Abstract
Sarcodifurines A and B are two original dihydrofuroquinolines isolated from Sarcomelicope follicularis, a New Caledonian tree. The cytotoxicity and antiproliferative activity of these two alkaloids were investigated against 8 distinct cell lines representative of the most frequent solid tumors developing in human. Cytotoxicity of sarcodifurines was low on the 8 cell lines, with, for example, less than 10% of the total cells killed after 24 h exposure at 10 μM and IC50 ≈ 7.10− 5 M (MCF-7 and MDA MB 231 cell lines). Proliferation studies confirmed that sarcodifurines had a weak effect on cancer cells growth, with less than 5% growth inhibition at 10 μM. Sarcodifurine A activity was comparable to that of Sarcodifurine B, in term of cytotoxicity and antiproliferative activity on all cell lines. In spite of the weak activity of sarcodifurines and furoquinolines, rationalized pharmacomodulations to obtain planar analogs could lead to efficient topoisomerases inhibitors and DNA intercalants.
Introduction
Despite the important improvement of anticancer chemotherapy, cancer remains one of the major causes of death in developed countries, at the beginning of the twenty-first century.
Several phytochemicals are capital and undeniable drugs in current use against this pathology (e.g. Vinca alkaloids, taxol derivatives, camptothecins …).
Although plant-derived formulations account for one-fourth of the available anticancer treatments, natural products still represent an underexploited resource in this area, if we consider that only almost 10% of natural sources have been phytochemically studied [Citation1].
Numerous cytotoxic compounds were isolated from diverse higher plant families; particularly, the Rutaceae family constitutes one of the richest sources of cytotoxic alkaloids. This family of dicotyledon plants, commonly known as the Rue or Citrus family, contains 900 species, mostly tropical or subtropical woody plants, extremely polymorphous, especially characterized by schizolysigenous secretory cavities containing essential oils. Rutaceous alkaloids belong to different classes; among them, acridine, acridone and pyranoacridone derivatives have been described and well-studied because of their potential use as DNA intercalating agents. Among all acridones, acronycine received most attention [Citation2]. Acronycine was the first antitumor pyranoacridone isolated in the 1950s from Sarcomelicope simplicifolia ssp simplicifolia (Acronychia baueri), a New Caledonian endemic species belonging to the Rutaceae family. Because acronycine derivatives displayed promising antitumor activities including a wide range of solid tumors, the genus Sarcomelicope has captured the attention of phytochemists. This genus consists in 9 species nearly all endemic to New Caledonia [Citation3].
From 1948 to 2007, 53 alkaloids were isolated from the genus Sarcomelicope, belonging to 5 chemical classes: 3 pyranofuroquinolines, 10 quinolinones, 11 pyranoacridones, 14 furoquinolines and 15 acridones Citation4Citation5Citation6Citation7Citation8Citation9Citation10Citation11Citation12Citation13Citation14Citation15.
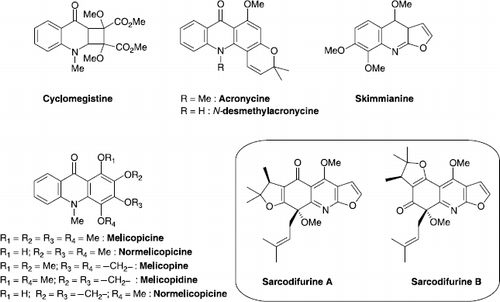
Among the twelve alkaloids [Citation13,Citation14] recently isolated by our group from stem barks and leaves of Sarcomelicope follicularis, two novel dihydrofuroquinolines: sarcodifurines A and B appeared particularly attractive (Scheme ).
As observed by J.P. Michael in his recent review on quinoline, quinazoline and acridone alkaloids:[Citation16] “ The genus Sarcomelicope continues to yield new metabolites with surprising structures. Sarcodifurines A and B […], also turned out to be 8-methoxy-8-prenyl derivatives of the furo[2,3-b]quinoline class, but with the additional fusion of an extraordinary 2,2,3-trimethyldihydrofuran ring”.
Even if quinolines are generally less cytotoxic than acridone derivatives [Citation17,Citation18], the unique and unusual skeleton of sarcodifurines A and B incited us to assess their cytotoxicity. Thus, these two alkaloids were evaluated against a panel of human and animal cancer cell lines, commonly used for primary screenings [Citation1].
Materials and methods
Plant material
Sarcomelicope follicularis Hartley is a pretty New Caledonian little tree 3-8 m high belonging to the Rutaceae family. The main botanical features are: unifoliolate leaves, white tetramerous flowers and glabrous white fruits.
Leaves were collected in January 2001 and stored dried until alkaloid extraction.
Extraction, isolation and structure determination
The cyclohexanic extract of dried and powdered leaves was chromatographied several times on silica gel to afford few mg of sarcodifurines A and B. Structures were elucidated on the basis of 1D and 2D NMR experiments and confirmed by X-Ray diffraction analyses.
