Abstract
The methanolic extract of Paeonia lactiflora roots significantly protected primary cultures of rat cortical cells exposed to oxidative stress induced by H2O2. Seven monoterpenes, paeonilactone-B (1), paeonilactone-C (2), paeoniflorigenone (3), benzoylpaeoniflorin (4), paeoniflorin (5), oxypaeoniflorin (6) and albiflorin (7), were isolated by bioactivity-guided fractionation and further separation using chromatographic techniques. Among them, compounds 2 and 4 significantly protected primary cultures of rat cortical cells against H2O2-induced neurotoxicity.
Introduction
Oxidative stress, namely the imbalances between the production of reactive oxygen species and their removal, has been generally recognized as a cause of various diseases. In the central nervous system, a variety of neurodegenerative disorders including Alzheimer’s disease and Parkinson’s disease, as well as ischemia and aging process may all have a common pathology involving oxidative stress [Citation1, Citation2]. Therefore, diverse antioxidants have been investigated as potential therapeutic agents against oxidative stress related diseases.
Thus we tried to find neuroprotective compounds from natural products using H2O2-injured primary cultures of rat cortical cells. In this screening system, the methanolic extract of the roots of Paeonia lactiflora (Ranunculaceae) exhibited significant neuroprotective activity.
P. lactiflora has been used to treat wounds, fungal infection, pain and dementia in some Asian countries including China and Korea [Citation3]. Monoterpenes, triterpenes and flavonoids have been reported to be isolated from the roots of Paeonia species [Citation4, Citation5].
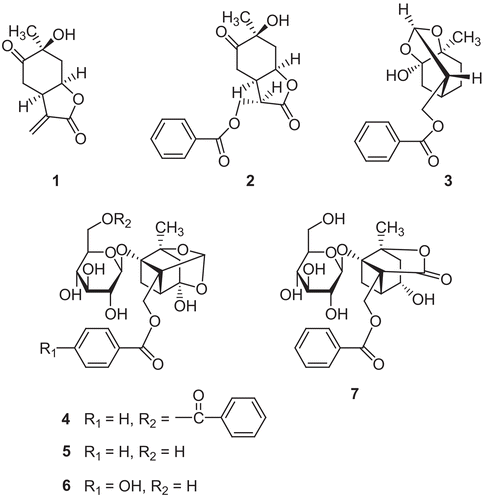
In the present study, bioassay-guided fractionation of the extract of P. lactiflora led to the isolation of seven monoterpenes (1−7). We describe the isolation and the identification of these compounds, and the evaluation of their neuroprotective activity in H2O2-injured primary cultured rat cortical cells.
Methods and materials
Plant material
The root of P. lactiflora was purchased from Kyungdong traditional herbal market (Seoul, Korea) and authenticated by Dr. Jong Hee Park, professor of Pusan National University. A voucher specimen (SNUPH-0606) has been deposited at the Herbarium of the Medicinal Plant Garden, College of Pharmacy, Seoul National University.
Isolation of neuroprotective monoterpenes
The dried root of P. lactiflora (8.5 kg) was ground and extracted with 80% methanol at room temperature in an ultrasonic apparatus. The methanol extract was concentrated in vacuo to give crude extract (1.3 kg). The methanol extract was then suspended in H2O and partitioned successively with n-hexane, CHCl3, ethylacetate (EtOAc) and n-butanol. The CHCl3 fraction (17 g) was subjected to silica gel column chromatography (CC) with a mixture of CHCl3-methanol as an eluent to yield five fractions (C1−C5). Compound 1 (11 mg) was isolated from C3 by CC over Sephadex LH-20 using methanol and the semipreparative HPLC using acetonitrile-methanol-H2O (5: 45: 50, flow rate 2 mL/min, retention time: 18.2 min). C2 was subjected to Sephadex LH-20 eluted with methanol to yield four subfractions (C2-1−C2-4). Compound 2 (25 mg) was obtained from C2-2 by the semipreparative HPLC using acetonitrile-methanol-H2O (5: 45: 50, flow rate 2 mL/min, retention time: 18.8 min). Compound 3 (42 mg) was isolated from C2-3 using the semipreparative HPLC using acetonitrile-methanol-H2O (5: 40: 55, flow rate 2 mL/min, retention time: 21.5 min). In addition, the EtOAc fraction (80 g) was fractionated into six fractions (E1−E6) by silica gel CC eluted with a mixture of CHCl3-methanol. E2 was applied to CC over silica gel using n-hexane-EtOAc-methanol mixture followed by CC over Sephadex LH-20 using methanol and afforded compound 4 (36 mg). Compound 5 (5800 mg) and 6 (55 mg) were isolated from E3 and E4, respectively, by CC over Sephadex LH-20 eluted with methanol. Compound 7 (189 mg) was isolated from E5 by sequentially using Sephadex LH-20 CC eluted with methanol and the semipreparative HPLC eluted with acetonitrile-methanol-H2O (8: 11: 81, flow rate 2 mL/min, retention time: 19.9 min).
