a′, -b′), 4.23-4.28 (4H, m, PhCH2CH), 7.18-7.27 (20H, m, Ph).
1 G3 Pamam: 32 Tert-butyl 1-Amino-1-oxo-3-phenylpropan-2-ylcarbamate (1Pamam:32phenylalanine)
2,5-Dioxopyrrolidin-1-yl 2-(tert-butyloxycarbonylamino)-3-phenylpropanoate (3) (1.530 g, 4.24 mmol) was dissolved in anhydrous dioxane (20 mL) under a nitrogen atmosphere. To the solution was added an anhydrous MeOH solution (10 mL) containing G3 PAMAM dendrimer (0.610 g, 0.088 mmol) and triethylamine (0.858 g, 8.48 mmol). The mixture was stirred for 48 h and then the solvent evaporated in vacuo. The resultant solid (2.03 g) was purified using a Sephadex LH-20 column eluting with a MeOH mobile phase. This yielded the fully conjugated G3 dendrimer (1.136 g, 0.077 mmol, 87.5% yield). 1H NMR: (300 MHz d4-MeOD) δ ppm 1.34 (288H, bs, But), 2.25-2.40 (120 H, m, PAMAM-d), 2.46-2.64 (56H, m, PAMAM-a), 2.69-2.88 (120H, m, PAMAM-c), 3.09-3.16 (32H, m, PhCHACHB), 3.19-3.33 (216H m, PAMAM-a′, -b′, -b and PhCHACHB), 4.14-4.31 (32H, m, PhCH2CH), 7.12-7.29 (160 H, m, Ph).
Partial G3 PAMAM 2-(tert-butyloxycarbonylamino)-3-phenylpropanoate conjugates
PAMAM dendrimers with partially conjugated surfaces were synthesized by using different reactant ratios (16 or 21 equivalents) of (3) to G3 PAMAM dendrimer (32 reaction sites). The synthesis followed the procedure outlined above.
For 21 equivalent reaction
1H NMR: (300 MHz d4-MeOD) δ ppm 1.33 (189H, bs, But), 2.26-2.42 (120H, m, PAMAM-d), 2.49-2.61 (78H, m, PAMAM-a and the -a′ of non-conjugated branches), 2.69-2.86 (120H, m, PAMAM-c), 3.06 (21H, dd, Jgem 13.35 JHH 5.31, PhCHAHB), 3.16-3.33 (183H, m, PAMAM-a′ of conjugated branches, -b′ of conjugated and non-conjugated branches, -b and PhCHAHB), 4.20-4.31 (21H, m, PhCH2CH), 7.14-7.29 (105H, m, Ph).
For 16 equivalent reaction
1H NMR: (300 MHz d-4MeOD) δ ppm 1.31 (144H, bs, But), 2.23-2.41 (120H, m, PAMAM-d), 2.46-2.61 (88H, m, PAMAM-a and the -a′ of non-conjugated branches), 2.67-2.86 (120H, m, PAMAM-c), 3.04 (16H, dd, Jgem 13.52 JHH 5.66, PhCHAHB), 3.13-3.33 (168H, m, PAMAM-a′ of conjugated branches, -b′ of conjugated and non-conjugated branches, -b and PhCHAHB), 4.20-4.31 (16H, m, PhCH2CH), 7.14-7.29 (80H, m, Ph).
1 G3 PAMAM: 32 2-amino-3-phenylpropanamide
To the Boc-protected PAMAM:phenylalanine conjugate (0.700 g of 1:32 conjugate) was added 4M HCl in dioxane (20 mL) with thioanisole (5 mL) as a scavenger. This was stirred for 30 min before purification by dialysis against distilled water (3 days, MWt cut off 3500) gave the deprotected PAMAM:phenylalanine conjugate. 1H NMR: (300 MHz d4-MeOD) δ ppm 2.27-2.42 (120H, m, PAMAM-d), 2.53-2.66 (56H, m, PAMAM-a), 2.71-2.88 (120H, m, PAMAM-c), 2.96 (32H, bdd, Jgem 13.3 JHH 7.9 PhCHAHB), 3.13 (32H, bdd, Jgem 13.7 JHH 5.9, PhCHAHB), 3.18-3.31 (184H, m, PAMAM-a′, -b′ and -b), 3.84-3.95 (32H, m, PhCH2CH), 7.21-7.35 (160H, m, Ph).
For 21 equivalent deprotection (protonated primary amines on non-conjugated branches)
1H NMR: (300 MHz d4-MeOD) δ ppm 2.56-2.75 (120H, m, PAMAM-d), 3.03-3.52 (401H, m, PAMAM-a, -c, PhCHAHB, -a′ of conjugated branches, -b′ of conjugated and non-conjugated branches, -b, and PhCHAHB), 3.52-3.79 (22H, m, PAMAM-a′ of non-conjugated branches), 4.14-4.28 (21H, m, PhCH2CH), 7.32-7.42 (105H, m, Ph).
