Abstract
The dichloromethane fraction of the bark of Machilus thunbergii Sieb. et Zucc. (Lauraceae) significantly protected primary cultures of rat cortical cells exposed to the excitotoxic amino acid, L-glutamate. Through the activity-guided isolation from the CH2Cl2 fraction, (+)-9′-hydroxygalbelgin (1), isogalcatin B (2), (7S,8S,8′R)-3′,4′-dimethoxy-3,4,-methylenedioxylignan-7-ol (3), 1-hydroxy-7-hydroxymethyl-6-methoxyxanthone (4), 5,7-dimethoxy-3′,4′-methylenedioxyflavan-3-ol (5), (+)-(3S,4S,6R)-3,6-dihydroxypiperitone (6), protocatechuic acid methyl ester (7) and tyrosol (8) were obtained. All of them had significant neuroprotective activities against glutamate-induced neurotoxicity in primary cultures of rat cortical cells at concentrations ranging from 0.1 μM to 10.0 μM and were comparable to MK-801, a well-known inhibitor of glutamate receptor.
Introduction
Glutamate is an excitatory amino acid and activates different types of ion channel-forming receptors and G-protein-coupled receptors. In the central nervous system, glutamate plays its essential roles such as neuronal survival, synaptogenesis, neuronal plasticity, memory, learning and behavior [Citation1, Citation2]. However, one of the pathogenetic mechanisms discussed to be relevant for the etiology of Alzheimer’s disease (AD) are the glutamate induced excitotoxic cascades [Citation3]. Thus, glutamate excitotoxicity has also become a target for AD treatment. We previously applied glutamate induced neurotoxicity in primary cultures of rat cortical cells as an in vitro assay system to isolate neuroprotective compounds from natural products [Citation4]. The methanolic extract of the bark of Machilus thunbergii Sieb. et Zucc. (Lauraceae) was found to significantly protect primary cultures of rat cortical cells against glutamate-induced neurotoxicity.
Machilus thunbergii (Lauraceae) is widely distributed in Korea. The cortex of M. thunbergii, which has been consumed as traditional herbal medicine for a long period of time, has provided Korean farmers with significant income. The cortex are used in Korean folk medicine for treatment of leg edema and abdominal distension and pain [Citation5]. Many lignans, flavonoids and essential oils have all been previously reported as components of the bark of M. thunbergii [Citation6]. In our previous study, subsequent phytochemical studies coupled with our bioassay were performed to elucidate active principles responsible for the neuroprotective activity of M. thunbergii. We reported that meso-dihydroguaiaretic acid, licarin A, isoguaiacin and guaiacin isolated from M. thunbergii had the significant neuroprotective activity against glutamate-induced neurotoxicity [Citation7]. In addition to lignans such as meso-dihydroguaiaretic acid, licarin A, isoguaiacin and guaiacin, we attempted to isolate more neuroprotective compounds from the CH2Cl2 fraction of M. thunbergii methanolic extract against glutamate-induced neurotoxicity as measured in vitro.
Materials and methods
General experimental procedures
The 1H- and 13C-NMR measurements were carried out in a Bruker AMX 400 spectrometer operating at 400 and 100 MHz, respectively. TMS or solvent signals were used as internal standard. FT-IR spectra were recorded on a Perkin-Elmer 1710 spectrophotometer. UV spectra were recorded on a Shimadzu UV-2100 spectrophotometer. EIMS spectra were obtained on a VG Trio II spectrometer. Column chromatography was performed on Merck (9025) silica gel 60 (0.04-0. 063 mm). Analytical TLC was performed on precoated Merck F254 silica gel plates and visualized by spraying with anisaldehyde-H2SO4. An HPLC system (Hitachi L-6200, Japan) equipped with a UV-visible detector and Microsorb C18 semipreparative column (Rainin Inst. Co.) was used for isolation. CD data were recorded in MeOH on a JASCO-J715 spectrophotometer.