Cortical cell culture
Primary cultures of mixed cortical cells containing both neurons and glia were prepared from 17 to 19 day old fetal rats (Sprague Dawley) as described previously [Citation6]. Sprague Dawley rats (200 ± 50 g body weight) were provided by the Laboratory Animal Center, Seoul National University. Rats were kept on standard rat chow with free access to tap water in temperature- and humidity-controlled animal quarters under a 12 h light-dark cycle. All experiments were conducted according to the guidelines of the Committee on Care and Use of Laboratory Animals of Seoul National University. The cortical cells were grown in DMEM containing 10 % heat-inactivated FBS with penicillin (100 IU/mL) and streptomycin (10 mg/mL) at 37°C in a humidified atmosphere of 95 % air-5 % CO2. Cultures were allowed to mature for 10 days before being used for experiments.
Assessment of neuronal cell viability
The tested compounds were dissolved in dimethylsulfoxide (final concentration in culture 0.1 %). The 10 day old cortical cell cultures were pretreated with a test sample for 1 h. Then the cultures were exposed to 50 μM H2O2 and maintained for 24 h. After the incubation, MTT (0.2 mg/mL) was directly added to cultures, followed by incubation at 37°C for 3 h. The supernatant was then aspirated and 300 μL of DMSO was added to dissolve the formazan. The absorbance (abs) at 540 nm was measured using a microplate reader. Data were expressed as percent cell viability relative to control cultures [Citation6].
Statistical analysis
All data was expressed as mean ± standard deviation (SD). The evaluation of statistical significance was determined by an “one-way ANOVA” test using computerized statistical package. The data were considered to be statistically significant if the probability had a value of 0.05 or less.
Results and discussion
Oxidative stress has been generally recognized as a cause of various diseases. In particular, central nervous system neurons are unusually vulnerable to oxidative stress because of their high rate of oxygen consumption in the brain, the high content of polyunsaturated fatty acids as substrates for lipid peroxidation and the presence of iron [Citation7]. H2O2 is well known as a cellular toxin. Since H2O2 has remarkable membrane permeability, intracellular H2O2 can induce detrimental effects on cells [Citation8]. Therefore, we tried to find neuroprotective compounds with antioxidative activity from natural products using primary cultures of rat cortical cells injured by H2O2 as a screening system. In this screening system, the methanol extract of P. lactiflora roots significantly protected cortical neurons against H2O2-induced toxicity at the concentration of 100 μg/mL. As shown in , among the partitioned fractions, the EtOAc fraction showed the most potent neuroprotective activity. The CHCl3 fraction also exerted significant neuroprotective activity. Both fractions rich in monoterpenes as determined by TLC were used for the isolation of active compounds. Three monoterpenes, paeonilactone-B (1), paeonilactone-C (2) and paeoniflorigenone (3), were isolated from the CHCl3 fraction. The EtOAc fraction yielded four monoterpenes, benzoylpaeoniflorin (4), paeoniflorin (5), oxypaeoniflorin (6) and albiflorin (7). The chemical structures of these compounds were determined by comparison of their spectroscopic data with those previously reported [Citation9, Citation10, Citation11] (). Neuroprotective activity of these compounds was evaluated using MTT assay which reflects mitochondrial succinate dehydrogenase function at the concentrations ranging from 0.1 μM to 10.0 μM. As shown in , compounds 2 and 4−7 significantly protected cultured neuronal cells against H2O2-induced toxicity. All these active compounds contained benzoyl moiety in their structures. Moreover, among them, compounds 2 and 4, benzoylated compounds of 1 and 5, respectively, showed the most potent activity. These results suggest that benzoyl moiety might contribute to the neuroprotective activity of the active monoterpenes at least in part. Neuronal cell death induced by H2O2 is primarily caused by the highly toxic reactive oxygen species (ROS) generated from H2O2. These ROS oxidatively modify nucleic acid, lipid, sugar and protein. Otherwise, oxidative stress also stimulates astrocytes and microglia to yield and secrete cytokines causing glial inflammatory response [Citation12]. Since mixed cortical cell cultures containing both neuronal and glial cells were used in this study, it cannot be ruled out that both cell types might contributes to the neuroprotective activity of the isolated compounds. In addition, compounds 4−6 reported to exhibit almost no potency in DPPH radical scavenging assay [Citation13]. It suggests that the mechanism of their antioxidant activity might not involve the direct scavenging of free radicals. Taken together, further investigation will be needed to evaluate the underlying mechanism of the antioxidative effect of these compounds.
