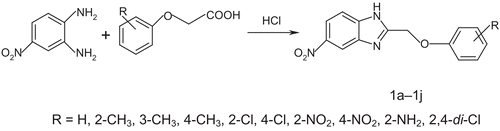
Abstract
The efficient and rapid synthesis of 5-nitro-2-aryl substituted-1H-benzimidazole libraries (1a-1j) has been established. Thus, both microwave and conventional cyclo-condensation of 4-nitro ortho-phenylenediamine with various phenoxyacetic acids were carried out in the presence of HCl catalyst. The microwave synthesis route afforded advantages, such as good to excellent yields, shorter reaction time (2.5–3.5 min), readily available starting material, and simple purification procedure, which distinguish the present protocol from other existing methods used for the synthesis of benzimidazole libraries. Bioassay indicated that all the compounds showed in vitro antimicrobial activity against Vancomycin resistant enteroccoccus, Staphylococcus aureus, Micrococcus, Bacillus subtilis (gram-positive bacteria) and Shigella dysentery, Escherichia coli (gram-negative bacteria) and Candida albicans, Aspergillus niger, Penicillium (fungi). The minimum inhibitory concentration (MIC) was determined for test compounds as well as for reference standards.
Introduction
Benzimidazole has been an important pharmacophore and privileged structure in medicinal chemistry [Citation1] e.g., as antifungal, antimicrobial, anticancer, anaesthetic, HIV-Rt inhibitor, antihistaminic [Citation2–6], Mean while 5-nitrobenzimidazole with varying substituents at 2-position used as angiotensin II receptor antagonists [Citation7]. Many approaches for the synthesis of benzimidazoles continue to utilize the condensation reactions of o-phenylenediamine with carboxylic acids, carboxylic acid esters, lactones, anhydrides and aldehydes [Citation8].
Modern drug discovery uses techniques such as combinatorial and parallel synthesis, as well as automated library production, to accelerate the lead identification process [Citation9]. There is, however, an acute need to implement more sustainable methods, not only for large-scale production but also for lab-scale medicinal chemistry research [Citation10]. A set of environmentally friendly approaches and technologies are available that can help to make progress in this somewhat forgotten research area [Citation11]. Beyond the development of a environmentally friendlier synthetic methods, there is a need for decreased reaction times. Thus, microwave irradiation has become a helpful processing tool because it allows rapid and convenient super heating to high temperatures in combination with excellent reaction control and low-energy consumption [Citation12,Citation13]. Hence the decomposition of reactants and/or products is diminished in these reactions leading to enhanced yields. Often, microwave assisted organic reactions proceed rapidly in the absence of solvent also [Citation14]. This expeditious and solvent-free approach involves the exposure of neat reactants to microwave irradiation in conjunction with the use of supported reagents or catalysts, which are primarily of mineral origin. The salient features of these high yield protocols are the enhanced reaction rates, greater selectivity and the experimental ease of manipulation, which is in many ways superior to traditional heating [Citation15,Citation16]. This novel synthetic route did not lead to formation of some carcinogenic by-products as reported with the literature methods [Citation17–20]. But in our preliminary in vitro bioassay against six strains of bacteria [Vancomycin resistant enteroccoccus (ATCC-51299), Staphylococcus aureus (ATCC-29213), Micrococcus (natural isolates), Bacillus subtilis (natural isolates), Shigella dysentery (natural isolates) and Escheria coli (ATCC-25922)] and three strains of fungi [Candida albicans, Aspergillus niger and Penicillium ] indicated that these benzimidazole libraries displayed significant antimicrobial activities.
As part of our ongoing work devoted toward the development of a rapid synthesis of heterocyclic molecules of biological interest, we explored the possibility of synthesizing 5-nitro-2-aryl substituted phenoxymethyl-1H-benzimidazole libraries from 4-nitro-o-phenylenediamine and substituted phenoxyacetic acids using mineral acid as a catalyst in solvent-free conditions under microwave irradiation to facilitate the rapid synthesis of drugs from commercially available building blocks.