For more details concerning phytochemical characteristic data and physico-chemical properties of sarcodifurines A and B, see references 13 and 14.
Cell lines and cell cultures
The cytotoxicity and antiproliferative activity of sarcodifurines were studied on 8 distinct cell lines representative of the most frequent solid tumors developing in human. All of them were obtained from the european ECAC collection except MCF-7 and MDA-MB-231 breast cancer cell lines that came from LGC Promochem and skin diploid fibroblasts that were provided by BIOPREDIC International Company. They included two human colon carcinoma cells Caco2 and HCT 116 representative of two distinct differentiated and highly colon tumorigenic tumors respectively, two breast tumor lines MCF-7 and MDA-MB-231 representative of steroid responding or not responding tumor cells, a differentiated highly growing HUH7 hepatocarcinoma cells, the NCI lung and PC3 prostate tumor cells. They all have adherent cell properties. They were grown according to the providers recommendations. MCF-7 is a hormone dependent epithelial breast cancer cell line tumorigenic in immunocompromised mice. MDA-MB-231 is classified both as a hormone-independent and a highly invasive breast cancer cell line.
Cytotoxicity assays
Two complementary strategies were performed, the first one consisting in determining viability and IC50 using the CellTiter 96® Non-radioactive cell proliferation assay (Promega) which allows to determine the fraction of viable cells remaining after drug treatment [Citation15]. Sarcodifurines were dissolved in dimethylsulfoxide (DMSO, Sigma-Aldrich) to give 10− 3 M stock solutions from which further dilutions were made in culture medium. 2.2 × 105 cells were seeded in each well of a 96-wells microplate and grown for 24 h in absence of sarcodifurines. Cells were then exposed for 24 h to control culture medium, lysis medium (medium containing 1% SDS) or medium containing 10− 10 to 10− 6 M sarcodifurine. Viability and IC50 were determined comparing treated to control cells (0 and 100% cytotoxicity), in 24 assays from three independent experiments.
The second strategy is based on an automated imaging analysis. The toxicity test of the compounds on cells was as followed: 4 × 103 cells are seeded in 96 multiwell plates and led for 24 h for attachment, spreading and growth. Then, they were exposed for 24 and 48 h to increasing concentrations of the compounds, ranging from 0.1 to 25μM in a final volume of 80μl of culture medium. They were fixed with 4% paraformaldehyde solution and nuclei were stained with Hoechst 3342 and counted according to automated imaging quantification. 4 pictures per well were obtained with a high speed camera and statistical analyses were established using the Simple PCI software. In addition, imaging analysis allowed detection of possible cell morphology changes.
Antiproliferative activity
Cells were seeded in 96-wells culture plates (5000 cells/well) in culture medium containing sarcodifurines 10− 10 to 10− 6 M. After72 h growth and exposure to sarcodifurines, viable cells were quantified using the CellTiter 96® Non-radioactive cell proliferation assay (Promega). Antiproliferative activity was expressed as a percentage of growth inhibition in 24 assays from 3 independent experiments. In parallel, nuclei counting was performed by automated imaging analysis. The assay included the comparative tumor cell responses to the molecules according to distinct tissular origins and also with the poorly growing normal diploid cells from human skin origin.
Results and discussion
Cytotoxicity of sarcodifurines was low on MCF-7 and MDA-MB-231 cell lines, with less than 10% of the total cells killed after 24 h exposure at 10 μM and IC50 ≈ 7. 10− 5 M. Proliferation studies confirmed that sarcodifurines had a weak effect on cancer cells growth, with less than 5% growth inhibition at 10 μM. Sarcodifurine A activity was comparable to that of Sarcodifurine B, in term of cytotoxicity and antiproliferative activity on all cancer cell lines. No effect was observed on normal skin fibroblasts. No evidence of morphological changes could be detected. Together these results argue for bad cytotoxic, bad antiproliferative and bad apoptotic effects of the molecules up to 10-20μM.
These results are close to those recently published by Frédérich and co-workers [Citation18]. They described the screening for cytotoxicity of 14 alkaloids. Among them, furoquinolines were tested. It was observed that no compounds of this class exerted a good cytotoxicity against the cell lines chosen finally (IC50 ≥ 100μM).
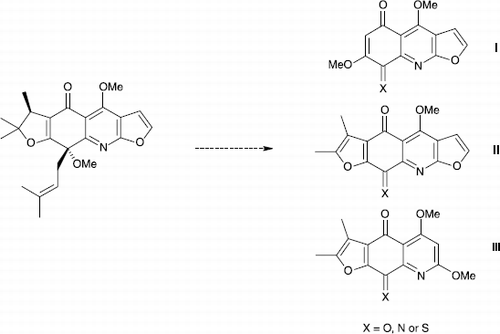
The values obtained with our products seem more significant (IC50 ≤ 70μM) and allowed us to expect to increase their biological activities by rationalized pharmacomodulations. For example, we are studying the synthesis of planar derivatives (e.g. compound I, II, and III in Scheme ) which can be able to intercalate with DNA and thus become potent inhibitors of topoisomerases.
