Abstract
In the present study we have synthesized (4-nitrophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]-methanones, (2-bromophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]-methanone analogues (1–14) and evaluated them for their antimicrobial and antiviral potential. The results of antimicrobial screening indicated that none of the synthesized compounds were effective against the tested bacterial strains. Compounds 3, 11, 13 and compounds 5, 11, 12 were found to be active against Aspergillus niger and Candida albicans respectively, and may be further developed as antifungal agents. Furthermore, evaluation against a panel of different viruses pointed out the selective activity of compounds 5 and 6 against vaccinia virus and Coxsackie virus B4.
Introduction
The herpes simplex viruses HSV-1 and HSV-2 are of clinically relevance among the eight known human herpes viruses belongs to the family of the Herpesviridae [Citation1]. HSV virions are 180–200 nm in diameter and contain an icosahedral capsid surrounding the linear double-stranded DNA genome. Cellular infection is initiated when the virus binds to heparin sulfate chains on cell surface proteoglycans [Citation2]. HSV-1 is responsible for the lesions at orofacial sites that are commonly known as cold sores. HSV-2 is responsible for mucocutaneous genital infections [Citation3]. Much research has been focused on HSV-1 and HSV-2 as these viruses have a high incidence rate (1.6 million new cases of HSV-2 predicted per year in the United States) and prevalence [50–95% (HSV-1) and 6–50% (HSV-2)] [Citation4].
The alpha viruses are pathogenic viruses with worldwide distribution and causes fever, rash, arthralgia or arthritis, lassitude, headache, and myalgia. There is presently no treatment available. Alphaviruses are transmitted to humans through mosquito bites [Citation5]. VSV is one of the most carefully studied virus and its replication strategy forms a valid model for the replication of all Mononegavirales viruses and provides important insights for the study of replication of other viruses with negative-sense RNA genomes [Citation6].
Variola, monkeypox, cowpox, and vaccinia viruses are orthopoxviruses, which can infect humans. Among orthopoxviruses, variola virus, that causes smallpox, is of clinical relevance. Smallpox virus was responsible for serious illness and death until the development of successful vaccine [Citation7].
Benzimidazole derivatives are structural isosteres of naturally occurring nucleotides, which allows them to interact easily with the biopolymers of the living systems [Citation8]. Numerous biological activities and functions have been described for benzimidazole nucleus viz. antihypertensive [Citation9], antifungal [Citation10], antioxidant [Citation11], antiviral [Citation12] and topoisomerase inhibitory [Citation13] activities. Literature reports revealed that the benzimidazole derivatives also possessed antiviral activity against various strains of pathogenic viruses viz. hepatitis virus [Citation14], human immunodeficiency virus (HIV) [Citation15], enteroviruses [Citation16], respiratory syncytial virus (RSV) [Citation17], human cytomegalovirus virus (HCMV) and varicella-zoster virus (VZV) [Citation18].
Disease-causing microbes that have become resistant to drug therapy are an increasing public health problem nowadays [Citation19]. There is a real need for new chemical entities endowed with antimicrobial activity, possibly acting through mechanisms, which are distinct from the well known classes of antimicrobial agents, to which many clinically relevant pathogens have become resistant [Citation20]. Benzimidazole derivatives have been reported to possess antimicrobial activities [Citation21–23].
Prompted by the chemotherapeutic importance of benzimidazole derivatives especially in treating microbial and viral infections as well in continuation of our ongoing search for novel anti-infective agents [Citation24–34], in the present study we hereby report the synthesis, antimicrobial and antiviral activity of (4-nitrophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]-methanones, (2-bromophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]-methanone analogues (1–14). Further we have mentioned the antiviral potential of [2-(substituted phenyl)-benzimidazol-1-yl]-pyridin-3-yl-methanones (15–23) synthesized in our previous report [Citation35].
Experimental
Melting points were determined in open capillary tubes on a Sonar melting point apparatus and are uncorrected. Reaction progress was monitored by thin layer chromatography on silica gel sheets (Merck silica gel-G) and the purity of the compounds was ascertained by single spot TLC. 1H-Nuclear magnetic resonance (1H NMR) spectra were recorded on a Bruker Avance II 400 NMR spectrometer using appropriate deuterated solvents and are expressed in parts per million (δ, ppm) downfield from tetramethylsilane (internal standard). Infrared (IR) spectra were recorded on a Shimadzu FTIR spectrometer.
