Abstract
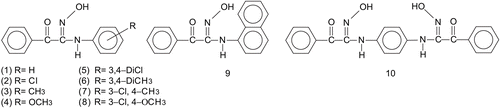
The antioxidant activity of some amido-carbonyl oximes containing a C=O and –NH–R adjacent to the oxime group, [Phenyl-C(=O)-C(=N-OH)-N(-H)-Phenyl(-R)] where R= H, 4-chloro, 4-methyl, 4-methoxy, 3,4-dichloro, 3,4-dimethyl, 3-chloro-4-dimethyl, 3-chloro-4-methoxy, naphthyl and an amido-carbonyl dioxime were investigated in vitro by ferric thiocyanate, total reducing power by potassium ferricyanide reduction, 1,1-diphenyl-2- picryl-hydrazyl (DPPH·) free radical scavenging, ferrous ions chelating, superoxide anion radical scavenging and hydrogen peroxide scavenging activity assays. The results indicated that the amido-carbonyl oximes have powerful antioxidant capacity.
Introduction
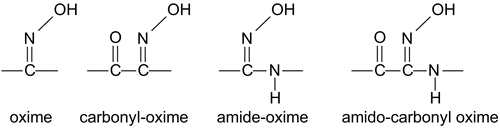
Organic compounds containing the –C=N–OH group have been named as an oxime compounds () [Citation1]. Oxime derivatives are very important compounds because of their biological activity, such as insecticidal, miticidal, nematocidal, and antidote activities towards organophosphorous poisons. Some oxime complexes have anti cancerogenic activities [Citation2–4].
Also, they have been used as anti-skinning agents in paint, blocking agents in the polymer industry and chelators in variable industrial applications. Some oxime derivatives clinical used as anti-inflammatory and anti-allergic agents [Citation5–7].
Recent studies showed the oximes have powerful antioxidants [Citation8–11] antiepileptic drug [Citation12], anti-inflammatory [Citation13–15], antimicrobial [Citation16,Citation17], oxidative effect [Citation18], antihyperglycemic agent [Citation19] and hepatoprotective [Citation20] activities.
In addition to these, the oximes and their derivatives have been used in analytical applications, such as determination and extraction of the metals [Citation21–26].
Oximes have been named as carbonyl oximes or amidoximes relating to position of the groups. While carbonyl oximes have a carbonyl (C=O) group in the α- position to the oxime group (), amide-oximes have a –NH–R group in the α- position to the oxime group (). [1,2,4] Carbonyl oximes and amide-oximes have been studied extensively, but work on amido-carbonyl oximes (), which have –C=O and –NH–R’ groups in the same molecule in the α-position to the oxime group is scantily described [Citation27].
The reactive oxygen species (ROS) are produced as a normal consequence of biochemical processes in the body and due to increased exposure to environmental and/or dietary xenobiotics. It is an imbalance in these oxidants versus antioxidant processes (oxidative stress) that is thought to cause the subsequent cellular damage that leads to the some biological disease processes [Citation28]. Neurodegenerative diseases, such as Alzheimers and Parkinson’s disease, are also linked to damage from ROS as a result of an imbalance between the rates of radical generation and scavenging [Citation29].
Antioxidants interfere with the oxidation process by reacting with free radicals, chelating, catalytic metals, and also by acting as oxygen scavengers [Citation30, Citation31]. Antioxidant supplements or foods containing synthetic antioxidants such as BHA (butylated hydroxyanisole), BHT (butylated hydroxytoluene), trolox, use to help the human body reduce oxidative damage [Citation32–34]. However, they may cause for liver damage and carcinogenesis in laboratory animals [Citation35]. Therefore, the preparing of more effective new antioxidants is very important research area.
So, the aim of this work were to systemically investigate the total antioxidant activities, reducing power, free radical scavenging, superoxide anion radical scavenging, hydrogen peroxide scavenging, and metal chelating activities of the some amido-carbonyl oximes (Subsequently referred to as oxime) and to determine the substitutent effect on this activities (). An important goal of this research was to determinate in vitro antioxidant effects of oximes against to commercial and standard antioxidants such as trolox, BHA and BHT, commonly used by the pharmaceutical industry.