Plant materials
The bark of M. thunbergii Sieb. et Zucc. was purchased in a local market for Oriental medicine in Gyeongdong, Seoul, Korea, in 2001. Voucher specimens (SNUPH-0521) have been deposited in the Herbarium of the College of Pharmacy, Seoul National University.
Extraction and isolation
The dried bark of M. thunbergii (30 kg) was ground into a powder and extracted 3 times with 80% MeOH using the reflux apparatus. Upon removal of the solvent in vacuo, the methanolic extract yielded 2.9 kg of material (9.7% by dry weight). The methanolic extract was suspended in H2O and partitioned successively with CH2Cl2. The CH2Cl2 fraction (980 g) which showed significant neuroprotective activity was subjected to column chromatography (cc) over silica gel (12 × 80 cm) eluted with n-hexane-EtOAc (20:1, 10:1, 5:1, 2:1, 1:1, EtOAc, MeOH) to afford seven fractions (F01-F07). F03 was further applied to a silica gel cc (3.5 × 45 cm) eluting with n-hexane-EtOAc (20:1, 10:1, 5:1, 2:1, 1:1, EtOAc, MeOH), affording ten fractions (F03-I to F03-X). F03-V was subjected to a Sephadex LH-20 column eluting with MeOH to afford seven fractions (F03-V-01 to F03-V-07). Among the 7 subfractions, F03-V-04 (125 mg) yielded compound 3 (3.3 mg) and 4 (13.2 mg) by additional purification steps on RP-18 HPLC (H2O-AcCN, 65:35). F04 was further applied to a silica gel cc (3.5 × 45 cm) eluting with n-hexane-EtOAc (20:1, 10:1, 5:1, 2:1, 1:1, EtOAc, MeOH), affording three fractions (F04-I to F04-III). Among the 3 subfractions, F04-III (85 mg) yielded compound 1 (3.6 mg) by additional purification steps on RP-18 HPLC (H2O-AcCN, 60:40) and compound 5 (6.3 mg). F05 was further applied to a silica gel cc (3.5 × 45 cm) eluting with n-hexane-EtOAc (20:1, 10:1, 5:1, 2:1, 1:1, EtOAc, MeOH), affording five fractions (F05-I to F05-V). From the subfraction F05-II, compounds 2 (3.4 mg), 6 (35.7 mg), 7 (5.0 mg) and 8 (2.1 mg) were isolated by additional purification steps on RP-18 HPLC (H2O-AcCN, 60:40). Optical rotations were determined on a polarimeter at 25°C.
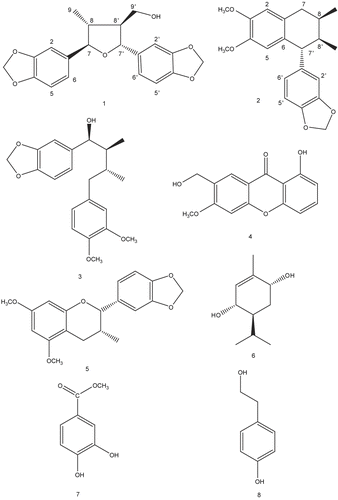
(+)-9′-hydroxygalbelgin (1): White amorphous powder. [α]25D +19.0° (CHCl3, 2.44). IR (KBr) vmax: 3440, 1510, 1450, 1271, 1118 cm−1. 1H-NMR (300 MHz, CDCl3) δ: 1.08 (3H, d, J = 6.6 Hz, H-9), 1.99-2.07 (1H, m, H-8), 2.25-2.35 (1H, m, H-8′), 3.71 (2H, d, J = 7.1 Hz, H-9′), 4.18 (1H, d, J = 9.0 Hz, H-7), 4.59 (1H, d, J = 8.9 Hz, H-7′), 5.93 (2H, s, OCH2O), 5.94 (2H, s, OCH2O), 6.73 (1H, d, J = 8.1 Hz, H-5), 6.74 (1H, d, J = 8.0 Hz, H-5′), 6.76 (1H, dd, J = 1.7, 8.0 Hz, H-6), 6.77 (1H, dd, J = 1.7, 8.0 Hz, H-6′), 6.85 (1H, d, J = 0.8 Hz, H-2), 6.86 (1H, d, J = 0.8 Hz, H-2′) ppm, 13C-NMR (100 MHz, CDCl3) δ: 16.8 (C-9), 46.3 (C-8), 54.0 (C-8′), 70.0 (C-9′), 77.2 (C-7′), 89.4 (C-7), 100.9 (OCH2O), 101.1 (OCH2O), 106.7 (C-2′), 106.7 (C-2), 107.9 (C-5′), 108.1 (C-5), 119.9 (C-6), 120.0 (C-6′), 134.9 (C-1′), 137.3 (C-1), 147.1 (C-4′), 147.3 (C-4), 147.8 (C-3′), 147.9 (C-3) ppm. EIMS m/z (rel int) 356 [M]+ (52%), 255 (44%), 250 (22%), 208 (87%), 192 (70%), 173 (100%). HREIMS m/z 356.1258 (calcd for C20H20O6: 356.1259).