Materials and methods
Melting points were determined on a open capillary apparatus and are uncorrected. Elemental analyses were recorded as both experimental as well as theoretical. Infrared spectra were determined in KBr on Nicolet 5700 FTIR instrument. The 1H and 13C NMR spectras were recorded on a 300MHz Bruker-Avanace NMR instrument in CDCl3 and the chemical shifts were expressed in parts per million (ppm) with tetramethylsilane (TMS) as an internal standard. All compounds were routinely checked by thin-layer chromatography (TLC) on aluminium-backed silica gel plates. Microwave synthesis was carried out on a Ken Star synthesizer (Model: OM-25DGQ).
Chemistry
The synthesis of the target compounds was carried out as outlined in . Cyclo-condensation of 4-nitro ortho-phenylenediamine with various phenoxyacetic acids were carried out in the presence of HCl catalyst, afforded 5-nitro-2-aryl substituted-1H-benzimidazole libraries under microwave conditions.
The new benzimidazole libraries were characterized by their spectral data. IR spectra of the target substituted benzimidazoles showed NH stretching bands at 3350- 3500 cm−1, NO2 asymmetric stretching bands at 1510- 1550 cm−1 & symmetric stretching bands at 1335- 1365 cm−1, respectively. The absorption bands associated with other functional groups appeared in the expected regions. In the 1H NMR spectra of the target substituted benzimidazoles, the NH proton appeared at 3.5 ppm as a broad D2O exchangeable singlet, whereas sharp singlet at 4.5 ppm was characterstic of the CH2-O group. The other protons appeared at the expected chemical shifts.
General method for the synthesis of 5-nitro-2-aryl substituted-1H-benzimidazole libraries
Microwave synthesis of 5-nitro-2-aryl substituted-1H- benzimidazole libraries.
The mixture of 4-nitro-o-phenylenediamine (0.01 mole) and substituted phenoxyacetic acids (0.01 mole) in presence of 1 or 2 mL of HCl 6N, was irradiated in a sealed vessel under microwave oven (Model: Ken Star OM-25DGQ) for 2.5–3.5 min at 400W output power and the reaction progress examined under thin layer chromatography (TLC). After the reaction was completed the mixture was cooled to room temperature and then poured into ice cold water. Stirring was continued for few minutes and the mixture was neutralized with aqueous ammonia, then pure product is obtained in most of the cases as evident from 1H NMR. Otherwise the products were recrystalized from water-ethanol system. The yield and m.p. of compounds are given in .
Table 1. Comparison between microwave-assisted and thermal method synthesis of 5-nitro-2-aryl substituted phenoxymethyl-1H-benzimidazole libraries.
Conventional synthesis of 5-nitro-2-aryl substituted-1H-benzimidazole libraries.
A mixture of 4-nitro-o-phenylenediamine (0.01 mole) and substituted phenoxyacetic acids (0.01 mole) was heated at 100°C for 3–4 h in 15mL of 6N HCl and the reaction progress examined under thin layer chromatography (TLC). After the reaction was completed the mixture was cooled to room temperature and then poured into ice cold water. Stirring was continued for a few minutes and the mixture was neutralized with aqueous ammonia and the products were recrystalized from water-ethanol system. The yield and m.p. of compounds are given in .
5-nitro-2-phenoxymethyl-1H-benzimidazole (1a):
IR (KBr): γ (cm−1) 3422 br (ArNH), 1533 and 1344 (NO2 asymmetric and symmetrical stretching), 1629 and 1455 (C=C and C=N ring stretching); 1H NMR (CDCl3, 300 MHz,): δ (ppm) 2.74 (br, s, 1H, NH-benzimidazole), 3.5 (s, 1H, OCH3), 7.27 (s, 1H, Ar-H), 7.28 (s, 2H, Ar-H), 7.63 (s, 1H, Ar-H), 8.22 (s, 1H, Ar-H), 8.51 (s, 1H, Ar-H); CIMS: m/z 269.24: Anal. Calcd. For C14H11N3O3: C 62.45, H 4.12, N 15.61. Found: C 62.43, H 4.11, N 15.60%.
