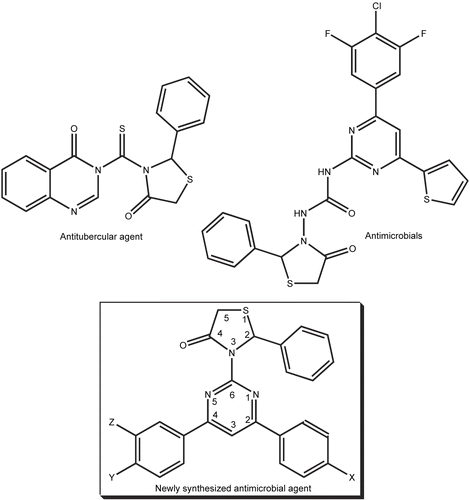
Abstract
A series of novel 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones 23-33 were synthesized, and studied for their in vitro antibacterial and antifungal activities against clinically isolated strains. Generally compounds possessing electron donating groups showed good antibacterial activity. Compound 31, which contain both electron withdrawing chloro and electron donating methyl groups showed potent activity against all the tested Gram positive and Gram negative bacterial strains whereas compounds 32 and 33 which contain electron donating methoxy functional group at the para position of the phenyl ring attached to pyrimidine ring showed promising activity against S.aureus, S.typhii and E.coli. Compounds 32 and 33, both containing electron withdrawing groups (-Cl, -F) showed excellent activities against all the tested A. flavus, Mucor, Rhizopus and M.gypsuem fungal strains. while against Mucor, compound 27 which contains an electron donating methyl group at the para position of the phenyl ring attached to pyrimidine ring showed promising activity. Also compound 31, which contains both electron withdrawing chloro and electron donating methyl groups showed potent activity against A. flavus and Rhizopus.
Introduction
Various 4-thiazolidinones have attracted considerable attention as they are endowed with wide range of pharmacological activities. Peptidoglycan is an essential component of the cell wall of both Gram-positive and Gram-negative bacteria. 4-Thiazolidinones have been reported as novel inhibitors of the bacterial enzyme Mur B which is a precursor, acting during the biosynthesis of peptidoglycan [Citation1]. A wide variety of biological properties such as hypolipidaemic [Citation2], antidegenerative [Citation3], muscarinic receptor 1 agonist [Citation4], antiproteolytic[Citation5], anti-inflammatory [Citation6], antiviral [Citation7], antifungal [Citation8], antibacterial [Citation9], antitubercular [Citation10], anticonvulsant [Citation11], respiratory [Citation12] and hypnotic [Citation13] activities have been reported for 4-thiazolidinones.
Pyrimidines are the basic nucleus in nucleic acids and have been associated with a number of biological activities. Substituted aminopyrimidine nuclei are common in marketed drugs such as anti-atherosclerotic aronixil, anti-histaminic thonzylamine, anti-anxielytic buspirone, anti-psoriatic enazadrem, and other medicinally relevant compounds. Some notable biological activity of pyrimidine derivatives include adenosine receptor antagonists [Citation14], kinase inhibitors [Citation15], analgesic [Citation16], anti-inflammatory [Citation16], inhibitors of cyclin-Dependent kinases 1 and 2 [Citation17], calcium channel antagonist [Citation18], antihistaminic [Citation19], antitubercular [Citation20] activities.
Recently, we exploited the synthesis of 6-aryl-1,2,4,5-tetrazinane-3-thiones [Citation21], fused indazoles [Citation22], 2,6-diarylpiperidin-4-one derivatives [Citation23–25] with a view to incorporate various other bioactive heterocyclic nucleus such as 1,2,3-selenadiazoles, 1,2,3-thiadiazoles, diazepans intact for evaluation of associated antibacterial and antifungal activities.
Among the various substituted thiazolidin-4-one compounds, pyrimidine moiety present thiazolidine-4-one compound exhibit strong tuberculosis [Citation26] and antimicrobial activity [Citation27]. In the interest of above, we planned to synthesize a system, which comprises both 4-thiazolidinones and 2-amino-4,6-diarylpyrimidine components together to give a compact structure like title 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones ().