Materials and methods
Chemicals
Ammonium thiocyanate, ferrous chloride and 1,1-diphenyl-2-picryl-hydrazl (DPPH·), nicotine adenine dinucleotide (NADH), BHA, BHT, trolox, trichloracetic acid (TCA), polyoxycthylenesorbitan monolaurate (Tween-20), nitroblue tetrazolium (NBT), nicotinamide adenine dinucleotide (NADH), phenazine methosulphate (PMS), potassium ferricyanide and linoleic acid were commercially available and were reagent grade.
Synthesis of the oximes
The syntheses of the oxime derivatives 1-5, 7-10 used in this work, have been described previously by Tas˛ and et al. [Citation27, Citation36–42]. Compound 6 was newly prepared for this work from the reaction of ω-chloroisonitrosoacetophenone with 3,4 dimethylaniline using Tas˛’ method [Citation27].
Assay of total antioxidant activity
The ferric thiocyanate method (FTC) was adapted from the model of Mitsuda [Citation43]. The solutions which contains the same concentration oximes and standard antioxidants (5 and 50 μM) in 2.5 mL of potassium phosphate buffer (0.04 M, pH 7.0) was added to linoleic acid emulsion in potassium phosphate buffer (2.5 mL, 0.04 mM, pH 7.0). In addition to these solutions, 5 mL control solution was prepared with linoleic acid emulsion (2.5 mL) and potassium phosphate buffer (2.5 mL, 0.04 M, pH 7.0). The all solutions were shaked and periodical reaction mixture was incubated at 37°C in dark. The peroxide values were determined by reading the absorbance at 500 nm after reaction with FeCl2 and thiocyanate (SCN−) at intervals during incubation. During the linoleic acid oxidation, peroxides forms and these compounds oxidize Fe2+ to Fe+3 and give complex compound with SCN− ligands which has an absorbance maximum at 500 nm. This step was repeated every 5 h until the control reached its maximum absorbance value. Higher absorbance values indicate higher linoleic acid oxidation. The solutions without oximes or standards were used as a blank sample. The inhibition of lipid peroxidation in percent was calculated by the following equation:
Inhibition of lipid peroxidation (%) = [(A0−A1)/A0 × 100]
where A0 was the absorbance of control incubated with linoleic acid but without the samples (control) and A1 was absorbance of oximes or the standards which are BHA, BHT and trolox.
Assay of reducing power
The reducing power capacity of oximes was determined according to the method of Oyaizu [Citation44]. The oximes and standards 5 or 50 μM in 1 mL of the corresponding solvent mixed with buffer (2.5 mL, 0.2 M, pH 6.6) and K3Fe(CN)6 (2.5 mL, 1%), then the mixture was incubated at 50°C for 20 min. Afterwards, TCA (2.5 mL, 10%) was added to the mixture, which was then centrifuged at 3000 rpm for 15 min. The upper layer of solution (0.5 mL) was mixed 1 mL of distilled water and FeCl3 (0.5 mL, 0.1%), and the absorbance was measured at 700 nm Higher absorbance of the reaction mixture indicated increased reducing power.
Assay of antiradical activity
The effect of oximes on DPPH· radical was estimated according to the method of Blois [Citation45] wherein the bleaching rate of a stable free radical, DPPH·, is monitored at a characteristic wavelength (517 nm) in the presence of samples. An amount of 0.5 mL of 0.1 mM ethanolic solution of DPPH· was added to 3.0 mL of oximes or standard antioxidants. The mixture was shaken vigorously and waited at room temperature for 30 min. Then the absorbance was measured at 517 nm. The decrease in the absorbance of the DPPH· solution indicates an increasing of DPPH· radical-scavenging activity. The DPPH· concentration (mM) in the reaction medium was calculated from the calibration curve determined by linear regression (R2:0.9998):
Absorbance = 6.5781 × [DPPH·] + 0.058
This activity was calculated by;
DPPH· Scavenging Effect (%) = [(A0−A1)/A0 × 100],
where A0 was the absorbance of control and A1 was absorbance of oximes or standards. The radical scavenging activity was expressed as IC50 which was determined from a calibration curve for each compound.
