Abstract
A number of 1,8-naphthyridine derivatives (22–62) have been synthesized and screened for their in vitro cytotoxicity against eight tumors and two non-tumor cell lines. Halogen substituted 1,8-naphthyridine-3-caboxamide derivatives showed potent activity with compound 47 having IC50 of 0.41 and 0.77 μM on MIAPaCa and K-562 cancer cell lines, respectively while, compound 36 had IC50 of 1.19 μM on PA-1 cancer cell line. However, one of the unsubstituted 1,8-naphthyridine-C-3’-heteroaryl derivative 29 showed potent cytotoxicity with IC50 of 0.41 and 1.4 μM on PA-1 and SW620 cancer cell lines, respectively. These compounds were also evaluated for anti-inflammatory activity as suggested by downregulation of proinflammaotory cytokines.
Keywords::
Introduction
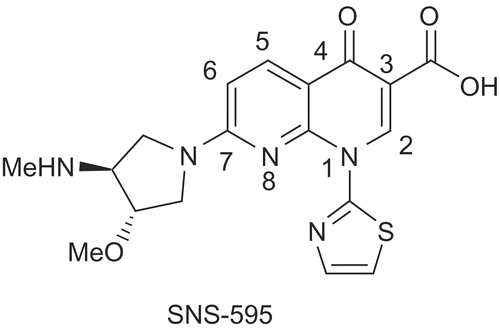
Cancer is a group of diseases characterized by uncontrolled growth and spread of abnormal cells. Recently 1,8-naphthyridine is being exploited in cancer chemotherapy like SNS-595 (), which is in second phase of clinical trial [Citation1–4]. In our efforts to find out a potent molecule, we have modified the C-3 carboxylic acid of 1,8-naphthyridine with different non-conventional functionalized amino acids, which were synthesized “in house” to afford 1,8-naphthyridine-3-carboxamide derivatives (22–62) [Citation5].
Mammalian Topoisomerase II is one of the known target for antitumor agents like doxorubicin, etoposide, ellipticine and amascrine [Citation6]. 1,8-Naphtyridine derivatives were found to display moderate cytotoxic activity against murine P388 leukemia, when changes were carried out at N-1 and C-7 position [Citation3,Citation4]. We have carried out further changes at C-3 position and synthesized 1,8-naphthyridine-3-carboxamide derivatives (22–62), and tested them against different cancer cell lines. These compounds have shown promising anticancer activities and were further tested for their potential anti-inflammatory activity based on the molecular link between cancer and inflammation [Citation7–9]. An in vitro septic shock assay based on murine bone marrow-DCs has been used to evaluate potential anti-inflammatory activity as indicated by resultant down regulation of various pro-inflammatory cytokines.
Materials and methods
All the solvents and reagents were purchased from Aldrich, Lancaster or Across & Rankem and were used as supplied. All TLC data (Rf values) were determined on aluminum sheets coated with silica gel 60 F254 (Merck) and visualization was achieved with UV light and iodine vapors. Column chromatography was performed using silica gel (100–200 mesh) and the synthesized compounds were characterized using 1H NMR and mass spectroscopy. Proton Magnetic Resonance (1H NMR) spectra were recorded on a Bruker 300 MHz instrument using tetramethylsilane (TMS) as an internal standard and mass spectra were recorded on a Micromass Quattro Micro™ instrument. The purity of the synthesized compounds was determined on a Shimadzu HPLC LC-2010 C HT instrument using a gradient system. Melting points were obtained in a capillary tube with a thermal scientific melting point apparatus Mettler Toledo and are uncorrected.
Chemistry
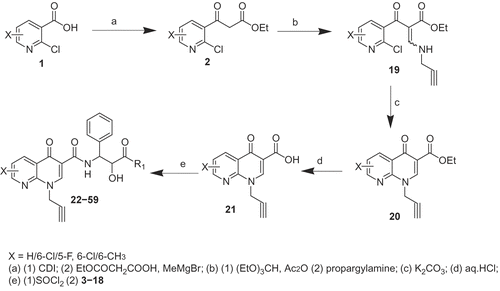
Commercially available, 2-chloro nicotinic acid 1 was reacted with 1,1’-carbonyldiimidazole (CDI), ethyl hydrogen malonate and methyl magnesium bromide in dry THF to afford nicotinoylacetate 2. Compounds 2 upon treatment with triethyl orthoformate and acetic anhydride followed by reaction with propargyl amine, afforded ethyl nicotinoylacrylate 19. Further cyclization of the compound 19 using K2CO3 in ethyl acetate provided ethyl 1,8-naphthyridine-3-carboxylate (20), Compound 20 on acidic hydrolysis resulted in 1,8-naphthyridine-3-carboxylic acids (21). The acid 21 was treated with different functionalized amino acids 3–18 (), prepared in-house, to afford 1,8-naphthyridine-3-carboxamide derivatives 22–62 () as shown in and .
