Abstract
Seventeen compounds with either an imidazolin-2-one or a tetrahydropyrimidin-2(1H)-one scaffold were synthesized and evaluated for their immunosuppressive activity in a concanavallin A (ConA)-stimulated mouse splenocytes proliferation test. Three of these molecules exerted a significant activity at 90 μM. All the compounds of the tetrahydropyrimidin-2(1H)-one series have turned out to be inactive showing the crucial role of the imidazolidin-2-one scaffold in the induction of an immunosuppressive activity.
Introduction
The control of pathological or deleterious immune responses is very often achieved by an immunosuppressive therapy. Immunosuppressant drugs are mainly used in organ transplantation for the prevention and the treatment of allograft rejection. At the present time, these molecules are also part of autoimmune diseases therapy. Some of these agents can, for example, be used in type I diabetes mellitus [Citation1], arthritis [Citation2,Citation3] and dermatological pathology like psoriasis [Citation4] or systemic lupus erythematosus [Citation5]. In absence of immunosuppression, transplanted organs invariably undergo progressive immune-mediated injury. Acute allograft rejection is primarily mediated by immunological mechanisms implying the activation of T lymphocytes by antigen-presenting cells (APCs). Indeed, recipient T cells have the ability to recognize, through their antigen receptor, donor alloantigens presented by APCs. Once activated, T-cells differentiate, proliferate and become able to damage graft target tissues. T cells also secrete cytokines that directly cause tissue destruction (e.g., tumor necrosis factor-β) or recruit and activate cells of the innate immune system (e.g., macrophages), which participate to the graft rejection. Current immunosuppressive agents inhibit T-cell responses either directly or through actions on APCs. These drugs can be classified to five groups in regard to their mechanism of action: inhibitors of cytokine production (calcineurin inhibitors such as cyclosporine and tacrolimus), inhibitors of cytokine binding (IL-2 receptor α chain specific monoclonal antibody), inhibitors of cytokine receptor signal transduction (rapamycin), inhibitors of DNA synthesis (cyclophosphamide, azathioprine, mycophenolate mofetil, leflunomide, brequinar sodium, methotrexate) and inhibitors of APC development and maturation (glucocorticoids, rapamycin). Although these agents, over the past 40 years, have transformed solid organ transplantation into a routine clinical procedure with a satisfactory control of acute rejection and adequate short-term graft survival [Citation6], several problems remain. First, these drugs exhibit important side effects due to their intrinsic toxicity (e.g., nephrotoxicity of calcineurin inhibitors, hematotoxicity of mycophenolate mofetil, myelotoxicity of rapamycin, etc.) and to their lack of specificity, which triggers a general immunodepression state responsible for an enhanced risk of opportunistic infections and neoplasic complications [Citation7]. These adverse effects often compromise patient and graft survival. Moreover these drugs have low efficiency on chronic graft rejection, which is often responsible for long-term graft loss [Citation8].
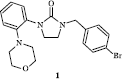
In a previous work, we described the synthesis and SAR of a series of imidazolidin-2-ones, which exhibit immunosuppressive properties [Citation9]. These studies permitted us to identify a lead compound 1 (), which has shown maximal inhibition of the mouse splenocytes Con-A-induced proliferation at 30 μM. These results are comparable to those obtained with the positive control, cyclosporine A, at 5 μM (optimal dose). However, this molecule exerts cytotoxic effects on human MRC5 fibroblasts used in our cytotoxicity assay with an IC50 of 21 μM and, so, an unsatisfactory toxicity/activity index of 0.7. These interesting results prompted us to synthesize some derivatives of 1. To explore the role of the imidazolidin-2-one scaffold in the emergence of immunosuppressive activity, we first decided to expand the ureic cycle by preparing some tetrahydropyrimidin-2-(1H)-one derivatives. We then synthesized some analogues of 1 with an imidazolidin-2-one moiety N-substituted by a phenyl or azaheterocyclic groups like phthalimidic moieties by analogy with some thalidomide analogues which exert TNF-α production inhibitory properties.
Materials and methods
General
Melting points were determined on a Tottoli-Büchi apparatus (Büchi, Flawil, Switzerland) and are uncorrected. Structures of the described compounds were supported by IR, 1H-NMR and microanalytical data. IR spectra were run with KBr pellets on a Perkin-Elmer FT-IR Paragon 1000 grating infrared spectrometer (Perkin-Elmer, St-Quentin-en-Yvelines, France). 1H-NMR spectra were recorded on a Bruker AC 250 spectrometer (250 MHz) (Bruker, Wissembourg, France), using CDCl3 or DMSO as a solvent; chemical shifts (δ) are reported in parts per million (ppm), from internal Me4Si. Mass spectra were recorded on an ESQUIRE-LC spectrometer (Bruker) (electrospray ionisation with ion trap system). Purification of synthesized compounds was made using columns of silica gel (Silica gel 60, 70-230 mesh, E. Merck, Darmstadt, Germany), with appropriate solvents. Anhydrous Na2SO4 was always used as the drying agent. Chemicals were purchased from Sigma-Aldrich Fluka (St Quentin Fallavier, France), Lancaster Synthesis (Bischeim, France) or Avocado (La Tour du Pin, France).
Chemistry
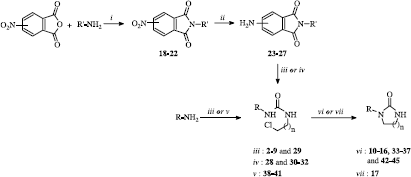
The synthesis of N-substituted imidazolidin-2-ones 10-17 and 33-37, from ureas 2-9 and 28-32 respectively, and of N-substituted tétrahydropyrymidin-2(1H)-ones 42-45, from ureas 38-41, is shown in Scheme .
Scheme 1 Synthesis of imidazolidin-2-ones 10-17 and 33-37 and tetrahydropyrimidin-2(1H)-ones 42-45. Reaction reagents and conditions: (i) AcOH, reflux; (ii) Pd/C 5%, H2, THF, 50°C; (iii) 2-chloroethyl isocyanate, CHCl3, reflux; (iv) 2-chloroethyl isocyanate (8 éq), microwaves, 82°C, 20 W; (v) 3-chloropropyl isocyanate, CHCl3, reflux; (vi) Cs2CO3, CH3CN, reflux; (vii) Na2CO3, CH3CN, reflux.