Experimental
Chemistry
TLC was performed to assess the reactions and the purity of the products. All the reported melting points were taken in open capillaries and were uncorrected. IR spectra were recorded in KBr (pellet forms) on a Nicolet-Avatar–330 FT-IR spectrophotometer and noteworthy absorption values (cm−1) alone are listed. 1H and 13C NMR spectra were recorded at 400 MHz and 100 MHz respectively on Bruker AMX 400 NMR spectrometer using DMSO-d as solvent. The ESI +ve MS spectra were recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalysis was obtained on Carlo Erba 1106 CHN analyzer.
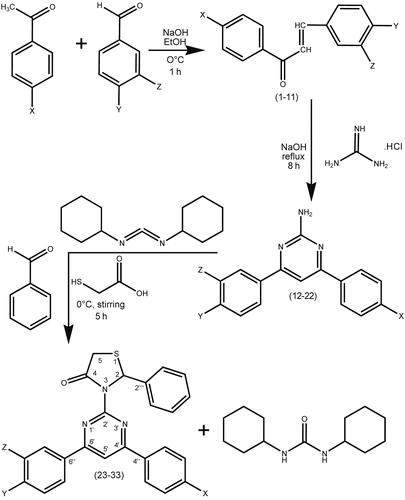
Adopting the literature reports 1,3-diaryl-prop-2-en-1-ones 1-11 [Citation28] and 2-amino-4,6-diarylpyrimidines 12-22 [Citation29], were synthesized.
General method for the synthesis of 2-phenyl-3- (4,6-diphenylpyrimidin-2-yl)thiazolidin-4-one 23
To an ice cold stirred solution of 2-amino-4,6-diphenylpyrimidine in dry dichloromethane was added benzaldehyde in drops followed by dicyclohexylcarbodiimide (DCC). After five min. thioglycolic acid was added and stirring was continued for additional 5 h at 0°C. Then the reaction mixture was filtered to remove dicyclohexyl urea followed by washing with 5% citric acid, 10% sodium bicarbonate, brine solution, finally with water and dried over anhydrous sodium sulfate. After evaporation of the solvent under reduced pressure, a gummy mass was obtained, which was solidified on treatment of petroleum ether (bp40°–60°). Final purification of 2-phenyl-3-(4,6-diphenylpyrimidin-2-yl)thiazolidin-4-one 23 was done by column chromatography using silica gel (100-200 mesh), with ethyl acetate -Petroleum ether (bp40–60) in the ratio (2:8) as eluent. IR (KBr) (cm−1): 3125, 3033, 2927, 2851, 1716, 1627, 1576, 1350, 710, 698, 649; 1H NMR (δ ppm): 3.21 (d, 1H, CH2a at H5a, J = 15.37Hz), 3.38 (d, 1H, CH2e at H5e, J = 15.37Hz), 5.25 (s, 1H, CH at H2), 7.19-8.37 (m, 16H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.0 C-5, 62.5 C-2, 108.1 C-5′, 131.4 C-2‴, 125.9-128.8 –Carom, 139.1 C-4′, 139.1 C-6″, 161.3 C-4′, 161.3 C-6′, 163.8 C-2′, 170.6 C-4.
The compounds 24-33 were similarly synthesized.
3-(4-(4″-chlorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one. 24 IR (KBr) (cm−1): 3120, 3033, 2927, 2851, 1696, 1627, 1575, 1310, 894, 710, 650, 647; 1H NMR (δ ppm): 3.22 (d, 1H, CH2a at H5a, J = 15.37Hz), 3.39 (d, 1H, CH2e at H5e, J = 15.34Hz), 5.27 (s, 1H, CH at H2), 7.31-8.44 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 33.9 C-5, 62.6 C-2, 108.8 C-5′, 127.5-133.1 –Carom, 131.4 C-2‴, 135.9 ipso C, 139.1 C-4″, 139.7 C-6″, 164.9 C-4″, 165.0 C-6′, 162.9 C-2′, 170.6 C-4.