isogalcatin B (2): White amorphous powder. [α]25D -21.1° (CHCl3, 1.25). IR (KBr) vmax: 2900, 1610, 1510, 1495, 1450 cm−1. 1H-NMR (400 MHz, CDCl3) δ: 0.89 (3H, d, J = 6.7 Hz, H-9), 0.90 (3H, d, J = 6.9 Hz, H-9′), 1.88-1.91 (1H, m, H-8′), 2.00-2.02 (1H, m, H-8), 2.45 (1H, dd, J = 8.1, 16.5 Hz, H-7a), 2.84 (1H, dd, J = 5.4, 16.5 Hz, H-7b), 3.66 (1H, d, J = 5.6 Hz, H-7′), 3.68 (3H, s, OCH3), 3.86 (3H, s, OCH3), 5.91 (2H, s, OCH2O), 6.33 (1H, s, H-5), 6.48 (1H, dd, J = 1.5, 8.1 Hz, H-6′), 6.49 (1H, d, J = 1.5 Hz, H-2′), 6.58 (1H, s, H-2), 6.70 (1H, d, J = 8.3 Hz, H-5′), 13C-NMR (100 MHz, CDCl3) δ: 15.3 (C-9), 16.6 (C-9′), 28.4 (C-8), 34.6 (C-7), 40.9 (C-8′), 51.0 (C-7′), 55.7 (OCH3×2), 55.8 (OCH3×2), 100.8 (OCH2O), 107.6 (C-2), 109.4 (C-2′), 111.2 (C-5′), 113.2 (C-5), 122.1 (C-6′), 128.4 (C-1), 129.3 (C-6), 141.3 (C-1′), 145.5 (C-4), 147.1 (C-4′), 147.3 (C-3), 147.4 (C-3′). EIMS m/z (rel int): 340 [M]+ (34%), 309 (41%), 283 (64%), 269 (37%), 253 (100%), 223 (76%), 187 (65%). HREIMS m/z 340.1672 (calcd for C21H24O4: 340.1675).
In vitro neuroprotective activity
Female Sprague-Dawley rats (20-23 °C; 12 h light cycle from 09:00 to 21:00; food, Agribrand Purinar Korea, and water ad libitum) were provided by the Laboratory Animal Center, Seoul National University. All experiments were conducted according to the guidelines of the Committee on Care and Use of Laboratory Animals of the Seoul National University. Primary cultures of mixed cortical cells containing both neuronal and glial cells were prepared from late fetal SD rats (17-19-days gestation in utero) as described previously [Citation8]. All compounds were dissolved in DMSO (final culture concentration, 0.1 %). Preliminary studies indicated that the solvent had no effect on cell viability at the concentration used (data not shown). Two known glutamate receptor antagonists, MK-801 (dizocilpine maleate, a non-competitive antagonist of the NMDA receptor) and CNQX (6-cyano-7-nitroquinoxaline-2,3-dione, a non-NMDA receptor antagonist) were used as positive controls for the assessment of neuroprotective activity [Citation8, Citation9]. Cortical cell cultures were washed with DMEM and incubated with the test compounds for 1 h. The cultures were then exposed to 100 μM glutamate. After 24 h incubation, the cultures were assessed for the extent of neuronal damage. Neuronal viability and integrity were quantified by MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) and/or LDH (lactate dehydrogenase) assay described in our previous report [Citation8]. Data are expressed as the percentage protection relative to control cultures.