1-(4-Bromophenyl)-3-(2-chloroethyl)urea (2)
To a solution of 4-bromoaniline (3 g, 17.40 mmol) in chloroform (50 mL) was added 2-chloroethyl isocyanate (1.51 mL, 17.40 mmol). The mixture was refluxed for 40 min, and then the solvent was removed under reduced pressure. The crystalline residue was recrystallized from diethyl ether to give compound 2 as a white powder. M.p. = 177°C, Yield = 96%. IR (KBr) (ν, cm− 1) 3317 (NH), 1631 (C = O), 825 (C-Cl), 1071 (C-Br). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.40-3.52 (m, 2H, CH2N), 3.69 (t, 2H, CH2Cl, 3J = 5.8), 6.48 (t, 1H, NH3, 3J′ = 5.8), 8.84 (s, 1H, NH1), 7.30-7.50 (m, 4H, Harom).
Ureas 3-9 and compound 29 were also synthesized according to this procedure with a reflux time in a range of 5 min to 39 h.
1-(2-Chloroethyl)-3-(3-chlorophenyl)urea (3)
Recrystallized from diethyl ether. M.p. = 99°C, Yield = 65%. IR (KBr) (ν, cm− 1) 3357 (NH), 1638 (C = O), 1076 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.55-3.64 (m, 4H, CH2-CH2Cl), 5.89 (bs, 1H, NH3), 7.52 (s, 1H, NH1), 6.97-7.02 (m, 1H, H4′), 7.14-7.17 (m, 2H, H5′ and H6′), 7.34 (dd, 1H, H2′, 4J = 4J′ = 1.8).
1-(2-Chloroethyl)-3-(4-methylthiophenyl)urea (4)
Recrystallized from chloroform. M.p. = 123°C, Yield = 48%. IR (KBr) (ν, cm− 1) 3334 (NH), 1636 (C = O), 818 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 2.47 (s, 3H, CH3), 3.56-3.68 (m, 4H, CH2-CH2Cl), 5.53 (bs, 1H, NH3), 6.95 (s, 1H, NH1), 7.21-7.27 (m, 4H, Harom).
1-(2-Chloroethyl)-3-(2-phenoxyphenyl)urea (5)
Recrystallized from diethyl ether. M.p. = 130°C, Yield = 74%. IR (KBr) (ν, cm− 1) 3341 (NH), 1641 (C = O), 750 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.55-3.65 (m, 4H, CH2-CH2Cl), 5.40 (bs, 1H, NH3), 6.84 (dd, 1H, H3′, 3J = 8.0, 4J = 1.6), 6.94 (dd, 1H, H4′, 3J = 3J′ = 8.0), 6.98 (d, 2H, H2″ and H6″, 3J‴ = 8.0), 7.03 (s, 1H, NH1), 7.08-7.14 (m, 2H, H5′ and H4″), 7.33 (dd, 2H, H3″ and H5″, 3J‴ = 3J″″ = 8.0), 8.11 (dd, 1H, H6′, 3J″ = 8.0, 4J = 1.2).
1-(2-Chloroethyl)-3-(4-chloro-3-trifluoromethylphenyl)urea (6)
Recrystallized from diisopropyl ether. M.p. = 123°C, Yield = 50%. IR (KBr) (ν, cm− 1) 3366 (NH), 1654 (C = O), 1029 and 829 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.52-3.72 (m, 4H, CH2-CH2Cl), 5.96 (bs, 1H, NH3), 7.81 (s, 1H, NH1), 7.29-7.41 (m, 2H, H5′ and H6′), 7.58 (d, 1H, H2′, 4J = 2.1).
1-(2-Chloroethyl)-3-(1,3-dimethyl(1H)pyrazol-5-yl)urea (7)
Recrystallized from dichloromethane/diethyl ether (50/50). M.p. = 128°C, Yield = 35%. IR (KBr) (ν, cm− 1) 3327 (NH), 1687 (C = O), 782 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 2.25 (s, 3H, CH3), 3.51-3.63 (m, 2H, CH2N), 3.64 (t, 2H, CH2Cl, 3J = 5.8), 3.71 (s, 3H, NCH3), 5.30 (bs, 1H, NH3), 5.97 (s, 1H, Hpyraz), 6.66 (s, 1H, NH1).
1-(2-Chloroethyl)-3-(quinolin-8-yl)urea (8)
Recrystallized from diethyl ether. M.p. = 157°C, Yield = 84%. IR (KBr) (ν, cm− 1) 3309 (NH), 1648 (C = O), 823 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.68-3.78 (m, 4H, CH2-CH2Cl), 5.61 (bs, 1H, NH3), 7.39-7.45 (m, 2H, H3′ and H5′), 7.52 (dd, 1H, H6′, 3J = 3J′ = 7.6), 8.15 (dd, 1H, H4′, 3J″ = 8.3, 4J = 1.6), 8.53 (dd, 1H, H7′, 3J = 7.6, 4J = 1.2), 8.74 (dd, 1H, H2′, 3J‴ = 4.2, 4J = 1.6), 9.10 (s, 1H, NH1).
1-(2-Chloroethyl)-3-(1H-indol-5-yl)urea (9)
Recrystallized from diethyl ether. M.p. = 153°C, Yield = 90%. IR (KBr) (ν, cm− 1) 3421 (NHindol), 3315 (NH), 1626 (C = O), 734 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.39-3.51 (m, 2H, CH2N), 3.69 (t, 2H, CH2Cl, 3J = 5.8), 6.24 (s, 1H, H3′), 6.30 (t, 1H, NH3, 3J′ = 5.8), 7.14-7.18 (m, 1H, H6′), 7.24-7.28 (m, 2H, H2′ and H7′), 7.87 (s, 1H, H4′), 8.37 (s, 1H, NH1), 10.92 (s, 1H, NHindol).