General procedure for the synthesis of (4-nitrophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]- methanones, (2-bromophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]-methanones (1–14)
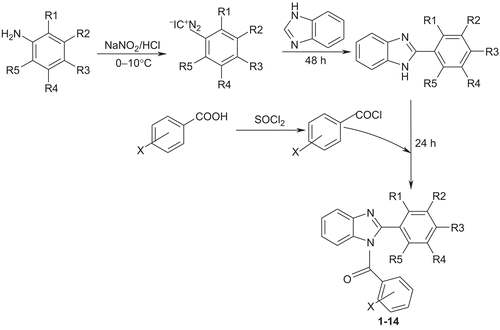
Substituted anilines (0.13 mol) in hydrochloric acid/water mixture (1:1) were diazotized using a solution of sodium nitrite at 0–10°C. To the diazotized mixture, benzimidazole (0.004 mol) was added with vigorous shaking. A solution of sodium acetate (40g in 100 mL of distilled water) was added drop wise to the above mixture by maintaining the temperature at 5–10°C. The above solution was stirred initially for 3 h under cold conditions followed by continuation of stirring at room temperature for 48 h. The product thus obtained was filtered, dried and recrystallized from alcohol to yield the intermediate compounds 2-(substituted phenyl)-1-H-benzimidazoles. A solution of 2-(substituted phenyl)-1-H-benzimidazoles (0.002 mol) in diethyl ether (50 mL) was added to a solution of corresponding acid chloride (0.002 mol) in diethyl ether (50 mL). The above mixture was stirred for 24 h at room temperature. The resultant product was isolated by evaporation of ether and purified by recrystallisation from methanol ().
Table 1. Physicochemical characteristics of synthesized benzimidazole derivatives.
(4-nitrophenyl)-[2-(2-nitrophenyl)-benzoimidazol -1-yl]-methanone (1)
mp (8C) – 154–156; Yield – 52.73 %; 1H NMR spectra CDCl3 δ ppm: 7.26–7.70 (m, 4H, ArH of benzimidazole), 7.71–8.05 (m, 4H, ArH of ArNO2 attached to 2nd position of benzimidazole), 8.07–8.38 (m, 4H, ArH of ArNO2 attached to N1 of benzimidazole).; IR (KBr pellets): cm−1 1427.6, 1540.4 (Skeletal bands), 1693.8 (C=O, str. tertiary amide), 1349.4 (NO2 sym str., ArNO2), 1492.3 (NO2 asym. str., ArNO2); CHN analysis calc.(found): C20H12N4O5: C, 61.86 (60.81); H, 3.11 (3.05); N, 14.43 (14.19); O, 20.60 (20.32) %.
2-[1-(4-nitrobenzoyl)-1H-benzoimidazol-2-yl]- benzoic acid (2)
mp (8C) – 209–211; Yield – 14.09 %; 1H NMR spectra CDCl3 δ ppm: 7.22–7.61 (m, 4H, ArH of benzimidazole), 7.73–8.02 (m, 4H, ArH of ArCOOH), 8.09–8.33(m, 4H, ArH of ArNO2 attached to N1 of benzimidazole), 11.0 (s, 1H, COOH of ArCOOH).; IR (KBr pellets): cm−1 1427.5, 1540.6 (Skeletal bands), 1693.0 (C=O, str. tertiary amide), 1349.9 (NO2 sym. str., ArNO2), 1496.3 (NO2 asym. str., ArNO2), 1106.9 (C-O str., COOH); CHN analysis calc.(found): C21H13N3O5: C, 65.12 (65.01); H, 3.38 (3.05); N, 10.85 (10.19); O, 20.65 (20.30) %.
[2-(4-Hydroxyphenyl)-benzoimidazol-1-yl]-(4-nitrophenyl) methanone (3)
mp (8C) – 229–231; Yield – 16.09 %; 1H NMR spectra CDCl3 δ ppm: 7.24–7.65 (m, 4H, ArH of benzimidazole), 6.79–7.21 (m, 4H, ArH of ArOH), 8.07–8.22 (d, 2H, ArH of ArNO2 attached to N1 of benzimidazole), 5.07 (s, 1H, OH of ArOH).; IR (KBr pellets): cm−1 1428, 1541.5 (Skeletal bands), 1692.5 (C=O, str. tertiary amide),1350.2 (NO2 sym. str., ArNO2), 1495.2 (NO2 asym. str., ArNO2), 3615.2 (O-H str, ArOH); CHN analysis calc.(found): C20H13N3O4: C, 66.85 (66.01); H, 3.65 (3.05); N, 11.69 (11.09); O, 17.81 (17.30) %.