5-nitro-2-o-toloxymethyl-1H-benzimidazole (1b):
IR (KBr): γ (cm−1) 3423 br (ArNH), 1540 and 1344 (NO2 asymmetric and symmetrical stretching), 1630 and 1388 (C=C and C=N ring stretching); 1H NMR (CDCl3, 300 MHz,): δ (ppm) 1.70 (s, 1H, CH3), 2.78 (br, s, 1H, NH-benzimidazole), 3.87 (s, 1H, OCH3), 7.41 (s, 5H, Ar-H), 8.20 (s, 1H, Ar-H), 8.41 (s, 1H, Ar-H); CIMS: m/z 283.27: Anal. Calcd. For C15H13N3O3: C 63.60, H 4.63, N 14.83. Found: C 63.59, H 4.62, N 14.81%.
5-nitro-2-m-toloxymethyl-1H-benzimidazole (1c):
IR (KBr): γ (cm−1) 3420 br (ArNH), 1533 and 1344 (NO2 asymmetric and symmetrical stretching), 1630 and 1388 (C=C and C=N ring stretching); 1H NMR (CDCl3, 300 MHz,): δ (ppm) 2.19 (s, 1H, CH3), 2.70 (br, s, 1H, NH-benzimidazole), 3.87 (s, 1H, OCH3), 7.27 (s, 4H, Ar-H), 8.21 (d, 2H, J= 9.0, Ar-H), 8.50 (s, 1H, Ar-H); CIMS: m/z 283.27: Anal. Calcd. For C15H13N3O3: C 63.60, H 4.63, N 14.83. Found: C 63.59, H 4.62, N 14.81%.
5-nitro-2-p-toloxymethyl-1H-benzimidazole (1d):
IR (KBr): γ (cm−1) 3422 br (ArNH), 1533 and 1345 (NO2 asymmetric and symmetrical stretching), 1631 and 1387 (C=C and C=N ring stretching); 1H NMR (CDCl3, 300 MHz,): δ (ppm) 2.18 (s, 1H, CH3), 2.79 (br, s, 1H, NH-benzimidazole), 3.67 (s, 1H, OCH3), 7.27 (s, 3H, Ar-H), 7.30 (d, 2H, J= 6.1, Ar-H), 8.41 (s, 2H, Ar-H); CIMS: m/z 283.27: Anal. Calcd. For C15H13N3O3: C 63.60, H 4.63, N 14.83. Found: C 63.59, H 4.62, N 14.81%.
2-(2-chloro-phenoxymethyl)-5-nitro-1H-benzimidazole (1e):
IR (KBr): γ (cm−1) 3425 br (ArNH), 1517 and 1343 (NO2 asymmetric and symmetrical stretching), 1627 and 1388 (C=C and C=N ring stretching), 1284 and 733 (ArCl); 1H NMR (CDCl3, 300 MHz,): δ (ppm) 2.72 (br, s, 1H, NH-benzimidazole), 3.41 (s, 1H, OCH3), 7.27 (s, 4H, Ar-H), 7.58 (s, 1H, Ar-H), 8.18 (s, 1H, Ar-H), 8.49 (s, 1H, Ar-H); CIMS: m/z 303.68: Anal. Calcd. For C14H10N3O3Cl: C 55.37, H 3.32, N 13.84. Found: C 55.38, H 3.34, N 13.85%.
2-(4-chloro-phenoxymethyl)-5-nitro-1H-benzimidazole (1f):
IR (KBr): γ (cm−1) 3425 br (ArNH), 1517and 1345 (NO2 asymmetric and symmetrical stretching), 1631 and 1387 (C=C and C=N ring stretching), 1283 and 734 (ArCl); 1H NMR (CDCl3, 300 MHz,): δ (ppm) 2.72 (br, s, 1H, NH-benzimidazole), 3.67 (s, 1H, OCH3), 7.28 (s, 4H, Ar-H), 8.23 (s, 2H, Ar-H), 8.50 (s, 1H, Ar-H); CIMS: m/z 303.68: Anal. Calcd. For C14H10N3O3Cl: C 55.37, H 3.32, N 13.84. Found: C 55.38, H 3.34, N 13.85%.