1-(2-Chloroethyl)-3-(2-morpholin-4-yl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)urea (29)
Recrystallized from diethyl ether. M.p. = 207°C, Yield = 70%. IR (KBr) (ν, cm− 1) 3354 (NH), 1773 and 1716 (C = Oimide), 1653 (C = O), 738 (C-Cl). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.33-3.38 (m, 4H, CH2Nmorphol), 3.44-3.61 (m, 2H, CH2N), 3.70-3.81 (m, 6H, CH2Cl and CH2O), 6.74 (bs, 1H, NH3), 7.68 (d, 1H, H6′, 3J = 8.2), 7.81 (d, 1H, H7′, 3J = 8.2), 8.07 (s, 1H, H4′), 9.49 (s, 1H, NH1).
1-(2-Chloroethyl)-3-(2-morpholin-4-yl-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl)urea (28)
In a sealed tube containing 3-aminophthalimide (0.51 g, 2.06 mmol) was added 2-chloroethyl isocyanate (1.43 mL, 16,48 mmol). The mixture was stirred and heated by microwaves at 82°C with a 20 W power during 40 min and taken up into acetone. The solvent was then evaporated under reduced pressure and purification was accomplished by column chromatography over silica gel with dichloromethane. The residue was recrystallized from diethyl ether to give urea 28 as a white powder. M.p. = 226°C, Yield = 79%. IR (KBr) (ν, cm− 1) 3328 (NH), 1772 and 1712 (C = Oimide), 1700 (C = O), 745 (C-Cl). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.33-3.38 (m, 4H, CH2Nmorphol), 3.42-3.54 (m, 2H, CH2N), 3.60-3.85 (m, 6H, CH2O and CH2Cl), 7.39 (d, 1H, H7′, 3J = 7.0), 7.73 (dd, 1H, H6′, 3J = 7.0, 3J′ = 8.5), 8.13 (bs, 1H, NH3), 8.57 (d, 1H, H5′, 3J′ = 8.5), 8.94 (s, 1H, NH1).
Ureas 30-32 were prepared according to the same procedure.
1-(2-Chloroethyl)-3-(2-phenyl-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl)urea (30)
Recrystallized from diethyl ether. M.p. = 193°C, Yield = 66%. IR (KBr) (ν, cm− 1) 3391 (NH), 1753 and 1700 (C = Oimide), 1682 (C = O), 767 (C-Cl). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.43-3.55 (m, 2H, CH2N), 3.73 (t, 2H, CH2Cl, 3J = 5.8), 7.40-7.80 (m, 6H, Harom and H7′), 7.80 (dd, 1H, H6′, 3J′ = 7.3, 3J″ = 8.5), 8.16 (t, 1H, NH3, 3J = 5.8), 8.60 (d, 1H, H5′, 3J″ = 8.5), 9.04 (s, 1H, NH1).
1-(2-Chloroethyl)-3-(2-phenyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)urea (31)
Recrystallized from diethyl ether. M.p. = 230°C, Yield = 75%. IR (KBr) (ν, cm− 1) 3359 (NH), 1774 and 1717 (C = Oimide), 1703 (C = O), 748 (C-Cl). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.48-3.57 (m, 2H, CH2N), 3.74 (t, 2H, CH2Cl, 3J = 5.8), 6.77 (t, 1H, NH3, 3J = 5.8), 7.43-7.59 (m, 5H, Harom), 7.71 (dd, 1H, H6′, 3J′ = 8.2, 4J = 1.8), 7.86 (d, 1H, H7′, 3J′ = 8.2), 8.19 (d, 1H, H4′, 4J = 1.8), 9.55 (s, 1H, NH1).
1-(2-Chloroethyl)-3-(2-benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl)urea (32)
Recrystallized from dichloromethane. M.p. = 186°C, Yield = 41%. IR (KBr) (ν, cm− 1) 3317 (NH), 1760 and 1708 (C = Oimide), 1659 (C = O), 740 (C-Cl). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.42-3.54 (m, 2H, CH2N), 3.71 (t, 2H, CH2Cl, 3J = 5.2), 4.78 (s, 2H, CH2Ph), 7.20-7.40 (m, 5H, Harom), 7.44 (d, 1H, H7′, 3J′ = 6.7), 7.73 (dd, 1H, H6′, 3J′ = 6.7, 3J″ = 8.5), 8.11 (t, 1H, NH3, 3J = 5.2), 8.58 (d, 1H, H5′, 3J″ = 8.5), 8.97 (s, 1H, NH1).
1-(4-Chlorophenyl)-3-(3-chloropropyl)urea (38)
To a solution of 4-chloroaniline (1 g, 7.84 mmol) in chloroform (50 mL) was added dropwise 3-chloropropyl isocyanate (0.81 mL, 7.84 mmol). The mixture was refluxed for 1 h, and then the solvent was removed under reduced pressure. The crystalline residue was recrystallized from diethylether to give compound 38 as a white powder. M.p. = 143°C, Yield = 95%. IR (KBr) (ν, cm− 1) 3331 (NH), 1635 (C = O), 828 (C-Cl). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 1.86-1.98 (m, 2H, CH2CH2CH2), 3.18-3.30 (m, 2H, CH2NH), 3.70 (t, 2H, CH2Cl, 3J = 6.6), 6.33 (t, 1H, NH3, 3J′ = 6.6), 7.29 (d, 2H, H3′ and H5′, 3J″ = 8.9), 7.45 (d, 1H, H2′ and H6′, 3J″ = 8.9), 8.63 (s, 1H, NH1).
Ureas 39-41 were synthesized according to this method.
1-(3-Chloro-4-fluorophenyl)-3-(3-chloropropyl)urea (39)
Recrystallized from diethyl ether. M.p. = 107°C, Yield = 84%. IR (KBr) (ν, cm− 1) 3336 (NH), 1651 (C = O), 1217 (C-F), 1052 and 797 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 1.87-1.99 (m, 2H, CH2CH2CH2), 3.29-3.41 (m, 2H, CH2NH), 3.56 (t, 2H, CH2Cl, 3J = 6.2), 6.02 (t, 1H, NH3, 3J′ = 6.2), 6.91-7.00 (m, 2H, H5′ and H6′), 7.37 (dd, 1H, H2′, 4JHF = 6.5, 4J = 2.4), 7.95 (s, 1H, NH1).