[2-(2-Methoxyphenyl)-benzoimidazol-1-yl]-(4-nitrophenyl) methanone (4)
mp (8C) – 149–151; Yield –56.72 %; 1H NMR spectra CDCl3 δ ppm: 7.30–7.70 (m, 4H, ArH of benzimidazole), 6.83–7.23 (m, 4H, ArOCH3 attached to 2nd position of benzimidazole), 8.10–8.32 (m, 4H, ArH of ArNO2 attached to N1 of benzimidazole), 3.90 (s, 3H, OCH3).; IR (KBr pellets): cm−1 1427.9, 1539.7 (Skeletal bands), 1694.0 (C=O, str. tertiary amide), 1349.5 (NO2 sym. str., ArNO2), 3063.5 (aralkyl ethers); CHN analysis calc.(found): C21H15N3O4: C, 67.56 (67.09); H, 4.05 (3.95); N, 11.25 (11.19); O, 17.14 (17.00) %.
(2-Bromophenyl)-[2-(4-nitrophenyl)-benzoimidazol- 1-yl]-methanone (11)
mp (8C) – 149–151; Yield – 34.23 %; 1H NMR spectra CDCl3 δ ppm: 7.36–7.67 (m, 4H, ArH of benzimidazole), 7.74–8.25 (m, 4H, ArH of ArNO2 attached to 2nd position of benzimidazole), 7.09–7.25 (m, 4H, ArH of ArBr).; IR (KBr pellets): cm−1 1430.2, 1540.6 (Skeletal bands), 1684.1 (C=O, str. tertiary amide), 1498.2 (NO2 asym. str., ArNO2), 553.2 (C-Br, str.); CHN analysis calc.(found): C20H12N3O3Br: C, 56.89 (56.01); H, 2.86 (2.76); N, 9.95 (9.19); O, 11.37 (11.30); Br 18.92 (18.54) %.
(2-Bromophenyl)-[2-(4-hydroxyphenyl)- benzoimidazol-1-yl]-methanone (12)
mp (8C) - 149–151; Yield – 47.65 %; 1H NMR (CDCl3) δ ppm: 7.39–7.70 (m, 4H, ArH of benzimidazole), 7.05–7.22 (m, 4H, ArH of ArBr), 6.79–6.99 (m, 4H, ArH of ArOH), 5.0 (s, 1H, OH of ArOH); IR (KBr pellets): cm−1 1432.0, 1535.2 (Skeletal bands), 1684.8 (C=O, str. tertiary amide), 3618.5 (O-H str., ArOH), 552.9 (C-Br, str.); CHN analysis calc.(found): C20H13N2O2Br: C, 61.09 (60.91); H, 3.33 (2.96); N, 7.12 (7.02); O, 8.14 (8.07); Br 20.32 (20.06) %.
2-[1-(2-Bromobenzoyl)-1H-benzoimidazol-2-yl]- benzoic acid (13)
mp (8C) – 139–141; Yield – 57.68 %; 1H NMR (CDCl3) δ ppm: 7.33–7.62 (m, 4H, ArH of benzimidazole), 7.78–8.19 (m, 4H, ArH of ArCOOH), 7.04–7.25 (m, 4H, ArH of ArBr), 11.0 (s, 1H, COOH of ArCOOH).; IR (KBr pellets): cm−1 1430.9, 1540.2 (Skeletal bands), 1683.6 (C=O str., tertiary amide), 1116.0 (C-O str., COOH), 551.1 (C-Br, str.); CHN analysis calc.(found): C21H13N2O3Br: C, 59.88 (59.01); H, 3.11 (2.79); N, 6.65 (6.54); O, 11.39 (11.20); Br 18.97 (18.34) %.
Evaluation of antimicrobial activity
Determination of MIC
The antimicrobial activity was performed against the Gram-positive bacteria Staphylococcus aureus, Bacillus subtilis, Gram-negative bacterium Escherichia coli and the fungal species Candida albicans and Aspergillus niger. The standard and test samples were dissolved in DMSO and the minimum inhibitory concentration (MIC) was determined by tube dilution method. Dilutions of test and standard compounds were prepared in double strength nutrient broth – I.P. (bacteria) or Sabouraud dextrose broth – I.P. [Citation36] (fungi). The samples were incubated at 37 °C for 24 h (bacteria), 25 °C for 7 d (A. niger) and 37 °C for 48 h (C. albicans) respectively and the results were recorded in terms of MIC (the lowest concentration of test substance which inhibited the growth of microorganism).