3-(4′-(3″-chlorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one. 25 IR (KBr) (cm−1): 3115, 3033, 2927, 2850, 1714, 1627, 1575, 1344, 894, 767, 690, 648; 1H NMR (δ ppm): 3.22 (d, 1H, CH2a at H5a, J = 15.36Hz), 3.39 (d, 1H, CH2e at H5e, J =15.37Hz), 5.27 (s, 1H, CH at H2), 7.21-8.24 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 33.9 C-5, 62.4 C-2, 108.9 C-5′, 124.6-129.0 –Carom, 130.6 ipso C, 131.5 C-2‴, 139.0 C-4″, 141.8 C-6″, 164.9 C-4′, 165.5 C-6′, 162.7 C-2′, 170.6 C-4.
3-(4′-(4″-methoxyphenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one. 26 IR (KBr) (cm−1): 3065, 3038, 2927, 2851, 1714, 1627, 1577, 1351, 700, 650, 649; 1H NMR (δ ppm): 3.23 (d, 1H, CH2a at H5a, J = 15.35Hz), 3.39 (d, 1H, CH2e at H5e, J = 15.27Hz), 3.84 (s, 3H, OCH3), 5.28 (s, 1H, CH at H2), 7.21-8.21 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.5 C-5, 54.9 OCH3 on aryl ring, 62.5 C-2, 108.7 C-5′, 126.0-128.6 –Carom, 129.1 C-2‴, 139.1 C-4″, 141.5 C-6″, 164.0 C-4′, 165.0 C-6′, 162.3 C-2′, 170.6 C-4.
3-(4′-(4″-methylphenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one. (27) IR (KBr) (cm−1): 3060, 3033, 2926, 2852, 1715, 1627, 1579, 1350, 714, 700, 643.; 1H NMR (δ ppm): 2.32 (s, 3H, CH3), 3.23 (d, 1H, CH2a at H5a, J = 15.34Hz), 3.40 (d, 1H, CH2e at H5e, J = 15.36Hz), 5.27 (s, 1H, CH at H2), 7.20-8.24 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 24.5 CH3 on aryl ring, 34.1 C-5, 62.6 C-2, 108.4 C-5′, 126.0-131.4 –Carom, 133.1 C-2‴, 135.9 ipso C, 138.7 C-4″, 139.1 C-6″, 164.9 C-4′, 165.5 C-6′, 162.6 C-2′, 170.8 C-4.
3-(4′-(4″-fluorophenyl)-6′-phenylpyrimidin-2′-yl)-2-phenylthiazolidin-4-one. 28 IR (KBr) (cm−1): 3071, 3027, 2928, 2852, 1712, 1626, 1575, 1352, 836, 769, 698; 1H NMR (δ ppm): 3.20 (d, 1H, CH2a at H5a, J = 15.24Hz), 3.37 (d, 1H, CH2e at H5e, J = 15.28Hz), 5.26 (s, 1H, CH at H2), 6.64-8.19 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.5 C-5, 62.9 C-2, 108.9 C-5′, 127.3-143.1 –Carom, 143.6 C-2‴, 145.1 C-4″, 146.1 C-6″, 166.8 C-4′, 167.0 C-6′, 163.9 C-2′, 171.4 C-4.
3-4′-phenyl-(6′-(4″-chlorophenyl)pyrimidin-2′-yl)-2-phenylthiazolidin-4-one. 29 IR (KBr) (cm−1): 3071, 3027, 2926, 2852, 1721, 1627, 1576, 1398, 782, 730, 693, 582; 1H NMR (δ ppm): 3.21 (d, 1H, CH2a at H5a, J = 15.33Hz), 3.38 (d, 1H, CH2e at H5e, J = 15.32Hz), 5.25 (s, 1H, CH at H2), 7.15-7.93 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.1 C-5, 62.9 C-2, 108.9 C-5′, 126.5-128.6 –Carom, 129.2 C-2‴, 139.1 C-4″, 141.9 C-6″, 164.4 C-4′, 165.3 C-6′, 162.5 C-2′, 170.8 C-4.