Assay of metal chelating activity
The chelating of ferrous ions by oximes was determined by the method of Dinis [Citation46]. Briefly, the samples (oximes or standard antioxidants; 5 or 50 μM) were added to a solution of 2 mM FeCl2 (0.05 mL). The reaction was initiated by the addition of 5 mM ferrozine (0.2 mL) and the mixture was shaken vigorously and left standing at room temperature for 10 min. The absorbance of the resulting solution was then measured at 562 nm. The metal chelating activities were calculated by the given formula:
Metal chelating effect (%) = [(A0−A1)/A0 × 100],
where A0 was the absorbance of control and A1 was absorbance of oximes or standards. The control contains FeCl2 and ferrozine.
Assay of superoxide anion scavenging activity
The determination of superoxide anion scavenging activity of oximes was measured according to slightly modified Nishimiki’s method [Citation47]. Superoxide radicals are generated in phenazine methosulphate (PMS)-nicotinamide adenine dinucleotide (NADH) systems by oxidation of NADH and assayed by the reduction of nitroblue tetrazolium (NBT) [Citation48]. One milliter of oximes solution and standard antioxidants (5 or 50 μM), 1.0 mL NBT solution (156 μM NBT in 100 mM phosphate buffer, pH 7.4) and 1.0 mL NADH solution (468 μM in 100 mM phosphate buffer, pH 7.4) were mixed. The reaction was started by adding 100 μl of PMS solution (60 μM PMS in 100 mM phosphate buffer, pH 7.4) to the mixture. The mixture was incubated at 25 ºC temperature for 5 min, and its absorbance was measured at 560 nm wavelength against blank samples. L-ascorbic acid was used as a control. The decreasing of the absorbance for the mixtures indicates an increasing superoxide anion scavenging activity. The percentage inhibition of superoxide anion generation was calculated using the following formula:
Inhibition of superoxide anion generation (%) = [(A0−A1)/A0 × 100],
where A0 was the absorbance of control, and A1 was absorbance of oximes or standards.
Assay of hydrogen peroxide scavenging activity
The ability of oximes to scavenge hydrogen peroxide was determined according to the method of Ruch [Citation49]. A solution of H2O2 (40 mM) was prepared in phosphate buffer (100 mM, pH 7.4). The concentration H2O2 was determined from absorption at 230 nm. Samples (oximes or standard antioxidants; 5 or 50 μM, 3.4 mL) were added to the H2O2 solution of 0.6 mL. The absorbance of H2O2 at 230 nm was determined after 10 minute against a blank solution containing the phosphate buffer without hydrogen peroxide. The percentage of scavenging H2O2 of oximes and standard antioxidants was calculated using the following equation:
Percent Scavenged (H2O2) = [(A0−A1)/A0 × 100],
where A0 was the absorbance of control and A1 was absorbance of oximes or standards.
Statistical analysis
The assays were performed in triplicate. The data were recorded as mean ± standard deviation. They were analysed by SPSS. One-way analysis of variance was performed by ANOVA and Duncan’s Multiple Range tests. All results was regarded as p < 0.05 (significant) and p < 0.01 (very significant).
Results and discussion
Characterization of compound 6.
In the 1H NMR spectrum of 6 (R= 3,4-dimethyl, ) a singlet peak for the OH proton of oxime group was observed at 11.02 ppm. The N–H protons adjacent to the oxime groups in the ligands resonate at 8.36 ppm. The aromatic C–H protons resonate at 7.90–6.50 ppm while aliphatic CH3 protons at 2.12–2.06 ppm. The O–H and N–H peaks of the ligands disappeared with the addition of deuterium oxide to the solutions. In the IR spectra of 6, bands at 3359, 3263, 1687, 1646, and 916 cm−1 belong to N–H, O–H, C=O, C=N, and N–O vibrations, respectively. These results are in good agreement with those of related oximes and indicate that the compound 6 have similar structure with previously reported ones ().
Total antioxidant activity
The ferric thiocyanate method (FTC) measures the amount of peroxide produced during the initial stages of oxidation, which is the primary product of oxidation. The mechanism of bleaching of the Fe3+-SCN− complex is a free radical-mediated phenomenon, resulting from the hydro-peroxides formed from linoleic acid. Fe3+-SCN− complex, in this model system, undergoes rapid discoloration in the absence of an antioxidant. As Fe3+-SCN− complex loses their electron by oxidation, the compound loses its electron and characteristic red color, which is monitored spectrophotometrically at 500 nm [Citation48].