5-Nitro-2-(2-nitro-phenoxymethyl)-1H-benzimidazole (1g):
IR (KBr): γ (cm−1) 3425 br (ArNH), 1517 and 1344 (NO2 asymmetric and symmetrical stretching), 1629 and 1387 (C=C and C=N ring stretching); 1H NMR (CDCl3, 300 MHz,): δ (ppm) 2.71 (br, s, 1H, NH-benzimidazole), 4.81 (s, 1H, OCH3), 7.27 (s, 3H, Ar-H), 7.60 (s, 1H, Ar-H), 8.19 (d, 2H, J= 8.8, Ar-H), 8.49 (s, 1H, Ar-H); 13C NMR (CDCl3, 75 MHz,): δ (ppm) 41.21 (s, C, OCH3), 110.05 (s, C, Ar-C), 118.07 (s, C, Ar-C) and 143.0 (s, C, Ar-C); CIMS: m/z 314.23: Anal. Calcd. For C14H10N4O5: C 53.51, H 3.21, N 17.83. Found: C 53.49, H 3.19, N 17.82%.
5-nitro-2-(4-nitro-phenoxymethyl)-1H-benzimidazole (1h):
IR (KBr): γ (cm−1) 3422 br (ArNH), 1516 and 1343 (NO2 asymmetric and symmetrical stretching), 1627 and 1386 (C=C and C=N ring stretching); 1H NMR (CDCl3, 300 MHz,): δ (ppm) 2.72 (br, s, 1H, NH-benzimidazole), 3.61 (s, 1H, OCH3), 7.60 (s, 1H, Ar-H), 7.97 (s, 2H, Ar-H), 8.30 (d, 2H, J= 8.7, Ar-H), 8.50 (s, 2H, Ar-H); CIMS: m/z 314.23: Anal. Calcd. For C14H10N4O5: C 53.51, H 3.21, N 17.83. Found: C 53.49, H 3.19, N 17.82%.
2-(5-nitro-1H-benzimidazol-2-ylmethoxy)-phenyl amine (1i):
IR (KBr): γ (cm−1) 3562 s (ArNH2); 3327br (ArNH), 1518 and 1332 (NO2 asymmetric and symmetrical stretching), 1627 and 1390 (C=C and C=N ring stretching); 1H NMR (CDCl3, 300 MHz,): δ (ppm) 2.72 (s, 1H, NH2), 3.5 (br, 1H, NH-benzimidazole), 7.27 (s, 4H, Ar-H), 8.06 (d, 2H, J= 8.1, Ar-H), 8.50 (s, 2H, Ar-H); 13C NMR (CDCl3, 75 MHz,): δ (ppm) 41.19 (s, C, OCH3), 112.106 (s, C, Ar-C), 114.66 (s, C, Ar-C), 118.09 (s, C, Ar-C) and 143.017 (s, C, Ar-C); CIMS: m/z 284.28: Anal. Calcd. For C14H12N4O3: C 59.15, H 4.25, N 19.71. Found: C 59.15, H 4.23, N 19.70%.
2-(2,4-Dichloro-phenoxymethyl)-5-nitro-1H-benzimidazole (1j):
IR (KBr): γ (cm−1) 3416 br (ArNH), 1517 and 1344 (NO2 asymmetric and symmetrical stretching), 1629 and 1388 (C=C and C=N ring stretching), 1285 and 734s (ArCl); 1H NMR (CDCl3, 300 MHz,): δ (ppm) 2.69 (br, 1H, NH-benzimidazole), 4.82 (s, 1H, CH3), 7.77 (d, 2H, J= 5.5, Ar-H), 7.80 (s, 1H, Ar-H), 8.18 (d, 2H, J= 8.4, Ar-H), 8.47 (s, 1H, Ar-H); 13C NMR (CDCl3, 75 MHz,): δ (ppm) 41.20 (s, C, OCH3), 111.66 (s, C, Ar-C), 115.10 (s, C, Ar-C), 119.56 (s, C, Ar-C) and 144.113 (s, C, Ar-C); CIMS: m/z 338.14: Anal. Calcd. For C14H9N3O3Cl2: C 49.73, H 2.68, N 12.43. Found: C 49.72, H 2.67, N 12.41%.