3-4′-phenyl-(6′-(4″-methoxyphenyl)pyrimidin-2′-yl)-2-phenylthiazolidin-4-one. 30 IR (KBr) (cm−1): 3065, 3033, 2928, 2851, 1715, 1627, 1590, 1370, 835, 770, 699, 656; 1H NMR (δ ppm): 3.20 (d, 1H, CH2a at H5a, J = 15.08Hz), 3.37 (d, 1H, CH2e at H5e, J = 15.34Hz), 3.86 (S, 3H, OCH3), 5.26 (s, 1H, CH at H2), 6.97-8.20 (m, 15H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.1 C-5, 55.2 OCH3 on aryl ring, 62.5 C-2, 108.6 C-5′, 114.1-129.1 –Carom, 129.5 C-2‴, 130.2 ipso C, 139.2 C-6″, 146.1 C-4″, 163.8 C-4′, 164.4 C-6′, 161.2 C-2′, 170.7 C-4.
3-(4′-(4″-chlorophenyl)-6′-(p-tolylpyrimidin-2′-yl))-2-phenylthiazolidin-4-one. 31 IR (KBr) (cm−1): 3060, 3027, 2927, 2851, 1721, 1626, 1576, 1398, 781, 728, 694, 650; 1H NMR (δ ppm): 2.40 (s, 3H, CH3), 3.20 (d, 1H, CH2a at H5a, J = 15.04Hz), 3.37 (d, 1H, CH2e at H5e, J = 15.11Hz), 5.26 (s, 1H, CH at H2), 7.18-8.33 (m, 14H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 25.2 CH3 on aryl ring, 34.5 C-5, 62.9 C-2, 108.9 C-5′, 131.4 C-2‴, 126.1-130.4 –Carom, 133.1 ipso C, 138.7 C-6″, 139.1 C-4″, 164.8 C-4′, 165.0 C-6′, 161.2 C-2′, 170.8 C-4.
3-(4′,6′-bis(p-chlorophenyl)pyrimidin-2′-yl)-2-phenylthiazolidin-4-one. 32 IR (KBr) (cm−1): 3060, 3027, 2927, 2852, 1727, 1627, 1575, 1400, 897, 787, 730, 693; 1H NMR (δ ppm): 3.20 (d, 1H, CH2a at H5a, J = 15.21Hz), 3.36 (d, 1H, CH2e at H5e, J = 15.23Hz), 5.26 (s, 1H, CH at H2), 7.28-8.20 (m, 14H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.5 C-5, 62.5 C-2, 108.7 C-5′, 129.1 C-2‴, 126.0-128.6 –Carom, 139.1 C-6″, 141.8 C-4″, 164.1 C-4′, 161.3 C-6′, 165.3 C-2′, 170.9 C-4.
3-(4′-(p-chlorophenyl)-6′-(p-fluorophenyl)pyrimidin-2′-yl)-2-phenylthiazolidin-4-one. 31 IR (KBr) (cm−1): 3065, 3027, 2926, 2853, 1719, 1627, 1576, 1394, 897, 833, 776, 728, 695; 1H NMR (δ ppm): 3.18 (d, 1H, CH2a at H5a, J = 14.92Hz), 3.35 (d, 1H, CH2e at H5e, J = 14.92Hz), 5.26 (s, 1H, CH at H2), 7.18-8.19 (m, 14H, Harom), A singlet for CH proton at position 5′ of pyrimidine moiety is merged with aromatic protons. 13C NMR (δ ppm): 34.1 C-5, 62.6 C-2, 110.1 C-5′, 133.2 C-2‴, 127.3-143.1 –Carom, 145.1 C-6″, 146.8 C-4″, 166.8 C-4′, 167.0 C-6′, 163.4 C-2′, 171.8 C-4.
Microbiology
Materials
All the clinically isolated bacterial strains namely Staphylococcus aureus, β-Haemolytic streptococcus, Vibreo cholerae, Salmonella typhii, Shigella felxneri, Escherichia coli, Klebsiella pneumonia, Pseudomonas aeruginosa and fungal strains namely Aspergillus flavus, Mucor, Rhizopus and Microsporum gypsuem were obtained from Faculty of Medicine, Annamalai University, Annamalainagar-608 002, Tamil Nadu, India.