The antioxidant activities of oximes, trolox, BHA and BHT were evaluated at 5 and 50 μM using the linoleic acid emulsion model system. Antioxidant activity of the concentration of oximes, trolox, BHA and BHT is shown in .
Table 1. Total antioxidant activities (%) of the oximes, BHA, BHT and trolox at different concentration (5 and 50 μM) in the linoleic acid emulsion system by the FTC method.
The results clearly showed the substitutent and concentration effects on the antioxidant activities of the oximes. The increasing of the oxime functional group numbers indicated the best antioxidant activity. The ligands inductively pulling electron from the benzene ring showed increasing on antioxidant activity. The increasing of the concentration gave whole different lining up.
Increasing the concentration of oximes, trolox, BHA and BHT up to 50 μM resulted in a significant (p<0.05) increase in antioxidant activity. The standard antioxidant (Trolox) showed a maximum activity as ~80% whereas oxime (10) showed more activity (~85%) at the concentration of 50 μM. Oxime (10) showed ~79% inhibition at 5 μM concentration while standard antioxidant (BHA) showed ~72% inhibition.
Reducing power
The reducing capacity of a compound may help to decide as a significant indicator of its potential antioxidant activity. The presence of reductants such as antioxidant substances in the antioxidant samples causes the reduction of the Fe3+/ferricyanide complex to the Fe2+ monitored by measuring the formation of blue color at 700 nm [Citation50]. The antioxidant activity of oximes and standard antioxidants as reflected in their reducing power is presented in (as indicated by absorbance at 700 nm). The reducing power of oximes and standard antioxidants increased with increasing concentration of samples. At different concentrations, oximes showed an effective reducing power and these differences were statistically significant (p< 0.05). The reducing power of oximes was in a concentration-dependent manner and substitutent. At the high concentration, reductive capabilities of oximes were lower than the standard BHT except oxime 6. At the lower concentration, reductive capabilities of oximes were lower than the standard BHT except oximes 1, 2, 5, 6 and 1. However, the data clearly indicated that the oximes have significant reducing power activity. There was no correlation found between the reducing capabilities and substitutents.
Table 2. Reducing power of different concentrations (5 and 50 μM) of oximes, BHA, BHT and trolox.
Antiradical activity
Radical scavenging properties are very important because of the decomposition role of free radicals in foods and in biological system. Free radicals induce the oxidation of lipids in foods and decreasing food quality [Citation51]. The effect of antioxidants on DPPH· radical scavenging is thought to be due to their hydrogen donating ability. DPPH· is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule [Citation48]. Compounds react with DPPH· which is a nitrogen centered radical with a characteristic absorption at 517 nm, and convert it to stable diamagnetic molecule 1,1-diphenyl-picryl hydrazine, due to its hydrogen donating ability at a very rapid rate. It is visually noticeable as a discoloration from purple to yellow colored 1,1-diphenyl-2-picryl-hydrazine. DPPH· is usually used as a substrate to evaluate antioxidative and antiradical activities of antioxidants. The entire synthesized compounds scavenged DPPH· radical significantly in a concentration-dependent manner (p< 0.01). Their comparable scavenging activities were expressed in IC50 (concentration required for 50% inhibition of 0.1 mM DPPH· concentration) value. Trolox, BHT and BHA were used as the positive standard. The radical scavenging activities of the synthesized compounds are summarized in . Compounds 4, 5, 6, 9 and 10 showed appreciable radical scavenging activity higher than BHT and Trolox. These results indicated that oximes have a noticeable effect on scavenging free radicals. These data clearly indicate that 10 is a powerful free radical inhibitor or scavenger and the radical scavenging activity increased by the electron donor substitiuents.
Table 3. DPPH· free radical scavenging activity (IC50: μg/mL) of oximes. BHA. BHT and Trolox.