1-(3-Chloropropyl)-3-(2-methoxy-5-trifluoromethylphEnyl)urea (40)
Recrystallized from diethyl ether. M.p. = 158°C, Yield = 68%. IR (KBr) (ν, cm− 1) 3370 (NH), 1653 (C = O), 810 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 1.90-2.15 (m, 2H, CH2CH2CH2), 3.45-3.65 (m, 4H, CH2CH2CH2), 3.91 (s, 3H, OCH3), 5.01 (bs, 1H, NH3), 6.89 (d, 1H, H3′, 3J = 7.0), 6.99 (s, 1H, H6′), 7.25 (d, 1H, H4′, 3J = 7.0), 8.47 (s, 1H, NH1).
1-(3-Chloro-4-cyanophenyl)-3-(3-chloropropyl)urea (41)
Recrystallized from diethyl ether. M.p. = 111°C, Yield = 89%. IR (KBr) (ν, cm− 1) 3321 (NH), 2226 (C ≡ N), 1677 (C = O), 828 and 1045 (C-Cl). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 1.80-2.00 (m, 2H, CH2CH2CH2), 3.20-3.30 (m, 2H, CH2NH), 3.65-3.75 (m, 2H, CH2Cl), 6.65 (bs, 1H, NH3), 7.40 (d, 1H, H6′, 3J = 8.8), 7.79 (d, 1H, H5′, 3J = 8.8), 7.94 (s, 1H, H2′).
1-(4-Bromophenyl)imidazolidin-2-one (10)
Urea 2 (2 g, 7.21 mmol) was dissolved in acetonitrile (50 mL) and cesium carbonate (2.35 g, 7.21 mmol) was added. The reaction mixture was stirred and refluxed for 20 h and then filtered. The filtrate solvent was evaporated in vacuo. The crystalline residue was recrystallized from diethyl ether to give compound 10 as a white powder. M.p. = 185°C, Yield = 75%. MS-ES+ (CH3OH) (m/z) 241 (81Br), 239 (79Br). IR (KBr) (ν, cm− 1) 1681 (C = O), 1071 (C-Br). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.58 (t, 2H, CH2NH, 3J = 8.2), 3.90 (t, 2H, CH2N, 3J = 8.2), 5.53 (bs, 1H, NH), 7.40-7.45 (m, 4H, Harom).
All imidazolidin-2-ones and tetrahydropyrimidin-2(1H)-ones, compound 17 excepted, were prepared according to this procedure, with a reflux time of 30 min to 19 h.
1-(3-Chlorophenyl)imidazolidin-2-one (11)
Recrystallized from diethyl ether. M.p. = 121°C, Yield = 47%. MS-ES+ (CH3OH) (m/z) 197 (37Cl), 195 (35Cl). IR (KBr) (ν, cm− 1) 1703 (C = O), 1080 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.59 (t, 2H, CH2NH, 3J = 8.5), 3.91 (t, 2H, CH2N, 3J = 8.5), 5.65 (bs, 1H, NH), 7.02 (dd, 1H, H4, 3J′ = 8.2, 4J = 1.2), 7.25 (dd, 1H, H5, 3J′ = 3J″ = 8.2), 7.43 (dd, 1H, H6, 3J″ = 8.2, 4J′ = 1.2), 7.60 (dd, 1H, H2, 4J = 4J′ = 1.2).
1-(4-Methylthiophenyl)imidazolidin-2-one (12)
Recrystallized from diisopropyl ether. M.p. = 187°C, Yield = 32%. MS-ES+ (CH3OH) (m/z) 208. IR (KBr) (ν, cm− 1) 1707 (C = O). 1H NMR (250 MHz, CDCl3) (δ, ppm) 2.47 (s, 3H, CH3), 3.57 (t, 2H, CH2NH, 3J = 7.3), 3.87-3.95 (m, 2H, CH2N), 5.08 (bs, 1H, NH), 7.28 (d, 2H, H3 and H5, 3J′ = 8.8), 7.48 (d, 2H, H2 and H6, 3J′ = 8.8).
1-(2-Phenoxyphenyl)imidazolidin-2-one (13)
Recrystallized from diethyl ether. M.p. = 124°C, Yield = 48%. MS-ES+ (CH3OH) (m/z) 257 ([M + 2H]+). IR (KBr) (ν, cm− 1) 1682 (C = O). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.42 (t, 2H, CH2NH, 3J = 8.4), 3.89 (t, 2H, CH2N, 3J = 8.4), 5.18 (bs, 1H, NH), 6.96-7.01 (m, 3H, H3, H2′ and H6′), 7.09 (t, 1H, H4′, 3J′ = 7.5), 7.15-7.23 (m, 2H, H4 and H5), 7.32 (dd, 2H, H3′ and H5′, 3J′ = 3J″ = 7.5), 7.52 (dd, 1H, H6, 3J‴ = 7.2, 4J = 2.0).
1-(4-Chloro-3-trifluoromethylphenyl)imidazolidin-2-one (14)
Recrystallized from diisopropyl ether. M.p. = 148°C, Yield = 76%. MS-ES+ (CH3OH) (m/z) 265. IR (KBr) (ν, cm− 1) 1719 (C = O), 1025 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.55-3.70 (m, 2H, CH2NH), 3.90-4.00 (m, 2H, CH2N), 5.44 (bs, 1H, NH), 7.44 (d, 1H, H5, 3J = 8.8), 7.76 (dd, 1H, H6, 3J = 8.8, 4J = 2.8), 7.82 (d, 1H, H2, 4J = 2.8).
1-(1,3-Dimethyl(1H)pyrazol-5-yl)imidazolidin-2-one (15)
Recrystallized from diethyl ether. M.p. = 130°C, Yield = 49%. MS-ES+ (CH3OH) (m/z) 180. IR (KBr) (ν, cm− 1) 1704 (C = O). 1H NMR (250 MHz, CDCl3) (δ, ppm) 2.23 (s, 3H, CH3), 3.60 (t, 2H, CH2NH, 3J = 7.4), 3.74 (s, 3H, NCH3), 3.79 (t, 2H, CH2N, 3J = 7.4), 5.50 (bs, 1H, NH), 5.88 (s, 1H, Hpyraz).