Determination of MBC/MFC
The minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) were determined by subculturing 100 μL of culture from each tube that remained clear in the MIC determination into fresh agar medium. MBC and MFC values represents the lowest concentration of compound that produces a 99.9% end point reduction of microorganism [Citation37].
Evaluation of antiviral activity
Antiviral assay
The antiviral activity of the (4-nitrophenyl)- [2-(substituted phenyl)-benzoimidazol-1-yl]-methanones, (2-bromophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]-methanones (1–14) and [2-(substituted phenyl)-benzimidazol-1-yl]-pyridin-3-yl-methanones (15–23) was determined using a CPE reduction assay [Citation38] against herpes simplex virus-1 (KOS), herpes simplex virus-2 (G), vaccinia virus, vesicular stomatitis virus, herpes simplex virus-1 TK− KOS ACVr in HEL cell cultures, vesicular stomatitis virus (VSV), Coxsackie virus B4, respiratory syncytial virus (RSV) in HeLa cell cultures and parainfluenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4, Punta Toro virus in Vero cell cultures and the results were expressed as the 50% effective concentration (EC50). Cells, grown to confluency in 96-well plates, were infected with 100 CCID50 of virus, one CCID50 being the 50% cell culture infective dose. After an adsorption period of 2 h at 37 °C, the virus was removed and serial dilutions of the compounds were added. The cultures were further incubated at 37 8C for 3 days, until complete CPE was observed in the infected and untreated virus control.
Cytotoxic assay
The cytotoxicity of the compounds was evaluated in parallel with their antiviral activity in uninfected cells, and is expressed as minimum cytotoxic concentration that causes a microscopically detectable alteration of normal cell morphology (HEL cells, HeLa cells, and Vero cells).
Results and discussion
Chemistry
The synthesis of compounds 1–14 followed the general pathway depicted in . The key intermediates, 2-(substituted phenyl)-1H-benzimidazoles were prepared by the condensation of benzimidazoles with corresponding substituted aryl diazonium chlorides which in turn were prepared by the diazotization of substituted anilines. However, based on our experience, the application of cupric chloride for the condensation of aryldiazonium chloride with benzimidazole as suggested by Dahiya and Pathak [Citation39] resulted in resinous products. Therefore, the coupling was carried out using sodium acetate along with stirring under cold conditions for initial 3 h followed by 48 h stirring at room temperature, which resulted in a solid product. For the synthesis of (4-nitrophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]-methanones, (2-bromophenyl)- [2-(substituted phenyl)-benzoimidazol-1-yl]-methanones (1–14), the key intermediates were reacted with substituted benzoyl chloride which was prepared by the reaction of substituted benzoic acid with thionyl chloride. The physicochemical characteristics of the synthesized compounds are presented in . The synthesized compounds 1–14 were characterized by their IR and 1H-NMR as well by elemental analysis studies. The elemental analysis results were within ± 0.4% of the theoretical values.
The appearance of IR bands at 3615 cm−1 and 3618 cm−1 in the spectra of compounds 3 and 12 indicated the presence of an OH group on the aromatic ring substituted at the second position of the benzimidazole. The appearance of IR bands in the range of 555 cm−1 to 550 cm−1 corresponds to the C-Br stretch of Ar-Br of compounds 11, 12 and 13. The presence of aromatic nitro groups in compounds 1, 2, 3, 4 and 11 was indicated by the appearance of symmetric and asymmetric NO2 stretches in the range of 1350 cm−1- 1345 cm−1 and 1500 cm−1- 1495 cm−1, respectively, in their IR spectra. The presence of an aralkyl ether group (Ar-OCH3) in compound 4 was confirmed by the presence of an IR band at 3063.5 cm−1 in its IR spectra. The presence of COOH group in compound 2 and 13 was confirmed by the appearance of C=O stretching and C-O stretching at 1603 cm−1 and 1106 cm−1, respectively. Further, the appearance of strong C=O stretching bands at 1695- 1680 cm−1 in the IR spectra of (4-nitrophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]-methanones, (2-bromophenyl)- [2-(substituted phenyl)-benzoimidazol-1-yl]-methanones (1–14) demonstrated the presence of a tertiary amide linkage between the substituted benzoyl chloride and the N1 of benzimidazole nucleus.