In vitro antibacterial and antifungal activity
Minimum inhibitory concentration (MIC) in μg/mL values is carried out by two-fold serial dilution method [Citation30]. The respective test compounds 23-33 were dissolved in dimethyl sulphoxide (DMSO) to obtain 1 mg mL−1 stock solution. Seeded broth (broth containing microbial spores) was prepared in NB from 24 h old bacterial cultures on nutrient agar (Hi-media, Mumbai) at 37 ± 1 °C while fungal spores from 1 to 7 days old Sabourauds agar (Hi-media, Mumbai) slant cultures were suspended in SDB. The colony forming units (cfu) of the seeded broth were determined by plating technique and adjusted in the range of 104-105 cfu/mL. The final inoculums size was 105cfu/mL for antibacterial assay and 1.1-1.5 × 102 cfu/mL for antifungal assay. Testing was performed at pH 7.4 ± 0.2 for bacteria (NB) and at a pH 5.6 for fungi (SDB). Exactly 0.4 mL of the solution of test compound was added to 1.6 mL of seeded broth to form the first dilution. One milliliter of this was diluted with a further 1 mL of seeded broth to give the second dilution and so on till six such dilutions were obtained. A set of assay tubes containing only seeded broth was kept as control. The tubes were incubated in BOD incubators at 37 ± 1°C for bacteria and 28 ± 1°C for fungi. The minimum inhibitory concentrations (MICs) were recorded by visual observations after 24 h (for bacteria) and 72-96 h (for fungi) of incubation. Ciprofloxacin was used as standard for bacteria studies and Fluconazole was used as standards for fungal studies.
Results and discussion
Chemistry
Initially, attempted conversion of 2-amino-4,6-diarylpyrimidines 12-22 to 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones 23-33 was carried out in the absence of dicyclohexylcarbodiimide (DCC) but yields were achieved. Instead if DCC was used as a dehydrating agent, the yield of the product has been improved significantly (i.e., c. 65%) by stirring at about 0°C. The Claisen-Schmidt condensation of equimolar quantities of various p-substituted acetophenone with different m- and p- substituted benzaldehydes in the presence of sodium hydroxide base as a catalyst yields 1,3-diaryl-prop-2-en-1-ones 1-11. When 1,3-diaryl-prop-2- en-1-ones 1-11 are treated with guanidine nitrate in the presence of potassium hydroxide alkali in refluxing ethanol for 8 h gives 2-amino-4,6-diarylpyrimidines 12-22. Novel title compounds, 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones 23-33 are synthesized by the addition of benzaldehyde, followed by thioglycolic acid to 2-amino-4,6- diphenylpyrimidine in dry dichloromethane at 0°C catalyzed by dicyclohexylcarbodiimide (DCC). The schematic representation and the analytical data for compounds 23-33 are given in and , respectively. The importance of the title compounds is due to their diverse potential, broad-spectrum biological activity. The structure of the newly synthesized compounds 23-33 was confirmed by melting point, elemental analysis, MS, FT-IR, one- dimensional NMR (1H & 13C) spectroscopic data.
Table 1. Physical and analytical data of 2-phenyl-3-(4,6-diarylpyrimidin-2-yl) thiazolidin-4-ones 23-33.
Antibacterial activity
Novel 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones 23-33 were tested for their antibacterial activity in vitro against S.aureus, β-H.streptococcus, V.cholerae, S.typhii, S.felxneri, E.coli, K.pneumonia and P. aeruginosa. Ciprofloxacin was used as standard drug. Minimum inhibitory concentration (MIC) in μg/mL values is reproduced in . Compound 23 which has no substitution at the para position of phenyl rings only exerted moderate activities against all the used bacterial strains. Compounds 25 and 28 which contain electron withdrawing chloro and fluoro functional group respectively at the para position of phenyl ring attached to pyrimidine ring did not promote much activity against β-H.streptococcus, S.felxneri and E.coli. Structure-activity relationship results for the synthesized compounds have shown that 2-amino-4,6-diarylpyrimidino thiazolidinones with electron donating methoxy and methyl functional group at the para position of the phenyl ring attached to the pyrimidine ring 26, 27 and 30 exerted strong antibacterial activity against all the tested bacterial strains. Also compound 31, which contain both electron withdrawing chloro and electron donating methyl groups shows potent activity against all the tested bacterial strains whereas compounds 32 and 33 which contain electron donating methoxy functional group at the para position of phenyl ring attached to pyrimidine ring shows promising activity against S.aureus, S.typhii and E.coli.