Metal chelating activity
The production of highly ROS (reactive oxygen species) is also catalyzed by free iron through Haber–Weiss reaction (O2· +H2O2 → O2 + OH− + OH·) [Citation52]. Iron has the most important lipid pro-oxidant. It is known that the Fe+2 accelerates lipid peroxidation by breaking down hydrogen and lipid peroxides forms by Fenton free radicalic reaction;
(Fe2+ + H2O2 → Fe3+ + OH− + OH·). Fe+2 ion can form complexes with ferrozine. In the presence of chelating agents, the complex formation is prevented, resulting in a decrease in the red color of the complex. Measurement of color reduction allows determination of metal chelating activity. In this assay, oximes are interfered with the formation of Fe2+ and Fe3+-complex since they have chelating activity to capture Fe2+ ion before ferrozine (oxime + Fe2+ → oxime-Fe2+ complex). The oxime ligands are good chelating agents for metal ions [Citation1, Citation27]. The metal chelating effects of the oximes were found as concentration-dependent. As can be seen in , at the lower concentration, the metal chelating activity was almost similar with the standards. But at the higher concentration the most of oximes showed, significantly (p< 0.01) higher metal chelating activity than standards.
Table 4. Ferrous ion (Fe 2+) chelating activity (%) of oximes and the standard antioxidants BHA. BHT and trolox at different concentrations (5 and 50 μM).
These results indicate the good metal chelating capacity of oximes and reduction of the catalysis of lipid peroxidation by the transition metals.
Superoxide anion scavenging activity
Numerous biological reactions generate superoxide anion which is a highly toxic species. Superoxide anions are a precursor to active free radicals that have potential of reacting with biological macromolecules and thereby inducing tissue damage [Citation53]. Superoxide has also been accelerated to directly initiate lipid peroxidation [Citation54]. Superoxide anion plays an important role in the formation of other ROS such as hydrogen peroxide, hydroxyl radical, and singlet oxygen, which induce oxidative damage in lipids, proteins, and DNA [Citation55]. Superoxide anion formed by the reaction between dissolved oxygen with PMS/NADH coupling, is reduced NBT in this system. In this method, superoxide anion is reduced by the yellow dye (NBT) to produce the blue formazan (NBT-H2) which can measured spectrophotometrically at 560 nm. The test system is used for superoxide generation by PMS/NADH system and reducing superoxide by NBT [Citation47, Citation56]. It can be said the reaction occurs in two principal stages. The first is the oxidation of NADH by PMS to produce superoxide (1).
The second is the reduction of superoxide by NBT:
Overall, the stoichiometry of the reaction is as follows reaction:
Antioxidants are able to inhibit producing superoxide by PMS/NADH system and this activity can be monitored by decreasing blue NBT-H2 formation [58]. The decreasing of absorbance at 560 nm with antioxidants indicates the consumption of superoxide anion in the reaction mixture. shows the inhibition percentage of superoxide radical generation by 5 and 50 μM concentration by oximes and standards. The inhibition of superoxide radical generation by oximes, standards were found statistically significant (p <0.05). As can be seen in , the percentage inhibition of superoxide anion generation of oximes was higher than standards except for 1 and 10, at 50 μM and similar with standards at 5 μM concentrations.
Table 5. Superoxide anion scavenging activity (%) of oximes and the standard antioxidants BHA, BHT and trolox at different concentrations (5 and 50 μM).
Hydrogen peroxide scavenging activity
Hydrogen peroxide forms in vivo by many enzymes such as superoxide dismutase and hydrogen peroxide is a precursor to produce the hydroxyl radical (·OH). The hydroxyl radical (·OH) in the cells can easily cross cell membranes at specific sites, react with most bio-molecules and furthermore cause tissue damage and cell death [Citation35]. Thus, removing ·OH is very important for the protection of living, pharmaceutical and food systems.
shows the percentage hydrogen peroxide scavenging effect by the oximes and comparison with the effect of standards (trolox, BHT and BHA) at the dose of 5 and 50 μM. Compounds 2, 3, 4, and 7 showed appreciable hydrogen peroxide scavenging activity higher than standards at lower dose. 2, 3, 4, 6, 7, 9 and 10 showed higher hydrogen peroxide scavenging activity than standards at higher dose, not significantly.
Table 6. Hydrogen peroxide scavenging activity (%) of oximes and the standard antioxidants BHA, BHT and trolox at different concentrations (5 and 50 μM).
Conclusion
The results of this study clearly indicate that the oximes have a powerful antioxidant capacity against various antioxidant systems in vitro assays and the capacity dependent on the concentrations. They were shown to have better antioxidant and radical scavenging activity than the standards used. Therefore, they can be potentely useful as food additive material preventing oxidation, for foods or pharmaceuticals.
Acknowledgements
The authors are thankful to their graduates, Salih Çalık, Gül Dilaver and Ebru Gül Kutlu for their contributions.