The 1H- NMR spectra of the synthesized compounds showed a signal at δ 7.26–7.70 ppm corresponding to Ar-H of the benzimidazole nucleus. The appearance of a multiplet signal at δ 8.07–8.38 ppm indicated the presence of four aromatic protons in Ar-NO2 (1–4) attached to N1 of benzimidazole. The appearance of signal at δ 5 ppm corresponding to the proton of the OH group confirmed the formation of compound 3 and 12. The presence of a signal around δ 11 ppm in the 1H-NMR spectra indicated the presence of a free COOH group in compounds 2 and 13. Furthermore, the absence of a singlet signal corresponding to the COOH in the NMR spectra of other synthesized compounds supports the complete reaction of acid chlorides with N1 hydrogen of benzimidazole nucleus and also indicated the absence of free aromatic acids in the final products. The presence of a bromo substituted benzene ring (11, 12 and 13) was identified by the existence of multiplet signals at δ 7.39–7.70 ppm in the NMR spectra. The signal at δ 3.90 ppm in the proton spectra demonstrated the presence of an aralkyl ether group in compound 4.
Antimicrobial activity
The synthesized compounds were screened for their in vitro antimicrobial activities against two Gram positive bacteria - S. aureus, B. subtilis; Gram negative bacterium - E. coli and the fungal species A. niger and C. albicans by tube dilution method [Citation40], using norfloxacin and fluconazole as control drugs for antibacterial and antifungal activity, respectively. Based on the MIC values observed, the minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) were determined by subculturing 100 μL of culture from each tube that remained clear in the MIC determination into fresh medium. The results of antimicrobial studies are presented in and . The data shown in represents the minimum inhibitory concentration of synthesized substituted benzimidazoles against the representative organisms. With respect to antibacterial activity none of the synthesized compounds showed activity comparable to the standard norfloxacin in both MIC () and MBC () determination. Similar results were observed for the antifungal activity of synthesized compounds against C. albicans and A. niger on the basis of their MIC () i.e. none of the synthesized compounds were more active than standard fluconazole. However, in contrast to MBC results, the MFC results yielded better results against A. niger.
Table 2. Antimicrobial activity (MIC) of synthesized benzimidazole derivatives.
Table 3. MBC and MFC values of synthesized benzimidazole derivatives.
Compounds 3, 8, 10, 11, 13 and 14 demonstrated MFC values less than the standard fluconazole (). Similarly, in the case of C. albicans compounds 4–7 and 10–11 expressed MFC values lesser than the standard. Analysis of the structures of most active antifungal compounds indicated the fact that presence of an electron withdrawing group on the benzene ring substituted at second position as well on the acyl benzene ring substituted at N1 of benzimidazole favored the antifungal activity of the synthesized compounds. The role of electron withdrawing group in improving antimicrobial activities is supported by the studies of Sharma et al [Citation41]. The compounds (4, 5, 6, 7 and 12) which are effective against C. albicans were found to be inactive against A. niger and the reverse is true for compounds (3, 8, 10, 13 and 14) which were effective against A. niger. This indicated the fact that different structural requirements are needed for the binding of drug to different fungal targets such as A. niger and C. albicans [Citation42]. In general, the compounds showed better antifungal activity.
The MIC results are different from MFC/MBC results as MIC was determined by the absence of turbidity in the culture tubes which are inoculated with representative microorganism after the addition of test drug. It may be possible that the presence of one or few organisms cannot be detected by determining the turbidity of the solution. In this case, the determination of MBC and MFC may give useful information which involves subculturing of 100 μL of culture from each tube that remained clear in the MIC determination into fresh medium. This is the reason for the fact that MBC/MFC values () of test compounds are observed at high concentration than their MIC values (). In general, the MFC and MBC values of synthesized benzimidazole derivatives were 3-fold higher than the MIC values which indicated that the synthesized compounds were bacteriostatic and fungistatic in action. (A drug is considered to be bacteriostatic/fungistatic when its MFC and MBC values are 3-fold higher than its MIC value) [Citation43–44].
Antiviral activity
The antiviral evaluation of synthesized compounds (1–23) against Herpes simplex virus-1 (KOS) [HSV-1 KOS], Herpes simplex virus-2 (G) [HSV-2G], Vaccinia virus [VV], Vesicular stomatitis virus [VSV], Herpes simplex virus-1 TK− KOS ACVr [HSV-1 TK− KOS ACVr] in HEL cell cultures, Vesicular stomatitis virus (VSV), Coxsackie virus B4 [CV-B4], Respiratory syncytial virus [RSV] in HeLa cell cultures and Parainfluenza-3 virus [PI-3V], Reovirus-1 [RV-1], Sindbis virus [SV], Coxsackie virus B4 [CV-B4], Punta Toro virus [PTV] in Vero cell cultures was determined using CPE reduction assay.