Table 2. In vitro antibacterial activity (MIC) values for compounds 23-33.
Antifungal activity
The in vitro antifungal activity of 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones 23-33 was studied against the fungal strains viz., A.flavus, Mucor, Rhizopus and M.gypsuem. Fluconazole was used as a standard drug. Minimum inhibitory concentration (MIC) in μg/mL values is reproduced in . Compound 23 did not exhibited antifungal activity against A.flavus and M.gypsuem. Further introduction of electron withdrawing chloro functional group at the para position of phenyl ring attached to pyrimidine ring in compounds 24 and 29 also did not promote much activity against A.flavus and M.gypsuem while against Mucor and Rhizopus, it has registered maximum activity at 25 μg/mL. Instead if the chloro group is attached at the meta position of phenyl ring attached to pyrimidine ring in 25 promotes pronounced activity against Mucor at 12.5 μg/mL. Compounds 32 and 33 shows excellent activitites against all the tested clinically isolated fungal strains, while against Mucor, compound 27 which contain electron donating methyl group at the para position of phenyl ring attached to pyrimidine ring showed promisable activities. The antifungal activities of these two compounds are due to the presence of two electron withdrawing chloro and fluoro functional groups attached to para position of the two phenyl rings. Compounds 26 and 30 which contain electron donating methoxy functional group at the para position of phenyl ring attached to pyrimidine ring did not promote much activity against Mucor and Rhizopus. Also compound 31, which contain both electron withdrawing chloro and electron donating methyl groups shows potent activity against A. flavus and Rhizopus.
Table 3. In vitro antifungal activity (MIC) values for compounds 23-33
Conclusion
The microbiological screening studies carried out to evaluate the antibacterial and antifungal potencies of the newly synthesized 2-phenyl-3-(4,6-diarylpyrimidin-2-yl)thiazolidin-4-ones 23-33 are clearly known from and . A close inspection of the in vitro antibacterial and antifungal activity profile in differently electron donating (CH3 and OCH3) and electron withdrawing (-Cl and -F) functional group substituted phenyl rings of novel 2-aminopyrimidino thiazolidinones 23-33 exerted strong anti-bacterial activity against all the tested bacterial strains. Compound 31, which contain both electron withdrawing chloro and electron donating methyl groups shows potent activity against all the tested bacterial strains whereas compounds 32 and 33 which contain electron donating methoxy functional group at the para position of phenyl ring attached to pyrimidine ring shows promising activity against S.aureus, S.typhii and E.coli. Results of the anti-fungal activity study show that the nature of substituents on the phenyl ring viz., methyl, fluoro and chloro functions at the para positions of the aryl moieties are determinant for the nature and extent of the anti-fungal activity of all the synthesized compounds 23-33 over fungal strains namely A.flavus, Mucor, Rhizopus and M.gypsuem. Compounds 26 and 30 which contain electron donating methoxy functional group at the para position of phenyl ring attached to pyrimidine ring did not promote much activity against Mucor and Rhizopus. Also compound 31, which contain both electron withdrawing chloro and electron donating methyl groups shows potent activity against A. flavus and Rhizopus. The mode of action of these compounds is unknown. Further development of this group of 2-amino-4,6-diarylpyrimidino thiazolidinones may lead to compounds with better pharmacological profile than standard antibacterial and antifungal drugs.
Acknowledgement
Authors are thankful to NMR Research Centre, Indian Institute of Science, Bangalore for recording spectra. One of the authors (V. Kanagarajan) is grateful to Council of Scientific and Industrial Research (CSIR), New Delhi, Republic of India for providing financial support in the form of CSIR-Senior Research Fellowship (SRF) in Organic Chemistry. J.Thanusu wishes to thank Annamalai University authorities for providing financial support in the form of Research Fellowship.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Andres CJ, Bronson JJ, D’Andrea SV, Deshpande MS, Falk PJ, Grant-Young KA, Harte WE, Hsu-Tso Ho, Misco PF, Robertson JG, David Stock, Yaxiong Sun, Walsh AW. 4-Thiazolidinones: novel inhibitors of the bacterial enzyme Mur B. Bioorg Med Chem Lett (2000); 10:715–717.