Statistical analysis
Data were evaluated for statistical significance by “ANOVA” test using a computerized statistical package (control vs 0.1 μM, control vs 1.0 μM and control vs 10.0 μM, respectively). We performed all the statistics on the raw data prior to transformation to percentage against control. The confidence level for statistical significance was set at a probability value of 0.05.
Results and discussion
As a part of our continuing research seeking neuroprotective compounds from natural resources, the methanolic extract of the bark of Machilus thunbergii Sieb. et Zucc. (Lauraceae) was found to significantly protect primary cultures of rat cortical cells against glutamate-induced neurotoxicity. We attempted to isolate more neuroprotective compounds from the CH2Cl2 fraction of M. thunbergii methanolic extract against glutamate-induced neurotoxicity as measured in vitro. As a result, we isolated and identified two new lignans, (+)-9′-hydroxygalbelgin (1) and isogalcatin B (2), and six known compounds (). The methanolic extract of bark of M. thunbergii was found to exhibit a significant neuroprotective activity against glutamate-induced toxicity. The methanolic extract was suspended in H2O and partitioned with CH2Cl2. At a concentration of 10 μg/mL, neuroprotection against glutamate-induced toxicity of CH2Cl2 fraction was 65.7 % (p<0.01). Further fractionation and separation of the CH2Cl2 fraction by several chromatographic methods yielded eight compounds. Spectral data of compounds 3-8 matched those of (7S,8S,8′R)-3′,4′-dimethoxy-3,4,-methylenedioxylignan-7-ol (3), 1-hydroxy-7-hydroxymethyl-6-methoxyanthone (4), 5,7-dimethoxy-3′,4′-methylenedioxyflavan-3-ol (5), (+)-(3S,4S,6R)-3,6-dihydroxypiperitone (6), protocatechuic acid methyl ester (7) and tyrosol (8) [Citation10–16].
Compound 1 was obtained as amorphous powders. The molecular formula C20H20O6 was established by HREIMS, m/z 356.1258 (calcd for C20H20O6: 356.1259) and 13C NMR data. It contained two 3,4-methyenedioxyphenyl groups as established by the presence of two sets of ABX system for six aryl protons (one for H-2, H-5 and H-6, and the other for H-2′, H-5′ and H-6′) and two metylenedioxy singlets (δ 6.04 and 5.94) in its 1H NMR spectrum. The spectral data indicated 1 contained two relatively deshielded benzylic protons (δ 4.18 (1H, d, J = 9.0 Hz, H-7) and δ 4.59 (1H, d, J = 8.9 Hz, H-7′)), two aliphatic methanes (δ 1.99-2.07 (1H, m, H-8), δ 2.25-2.35 (1H, m, H-8′)), and two protons bound to hydroxyl group (δ 3.71 (2H, d, J = 7.1 Hz, H-9′)). On the basis of these data, 1 was inferred as 9′-hydroxy-3,4:3′,4′-bis(methylenedioxy)-7,7′-epoxylignan [Citation17]. In the NOESY spectrum, H-7 and H-8′ peaks correlated each other, and H-7′ and H-8 peaks correlated each other. We determined trans H-7/H-8, trans H-7/H-7′, trans H-7′/H-8′, and trans H-8′/H-8 relationships. Absolute configuration was determined by comparison of CD data of compound 1 and (+)-galbelgin, which has the similar stereochemistry of compound 1 () [Citation18, Citation19]. From the spectroscopic data above, the structure of 1 was concluded to be (+)-9′-hydroxygalbelgin.