For 16 equivalent deprotection (protonated primary amines on non-conjugated branches)
1H NMR: (300 MHz d4-MeOD) δ ppm 2.40-2.71 (120H, m, PAMAM-d), 2.79-2.97 (56H, m, PAMAM-a), 2.97-3.56 (m, 304H, PAMAM-c PhCHAHB, -a′ of conjugated branches, -b′ of conjugated and non-conjugated branches, -b and PhCHAHB), 3.34-3.46 (32H, m, PAMAM-a′ of non-conjugated branches), 4.01-4.11 (16H, m, PhCH2CH), 7.21-7.39 (80H, m, Ph).
Solubility studies
Aqueous dendrimer solutions
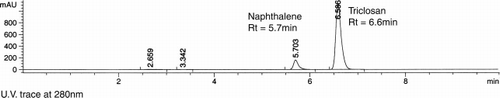
An aqueous stock solution of 5 mM G3 PAMAM dendrimer was diluted to 15 mM solutions (1 mM increments, 3 solutions at each concentration). An excess of TCN was added to each solution before sealing the vial and placing in a shaking water bath (60 rpm) at 27°C for 72 h. Excess TCN was then removed by filtration through a 0.45μm pore size filter paper. The resulting filtrate was analysed by HPLC (70% ACN: 30% phosphate buffer, 1 mL min− 1 on a 250 × 4.6 mm, 5 μm pore size, C18 column) against a naphthalene internal standard (0.1 mL of 0.05 mM naphthalene to 0.9 mL filtrate).
pH buffered dendrimer solutions
To investigate the pH dependence of the solubilising effect of dendrimers, a stock aqueous solution of G3 PAMAM dendrimer was diluted to 3 mM with a mixture of water and Teorell and Stenhagens (citrate-phosphate-borate) buffer solution to the required pH (5-12) [Citation15]. These solutions (3 at each pH) were then saturated with TCN, enclosed and left in a shaking water bath (60rpm) at 27°C for 72 h. Excess TCN was removed by filtration through a 0.45μm pore size filter paper. The filtrate was analysed by HPLC against a naphthalene standard.
pH 5-12 buffered solutions
To investigate whether the solubilising effect was due to encapsulation by the dendrimers or simply an ionization effect, the experiment was repeated with pure citrate-phosphate-borate buffer solutions (pH 5-12, no dendrimer). An identical protocol was followed as for the pH buffered dendrimer solutions, except that no dendrimer was added.
Solubility studies on modified dendrimers
The pH buffered solubility studies were repeated using G3 PAMAM dendrimers conjugated to 21 phenylalanine groups.
Results and discussion
A range of concentrations of un-substituted G3 PAMAM dendrimer (1–5 mM) were investigated for their ability to solubilise TCN. A HPLC procedure was developed for the analysis of TCN solutions involving the use of a naphthalene internal standard. This permitted analysis of TCN in dendrimer and control buffer solutions. Good separation between the naphthalene internal standard peak and the TCN peak was achieved ().
In the unbuffered solutions of dendrimer the results show () that the amount of TCN solubilised increases with dendrimer concentration in a linear manner over the 1–5 mM range. In an attempt to prove that this increase in solubility was not due to the ionization of the phenolic groups, some buffered dendrimer solutions were analysed for their ability to solubilise TCN against control solutions containing buffer alone. However, when the pH of the solution was controlled the dendrimer was unable to maintain the previously seen increase in solubilisation of Triclosan at lower pH's and, as shown in , no improvement was observed over buffered aqueous solutions. This indicates that the solubilising effect is likely to be due to the ionization of TCN (pKa 8.14) rather than any encapsulation by the dendrimer.
In view to increase its aromatic character, the unsubstituted G3 PAMAM dendrimer was modified. Thus it was proposed that an increase in aromaticity may increase the interaction between TCN and the dendrimer, through π–π stacking, thus enabling increased solubilisation. The coupling chemistry was developed on the basis of a G0 PAMAM dendrimer. The chemistry is depicted in Scheme . Initially one phenylalanine molecule was conjugated to the G0 dendrimer and then all 4 surface groups were conjugated. In the 1H NMR spectrum (peak labels shown in ), the integration ratio between dendrimer and phenylalanine peaks was 4, supporting complete conjugation. The integration of the dendrimer was difficult due to overlapping peaks, and separation of (4) from unreacted dendrimer on Sephadex columns was difficult due to their low molecular weight difference. The reaction was then scaled up to a G3 dendrimer and the product separated by using Sephadex (LH-20) enabling separation of product and other materials. Using the procedure described above, different reactant ratios of activated Boc-phenylalanine to G3 dendrimer were investigated: 16:1, 21:1 and 48:1 (with the G3 dendrimer having 32 surface groups available for conjugation). This produced dendrimers with 16, 21 and 32 equivalents of phenylalanine conjugated to the surface, respectively (as depicted in Scheme ). The fully conjugated G3 dendrimer (deprotected) was insoluble in water, however the partial conjugates (deprotected) were more water soluble, especially in their protonated forms. However, further HPLC solubility studies using the modified dendrimers to solubilise TCN, again showed no increase in TCN solubility over that of the buffer solutions.