Decleration of interest: The authors reports no conflicts of interest.
References
- Chakravorty A. Structural chemistry of transition metal complexes of oximes. Coord Chem Rev 1974; 13:1–46.
- Barybin MV, Diaconescu PL, Cummins CC. Coordination chemistry of a chelating amidoximato ligand. Inorg Chem 2001; 40:2892–2897.
- Sevagapandian S, Rajagopal G, Nehru K, Athappan P. Copper(II), nickel(II), cobalt(II) and oxovanadium(IV) complexes of substituted beta-hydroxyiminoanilides. Trans Metal Chem 2000; 25:388–393.
- Srivastava RM, Brinn IM, Machura-Herrera JO, Faria HB, Carpenter GB, Andrade D, Venkatesh CG, Morais LPF. Benzamidoximes: Structural, conformational and spectroscopic studies .1. J Mol Struct 1997; 406:159–167.
- Katagi T, Kataoka H, Takahashi K, Fujioka T, Kunitomo M, Yamaguchi Y, Fujiwara M, Inoi T. Synthesis and antiinflammatory activity of novel oximes and O- acyloximes. Chem Pharm Bull 1992; 40: 2419–2422.
- Katagi T, Kataoka H, Konishi Y, Takata Y, Kitano S, Yamaki M, Inoi T, Yamamoto K, Yamamoto S, Yamagata Y. Syntheses and anti-inflammatory activities of O-acyloximes .2. Chem Pharm Bull 1996; 44:145–149.
- Kataoka H, Horiyama S, Yamaki M, Oku H, Ishiguro K, Katagi T, Takayama M, Semma M, Ito Y. Antiinflammatory and anti-allergic activities of hydroxylamine and related compounds. Biol Pharm Bull 2002; 25:11, 1436–1441.
- Ley JP, Bertram HJ. Hydroxy- or methoxy-substituted benzaldoximes and benzaldehyde-O-alkyloximes as tyrosinase inhibitors. Bioorg Med Chem 2001; 9:1879–1885.
- Kılcıgil-Ayhan G, Çoban T, Tunçbilek M, Can-Eke B, Bozda–Dündar O, Ertan R, Iscan M. Antioxidant Properties of Flavone-6(4’)-Carboxaldehyde Oxime Ether Derivatives. Arch Pharm Res 2004; 27:6, 610–614.
- Sethi A, Maurya A, Tewari V, Srivastava S, Faridi S, Bhatia G, Khan MM, Khanna AK, Saxena JK. Expeditious and convenient synthesis of pregnanes and its glycosides as potential anti-dyslipidemic and anti-oxidant agents. Bioorg Med Chem 2007; 15: 4520–4527.
- Jousserandot A, Boucher JL, Henry Y, Niklaus B, Clement B, Mansuy D. Microsomal Cytochrome P450 Dependent Oxidation of N-Hydroxyguanidines, Amidoximes, and Ketoximes: Mechanism of the Oxidative Cleavage of Their CdN(OH) Bond with Formation of Nitrogen Oxides. Biochem 1998; 37: 17179–17191.
- Hainzl D, Loureiro AI, Parada A, Soares-da-silva P. Metabolism of 10,11-dihydro-10-hydroxyimino-5H-dibenz [b,f] azepine-5-carboxamide, a potent anti-epileptic drug. Xenobiotica 2002; 32: 131–40.
- Kataoka H, Horiyama S, Yamaki M, Oku H, Ishiguro K, Katagi T, Takayama M, Semma M, Ito Y. Anti-inflammatory and anti-allergic activities of hydroxylamine and related compounds. Biol Pharm Bull 2002; 25:1436–1441.
- Pillai AD, Rathod PD, Franklin PX, Padh H, Vasu KK, Sudarsanam V. Design, synthesis, and SAR studies of some 5-aliphatic oximino esters of thiophene as potential anti-inflammatory leads: comparative biological activity profile of aliphatic oximes vs aromatic oximes. Biochem Biophys Res Comm 2004; 317:1067–1074.
- Molvi KI, Vasu KK, Yerande SG, Sudarsanam V, Haque N. Syntheses of new tetrasubstituted thiophenes as novel anti-inflammantory agents. Eur J Med Chem 2007; 42:1049–1058.