Biology
Antimicrobial activity
All the test compounds were assayed in vitro for antibacterial activity against Vancomycin resistant enteroccus, S. aureus, micrococcus and B. subtilis representative for gram-positive bacterias. S. dysentery and E. coli representative for gram-negative bacterias, and the antifungal activities were evaluated against C. albicans, A. niger and Penicillium. The strains used in this study were maintained at the Department of Botany, Karnatak University, Dharwad. The antimicrobial activity was determined by using disk diffusion method [Citation21–23] and MIC by twofold serial dilution method. Streptomycin and Nystatin were used as reference standards to compare antibacterial and antifungal activities, respectively. For determining the antimicrobial activity, the synthesized compounds were dissolved in dimethyl sulphoxide (the stock solution 1 mg/mL). Further dilutions were prepared at the required quantities of 100, 50 and 25 μg/mL concentrations. In order to ensure that the solvent had no effect on bacterial growth, a control test was also performed containing disc loaded with only DMSO at the same dilution used in our experiment. For determining the MIC of the synthesized compounds, they were diluted at 100, 50, 25 and 12 μg/mL concentrations and expressed in μM/mL (0.318, 0.159, 0.0795 and 0.0381 respectively for compound 1 h). In order to ensure that the solvent had no effect on fungal growth, a control test was also performed containing broath supplemented with only DMSO at the same dilution used in our experiment. The MIC of the compounds was defined, as the lowest concentration at which there was 100% inhibition of growth compared with the growth for a drug free control. Every experiment in the antibacterial assay and MIC was replicated thrice.
Antibacterial assay.
The antibacterial activity of the benzimidazole derivatives was tested by the agar disc-diffusion method against Gram-positive and Gram-negative bacteria. Test compound solutions prepared in DMSO were serially diluted and loaded (10 μL) to Sterile filter paper discs (6 mm diameter), which finally contained (25, 50 and 100 μg/mL) of the compound per disc respectively. Impregnated disks were then dried for 1 h and placed on inoculated plates. The seeded plates were incubated at 37 °C for 16 h. The radii of inhibition zones (in mm) of triplicate sets were measured and the results are given in & , for the compound 1h. The MIC of the compounds was defined, as the lowest concentration at which there was 100% inhibition of growth compared with the growth for a drug free control. The standard MIC for Streptomycin is Vancomycin resistant enteroccoccus (0.85–1.71), S. aureus (≤0.85), Micrococcus (≤0.85), B. subtilis (0.85-1.71), S. dysentery (1.71-3.43) and E. coli (≤0.85) μmoles/mL. Every experiment in the antibacterial assay was replicated thrice in order to define the MIC values, as shown in .
Figure 1. Photographic comparison of compound 1h with standard for antibacterial activity in Staphylococcus aureus and antifungal activity in Penicillium.
A & B - Streptomycin (Standard) and Compound 1h for Staphylococcus aureus
C & D - Nystatin (Standard) and Compound 1h for Penicillium
A & C - c- control disc with DMSO, std- standard (Streptomycin-10mcg & Nystatin-100units)
B & D - a-1mcg, b-0.5mcg and c-0.25mcg
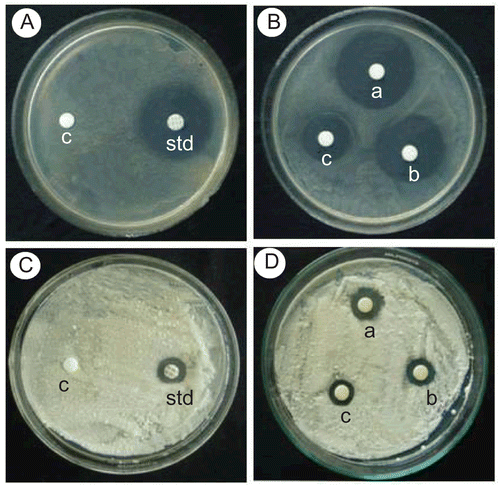
Figure 2. Bar curves for comparison of antibacterial activity of compound 1h with Streptomycin standard.
