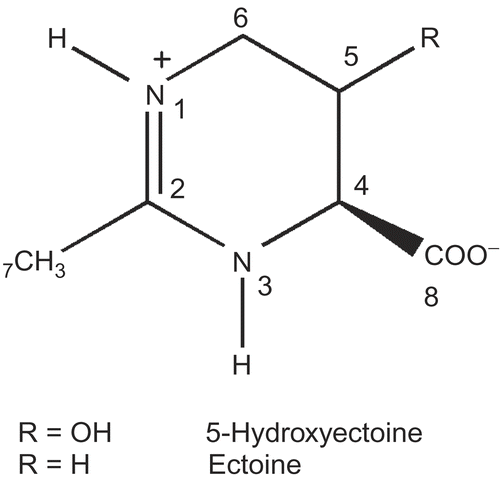
Abstract
The compounds pyrostatin A and B, isolated from Streptomyces sp. SA-3501 have been reported as N-acetyl-ß-D-glucosaminidase inhibitors with inhibition constants in the micromolar range. Recently, a comparison of NMR spectral data of the pyrostatins has led to a structural revision of the pyrostatins. It was shown that the pyrostatins A and B are identical to the ectoines 5-hydroxectoine and ectoine, respectively. Ectoines are known as compatible osmolytes in many halophilic and stress-tolerant bacteria. We have performed enzymatic experiments demonstrating that neither ectoine nor 5-hydroxyectoine exhibit an inhibitory effect on N-acetyl-ß-D-glucosaminidase. The previously reported inhibition of N-acetyl-ß-D-glucosaminidase by pyrostatins A and B may thus be due to the contamination of the compound preparations with a strong N-acetyl-ß-D-glucosaminidase inhibitor with an inhibition constant (Ki) in the nanomolar range, as has been reported in other Streptomyces species.
| Abbreviation: | ||
| GlcNAc-ase: | = | N-acetyl-ß-D-glucosaminidase |
Introduction
N-acetyl-beta-D-glucosaminidase (GlcNAc-ase; E.C. 3.2.1.30) catalyzes the hydrolytic release of N-acetyl-beta-D-glucosamine from glycoproteins. Previously, Aoyama et. al. (1995) purified and determined two GlcNac-ase inhibitors, pyrostatin A and B, from Streptomyces sp. SA-3501, which was isolated from a marine environment [Citation1]. The structures of pyrostatin A and B were determined by 1H and 13C-NMR analysis to be 4-hydroxy-2-amino-1-methylpyrrolidine-5-carboxylic acid and 2-imino-1-methylpyrrolidine-5-carboxylic acid respectively. It was reported that both pyrostatin A and B were competitive inhibitors on GlcNAc-ase with inhibition constants (Ki) of 1.7 × 10−6 M and 2.0 × 10−6 M respectively [Citation1].
As part of a search for biologically active secondary metabolites, Castellanos et. al. 2006 discovered 2-imino-1-methylpyrolidine-5-carboxylic acid in the organic extracts of the sponge Cliona tenius [Citation2]. The structure was determined using spectral analysis, however the data did not match those published for pyrostatin B by Aoyama et al. To clarify this discrepancy Castellanos et al. performed the total synthesis of 2-imino-1-methylpyrrolidine-5-carboxylic acid. The analysis of the synthesized compound confirmed their proposed structure and thus showed that the structure proposed by Aoyama et al. for pyrostatin B is incorrect.
Based on a comparison with the NMR data published in [Citation1], Castellanos et al. found that the data reported for pyrostatin A and pyrostatin B match those of 5-hydroxyectoine and ectoine respectively.
Ectoine and hydroxyectoine () are known as compatible solutes and are widespread in halotolerant and halophilic bacteria. Several species of the genus Streptomyces, from which the pyrostatins have been isolated, are known to produce ectoine and hydroxyectoine [Citation3–8]. Compatible solutes like the ectoines can be accumulated by microorganisms up to very high concentrations and are generally known as chemically inert. Consequently, no enzyme-inhibitory effect has been described for these compounds so far. We therefore re-examined whether pyrostatin A (5-hydroxyectoine) and pyrostatin B (ectoine) inhibit GlcNAc-ase, as described by Aoyama et al. [Citation1].
The enzymatic activity of GlcNAc-ase from bovine kidney in the presence and absence of various ectoine concentrations was measured. 2-acetamido-2-deoxy-D-glucono-1,5-lactone, a known strong inhibitor of GlcNAc-ase was used as a positive control for inhibition [Citation9].