- Ayhan-Kıllcıgil G, Bozda O, Tunçbilek M, Altanlar N, Ertan R. Synthesis and antimicrobial activity of flavone-6-carboxaldehyde oxime ether derivatives. Pharmazie 1999; 54: 228–229.
- Tunçbilek M, Bozda O, Ayhan-Kilcigil G, Altanlar N, Buyukbingol E, Ertan R. Synthesis and antimicrobial activity of some new flavonyl oxime ether derivatives. Arzneim.-Forsch./Drug Res 1999; 49:(II), 853–857.
- Sharma PK. Kinetics and mechanism of the oxidative regeneration of carbonyl compounds from oximes by pyridinium bromochromate. Int J Chem Kinet 2006; 38: 6, 364–368.
- Takamura M, Sakurai M, Yamada E, Fujita S, Yachi M, Takagi T, Isobe A., Hagisawa Y, Fujiwarad T, Yanagisawae H. Synthesis and biological activity of novel α-substituted β-phenylpropionic acids having pyridin-2-ylphenyl moiety as antihyperglycemic agents. Bioorg Med Chem 2004; 12: 2419–2439.
- Kulmagambetova EA, Yamovoi VI, Kusainova DD, Pak RN, Kulyyasov AT, Turdybekov KM, Adekenov S M, Gatilov YV. Synthesis and structure of pinostrobin oxime and its biological activity. Chem Nat Comp 2002; 38: 6, 527–531.
- Natarajan C, Hussain AN. Ni((II) And Cu(II) Complexes Of Some Isonitrosoacetophenones. Ind J Chem 1981; 20A: 307–309.
- Kim SY, Harada M, Tomiyasu H, Ikeda Y, Park YY. Structure and kinetic studies of U(VI)-benzamidoxime complex in non-aqueous solutions by H-1- and C-13-NMR. Prog Nucl Energy 2000; 37: 399–404.
- Talwar UB, Haldar BC. Rapid Extraction and Spectrophotometric Determınation Of Nickel (II) with Isonitrosoacetophenone. Analyt Chim Acta 1970; 51: 1, 53–59.
- More PS, Sawant AD. Isonitroso-4-methyl-2-pentanone as an analytical reagent for platinum(IV). J Ind Chem Soc 1996; 73: 377–378.
- Suzuki T, Saito K, Sugo T, Ogura H, Oguma K. Fractional elution and determination of uranium and vanadium adsorbed on amidoxime fiber from seawater. Analyt Sci 2000; 16: 429–432.
- Reddy PS, Reddy KH. Transition metal complexes of benzil- alpha-monoxime (BMO); X-ray structure determination of Co(BMO)(3). Polyhedron 2000; 19: 1687–1692.
- Tas˛ M, Batı H. Ligands containing of a C=O and an -NH-R adjacent to the oxime group and their cobalt(II), nickel(II) and copper(II) complexes. J Chem Res 2006; 87–92.
- Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Amer J Med 1991; 91:(Suppl. 3C), 14–22.
- Richardson SJ. Free radicals in the genesis of Alzheimers disease. Ann Acad Sci, 1993; 695: 73–76.
- Büyükokurogˇlu ME, Gülçin I, Oktay M, Küfreviogˇlu ÖI. In vitro antioxidant properties of dantrolene sodium. Pharmacol Res 2001; 44: 491–495.
- Shahidi F, Wanasundara PKJPD. Phenolic antioxidants. Crit Rev Food Sci Nutr 1992; 32: 67–103.
- Gülçin I, Oktay M, Küfreviogˇlu ÖI, Aslan A. Determination of antioxidant activity of lichen Cetraria islandica (L) Ach. J Ethnopharmacol 2002; 79: 325–329.
- Sherwin ER. Antioxidants. In: R. Branen, Editor. Food additives. New York: Marcel Dekker, 1990, pp 139–93.
- Huang Shu-Wen, Hopia A, Schwarz K, Frankel EN, German JB. Antioxidant Activity of α-Tocopherol and Trolox in Different Lipid Substrates: Bulk Oils vs Oil-in-Water Emulsions. J Agric Food Chem 1996; 44: 444–452.
- Oktay M, Gülçin I, Küfreviogˇlu ÖI. Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT-Food Sci Technol 2003; 36: 263–71.