1-(Quinolin-8-yl)imidazolidin-2-one (16)
Recrystallized from diethyl ether. M.p. = 151°C, Yield = 52%. MS-ES+ (CH3OH) (m/z) 213. IR (KBr) (ν, cm− 1) 1702 (C = O). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.53 (t, 2H, CH2NH, 3J = 8.3), 4.17 (t, 2H, CH2N, 3J = 8.3), 6.81 (bs, 1H, NH), 7.60 (dd, 1H, H3, 3J′ = 8.3, 3J″ = 4.1), 7.63 (dd, 1H, H6, 3J‴ = 8.2, 3J″″ = 7.2), 7.77 (dd, 1H, H7, 3J″″ = 7.2, 4J = 1.1), 7.90 (dd, 1H, H5, 3J‴ = 8.2, 4J = 1.1), 8.44 (dd, 1H, H4, 3J′ = 8.3, 4J′ = 1.7), 8.95 (dd, 1H, H2, 3J″ = 4.1, 4J′ = 1.7).
1-(2-Morpholin-4-yl-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl)imidazolidin-2-one (33)
Recrystallized from diethyl ether. M.p. = 193°C, Yield = 59%. MS-ES+ (CH3OH) (m/z) 318 ([M + 2H]+). IR (KBr) (ν, cm− 1) 1771 and 1722 (C = Oimide), 1661 (C = O). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.25-3.35 (m, 4H, CH2Nmorphol), 3.49 (t, 2H, CH2N, 3J = 7.0), 3.65-3.80 (m, 4H, CH2O), 4.06 (t, 2H, CH2NH, 3J = 7.0), 7.19 (bs, 1H, NH), 7.65 (d, 1H, H7, 3J′ = 6.7), 7.78-7.88 (m, 2H, H5 and H6).
1-(2-Morpholin-4-yl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)imidazolidin-2-one (34)
Recrystallized from diethyl ether. M.p. = 288°C, Yield = 61%. MS-ES+ (CH3OH) (m/z) 318 ([M + 2H]+). IR (KBr) (ν, cm− 1) 1774 and 1719 (C = Oimide), 1655 (C = O). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.25-3.35 (m, 4H, CH2Nmorphol), 3.49 (t, 2H, CH2N, 3J = 7.3), 3.65-3.80 (m, 4H, CH2O), 4.00 (t, 2H, CH2NH, 3J = 7.3), 7.48 (bs, 1H, NH), 7.81-7.89 (m, 2H, H6 and H7), 8.20 (s, 1H, H4).
1-(2-Phenyl-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl)imidazolidin-2-one (35)
Recrystallized from diethyl ether. M.p. = 201°C, Yield = 87%. MS-ES+ (CH3OH) (m/z) 309 ([M + 2H]+). IR (KBr) (ν, cm− 1) 1759 and 1714 (C = Oimide), 1654 (C = O). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.44-3.52 (m, 2H, CH2N), 4.09 (t, 2H, CH2NH, 3J = 8.1), 7.20 (bs, 1H, NH), 7.45-7.60 (m, 5H, Harom), 7.77 (dd, 1H, H7, 3J′ = 6.7, 4J = 1.5), 7.89 (dd, 1H, H6, 3J′ = 6.7, 3J″ = 8.3), 7.94 (dd, 1H, H5, 3J″ = 8.3, 4J = 1.5).
1-(2-Phenyl-1,3-dioxo-2,3-dihydro-1H-isoindol-5-yl)imidazolidin-2-one (36)
Recrystallized from diethyl ether. M.p. = 312°C, Yield = 78%. MS-ES+ (CH3OH) (m/z) 309 ([M + 2H]+). IR (KBr) (ν, cm− 1) 1774 and 1726 (C = Oimide), 1704 (C = O). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.52 (t, 2H, CH2N, 3J = 7.0), 4.05 (t, 2H, CH2NH, 3J = 7.0), 7.40-7.60 (m, 6H, NH and Harom), 7.90-7.95 (m, 2H, H6 and H7), 8.30 (s, 1H, H4).
1-(2-Benzyl-1,3-dioxo-2,3-dihydro-1H-isoindol-4-yl)imidazolidin-2-one (37)
Recrystallized from ethanol. M.p. = 175°C, Yield = 39%. MS-ES+ (CH3OH) (m/z) 323 ([M + 2H]+). IR (KBr) (ν, cm− 1) 1769 and 1709 (C = Oimide), 1656 (C = O). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.43-3.51 (m, 2H, CH2N), 4.08 (t, 2H, CH2NH, 3J = 7.0), 4.78 (s, 2H, CH2), 7.21 (bs, 1H, NH), 7.30-7.40 (m, 5H, Harom), 7.69 (d, 1H, H7, 3J′ = 6.7), 7.79-7.90 (m, 2H, H5 and H6).
1-(4-Chlorophenyl)tetrahydropyrimidin-2(1H)-one (42)
Recrystallized from diethyl ether. M.p. = 164°C, Yield = 86%. MS-ES+ (CH3OH) (m/z) 212 (37Cl), 210 (35Cl). IR (KBr) (ν, cm− 1) 1657 (C = O), 1092 (C-Cl). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 1.93-2.01 (m, 2H, CH2CH2CH2), 3.25 (td, 2H, CH2NH, 3J = 5.6, 4J = 2.5), 3.64 (t, 2H, CH2N, 3J′ = 5.6), 6.69 (bs, 1H, NH), 7.30-7.45 (m, 4H, Harom).
1-(3-Chloro-4-fluorophenyl)tetrahydropyrimidin-2(1H)-one (43)
Recrystallized from diethyl ether. M.p. = 153°C, Yield = 70%. MS-ES+ (CH3OH) (m/z) 230 (37Cl), 228 (35Cl). IR (KBr) (ν, cm− 1) 1663 (C = O), 1223 (C-F), 1055 (C-Cl). 1H NMR (250 MHz, CDCl3) (δ, ppm) 2.04-2.12 (m, 2H, CH2CH2CH2), 3.41 (td, 2H, CH2NH, 3J = 5.8, 4J = 2.4), 3.64 (t, 2H, CH2N, 3J′ = 5.8), 5.61 (bs, 1H, NH), 7.10 (dd, 1H, H5, 3J″ = 3JHF = 8.8), 7.19 (ddd, 1H, H6, 3J″ = 8.8, 4JHF = 4.3, 4J = 2.7), 7.37 (dd, 1H, H2, 4J′HF = 6.7, 4J = 2.7).