With the exception of compounds 5 and 6 against VV, no specific antiviral effects (MCC ≥ 5-fold higher than EC50) were noted for any of the compounds in HEL cells. Compounds 5 and 6 were active against VV at an EC50 of 4 and 2 μg/mL, respectively, while not being cytotoxic at a concentration as high as 100 μg/mL ().
Table 4. Antiviral activity of synthesized benzimidazole derivatives in HEL cell culture.
In HeLa cell cultures, compounds 3 and 7 showed an EC50 of 9–10 μg/mL () for Coxsackie B4 virus, but in Vero cells compound 3 was inactive against Coxsackie B4, and compound 7 was not found to be active (MCC < 5-fold higher than EC50) (). In fact, none of the compounds emerged as specifically antiviral compounds in Vero cell cultures ().
Table 5. Antiviral activity of synthesized benzimidazole derivatives in HeLa cell culture.
Table 6. Antiviral activity of synthesized benzimidazole derivatives in Vero cell culture.
Conclusion
In an effort to discover new (4-nitrophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]-methanone, (2-bromophenyl)-[2-(substituted phenyl)-benzoimidazol-1-yl]-methanone (1–14) analogues as antimicrobial agents we found that none of the synthesized compounds emerged as effective antibacterial compound. Compounds 3, 11 and 13 were found to be effective against A. niger and compounds 5, 11 and 12 were found to be effective against C. albicans. These compounds were effective at less than half the concentration of the standard drug fluconazole. Further, the antiviral evaluation results of the synthesized compounds indicated that compounds 5 and 6 have the potential to be selected as lead compounds for the development of novel antiviral agent against vaccinia virus.
Acknowledgements
We would like to thank Mrs. Leentje Persoons, Rega Institute for Medical Research, Belgium for her excellent technical assistance in the evaluation of antiviral activity.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Roizman B, Pellett PE. In Fields’ Virology; Knipe, D. M., Howley, P. M., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, 2001;Vol. 2, p 2381.
- Spear PG, Longnecker RJ. Herpesvirus Entry: an Update. J Virol 2003;77:10179–10185.
- Choo H, Beadle JR, Chong, Y, Trahan J, Hostetler KY. Synthesis of the 5-phosphono-pent-2-en-1-yl nucleosides: A new class of antiviral acyclic nucleoside phosphonates. Bioorg Med Chem 2007;15:1771–1779.
- Gudmundsson KS, Johns BA, Wang Z, Turner EM, Allen SH, Freeman GA, Boyd FL, Sexton CJ, Selleseth DW, Moniri KR, Creech KL. Synthesis of novel substituted 2-phenylpyrazolopyridines with potent activity against herpesviruses. Bioorg Med Chem 2005;13:5346–5361.
- Kim HY, Patkar C, Warrier R, Kuhn R, Cushman M. Design, Synthesis, and Evaluation of Dioxane-Based Antiviral Agents Targeted Against the Sindbis Virus Capsid Protein. Bioorg Med Chem Lett 2005;15:3207–3211.
- Romanutti C, Castilla V, Coto CE, Waschman MB. Antiviral effect of a synthetic brassinosteroid on the replication of vesicular stomatitis virus in Vero cells. Int J Antimicrob Agents 2007;29:311–316.
- Arumugham B, Kim HJ, Prichard MN, Kern ER, Chu, CK. Synthesis and antiviral activity of 7-deazaneplanocin A against orthopoxviruses (vaccinia and cowpox virus). Bioorg Med Chem Lett 2006;16:285–287.
- Starcevic K, Kralj M, Ester K, Sabol I, Grce M, Pavelic K, Karminski-Zamola G. Synthesis, antiviral and antitumor activity of 2-substituted-5-amidino-benzimidazoles. Bioorg Med Chem 2007;15:4419–4426.
- Xu JY, Zeng Y, Ran Q, Wei Z, Bi Y, He QH, Wang Q J, Hu S, Zhang J, Tang MY, Hua, WY, Wu, XM. Synthesis and biological activity of 2-alkylbenzimidazoles bearing a N-phenylpyrrole moiety as novel angiotensin II AT1 receptor antagonists. Bioorg Med Chem Lett 2007;17:2921–2926.
- Kerimov I, Ayhan-Kilcigil G, Can-Eke B, Altanlar N, Scan M. Synthesis, antifungal and antioxidant screening of some novel benzimidazole derivatives. J Enz Inhib Med Ch 2007;22(6):696–702.