- Büyükgüngör O, Hökelek T, Tas˛ M, Batı H. N-hydroxy-2-oxo-2,N’-diphenylacetamidine. Acta Cryst 2003; E59: o883–o885.
- Sarı U, Batı H, Güven K, Tas˛ M, Aksoy I˙. Srtucture of 1-(4-methylphenylamino)-2-phenyl-1,2-ethandione-1-oxime. Anal Sci 2003, Vol19, X61–X62.
- Hökelek T, Tas˛ M, Batı H. Crystal structure of N-(3,4-dichlorophenyl)-N’-hydroxy-2-oxo-2-phenylacetamidine. Cryst Res Technol, 2004; 39: No. 4, 363–367.
- Hökelek T, Büyükgüngör O, Tas˛ M, Batı H. N-(3-chloro-4-methylphenyl)-N ‘-hydroxy-2-oxo-2-phenylacetamidine. Acta Cryst (Section E-Structure Reports Online) 2004; E60:o406–o408.
- Soylu S, Tas˛ M, Andaç Ö, Batı H, Çalıs˛kan N, Büyükgüngör O. N-(3-Chloro-4-methoxyphenyl)-N’-hydroxy-2-oxo-2-phenylacetamidine N-(3-Chloro- 4-methoxyphenyl)-N ‘-hydroxy-2-oxo-2-phenylacetamidine. Acta Cryst (Section E-Structure Reports Online) 2003; E59:o1532–o1534.
- Hökelek T, Büyükgüngör O, Tas˛ M, Batı H. N-Hydroxy-N ‘-(1-naphthyl)-2-phenylacet-amidin-2-one. Acta Cryst (Section E-Structure Reports Online) 2004; E60:o109–o111.
- Tas˛ M, Batı H. Co(II), Ni(II) and Cu(II) complexes og 1,4-DI-(1-hydroxyimino-2-phenyl-2-oxo-ethylamino)benzene-Synthesis, characterization and thermal studies. J Therm Anal Calorim 2006; 85: 2, 295–299.
- Mitsuda H, Yuasumoto K, Iwami K. Antioxidation action of indole compounds during the autoxidation of linoleic acid. J Nutr 1966; 44: 307–315.
- Oyaizu M. Studies on products of browing reaction: Antioxidative activity of product of browing reaction preapared from glucosamine. Japanese J Nutr 1986; 44: 307–315.
- Nishimiki M, Rao NA, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem 1972; 46: 849–853.
- Blois MS. Antioxidant determinations by the use of a stable free radical. Nature, 1958; 26: 1199–1200.
- Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivatives (acetaminophen, salicylate and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation as peroxyl radical scavenging effects. Chem Pharm Bull 1994; 36: 2090–2097.
- Gülçin I˙, Das˛tan A. Synthesis of dimeric phenol derivatives and determination of in vitro antioxidant and radical scavenging activities, J Enz Inhib Med Chem 2007; 226: 685–695.
- Ruch RJ, Cheng SJ, Klaunig JE. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989; 10: 1003–1008.
- Chung YC, Chang CT, Chao WW, Lin CF, Chou ST. Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-K1. J Agr Food Chem 2002; 50: 2454–2458.
- Min DB. Lipid oxidation of edible oil. In: Akoh, C.C., Min, D.B. (Eds.), In Food Lipids Chemistry, Nutrition, and Biotechnology. Marcel Dekker, New York, 1998, pp. 283–296.
- Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proceedings of the Royal Society of London, Series A, 1934; 147: 332–351.
- Halliwell B, Gutteridge JM. Free Radicals in Biology and Medicine. Clarendon Press, Oxford, 1989, pp. 23–30.
- Wickens AP. Aging and the free radical theory. Resp Physiol Neurobi 2001; 128: 379–391.
- Pietta PG. Flavonoids as antioxidants. J Nat Prod 2000; 63: 1035–1042.
- Fried R. Enzymatic And Non-Enzymatic Assay of Superoxide-Dismutase. Biochemie 1975; 57: 657–660
- Parejo I, Viladomat F, Bastida J, Rosas-Romero A, Flerlage N, Burillo J, Codna C. Comparison between the radical scavenging activity and antioxidant activity of six distilled and nondistilled Mediterranean herbs and aromatic plants. J Agr Food Chem 2002; 50: 6882–6890.