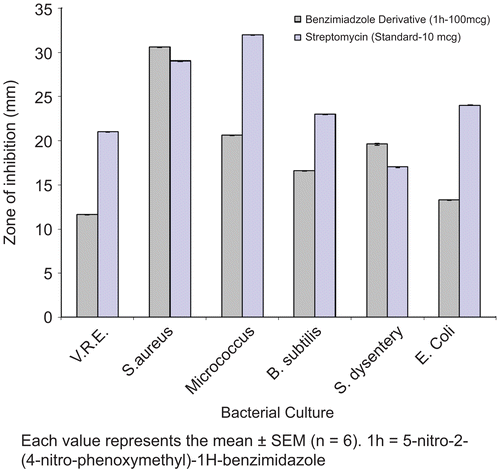
Table 2. The MIC values (μmoles/mL) of compounds 1a-1j for Antibacterial activity.
Antifungal assay.
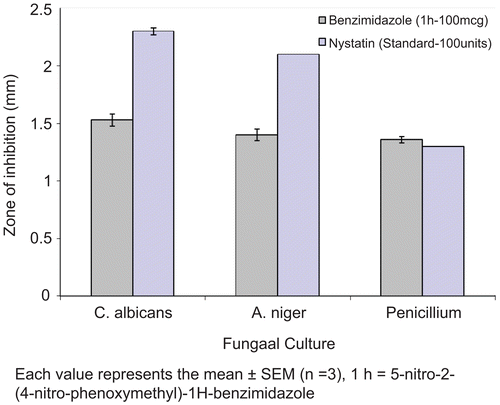
The synthesized Benzimidazole derivatives were tested for their antifungal activity in vitro, in comparison with Nystatin (Nystatin Ns 100, HIMEDIA, 100 units/disc) as a reference drug using the standard agar disk diffusion method against three strains of fungus (A. niger, Penicillium and C. albicans). A spore suspension in sterile distilled water was prepared from 3-5 days old culture of the test fungi growing on Potato Dextrose Agar (PDA) media. The final spore concentration was 5 × 10−4 spores mL−1. About 15 mL of the growth medium was placed into sterilized Petri dishes of 9 cm diameter and inoculated with 100 μL of the spore suspension. Sterile 6-mm filter paper disk (HiMedia Laboratories Pvt. Limited. India) was saturated with 10 μL of the test compound solution. Impregnated disks were then dried for 1 h and placed on inoculated plates. The seeded plates were incubated at 27 °C for 4 days. The radii of inhibition zones (in mm) of triplicate sets were measured and the results are given in . & . The MIC of the compounds was defined, as the lowest concentration at which there was 100% inhibition of growth compared with the growth for a drug free control. The standard MIC for Nystatin is A. niger (≤3.43), Penicillium (≤3.43) and C. albicans (≤1.71) at 100 units, as shown in
Table 3. The MIC values (μmoles/mL) of compounds 1a-1j Antifungal activity.
Results and discussion
Conventional synthesis sometimes has lower yields than microwave protocols. By using microwave irradiation 4-nitro ortho-phenylenediamine reacted with 1:1 equivalent of substituted phenoxyacetic acids at 400W for 2.5-3.5 min. to successfully afford the desirable 5-nitro-2-aryl substituted-1H-benzimidazole libraries in good to excellent isolate yields (82–92%). Whereas using conventional heating the yields are only 58–75%. The effects of time on the reaction were also studied and the results summarized in . All the compounds synthesized were adequately characterized by IR, 1H NMR, 13C NMR spectroscopies and by mass spectrometry.
The obtained results revealed that the nature of substituents and substitution pattern on the benzimidazole ring might have a considerable impact on the antimicrobial activities of the target substituted benzimidazole libraries of particular importance. A nitro group in the aromatic ring enhances the antimicrobial activity against most of the microorganisms.