Material and methods
Materials
GlcNAc-ase and 4-nitrophenyl N-acetyl-ß-D-glucosaminide were purchased from Sigma-Aldrich (Seelze, Germany). 2-Acetamido-2-deoxy-D-glucono-1,5-lactone was obtained from Chemos GmbH (Regenstauf, Germany). Ectoine and 5-hydroxyectoine (isolated from Halomonas elongata) were from bitop AG (Witten, Germany). All other chemicals were of standard analytical grade from Merck KGaA (Darmstadt, Germany).
Methods
The enzyme activity of GlcNAc-ase was determined by measuring the amount of liberated p-nitrophenol when using 4-nitrophenyl N-acetyl-ß-D-glucosaminide as a substrate [Citation10]. The reaction mixture contained 790 μL 0.1 M sodium citrate buffer (pH 5.0), 100 μL 4-nitrophenyl N-acetyl-ß-D-glucosaminide in final concentrations ranging from 0.3 mM to 2.5 mM. Ectoine, 5-hydroxyectoine and 2-acetamido-2-deoxy-D-glucono-1,5-lactone were added in a volume of 10 μL to the assay mixture, whereas water was used for reference measurements. After addition of 100 μL GlcNAc-ase (0.17 U/mL) the mixture was incubated in 1.5 mL semi-micro cuvettes. After 10 min of incubation the reaction was stopped by adding 10 μL 1 M glycine-sodium hydroxide buffer (pH 12) to the reaction mixture. The absorbance at 400 nm was measured using a Pharmacia Ultrospec 3000 Photometer.
Results and discussion
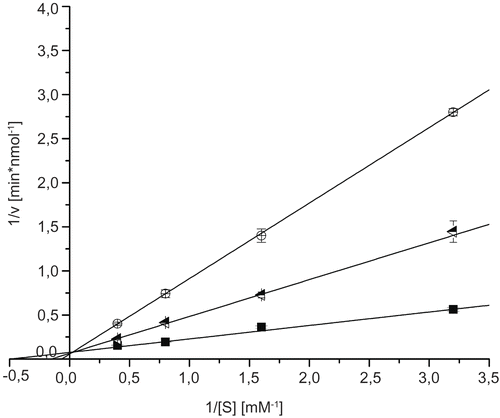
2-acetamido-2-deoxy-D-glucono-1,5-lactone shows competitive inhibition of GlcNAc-ase with an inhibition constant (Ki) of 0.036 μM at pH 4.25 [Citation9]. It was therefore chosen as a positive control to clarify if ectoine and 5-hydroxyectoine also show an inhibitory effect on GlcNAc-ase. For the inhibition of GlcNAc-ase two different concentrations (0.11 μM and 1.1 μM) of 2-acetamido-2-deoxy-D-glucono-1,5-lactone were used. As indicated in , 2-acetamido-2-deoxy-D-glucono-1,5-lactone shows typical characteristics of a competitive inhibitor, with a calculated Ki value of 0.18 μM at pH 5.0.
Figure 2. Lineweaver-Burk plots for inhibition of N-acetyl-ß-D-glucosaminidase by 2-acetamido-2-deoxy-D-glucono-1,5-lactone at different concentrations; 0 μM (▪), 0.11 μM (![]()
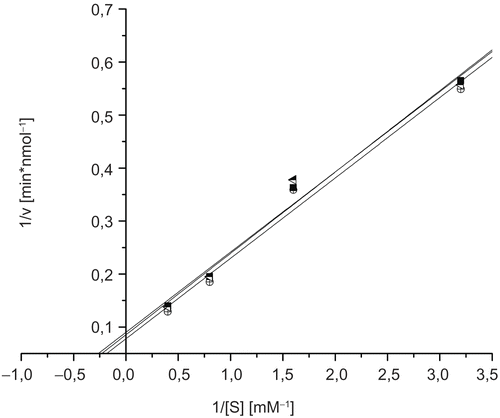
To measure the inhibition constants of ectoine and 5-hydroxyectoine on GlcNAc-ase, we used several concentrations in the range of 0.2 μM to 210 μM of ectoine and 5-hydroxyectoine. As shown in , even the highest concentration used does not induce an inhibitory effect of ectoine or 5-hydroxyectoine.
Figure 3. Lineweaver-Burk plots for inhibition of N-acetyl-ß-D-glucosaminidase by ectoine and 5-hydroxyectoine; blank (▪), 210 μM ectoine (![]()
Comparison of the 1H and 13C-NMR spectral data for pyrostatin B [Citation1], and ectoine [Citation11] as shown in and shows virtually no difference in either the proton or carbon chemical shifts.