In conclusion, the biological targets of sarcodifurines A and B remain unclear. Further studies are planed to improve the pharmaceutical interest of these so original heterocyclic alkaloids.
Acknowledgements
A large part of us thank the “Ligue Nationale Contre le cancer” for financial support: E.C. and T.B. for the. “Comité de Haute Normandie” and L.P. for the “Comité de Charente-Maritime.” C.G. and D.G. thank “Ouest Génopole” and the “Celchip/imaging platform” supported by the “Canceropôle Grand Ouest”.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- SE Kintzios. (2006). Terrestrial plant-derived anticancer agents and plant species used in anticancer research. Crit Rev Plant Sci 25:79–113.
- M Demeunynck, F Charmantray, and A Martelli. (2001). Interest of acridine derivatives in the anticancer chemotherapy. Curr Pharm Des 7:1703–1724.
- TG Hartley. (1986). Three new species of Sarcomelicope (Rutaceae) from New Caledonia (whith a key to the species of the genus). Bull Mus Natn Hist Nat 8:183–189.
- F Tillequin. (1997). Alkaloids in the genus Sarcomelicope. Recent Res Devel Phytochem 1:675–687.
- N Fokialakis, S Mitaku, E Mikros, AL Skaltsounis, F Tillequin, and T Sevenet. (1999). Megistosarcimine and megistosarconine, two alkaloids from Sarcomelicope megistophylla. Phytochemistry 52:1745–1748.
- N Fokialakis, P Magiatis, S Mitaku, F Tillequin, and T Sevenet. (2000). Two new 3-methoxy-4-quinolone alkaloids from the bark of Sarcomelicope megistophylla. Chem Pharm Bull 48:2009–2010.
- M Papageorgiou, N Fokialakis, S Mitaku, AL Skaltsounis, F Tillequin, and T Sevenet. (2000). Two new alkaloids from the bark of Sarcomelicope megistophylla. J Nat Prod 63:385–386.
- N Fokialakis, P Magiatis, AL Skaltsounis, F Tillequin, and T Sevenet. (2000). The structure of sarcomejine: an application of long-range (1)H-(15)N correlation at natural abundance. J Nat Prod 63:1004–1005.
- N Fokialakis, P Magiatis, AL Skaltsounis, F Tillequin, and T Sevenet. (2000). Megistolactone, a new alkaloid from Sarcomelicope megistophylla. J Biosci 50:874–876.
- N Fokialakis, P Magiatis, A Terzis, F Tillequin, and AL Skaltsounis. (2001). Cyclomegistine, the first alkaloid with the new cyclobuta[b]quinoline ring system from Sarcomelicope megistophylla. Tetrahedron Lett 42:5323–5325.
- N Fokialakis, P Magiatis, N Aligiannis, F Tillequin, and T Sevenet. (2001). Furomegistines I and II, two furanopyridine alkaloids from the bark of Sarcomelicope megistophylla. Phytochemistry 57:593–596.
- N Fokialakis, P Magiatis, I Chinou, S Mitaku, and F Tillequin. (2002). Megistoquinones I and II, two quinoline alkaloids with antibacterial activity from the bark of Sarcomelicope megistophylla. Chem Pharm Bull 50:413–414.
- E Chosson, P Vérité, A Blanckaert, E Seguin, M Litaudon, and T Sévenet. (2003). Non polar compounds from the bark of Sarcomelicope follicularis. Biochem Syst Ecol 31:1185–1188.
- E Chosson, AE Hay, A Chiaroni, A Favel, F Tillequin, M Litaudon, and E Seguin. (2004). Sarcodifurines A and B, two new furoquinolines from Sarcomelicope follicularis. Heterocycles 63:2043–2050.
- S Mitaku, N Fokialakis, P Magiatis, and F Tillequin. (2007). Alkaloids from Sarcomelicope megistophylla. Fitoterapia 78:169–170.
- JP Michael. (2007). Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep 24:223–246.
- B Cui, H Chai, Y Dong, F Horgen, B Hansen, D Madulid, D Soejarto, N Farnsworth, G Cordell, J Pezzuto, and A Kinghorn. (1999). Quinoline alkaloids from Acronychia laurifolia. Phytochemistry 52:95–98.
- O Jansen, V Akhmedjanova, L Angenot, G Balansard, A Chariot, E Ollivier, M Tits, and M Frédérich. (2006). Screening of 14 alkaloids isolated from Haplophyllum A Juss. for their cytotoxic properties. J Ethnopharmacol 105:241–245.