In general compounds 1a, 1c, 1d and 1h exhibited more pronounced antibacterial activities than the compounds 1b, 1e, 1f, 1g, 1i and 1j, with better activity against both gram-positive and gram-negative bacteria. Among all compounds tested 1h exhibited significant antibacterial activity against the gram-positive Staphylococcus aureus, as compared with antibiotic Streptomycin. On the other hand 1c, 1d and 1h against gram-negative Shigella dysentery as compared with antibiotic Streptomycin as showed in ( & .).
The antifungal screening results (MIC), showed in . It is evident from the screening data of the compounds, 1h was more effective against penicillium, compared with the standard Nystatin. Compounds 1a, 1b, 1c, 1d, 1e, 1f, 1g, 1i and 1j showed moderate antifungal activity against all fungal species with a MIC of 50 μg/mL (0.269, 0.176, 0.176, 0.176, 0.164, 0.164, 0.159, 0.176 and 0.147μM/mL respectively). Examination of the antifungal data it is seen that some of the compounds possess significant activity.
Conclusion
In summary we have described a simple, rapid, efficient and convenient protocol for the preparation of 5-nitro-2-aryl substituted phenoxymethyl-1H-benzimidazole libraries in one pot-synthesis. All these reactions were carried in microwave irradiation as well as conventional method under solvent free conditions. Furthermore, the procedure used commercially available reagents and equipment, and most of the reactions involved are efficient, giving the desired compounds in higher purity and yield. The versatility of this methodology is suitable for library synthesis in drug discovery efforts. Bioassay indicated that all the compounds showed in vitro antimicrobial activity against six strains of bacteria and three strains of fungus. Further in vivo biological evaluations are underway.
Acknowledgements
This research work is financially supported by the Department of Science & Technology (DST), New Delhi - 110 016. (Ref. No SR/S1/OC-08/2005 dated 05-09-2005).
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- (a) Preston PN. John Wiley & Son, New York, Vol. 40, Part 2, Chapter 10, 1980. According to Evans, a Privileged Structure is ‘a single molecular framework able to provide ligands for diverse receptors’, see; (b) Evans BE, Rittle KE, Bock MG, Dipardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DJ, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield J. Methods of drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J Med Chem 1988; 31: 2235–2246.
- Yesim U, Aysegul AY, Aysegul NI, Esin A, Likay Y, Sukrukeles. Investigating toxic effects of the HIV-RT inhibitor 2-phenoxymethyl-5-chloro-benzimidazole on rat liver. Turk J Med Sci 2005; 35: 5–12.
- Elnima EI, Zubair MU, Al-Badr AA. Antibacterial and antifungal activities of benzimidazole and benzoxazole derivatives. Antimicrob Agents Chemother 1981; 19(1): 29–32.
- Rashmi D, Syed A, Satyavan S, Chatterjee RK, Katiyar JC. Synthesis and anthelmintic activity of 5(6)-[(benzimidazol-2-yl)carboxamido]- and (4-substituted piperazin-1-yl) benzimidazoles. J Med Chem 1985; 28: 1748–1750.
- Yun-Fei L, Gui-Feng W, Pei-Lan H, Wei-Gang H, Feng-Hua Z, He-Yong G, Wei T, Yu L, Chun-Lan F, Li-Ping S, Yu-Dan R, Wei L, Jian-Ping Z. Synthesis and anti-hepatitis B virus activity of novel benzimidazole derivatives. J Med Chem 2006; 49: 4790–4794.
- Krzysztof B, Stawomir W, Anna B. Inhibition of DNA topoisomerase I and II, and growth inhibition of MDA-MB-231 human breast cancer cells by bis-benzimidazole derivatives with alkylating moiety. Pol J Pharm 2004; 56: 373–378.
- Alka B, Yogita B, Sugumaran M, Jatinder SS, Balakumar, Gurpreet K, Gulshan B, Ajay S, Manjeet S.Design, synthesis, and evaluation of novelly substituted benzimidazole compounds as angiotensin II receptor antagonists. Bioorg Med Chem Lett 2005; 15: 3962–3965.