Table 1. Comparison of 1H-NMR data of ectoine [Citation11] and pyrostatin B [Citation1]
Table 2. Comparison of 13C-NMR data of ectoine [Citation11] and pyrostatin B [Citation1]
In contrast comparison of the 1H and 13C-NMR data for the synthetic 2-imino-1-methylpyrrolidone-5-carboxylic acid [Citation2] does not match those reported for pyrostatin B (data not shown).
In summary it can be concluded that the substance pyrostatin B described by Aoyama et al. is in fact ectoine, and furthermore pyrostatin A is 5-hydroxyectoine, as proposed by Castellanos et al. (2006). Furthermore we demonstrated that these compounds do not have an inhibitory effect towards GlcNAc-ase. One possible explanation for the inhibition observed by Aoyama et al. is a contamination of the pyrostatin preparations with a highly potent GlcNAc-ase inhibitor, possibly similar to the Streptomyces compound nagstatin, with a Ki for GlcNAc-ase in the nanomolar range [Citation12].
Acknowledgment
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aoyama T, Koyima F, Imada C, Muraoka Y, Naganawa H, Okami Y, Takeuchi T, Aoyagi T. Pyrostatins A and B, new inhibitors of N-acetyl-beta-D-glucosaminidase, produced by Streptomyces sp.SA-3501. J Enz Inhib (1995);8:223–32.
- Castellanos L, Duque C, Zea S, Espada A, Rodríguez J, Jiménez C. Isolation and synthesis of (-)-(5S)-2-imino-1-methylpyrrolidine-5- carboxylic acid from Cliona tenuis: structure revision of pyrostatins. Org Lett (2006); 8:4967–4970.
- Bursy J, Pierik AJ, Pica N, Bremer E. Osmotically induced synthesis of the compatible solute hydroxyectoine is mediated by an evolutionarily conserved ectoine hydroxylase. J Biol Chem (2007);282:31147–31155.
- De Mot R, Schoofs G, Nagy I. Proteome analysis of Streptomyces coelicolor mutants affected in the proteasome system reveals changes in stress-responsive proteins. Arch Microbiol (2007);188:257–271.
- Inbar L, Lapidot A. The structure and biosynthesis of new tetrahydropyrimidine derivatives in actinomycin D producer Streptomyces parvulus.Use of 13C- and 15N-labeled L-glutamate and 13C and 15N NMR spectroscopy. J Biol Chem (1988);263:16014–16022.
- Killham K, Firestone MK. Salt Stress Control of Intracellular Solutes in Streptomycetes Indigenous to Saline Soils. Appl Environ Microbiol (1984);47:301–306.
- Malin G, Lapidot A. Induction of synthesis of tetrahydropyrimidine derivatives in Streptomyces strains and their effect on Escherichia coli in response to osmotic and heat stress. J Bacteriol (1996);178:385–395.
- Prabhu J, Schauwecker F, Grammel N, Keller U, Bernhard M. Functional expression of the ectoine hydroxylase gene (thpD) from Streptomyces chrysomallus in Halomonas elongata. Appl Environ Microbiol (2004);70:3130–3132.
- Legler G, Lüllau E, Kappes E, Kastenholz F. Bovine N-acetyl-beta-D-glucosaminidase: affinity purification and characterization of its active site with nitrogen containing analogs of N-acetylglucosamine. Biochim Biophys Acta (1991);1080:89–95.
- Bahl OP, Agrawal KML. Glycosidases of Phaseolus vulgaris. I.Isolation and characterization of beta-N-acetylglucosaminidase. J Biol Chem (1968);243:98–102.
- Galinski EA, Pfeiffer HP, Truper HG. 1,4,5,6-Tetrahydro-2-methyl-4-pyrimidinecarboxylic acid. A novel cyclic amino acid from halophilic phototrophic bacteria of the genus Ectothiorhodospira. Eur J Biochem (1985);149:135–139.
- Aoyagi T, Suda H, Uotani K, Kojima F, Aoyama T, Horiguchi K, Hamada M, Takeuchi T., Nagstatin, a new inhibitor of N-acetyl-beta-D-glucosaminidase, produced by Streptomyces amakusaensis MG846-fF3. Taxonomy, production, isolation, physico-chemical properties and biological activities. J Antibiot (Tokyo) (1992);45:1404–1408.