1-(2-Methoxy-5-trifluoromethylphenyl)tetrahydropyrimidin-2(1H)-one (44)
Recrystallized from diethyl ether. M.p. = 153°C, Yield = 20%. MS-ES+ (CH3OH) (m/z) 276 ([M + 2H]+). IR (KBr) (ν, cm− 1) 1665 (C = O). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 1.85-2.05 (m, 2H, CH2CH2CH2), 3.15-3.55 (m, 4H, CH2CH2CH2), 3.88 (s, 3H, OCH3), 6.58 (s, 1H, NH), 7.26 (d, 1H, H3, 3J = 8.6), 7.52 (s, 1H, H6), 7.62 (d, 1H, H4, 3J = 8.6).
1-(3-Chloro-4-cyanophenyl)tetrahydropyrimidin-2(1H)-one (45)
Recrystallized from diisopropyl ether. M.p. = 169°C, Yield = 88%. MS-ES+ (CH3OH) (m/z) 240 ([M + 2H]+., 37Cl), 238 ([M + 2H]+, 35Cl). IR (KBr) (ν, cm− 1) 2227 (C ≡ N), 1669 (C = O), 1047 (C-Cl). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 1.96-2.03 (m, 2H, CH2CH2CH2), 3.25 (td, 2H, CH2NH, 3J = 5.6, 4J = 2.4), 3.74 (t, 2H, CH2N, 3J′ = 5.6), 7.12 (bs, 1H, NH), 7.54 (dd, 1H, H6, 3J″ = 8.8, 4J = 2.0), 7.81 (d, 1H, H2, 4J = 2.0), 7.89 (d, 1H, H5, 3J″ = 8.8).
1-(1H-indol-5-yl)imidazolidin-2-one (17)
Urea 9 (0.5 g, 2.10 mmol) was dissolved in acetonitrile (50 mL) and sodium carbonate (0.22 g, 2.10 mmol) was added. The reaction mixture was stirred and refluxed for 6 h and then filtered. The filtrate solvent was removed under reduced pressure. Crystallization of the oily residue from dichloromethane/diethyl ether (50/50) gave the compound 17 as a white powder. M.p. = 256°C, Yield = 41%. MS-ES+ (CH3OH) (m/z) 202 ([M + H]+). IR (KBr) (ν, cm− 1) 3384 (NHindol), 1674 (C = O). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.78 (t, 2H, CH2NH, 3J = 8.2), 4.24 (t, 2H, CH2N, 3J = 8.2), 6.34 (s, 1H, H3), 7.16 (d, 1H, H6, 3J′ = 8.8), 7.24-7.28 (m, 2H, H2 and H7), 7.87 (s, 1H, H4), 8.70 (bs, 1H, NH), 10.88 (s, 1H, NHindol).
2-Morpholin-4-yl-4-nitro-1H-isoindole-1,3(2H)-dione (18)
To a solution of 3-nitrophthalic anhydride (2 g, 10.36 mmol) in glacial acetic acid (15 mL) was added N-aminomorpholine (1 mL, 10.36 mmol). The reaction mixture was stirred and refluxed for 19 h and then evaporated in vacuo. The crystalline residue was taken up into a solution of sodium hydrogenocarbonate (4%), filtered, washed with water, dried and recrystallized from ethanol to give 18 as a yellow powder. M.p. = 189°C, Yield = 79%. IR (KBr) (ν, cm− 1) 1795 and 1729 (C = Oimide), 1530 and 1360 (NO2). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.25-3.40 (m, 4H, CH2N), 3.70-3.80 (m, 4H, CH2O), 8.09 (dd, 1H, H6, 3J = 3J′ = 7.6), 8.17 (d, 1H, H7, 3J = 7.6), 8.31 (d, 1H, H5, 3J′ = 7.6).
Compounds 19-22 were synthesized according this method.
2-Morpholin-4-yl-5-nitro-1H-isoindole-1,3(2H)-dione (19)
Recrystallized from ethanol. M.p. = 221°C, Yield = 72%. IR (KBr) (ν, cm− 1) 1724 and 1721 (C = Oimide), 1540 and 1346 (NO2). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.42 (t, 4H, CH2N, 3J = 4.3), 3.89 (t, 4H, CH2O, 3J = 4.3), 8.06 (d, 1H, H7, 3J′ = 8.0), 8.63 (dd, 1H, H6, 3J′ = 8.0, 4J = 2.0), 8.67 (d, 1H, H4, 4J = 2.0).
2-Phenyl-4-nitro-1H-isoindole-1,3(2H)-dione (20)
Recrystallized from ethanol. M.p. = 121°C, Yield = 91%. IR (KBr) (ν, cm− 1) 1776 and 1734 (C = Oimide), 1545 and 1352 (NO2). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 7.48-7.62 (m, 5H, Harom), 8.16 (dd, 1H, H6, 3J = 3J′ = 7.6), 8.30 (dd, 1H, H7, 3J = 7.6, 4J = 0.9), 8.38 (dd, 1H, H5, 3J′ = 7.6, 4J = 0.9).
2-Phenyl-5-nitro-1H-isoindole-1,3(2H)-dione (21)
Recrystallized from ethanol. M.p. = 189°C, Yield = 94%. IR (KBr) (ν, cm− 1) 1780 and 1719 (C = Oimide), 1542 and 1342 (NO2). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 7.48-7.63 (m, 5H, Harom), 8.26 (d, 1H, H7, 3J = 8.3), 8.63 (d, 1H, H4, 4J = 1.8), 8.73 (dd, 1H, H6, 3J = 8.3, 4J = 1.8).