- For reviews on the chemistry of benzimidazoles, a) Wright JB. The chemistry of the benzimidazoles. Chem Rev 1951; 48: 397–541. b)Preston PN. Synthesis, reactions, and spectroscopic properties of benzimidazoles. Chem Rev 1974; 74: 279–314. c) Gray DN. Hydartes of 2,21-diphenyl-5,51-bibenzimidazole. J Heterocyclic Chem 1970; 7: 947–949.
- Dolle RE. Comprehensive survey of combinatorial library synthesis: 2003. J Comb Chem 2004; 6: 623–679.
- (a) Tucker JL. Green chemistry, a pharmaceutical perspective. Org Process Res Dev 2006; 10: 315–319. (b) Strauss CR. Invited review. A combinatorial approach to the development of environmentally benign organic chemical preparations. Aust J Chem 1999; 52: 83–96.
- Chandrasekhar S, Sultana SS, Yaragorla SR, Reddy NR. Copper-catalyzed N-arylation of aminesamides in poly(ethylene glycol) as recyclable solvent medium. Synthesis 2006; 5: 839–842.
- Lidstrom P, Tierney J, Wathey B, Westman J. Microwave assisted organic synthesis- a review. Tetrahedron 2001; 57: 9225–9283.
- Lew A, Krutzik PO, Hart ME, Chamberlin AR. Increasing rates of reaction: microwave-assisted organic synthesis for combinatorial chemistry. J Comb Chem 2002; 4: 95–105.
- Perumal S, Mariappan S, Selvaraj S. A microwave assisted synthesis of 2-aryl-1-arylmethyl-1H-1,3-benzimidazoles in the presences of K-10. Arkivoc 2004; 46–51.
- For recent reviews of microwave-assisted reactions see: (a) Bendale PM, Sun CM. Rapid microwave-assisted liquid-phase combinatorial synthesis of 2- (arylamino) benzimidazoles. J Comb Chem 2002; 4: 359–362; (b) David T, Kathy C, Christopher HP. Traceless solid-phase synthesis of substituted benzimidazoles via a base-cleavable linker. Org Lett 2001; 3(1): 83–86; (c) Krishna MKVV, Narender N, Kulkarni SJ. Zeolite catalyzed acylation of alcohols and amines with acetic acid under microwave irradiation. Green Chem 2006; 8: 368–372; (d) Kappe CO. Controlled microwave heating in modern organic synthesis. Angew Chem Int Ed 2004; 43: 6250–6284; (e) Burczyk A, Loupy A, Bogdal D, Petit A. Improvement in the synthesis of metallophthalocyanines using microwave irradiation. Tetrahedron 2005; 61: 179–188; (f) Larhed M, Hallberg A. Microwave-assisted high-speed chemistry: a new technique in drug discovery. Drug Discov Today 2001; 6: 406–416.
- Biswanath D, Harish H, Yalamalla S. Efficient (bromodimethyl) sulfonium bromide mediated synthesis of benzimidazoles. Tetrahedron Lett 2007; 48: 61–64.
- Fanta PE. The ullmann synthesis of biaryls, 1945-1963. Chem Rev 1964; 64: 613–632.
- Gomberg M, Bachmann WE. The synthesis of biaryl compounds by means of the diazo reaction. J Amer Chem Soc 1924; 46: 2339–2343.
- Meyers AI, Mihelich ED. Oxazolines. XXII. Nucleophilic aromatic substitution on aryl oxazolines. Efficient approach to unsymmetrically substituted biphenyls and o-alkyl benzoic acids. J Amer Chem Soc 1975; 97: 7383–7385.
- Lo YS, Rossano LT. Tetrazolylphenylboronic acid intermediates for the synthesis of all. Receptor Antagonists. U S Pat 1992; 5,130,439.
- National Committee for Clinical Laboratory Standards, NCCLS Approval Standard Document M2-A7. Villanova, PA, U.S.A. 2000.
- National Committee for Clinical Laboratory Standards, NCCLS Approval Standard Document M7-A5. Villanova, PA, U.S.A. 2000.
- William H. Microbiological assay. An Introduction to Quantitative Principles and Evaluation, Academic Press, New York 1977.