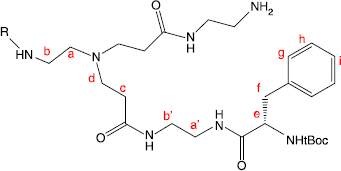
Figure 5 Partial structure of dendrimer branch showing peak labels for 1H NMR spectra of PAMAM dendrimer conjugates.
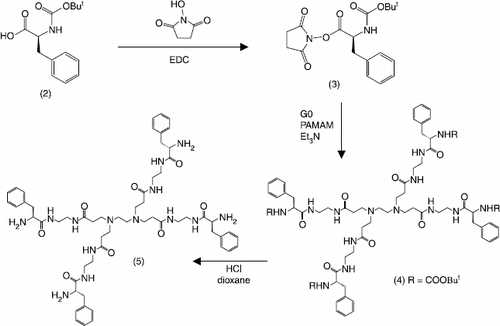
Scheme 2 Synthesis of G3 PAMAM Phenylalanine dendrimers. The number of conjugated branches in compounds (7) and (8) [32 for fully conjugated, 21 or 16 for partially conjugated] depends on the number of equivalents of (3) added in the conjugation step.
![Scheme 2 Synthesis of G3 PAMAM Phenylalanine dendrimers. The number of conjugated branches in compounds (7) and (8) [32 for fully conjugated, 21 or 16 for partially conjugated] depends on the number of equivalents of (3) added in the conjugation step.](/cms/asset/706b6bff-8d08-428d-b255-104390d37f96/ienz_a_320692_f0007_b.gif)
In summary, PAMAM dendrimers have been investigated for the solubilisation of the antibacterial TCN. Although initial results suggested that TCN solubility increased with increasing G3 dendrimer concentration, G3 PAMAM dendrimers were shown to increase TCN solubility due to ionization effects, rather than encapsulation. Modification of the dendrimer by the addition of aromatic phenylalanine groups to the terminal amine groups was undertaken in an attempt to increase the affinity of TCN for the dendrimer through π–π stacking interactions. The 21:1 conjugate was tested for its solubilising effect on TCN, however again it showed no increase over the control buffer solutions. Studies are now in progress to explore the potential of conjugating TCN directly to PAMAM via a hydrolysable linkage.
Acknowledgements
Unilever Research & Development, Port Sunlight and the BBSRC are thanked for an industrial case studentship.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- S Peter, DG Nayak, P Philip, and NS Bijlani. (2004). Antiplaque and antigingivitis efficacy of toothpastes containing triclosan and fluoride. Int Dent J 54 (5 Suppl 1):299–303.
- MG Brading, and PD Marsh. The oral environment: The challenge for antimicrobials in oral care products. Int Dent J 53 (6 Suppl 1):353–362.
- P Sreenivasan, and A Gaffar. (2002). Antiplaque biocides and bacterial resistance: A review. J Clin Periodontol 29:965–974.
- PD Marsh. (2003). Plaque as a biofilm: Pharmacological principles of drug delivery and action in the sub- and supragingival environment. Oral Dis (9 Suppl 1):16–22.
- PD Marsh, and DJ Bradshaw. (1993). Microbiological effects of new agents in dentifrices for plaque control. Int Dent J 43 (4 Suppl 1):399–406.
- DJ Bradshaw, PD Marsh, GK Watson, and D Cummins. (1993). The effects of triclosan and zinc citrate, alone and in combination, on a community of oral bacteria grown in vitro. J Dent Res 72:25–30.
- KA Saunders, J Greenman, and C McKenzie. (2000). Ecological effects of triclosan and triclosan monophosphate on defined mixed cultures of oral species grown in continuous culture. J Antimicrob Chemoth 45:447–452.
- MG Brading, VJ Cromwell, AK Green, S DeBrabander, T Beasley, and PD Marsh. (2004). The role of triclosan in dentifrice formulations, with particular reference to a new 0.3% triclosan calcium carbonate-based system. Int Dent J 54 (5 Suppl 1):291–298.
- A D'Emanuele, and D Attwood. (2005). Dendrimer-drug interactions. Adv Drug Deliv Rev 57:2147–2162.
- CN Moorefield, and GR Newkome. (2003). Unimolecular micelles: Supramolecular use of dendritic constructs to create versatile molecular containers. CR Chim 6:715–724.
- S-G Sampathkumar, and KJ Yarema. (2005). Targeting cancer cells with dendrimers. Chem Biol 12:5–6.
- DA Tomalia, and JMJ Frechet. Dendrimers and other dendritic polymersJMJ, Frechet, and DA Tomalia. John Wiley & Sons, Chichester, (2001).
- J Kopecek, P Kopeckova, T Minko, and ZR Lu. (2000). HPMA copolymer-anticancer drug conjugates: Design, activity, and mechanism of action. Eur J Pharm Biopharm 50:61–81.
- J Hao, and PWS Heng. (2003). Buccal delivery systems. Drug Dev Ind Pharm 29:821–832.
- T Teorell, and E Stenhagen. (1938). Universal buffer over the pH range 2.0 to 12.0. Biochem Z 299:416–419.