- Gurer-Orhan H, Orhan H, Suzen S, Orhan Puskullu M, Buyukbingol E. Synthesis and evaluation of in vitro antioxidant capacities of some benzimidazole derivatives. J Enz Inhib Med Ch 2006;21(2):241–247.
- Patel PD, Patel MR, Kaushik-Basu N, Talele TT. 3D QSAR and Molecular Docking Studies of Benzimidazole Derivatives as Hepatitis C Virus NS5B Polymerase Inhibitors. J Chem Inf Model 2008;48:42–45.
- Oksuzoglu E, Tekiner-Gulbas B, Alpher S, Temiz-Arpaci O, Ertan T, Yildiz I, Diril N, Sener-Aki E, Yalcin I. Some benzoxazoles and benzimidazoles as DNA topoisomerase I and II inhibitors. J Enz Inhib Med Ch 2008;23(1):37–42.
- Li Y, Wang G, Huang W, Tang W, Feng C, Shi L, Ren Y, Zuo J, Lu W. Identification of 1-isopropylsulfonyl-2-amine benzimidazoles as a new class of inhibitors of hepatitis B virus. Eur J Med Chem 2007;42:1358–1364.
- Middleton T, Lim HB, Montgomery D, Rockway T, Tang H, Cheng X, Lu L, Mo H, Kohlbrenner WE. Molla A, Kati WM. Inhibition of human immunodeficiency virus type I integrase by naphthamidines and 2-aminobenzimidazoles. Antiviral Res 2004;64:35–45.
- Bablanian R, Eggers HJ, Tamm I. Inhibition of Enterovirus Cytopathic Effects by 2-(α-Hydroxybenzyl)-Benzimidazole. J Bacteriol 1966;91(3):1289–1294.
- Andries K, Moermans M, Grevers T, Willebrords R, Sommen C, Lacrampe J, Janssens F, Wyde PR. Substituted benzimidazoles with nanomolar activity against respiratory syncytial virus. Antivir Res 2003;60:209–219.
- Fonseca T, Gigante B, Marques MM, Gilchrist TL, Clercq E De. Synthesis and antiviral evaluation of benzimidazoles, quinoxalines and indoles from dehydroabietic acid. Bioorg Med Chem 2004;12:103–112.
- Foroumadi A, Shahla M, Kiani Z, Rahmani A. Synthesis and in vitro antibacterial evaluation of N-[5-(5-nitro-2-thienyl)-1,3,4-thiadiazole-2-yl] piperazinyl quinolones. Eur J Med Chem 2003;38:851–854.
- Khalafi-Nezhad A, Rad MNS, Mohabatkar H, Asari Z, Hemmateenejad B, Design, synthesis, antibacterial and QSAR studies of benzimidazole and imidazole chloroaryloxyalkyl derivatives. Bioorg Med Chem 2005;13:1931–1938.
- Güven OO, Erdoğan T, Göker H, Yildiz S. Synthesis and antimicrobial activity of some novel phenyl and benzimidazole substituted benzyl ethers. Bioorg Med Chem Lett 2007;17:2233–2236.
- Ates-Alagoz Z, Yildiz S, Buyukbingol E. Antimicrobial Activities of Some Tetrahydronaphthalene-Benzimidazole Derivatives. Chemotherapy. 2007;53(2):110–113.
- Mohamed BG, Abdel-Alim AA, Hussein MA. Synthesis of 1-acyl-2-alkylthio-1, 2, 4-triazolobenzimidazoles with antifungal, anti-inflammatory and analgesic effects. Acta Pharm 2006;56(1):31–48.
- Narasimhan B, Kothwade UR, Pharande DS, Mourya VK, Dhake AS. Syntheses and QSAR studies of sorbic, cinnamic and ricinoleic acid derivatives as potential antibacterial agents. Indian J Chem 2003;42(B):2828–2834.
- Narasimhan B, Belsare D, Pharande P, Mourya VK, Dhake AS. Esters, amides and substituted derivatives of cinnamic acid: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 2004;39(10):827–834.
- Narasimhan B, Mourya VK, Dhake AS. Design, synthesis, antibacterial, and QSAR studies of myristic acid derivatives. Bioorg Med Chem Lett 2006;16:3023–3029.
- Narasimhan B, Mourya VK, Dhake AS. Studies of antibacterial ricinoleic acid derivatives. Khim Farm Zh 2007;41(3):133–139.