Table 1. CD data of compound 1 and (+)-galbelgin
Compound 2 was obtained as white amorphous powders. The molecular formula C21H24O4 was established by HREIMS, m/z 340.1672 (calcd for C21H24O4: 340.1675) and 13C NMR data. The spectral data were very similar to those of (-)-isoguaiacin, except for the presence of a methylenedioxy group (δ 5.91 (2H, s, OCH2O)) and the position of a methoxy group (δ 3.68 (3H, s, OCH3)) [Citation6]. The relative configuration of chiral protons was determined as cis H-8/H-8′ and trans H-7′/H-8′ relationships by comparison to 13C NMR data of reference [Citation20]. The position of methoxy group was also determined by comparison to reference [Citation21]. Optical rotation was determined on polarimeter at 25°C. The optical rotation value of the compound 2 was -21.1° (c 1.25 in CHCl3). From the spectroscopic data above, the structure of 2 was designed to be isogalcatin B as a stereoisomer of isogalcatin.
Compounds 1-8 were tested for protective activity against glutamate-induced toxicity. The relative protection of the eight compounds is compared in . All of these comounds showed significant neuroprotective activities at concentrations ranging from 0.1μM to 10.0 μM (; MTT assay showed same trend of LDH assay; data not shown). Of the four neuroprotective compounds, tyrosol (8) showed the most potent activity against glutamate-induced neurotoxicity. The potency of the tyrosol is similar to that of MK-801 or CNQX, the positive controls.
Table 2. The Protective activity of compounds isolated from the CH2Cl2 fraction of M. thunbergii bark against glutamate-induced neurotoxicity in primary cultures of rat cortical cells
Tyrosol is a major compound of extra virgin olive oil. Extra virgin olive oil, the typical added fat of the Mediterranean diet, has been related to a general health benefit and a reduced incidence of risk factors for coronary heart disease [Citation22]. Tyrosol inhibited cell-mediated oxidation of LDL and preserved cellular activities of antioxidative enzymes such as glutathione peroxidase and glutathione reductase reduced in the in J774 A.1 cells incubated with LDL. And tyrosol also was also effective at preserving the GSH content completely. Tyrosol inhibited the ROS production in J774 A.1 cells during cell mediated oxidation of LDL [Citation23]. Defections in GSH metabolism might cause oxidative stress, which has been implicated in several neurologic and neurodegenerative diseases [Citation24].
At present, the cellular and molecular mechanisms that underlie the action of tyrosol are not fully understood. However, our result and other previous reports indicate that tyrosol significantly protects primary cultured neuronal cells against glutamate-induced oxidative stress via antioxidative activities. Therefore, we conclude that tyrosol might offer useful therapeutic choice in treatment of neurodegenerative disorders caused by excitotoxicity.
Acknowledgements
This research was supported by a grant (M103KV010024-06K2201-02410) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Michaelis EK. Molecular biology of glutamate receptors in the central nervous system and their role in excitotoxicity, oxidative stress and aging. Prog Neurobiol 1998; 54: 369–415.
- Sucher NJ, Awobuluyi M, Choi YB, Lipton SA. NMDA receptors: from genes to channels. Trends Pharmacol Sci 1996; 17: 348–355.
- Jacob CP, Koutsilieria E, Bartla J, Neuen-Jacobb E, Arzbergere T, Zandera N, Ravidd R, Roggendorfe W, Riederera P, Grunblatt E. Alterations in expression of glutamatergic transporters and receptors in sporadic Alzheimer’s disease. J Alzheimers Dis 2007; 11: 97–116.
- Kim YC, Kim SR, Markelonis GJ, Oh TH. Ginsenoside Rb1 and Rg3 protect cultured rat cortical cells from glutamate-induced neurodegeneration. J Neurosci Res 1998; 53: 426–432.