Table 1. Neuroprotective activity of the methanol extract and the fractions of P. lactiflora roots against H 2O2-induced neurotoxicity in primary cultures of rat cortical cells.
Table 2. Neuroprotective activity of compounds 1−7 against H2O2-induced neurotoxicity in primary cultures of rat cortical cells.
Acknowledgements
This research was supported by a grant PF06200-00 from Plant Diversity Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Korea.
Declaration of interest: The authors report no conflicts of interest.
References
- Halliwell B, Gutteridge JMC, Cross CE. Free radicals, antioxidants and human disease: Where are we now? J Lab Clin Med 1992; 119: 598–620.
- Markesbery WR. Oxidative stress hypothesis in Alzheimer’s disease. Free Rad Biol Med 1997; 23: 134–147.
- Jung BS, Shin MK. Hyang-Yak-Dae-Sa-Jeon. Young Rim Sa Co., Seoul, 1990. pp 523–524.
- Kamiya K, Yoshioka K, Saiki Y, Ikuta A, Satake T. Triterpenoids and flavonoids from Paeonia lactiflora. Phytochemistry 1997; 44: 141–144.
- Dong H, Liu Z, Song F, Yu Z, Li H, Liu S. Structural analysis of monoterpene glycosides extracted from Paeonia lactiflora Pall. using electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry and high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 2007; 21: 3193–3199.
- Lee MK, Yeo H, Kim J, Markelonis GJ, Oh TH, Kim YC. Cynandione A from Cynanchum wilfordii protects cultured cortical neurons from toxicity induced by H2O2, L-glutamate, and kainite. J Neurosci Res 2000; 59: 259–264.
- Behl C, Davis JB, Lesly R, Shybert D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell 1994; 77: 817–827.
- Whittemore ER, Loo DT, Watt JA, Cotman CW. A detailed analysis of hydrogen peroxide-induced cell death in primary neuronal culture. Neurosci 1995; 67: 921–932.
- Hayashi T, Shinbo T, Shimizu M, Arisawa M, Morita N, Kimura M, Matsuda S, Kikuchi T. Paeonilactone-A, -B and –C, new monoterpenoids from paeony root. Tetrahedron Lett 1985; 26: 3699–3702.
- Shimazu M, Hayashi T, Morita N, Kimura K, Kimura M. Paeoniflorigenone, a new monoterpene from paeony roots. Tetrahedron Lett 1982; 22: 3069–3070.
- Kaneda M, Iitaka Y, Shibata S. Chemical studies on the oriental plant drugs-XXXIII. The absolute structures of paeoniflorin, albiflorin, oxypaeoniflorin and benzoylpaeoniflorin isolated from Chinese paeony root. Tetrahedron 1972; 28: 4309–4317.
- Shibata N, Kobayashi M. The role for oxidative stress in neurodegenerative diseases. Brain Nerve 2008; 60: 157–170.
- Lee SC, Kwon YS, Son KH, Kim HP, Heo MY. Antioxidative constituents from Paeonid lactiflora. Arch Pharm Res 2005; 28: 775–783.