- Narasimhan B, Narang R, Judge V, Ohlan S, Ohlan R. Synthesis, antimicrobial and QSAR studies of substituted anilides. ARKIVOC 2007;XV:112–126.
- Narasimhan B, Judge V, Narang R, Ohlan S, Ohlan R. Quantitative structure activity relationship studies for prediction of antimicrobial activity of synthesized 2,4-hexadienoic acid derivatives. Bioorg Med Chem Lett 2007;17:5836–5845.
- Narasimhan B, Kumari M, Jain N, Dhake AS, Sundaravelan C. Correlation of antibacterial activity of some N-[5-(2-furanyl)-2-methyl-4-oxo-4H-thieno[2,3-d]pyrimidin-3-yl]-carboxamide and 3-substituted-5-(2-furanyl)-2-methyl-3H-thieno[2,3-d]pyrimidin-4-ones with topological indices using Hansch analysis. Bioorg Med Chem Lett 2006;16:4951–4958.
- Kumar A, Narasimhan B, Kumar D. Synthesis, antimicrobial, and QSAR studies of substituted benzamides. Bioorg Med Chem 2007;15:4113–4124.
- Ohlan R, Ohlan S, Judge V, Narang R, Ahuja M, Narasimhan B. 2-(2,4-Difluorophenyl)-1,3-bis-(1,2,4-triazol-1-yl)-propan-2-ol derivatives: Synthesis, Antifungal evaluation and QSAR studies by Hansch analysis. ARKIVOC 2007;XIV:172–184.
- Narasimhan B, Ohlan S, Ohlan R, Judge V, Narang R. Hansch analysis of veratric acid derivatives as antimicrobial agents. Eur J Med Chem 2008 (In Press).
- Kumar P, Narasimhan B, Sharma D. Substituted benzoic acid benzylidene/furan-2-yl-methylene hydrazides: synthesis, antimicrobial evaluation and QSAR analysis. ARKIVOC 2008;XIII:159–178.
- Sharma D, Narasimhan B, Kumar P, Jalbout A. Synthesis and QSAR evaluation of 2-(substituted phenyl)-1H-benzimidazoles and [2-(substituted phenyl)-benzimidazol-1-yl]-pyridin-3-yl-methanones. Eur J Med Chem 2008 (In Press).
- Pharmacopoeia of India; Ministry of Health Department: Govt. of India: New Delhi, 2007, Vol-I, p 37.
- Cappucino JG, Sherman N, In Microbiology - A laboratory Manual, Addison Wesley Longman Inc.; California.1999, pp. 263.
- De Palma AM, Heggermont W, Leyssen P, Purstinger G, Wimmer E, De Clercq E, Rao A, Monforte AM, Chimirri A, Neyts J. Anti-enterovirus activity and structure–activity relationship of a series of 2,6-dihalophenyl-substituted-1H,3H-thiazolo[3,4-a]benzimidazoles Biochem Biophys Res Comm 2007;353:628–632.
- Dahiya R, Pathak D. Synthetic studies on novel benzimidazolopeptides with antimicrobial, cytotoxic and anthelmintic potential. Eur J Med Chem 2007;42:772–798.
- Rodriguez-Arguelles MC, Lopez-Silva EC, Sanmartin J, Pelagatti P, Zani F. Copper complexes of imidazole-2-, pyrrole-2- and indol-3-carbaldehyde thiosemicarbazones: Inhibitory activity against fungi and bacteria. J Inorg Biochem 2005;99:2231–2239.
- Sharma P, Rane N, Gurram VK. Synthesis and QSAR studies of pyrimido[4,5-d]pyrimidine-2,5-dione derivatives as potential antimicrobial agents. Bioorg Med Chem Lett 2004;14:4185–4190.
- Sortino M, Delgado P, Jaurez S, Quiroga J, Abonia R, Insuasey B, Nogueras M, Rodero L, Garibotto FM, Enriz RD, Zacchino SA. Synthesis and antifungal activity of (Z)-5-arylidenerhodanines. Bioorg Med Chem Lett 2007;15:484–494.
- Emami S, Falhati M, Banifafemi A, Shafiee A. Stereoselective synthesis and antifungal activity of (Z)-trans-3-azolyl-2-methylchromanone oxime ethers. Bioorg Med Chem 2004;12:5881–5889.
- Sharma D, Narasimhan B, Kumar P, Judge V, Narang R, Clercq E, De Balzarini J. Synthesis, antimicrobial and antiviral evaluation of substituted imidazole derivatives. Eur J Med Chem 2008 (In Press).