2-Benzyl-4-nitro-1H-isoindole-1,3(2H)-dione (22)
Recrystallized from ethanol. M.p. = 141°C, Yield = 87%. IR (KBr) (ν, cm− 1) 1778 and 1720 (C = Oimide), 1538 and 1331 (NO2). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 4.81 (s, 2H, CH2), 7.30-7.38 (m, 5H, Harom), 8.10 (dd, 1H, H6, 3J = 3J′ = 7.6), 8.22 (d, 1H, H7, 3J = 7.6), 8.32 (d, 1H, H5, 3J′ = 7.6).
4-Amino-2-morpholin-4-yl-1H-isoindole-1,3(2H)-dione (23)
To a solution of compound 18 (1.29 g, 4.65 mmol) in tetrahydrofurane (100 mL) was added catalytic quantity of 5% palladium on carbon. The reaction mixture was heated at 50°C and stirred under hydrogen atmosphere for 8 h. The catalyst was then filtered and the solvent evaporated under reduced pressure. The oily residue was purified by column chromatography over silica gel with dichloromethane and recrystallized from diethyl ether to give compound 23 as a yellow powder. M.p. = 264°C, Yield = 89%. IR (KBr) (ν, cm− 1) 3399 and 1623 (NH2), 1773 and 1705 (C = Oimide). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 3.30 (t, 4H, CH2N, 3J = 4.3), 3.72 (t, 4H, CH2O, 3J = 4.3), 6.50 (bs, 2H, NH2), 6.96 (dd, 1H, H5, 3J = 7.0, 4J = 0.6), 7.02 (dd, 1H, H7, 3J′ = 8.5, 4J = 0.6), 7.47 (dd, 1H, H6, 3J = 7.6, 3J′ = 8.5).
Compounds 24-27 were synthesized according this method.
5-Amino-2-morpholin-4-yl-1H-isoindole-1,3(2H)-dione (24)
Recrystallized from diethyl ether. M.p. = 249°C, Yield = 80%. IR (KBr) (ν, cm− 1) 3454, 3353 and 1617 (NH2), 1763 and 1703 (C = Oimide). 1H NMR (250 MHz, CDCl3) (δ, ppm) 3.40 (t, 4H, CH2N, 3J = 4.9), 3.87 (t, 4H, CH2O, 3J = 4.9), 4.40 (bs, 2H, NH2), 6.84 (dd, 1H, H6, 3J = 8.0, 4J = 2.1), 7.02 (d, 1H, H4, 4J = 2.1), 7.60 (d, 1H, H7, 3J = 8.0).
4-Amino-2-phenyl-1H-isoindole-1,3(2H)-dione (25)
Recrystallized from diethyl ether. M.p. = 180°C, Yield = 80%. IR (KBr) (ν, cm− 1) 3472, 3351 and 1630 (NH2), 1753 and 1704 (C = Oimide). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 6.60 (bs, 2H, NH2), 7.06-7.12 (m, 2H, H5 and H7), 7.42-7.58 (m, 6H, H6 and Harom).
5-Amino-2-phenyl-1H-isoindole-1,3(2H)-dione (26)
Recrystallized from diethyl ether. M.p. = 207°C, Yield = 68%. IR (KBr) (ν, cm− 1) 3492, 3373 and 1629 (NH2), 1769 and 1690 (C = Oimide). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 6.61 (bs, 2H, NH2), 6.90 (d, 1H, H6, 3J = 8.0), 7.04 (s, 1H, H4), 7.30-7.54 (m, 5H, Harom), 7.62 (d, 1H, H7, 3J = 8.0).
4-Amino-2-benzyl-1H-isoindole-1,3(2H)-dione (27)
Recrystallized from diethyl ether. M.p. = 147°C, Yield = 78%. IR (KBr) (ν, cm− 1) 3477, 3355 and 1634 (NH2), 1744 and 1690 (C = Oimide). 1H NMR (250 MHz, DMSO-d6) (δ, ppm) 4.73 (s, 2H, CH2), 6.53 (bs, 2H, NH2), 7.00-7.05 (m, 2H, H5 and H7), 7.20-7.40 (m, 5H, Harom), 7.47 (dd, 1H, H6, 3J = 3J′ = 7.9).
Pharmacology
Drugs
All compounds were solubilized in DMSO and further diluted in RPMI (“Roswell Park Memorial Institute”) medium (Sigma, St Quentin Fallavier, France) complemented with 1% L-glutamine (Gibco, BRL, Paisley, Scotland) and 10% heat inactivated fetal calf serum (FCS) (Sigma) referred as complete medium. Cyclosporine A (CsA) (Tocris, Illkirch, France) was dissolved in absolute ethanol containing 2% Tween 80 and this solution was further diluted in complete RPMI medium.
Splenocytes proliferation
Splenocytes were isolated from two spleens of 8-week-old female C57BL/6 mice (CR Janvier, Laval, France). Spleens were aseptically harvested and homogenized in a Petri dish containing HBSS medium (Sigma). Splenocytes suspension was then hemolysed by buffer containing 20 mM Tris-HCl and 140 mM NH4Cl. Cells were washed twice with RPMI, subsequently suspended in complete RPMI medium and seeded at densities of 1.5 × 105/well in U-bottom 96-well culture plates (Falcon). Cells were incubated with 1 μg/mL concanavalin A (Sigma) in the presence of the studied compounds (90 μM) or CsA (5 μM) and cultured at 37°C in 5% CO2 in a final volume of 150 μL of complete RPMI medium supplemented with 50 μM mercaptoethanol. Cell proliferation was assessed in sextuplicate after 72 h of culture, by colorimetric detection. Briefly, cells were incubated with 12.5 μg/well of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) for 4 h at 37°C. Formazan products were solubilized by 100 μL of lysis buffer (dimethylformamide (1V), SDS 20% (2V), pH 4.7) and overnight incubation at 37°C. Cell growth was assessed using a MRX microplate reader (Dynex Technologies, Chantilly, USA) with the test wavelength at 570 nm and expressed as optical density (OD) values. The inhibition of splenocytes proliferation was expressed as inhibitory rate [(OD value of control untreated cells – OD value of treated cells)/OD value of control untreated cells group] × 100.