- Chung BS, Shin MG. Dictionary of Korean Folk Medicine, Seoul:Young Lim Sa; 2000. p 458.
- Yu YU, Kang SY, Park HK, Sung SH, Lee EJ, Kim SY, Kim YC. Antioxidant lignans from Machilus thunbergii protect CCl4-injured primary cultures of rat hepatocytes. J Pharm Pharmacol2000; 52: 1163–1169.
- Ma CJ, Sung SH, Kim YC. Neuroprotective lignans from the bark of Machilus thunbergii. Planta Med 2004; 70: 79–80.
- Kim SR, Hwang SY, Jang YP, Park MJ, Markelonis GJ, Oh TH.Kim YC Protopine from Corydalis ternata has antiacetylcholinestrase and antiamnesic activities. Planta Med 1999; 65: 218–221.
- Kim SR, Koo KA, Sung SH, Ma CJ, Yoon JS, Kim YC. Iridoids from Scrophularia buergeriana attenuate glutamate-induced neurotoxicity in rat cortical cultures. J Neurosci Res 2003; 74: 948–955.
- Briante R, Febbraio F, Nucci R. Antioxidant properties of low molecular weight phenols present in the mediterranean diet. J Agri Food Chem 2003; 51: 6975–6981.
- Delgado G, Rios MY. Monoterpenes from Chrysactinia mexicana. Phytochem. 1991; 30: 3129–3131.
- Lin M, Chen L, Chen C, Liu K, Lee S. Chemical constituents from Drypetes littoralis. J Nat Prod 2001; 64: 707–709.
- Martinez JC, Torres R. Lignans from Virola Aff. pavonis Leaves. Phytochem 1997; 44: 1179–1182.
- Miyamura M, Nohara T, Tomimatsu U, Nishioka I. Seven aromatic compounds from bark of Cinnamomum cassia. Phytochem 1983; 22: 215–218.
- Miyazawa M, Oshima T, Koshio K, Itsuzaki Y, Anzai J. Tyrosinase inhibitor from black rice bran. J Agri Food Chem 2003; 51: 6953–6956.
- Mukherjee RK, Fujimoto Y, Kakinuma K. 1-(ω-hydroxyfattyacyl)glycerols and two flavonols from Cinnamomum camphora. Phytochem 1994; 37: 1641–1643.
- Huang YL, Chen CC, Hsu FL, Chen CF. A new lignan from Phyllanthus virgatus. J Nat Prod 1996; 59: 520–521.
- Holloway D ScheinmannF. Two lignans from Listea grandis and L. gracilipes. Phytochem 1974; 13: 1233–1236.
- Liu JS, Huang MF, Gao YL. The structure of chicanine, a new lignan from Schisandra sp. Can J Chem 1981; 59: 1680–1684.
- Agrawal PK, Pathak AK. Reference data review: Influence of skeletal alteration of lignoids on carbon-13 NMR chemical shifts. Magn Reson Chem 1994; 32: 753–773.
- Nemethy EK, Lago R, Hawkins D, Calvin M. Lignans of Myristica otoba. Phytochem 1986; 25: 959–960.
- Harwood JL, Yaqoob P. Nutritional and health aspects of olive oil. Eur J Lipid Sci Tech 2002; 104: 685–697.
- Di Benedetto R, Vari R, Scazzocchio B, Filesi C, Santangelo C, Giovannini C, Matarrese P, D’Archivio M, Masella R. Tyrosol, the major extra virgin olive oil compound, restored intracellular antioxidant defences in spite of its weak antioxidative effectiveness. Nutr Metab Cardiovasc Dis 2007; 17: 535–545.
- Ma CJ, Kim SR, Kim YC. Meso-dihydroguaiaretic acid and licarin A of Machilus thunbergii protect against glutamate-induced toxicity in primary cultures of rat cortical cells. Brit J Pharmacol 2005; 146: 752–759.