- Nampurath GK, Mathew SP, Vivek Khanna, Zachariah RT, Suman Kanji, Mallikarjuna Rao Chamallamudi. Assessment of hypolipidaemic activity of three thiazolidin-4-ones in mice given high-fat diet and fructose. Chemico-Biological Interactions, (2008); 171:363–368.
- Rosaria Ottanà, Rosanna Maccari, Rosella Ciurleo, Maria Gabriella Vigorita, Anna Maria Panico, Venera Cardile, Floriana Garufi, Simone Ronsisvalle. Synthesis and in vitro evaluation of 5-arylidene-3-hydroxyalkyl-2-phenylimino-4-thiazolidinones with antidegenerative activity on human chondrocyte cultures. Bioorg Med Chem (2007); 15:7618–7625.
- Narendra Sharath Chandra JN, Manish Malviya, Sadashiva CT, Subhash MN, Rangappa KS. Effect of novel arecoline thiazolidinones as muscarinic receptor 1 agonist in Alzheimer’s dementia models. Neurochem Internat (2008); 52, 376–383.
- Kishore V, Narain NK, Kumar S, Parmar SS. Relationship between antiinflamatory and antiproteolytic properties of substituted oxothiazolylacetic acids. Pharmacol Res Commun (1976); 8:43–51.
- Newbould BB. Suppression of adjuvant-induced arthritis in rats with 2-butoxycarbonylmethylene-4-oxothiazolidine. Br J Pharmacol Chemother (1965); 24:632–640.
- Schauer P, Likar M, Tisler M, Krbavcic A, Pollak A. Studies of some substances with antiviral activity in 2,4-dioxo-5-thiazolidine acetic acid derivatives (DFT) as an inhibitor of growth of herpes virus and poliovirus type in cell cultures of human embryonic kidneys. Path Microbiol (1965); 28:382–387.
- Chaubey VN, Singh H. Synthesis of some new fungicides. Bull Chem Soc Jpn (1970); 43:2233–2236.
- Akerblom EB. Synthesis and structure-activity relations of a series of antibacterially active 5-(5-nitro-2-furfurylidene)thiazolones, 5-(5-nitro-2- furylpropenylidene) thiazolones, and 6-(5-nitro-2-furyl)-4H-1,3-thiazinones. J Med Chem (1974); 17:609–615.
- Litvinchuk MD. Antitubercular activity with low toxicity associated with a few derivatives of 2-imino- 4-thiazolidinones. Farmakol Toksikol (1963); 26:725–728.
- Dimri AK, Parmar SS. Synthesis of 3-Aryl-4-oxothiazolin-2-yl(4-ethoxy-3-methoxy)phenyl hydrazones as possible anticonvulsants. J Heterocycl Chem (1978); 15:335–336.
- Parmar SS, Dwivedi C, Chaudhari A, Gupta TK. Substituted thiazolidinones and their selective inhibition of nicotinamide-adenine dinucleotide dependent oxidations. J Med Chem (1972); 15:99–101.
- Singh SP, Parmar SS, Krishna Raman, Stenberg VI. Chemistry and biological activity of thiazolidinones. Chem Rev (1981); 81:175–203.
- Jacobus PD, Van Veldhoven, Lisa CW, Chang, Künzel J, Thea Mulder-Krieger, Regina Struensee-Link, Beukers MW, Johannes Brussee, Ijzerman IP. A new generation of adenosine receptor antagonists: From di- to trisubstituted aminopyrimidines. Bioorg Med Chem (2008); 16:2741–2752.
- Hughes TV, Emanuel SL, Beck AK, Wetter SK, Connolly PJ, Prabha Karnachi, Michael Reuman, Jabed Seraj, Fuentes-Pesquera AR, Gruninger RH, Middleton SA, Ronghui Lin, Davis JM, Moffat DFC.Bioorg Med Chem Lett (2007); 17:3266–3270.