Statistics
All results were compared by ANOVA analysis followed by a Dunnett test when the ANOVA test gives a significant difference (p < 0.05) between the different groups.
Results and discussion
Access to the cyclic urea analogues was achieved by a two-step method including the preparation and characterization of intermediate ureas. We implemented a process previously described by Gabriel et al. [Citation10] consisting in the addition of a primary amine on a 2-chloroethyl or a 3-chloropropyl isocyanate. The resulting chloroalkylureas 2-9, 28-32 and 38-41 are then cyclised by a nucleophilic substitution reaction in alkaline conditions to give the corresponding cyclic ureic compounds 10-17, 33-37 and 42-45 (Schem, ). The choice of this method was justified by the fact that this process has generated very few failures in the series previously synthesized in our laboratory [Citation11,Citation12]. The amines used were commercially available except for the aminophthalimides, which were prepared from the corresponding nitrophthalimides by catalytic reduction. Synthesis of the nitrophthalimides was accomplished by the action of an amine on a nitrophthalic anhydride in acetic acid [Citation13,Citation14,Citation15].
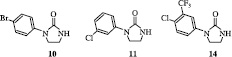
The effect of drugs on mouse splenocytes proliferation was examined in order to determine the immunosuppressive potential with a rapid low-cost in vitro test. Freshly isolated murine splenocytes were stimulated with 1 μg/mL ConA for 72 h in the presence of target cyclic ureas (90 μM). Splenocytes were also treated with CsA (5 μM) as a positive control. The results are shown in . The molecules, which exhibited a lymphocyte proliferation inhibition percentage lower than 30%, have been considered inactive. Among the 17 tested compounds, three of them exerted a moderate (10, 60% and 11, 43%) to potent (14, 91%) inhibitory activity. Generally speaking it seems that the molecules, which exert an immunosuppressive activity in this screening test show an imidazolidin-2-one scaffold N-substituted by a phenyl group. It seems however that the presence of a halogen on the phenyl moiety is favourable to the activity (10, 11 and 14). These observations are in agreement with the data previously observed in imidazolidin-2-one series [Citation9]. On the contrary the methylthio and the phenoxy group have demonstrated no interest (12 and 13 respectively). Moreover the replacement of the phenyl substituent by a heterocyclic group triggered a loss of potency as it can be testified by compounds 15, 16 and 17 and by phthalimidic derivatives 34, 35 and 37. Finally the elongation of the ureic cycle is responsible for a suppression of activity. Thus all the molecules of the tetrahydropyrimidin-2(1H)-one series (42-45) are inactive whereas some of their analogues in imidazolidin-2-one series were modestly to very potent [Citation9] suggesting the importance of the imidazolidin-2-one scaffold in the induction of an immunosuppressive activity.
Table I. Inhibition of the mouse splenocyte ConA-induced proliferation by N-substituted imidazolidin-2-ones 10-17 and 33-37 and tetrahydropyrimidin-2(1H)-ones 42-45 .
.
In conclusion, three new active molecules have been identified. The compound 14 exerts a potent activity in the ConA test with an inhibition of lymphocyte proliferation of 91%. Complementary studies are being investigated so as to confirm these results on human T lymphocytes. Other experiments must be realized in parallel on human MRC5 fibroblasts to determine the level of cytotoxicity of this molecule.
Acknowledgements
We would like to thank the “Région des Pays de la Loire” for financial support.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Z Yang, M Chen, LB Fialkow, JD Ellett, R WU, V Brinkmann, JL Nadler, and KR Lynch. (2003). The immune modulator FTY720 prevents autoimmune diabetes in nonobese diabetic mice small star, filled. Clin Immunol 107:30–35.
- ML Herrmann, R Schleyerbach, and BJ Kirschbaum. (2000). Leflunomide: An immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology 47:273–289.
- GS Alarcon. (2000). Methotrexate use in rheumatoid arthritis. A Clinician's perspective. Immunopharmacology 47:259–271.
- WW Epinette, CM Parker, EL Jones, and MC Greist. (1987). Mycophenolic acid for psoriasis. A review of pharmacology, long-term efficacy, and safety. J Am Acad Dermatol 17:962–971.
- MA Solsky, and DJ Wallace. (2002). New therapies in systemic lupus erythematosus. Best Pract Res Clin Rheumatol 16:293–312.
- http://test.efg.ddo.net.
- K Ross. (2007). For organ transplant recipients, cancer threatens long-term survival. J Natl Cancer Inst 99:421–422.
- P Libby, and JS Pober. (2001). Chronic rejection. Immunity 14:387–397.
- C Sabourin, JMH Robert, S Robert-Piessard, D Carbonnelle, and F Lang. (2004). Synthesis and evaluation of disubstituted N1- and N3-imidazolidin-2-ones acting as potential immunosuppressive agents. J Enz Inhib Med Chem 19:459–465.
- S Gabriel, and R Stelzner. (1895). Ueber vinylamin. Chem Ber 28:2938.
- H Abdala, JM Robert, P Le Pape, G Wielgosz, S Robert-Piessard, and G Le Baut. (2000). Synthesis and antileishmanial activity of new 1-(pyridin-2-yl)imidazolidin-2-ones derived from 2-amino-4,6-dimethylpyridine. Arzneim-Forsch/Drug Res 50:479–484.
- JM Robert, C Sabourin, N Alvarez, S Robert-Piessard, S Le Baut, and G Le Pape. (2003). Synthesis and antileishmanial activity of new imidazolidin-2-one derivatives. Eur J Med Chem 38:711–718.
- G Pagani, A Baruffini, P Borgna, and G Caccialanza. (1968). Different N-substutuents of phthalamic acid. Effect on geotropism of germinating roots of Lens esculenta Moench s.l. Il. Farmaco Ed Sci 23:448–467.
- G Pagani, G Caccialanza, L Vicarini, and A Baruffini. (1970). Effect of a series of substances related to N-alpha-naphthylphthalamic acid on root geotropism of Lens esculanta Moench s.1. seeds Il. Farmaco Ed Sci 25:203–225.
- Floc'h R. Contribution à l'étude des dérivés de la dihydro-2,3-1H-isoindolone-1 Thèse n 57, Université de Nantes, France 1979.