- Chhabria MT, Bhatt HG, Raval HG, Oza PM. Synthesis and biolo- gical evaluation of some 5-ethoxycarbonyl-6-isopropylamino-4-(substitutedphenyl)amino pyrimidines as potent analgesic and anti-inflammatory agents. Bioorg Med Chem Lett (2007); 17:1022–1024.
- Sayle KL, Bentley JF, Thomas Boyle, Calvert AH, Yuzhu Cheng, Curtin NJ, Endicott JA, Golding BT, Hardcastle IR, Philip Jewsbury, Veronique Mesguiche, Newell DR, Noble MEM, Parsons RJ, Pratt DJ, Wang LZ, Griffin RJ. Structure-Based design of 2-Arylamino-4-cyclohexylmethyl-5-nitroso-6-aminopyrimidine inhibitors of cyclin-Dependent kinases 1 and 2. Bioorg Med Chem Lett (2003); 13:3079–3082.
- Alfredo Pastor, Ramón Alajarin, Vaquero JJ, Julio Alvarez-Builla, Miguel Fau de Casa-Juana, Carlos Sunkel, Priego JG, Isabel Fonseca, Julia Sanz-Aparicio. Synthesis and Structure of New Pyrido[2,3-d]pyrimidine Derivatives with Calcium Channel Antagonist Activity. Tetrahedron (1994); 50:8085–8098.
- Youssouf MS, Kaiser P, Singh GD, Singh S, Bani S, Gupta VK, Satti NK, Suri KA, Johri RK. Anti-histaminic, anti-inflammatory and bronchorelaxant activities of 2, 7-dimethyl-3-nitro-4H pyrido [1,2-a] pyrimidine-4-one. Internat Immunopharmacol (2008); 8:1049–1055.
- Cécile Gasse, Dominique Douguet, Valérie Huteau, Gilles Marchal, Hélène Munier-Lehmann, Sylvie Pochet. Substituted benzyl-pyrimidines targeting thymidine monophosphate kinase of Mycobacterium tuberculosis: Synthesis and in vitro anti-mycobacterial activity. Bioorg Med Chem (2008); 16:6075–6085.
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J. Design, ‘one-pot’ synthesis, characterization, antibacterial and antifungal activities of novel 6-aryl-1,2,4,5-tetrazinan-3-thiones in dry media. J Sulf Chem (2007); 28:383–392.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V. Synthesis, spectral analysis, antibacterial and antifungal activities of some 4,6-diaryl-4,5-dihydro-3-hydroxy-2[H]-indazole—a novel fused indazole derivative. J Enz Inhib Med Chem (2008); 23:975–98.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V. Design, synthesis, characterization, antibacterial and antifungal activities of novel class of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-selenadiazoles. J Enz Inhib Med Chem (2008); 23:347–351.
- Gopalakrishnan M, Thanusu J, Kanagarajan V. Synthesis and biological evaluation of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-thiadiazoles. Med Chem Res (2007); 16:392–401.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V, Govindaraju R, Jayasri G. A convenient ‘one-pot’ synthesis and in vitro microbiological evaluation of novel 2,7-diaryl-[1,4]-diazepan-5-ones. J Enz Inhib Med Chem (2007); 22:709–715.
- Trivedi VP, Undaria NK, Trivedi PB. Synthesis and biological activity of novel thiazolidin-4-one derivatives. J. Indian Chem Soc (2004); 81:1506–1508.
- Shah TJ, Desai VA. Synthesis of some novel fluorinated 4-thiazolidinones containing amide linkages and their antimicrobial screening. ARKIVOC (2007); 14:218–228.
- Guthrie W, Wang XP. The aldol condensation of acetophenone with acetone. Can J Chem (1991); 69:339–340.
- Kothari S, Singhal M, Vijayvergia D, Vyas R, Verma BL. Synthesis and biocidal activity of some new 2-amino-4,6-diaryl-substituted-pyrimidines. J Indian Chem Soc (2000); 77:329–331.
- Dhar MH, Dhar, MM, Dhawan BN, Mehrotra BN, Ray C. Screening of Indian plants biological activity. Part I. Indian J Exp Biol (1968); 6:232–247.