Table 1. Functionalised amino acid derivatives (3–18).
Table 2. 1,8-Naphthyridine derivatives (22–62).
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-cyclopropylcarbamoyl-2-hydroxy-1- phenyl-ethyl)-amide (22). Thionyl chloride (313 mg, 2.6 mmol) was added drop wise to a stirred solution of unsubstituted-1,8-naphthyridine-3-carboxylic acid (21, 500 mg, 2.2 mmol) in dichloromethane (30 mL) and catalytic amount of dimethyl formamide (2–4 drops). The stirring was continued for 4h at room temperature. The acyl chloride intermediate formed was dried under vacuum and again diluted with dichloromethane (30 mL). Functionalized amino acid (3) was added to it and stirred for 2h. The reaction mixture was diluted with water (30 mL) and extracted with dichloromethane (30 mL). The organic layer was dried over anhydrous Na2SO4 and concentrated to provide the crude product. The crude product was purified over silica gel (mesh size 100–200) column using 2% MeOH/ DCM as eluent, to furnish compound (22).
Rf0.4 (10% MeOH/DCM); 1HNMR (CDCl3 + DMSO-d6) δ 10.62 (d, 1H, J = 7.6 Hz), 9.14 (s, 1H), 8.86–8.81 (m, 2H), 7.62–7.44 (m, 4H), 7.33–7.19 (m, 2H), 5.88–5.86 (m, 1H), 5.65 (d, 1H, J = 8.5 Hz), 5.39–5.33 (m, 3H), 4.32 (d, 1H, J = 5.5 Hz), 2.73–2.72 (m, 1H), 2.66-2.61 (m, 1H, partially merged with DMSO peak), 0.63–0.59 (m, 2H), 0.43–0.41 (m, 1H), 0.34–0.33 (m, 1H); MS (ES+) 431 (M++H), Yield 507 mg (53.6%), m.p. 152–154°C.
Compounds 23–59 were prepared in a similar to way to compound 22.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-cyclopentylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (23). Rf0.5 (10 % MeOH/DCM); 1HNMR (CDCl3) δ 10.83 (d, 1H, J = 8.2 Hz), 9.14 (s, 1H), 8.84–8.81 (m, 2H), 7.52–7.45 (m, 3H), 7.34–7.22 (m, 3H), 6.69 (d, 1H, J = 7.6 Hz), 5.66 (dd, 1H, J = 2.8, 8.2 Hz), 5.27 (s, 2H), 4.52–4.49 (m, 1H), 4.31–4.30 (m, 1H), 4.21–4.12 (m, 1H), 2.52 (t, 1H, J = 2.2 Hz), 1.91–1.76 (m, 4H), 1.59–1.37 (m, 4H); MS (ES+) 459 (M++H), Yield 850 mg (84.5%), m.p. > 250°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-cyclohexylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (24). Rf0.7 (10 % MeOH/DCM); 1HNMR (CDCl3) δ 10.88 (d, 1H, J = 8.4 Hz), 9.11 (s, 1H), 8.82 (d, 2H, J = 6.5 Hz), 7.50–7.44 (m, 3H), 7.30–7.18 (m, 3H), 6.72 (d, 1H, J = 8.5 Hz), 5.69 (dd, 1H, J = 2.8, 8.4 Hz), 5.27 (d, 2H, J = 2.4 Hz), 4.75 (d, 1H, J = 4.5 Hz), 4.51 (bs, 1H), 3.76–3.73 (m, 1H), 2.52 (t, 1H, J = 2.4 Hz), 2.10–2.09 (m, 1H), 1.83–1.49 (m, 5H), 1.27–1.08 (m, 4H); MS (ES+) 473 (M++H) (100), Yield 810 mg (78.1%), m.p. 122–124°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-hydroxy-1-phenyl-2-phenylcarbamoyl-ethyl)-amide (25). Rf0.6 (10% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.63 (d, 1H, J = 8.8 Hz), 9.73 (s, 1H), 9.13 (s, 1H), 9.02–9.0 (m, 1H), 8.81 (d, 1H, J = 6.5 Hz), 7.77–7.66 (m, 3H), 7.51–7.36 (m, 4H), 7.34–7.27 (m, 3H), 7.11–7.06 (m, 1H), 6.46 (d, 1H, J = 5.7 Hz), 5.72 (d, 1H, J = 8.8 Hz), 5.43 (s, 2H), 4.46 (d, 1H, J = 5.7 Hz), 3.55 (1H, merged with water peak); MS (ES+) m/z 466 (M++H), Yield 312 mg (30.5%), m.p. > 250°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-(4-fluoro-phenylcarbamoyl)-2-hydroxy-1-phenyl-ethyl]-amide (26). Rf0.6 (10% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.54 (d, 1H, J = 8.9 Hz), 9.78 (s, 1H), 9.05 (s, 1H), 8.94–8.92 (m, 1H), 8.72 (dd, 1H, J = 1.7, 7.9 Hz), 7.68–6.61 (m, 3H), 7.42–7.24 (m, 5H), 7.09–7.03 (m, 2H), 6.38 (d, 1H, J = 5.7 Hz), 5.63 (d, 1H, J = 8.9 Hz), 5.36 (s, 2H), 4.39 (dd, 1H, J = 2.1, 5.7 Hz), 3.48 (t, 1H, J = 2.3 Hz); MS (ES+) m/z 485 (M++H), Yield 325 mg (30.6%), m.p. > 250°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-2-(4-methoxy-phenylcarbamoyl)-1-phenyl-ethyl]-amide (27). Rf0.4 (7% MeOH/DCM); 1HNMR (CDCl3) δ 10.90 (d, 1H, J = 8.3 Hz), 9.09 (s, 1H), 8.80–8.76 (m, 2H), 8.67 (s, 1H), 7.49–7.39 (m, 5H), 7.30–7.16 (m, 3H), 6.73 (d, 2H, J = 8.8 Hz), 5.81 (dd, 1H, J = 2.3, 8.3 Hz), 5.22 (s, 2H), 5.13 (d, 1H, J = 5.2 Hz), 4.69–4.66 (m, 1H), 3.71 (s, 3H), 2.50 (t, 1H, J = 2.3 Hz); MS (ES+) m/z (relative intensity) 497 (M++H), (100), Yield 510 mg (46.8%), m.p. 203–205°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-benzylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (28). Rf 0.4 (10% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.56 (d, 1H, J = 8.7 Hz), 9.1 (s, 1H), 8.97 (d, 1H, J = 3.2 Hz), 8.70 (d, 1H, J = 6.9 Hz), 8.33–8.31 (m, 1H), 7.69–7.65 (m, 1H), 7.41–7.25 (m, 5H), 7.06–7.05 (m, 2H), 6.88–6.87 (m, 3H), 6.24 (d, 1H, J = 5.7 Hz), 5.62 (d, 1H, J = 8.7 Hz), 5.52–5.37 (m, 2H), 4.50–4.42 (m, 1H), 4.28 (d, 1H, J = 5.5 Hz), 4.10–4.04 (m, 1H), 3.54 (s, 1H); MS (ES+) m/z 481 (M++H), Yield 716 mg (67.9%), m.p. > 250°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(pyridin-2-ylcarbamoyl)-ethyl]-amide (29). Rf 0.5 (EtOAc); 1HNMR (DMSO-d6) δ 10.61 (d, 1H, J = 9.0 Hz), 9.7 (bs, 1H), 9.0 (s, 1H), 8.93 (d, 1H, J = 4.1 Hz), 8.74 (d, 1H, J = 7.4 Hz), 8.25 (d, 1H, J = 3.6 Hz), 8.07 (d, 1H, J = 8.4 Hz), 7.79–7.64 (m, 2H), 7.46–7.09 (m, 6H), 6.6 (d, 1H, J = 5.4 Hz), 5.7 (d, 1H, J = 8.8 Hz), 5.36 (s, 2H), 4.51 (d, 1H, J = 5.4 Hz), 3.48 (s, 1H); MS (ES+) m/z 468 (M++H), Yield 158 mg (15.4%), m.p. > 250°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(pyridin-3-ylcarbamoyl)-ethyl]-amide (30). Rf 0.7 (10% MeOH/DCM); 1HNMR (CDCl3) δ 10.94 (d, 1H, J = 8.3 Hz), 9.12 (s, 1H), 8.92 (s, 1H), 8.83–8.78 (m, 2H), 8.40 (s, 1H), 8.21–8.17 (m, 2H), 7.51–7.46 (m, 3H), 7.34–7.23 (m, 4H), 5.80 (dd, 1H, J = 2.3, 8.3 Hz), 5.73 (bs, 1H), 5.30–5.24 (m, 2H), 4.70 (d, 1H, J = 2.3 Hz), 2.53 (t, 1H, J = 2.4 Hz); MS (ES+) m/z 468 (M++H), Yield 605 mg (58.9%), m.p. > 250°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(pyridin-4-ylcarbamoyl)-ethyl]-amide (31). Rf 0.5 (7% MeOH/DCM); 1HNMR (CDCl3) δ 10.92 (d, 1H, J = 8.2 Hz), 9.13 (s, 1H), 8.96 (bs, 1H), 8.83–8.79 (m, 2H), 8.36–8.34 (m, 2H), 7.52–7.47 (m, 5H), 7.37–7.29 (m, 3H), 5.77 (d, 1H, J = 5.7 Hz), 5.26 (d, 2H, J = 2.1 Hz), 4.71 (d, 1H, J = 2.7 Hz), 2.52 (t, 1H, J = 2.4 Hz); MS (ES+) m/z 468 (M++H), Yield 234 mg (22.8%), m.p. > 250°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(thiazol-2-ylcarbamoyl)-ethyl]-amide (32). Rf 0.4 (10% MeOH/DCM); 1HNMR (CDCl3) δ 10.84 (d, 1H, J = 8.3 Hz), 10.69 (bs, 1H), 9.11 (s, 1H), 8.81–8.79 (m, 1H), 8.73–8.71 (m, 1H), 7.52–7.44 (m, 3H), 7.35–7.20 (m, 4H), 6.88 (d, 1H, J = 3.5 Hz), 6.33 (bs, 1H), 5.86 (d, 1H, J = 6.5 Hz). 5.24–5.21 (m, 2H), 4.80 (s, 1H), 2.51 (t, 1H, J = 2.4 Hz); MS (ES+) m/z 474 (M++H), Yield 578 mg (55.6%), m.p. > 250°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-hydroxy-3-oxo-1-phenyl-3-piperidin-1-yl-propyl)-amide (33). Rf 0.5 (7% MeOH/DCM); 1HNMR (CDCl3) δ 10.66 (d, 1H, J = 8.8 Hz), 9.10 (s, 1H), 8.9–8.8 (m, 2H), 7.54–7.46 (m, 3H), 7.39–7.26 (m, 3H), 5.57 (d, 1H, J = 8.2 Hz), 5.35–5.22 (m, 2H), 4.73 (d, 1H, J = 4.8 Hz), 4.49 (d, 1H, J = 6.4 Hz), 3.6-3.5 (m, 4H), 2.49 (t, 1H, J = 2.4 Hz), 1.65–1.35 (m, 6H); MS (ES+) m/z 459 (M++H), Yield 608 mg (60.4%), m.p. > 250°C.
4-Oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-hydroxy-3-oxo-1-phenyl-3-pyrrolidin-1-yl-propyl)-amide (34). Rf 0.4 (5% MeOH/DCM); 1HNMR (CDCl3) δ 10.72 (d, 1H, J = 8.6 Hz), 9.11 (s, 1H), 8.85–8.81 (m, 2H), 7.52–7.46 (m, 3H), 7.38–7.26 (m, 3H), 5.61 (dd, 1H, J = 2.5, 8.6 Hz), 5.26 (dd, 2H, J = 2.3, 14.8 Hz), 4.54 (dd, 1H, J = 2.5, 6.7 Hz), 4.28 (d, 1H, J =- 6.7 Hz), 3.57–3.42 (m, 4H), 2.5 (s, 1H), 1.97–1.85 (m, 4H); MS (ES+) m/z 445 (M++H), Yield 292 mg (29.9%), m.p. 197-199°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-cyclopentylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (35). Rf0.5 (10% MeOH/DCM); 1HNMR (CDCl3) δ 10.76 (d, 1H, J = 7.8 Hz), 9.14 (s, 1H), 8.75 (d, 1H, J = 8.2 Hz), 7.47–7.21 (m, 6H), 6.62 (d, 1H, J = 7.3 Hz), 5.65 (d, 1H, J = 7.0 Hz), 5.21 (s, 2H), 4.49 (s, 1H), 4.19–4.07 (m, 2H), 2.55 (s, 1H), 1.91–1.74 (m, 2H), 1.54–1.20 (m, 6H); MS (ES+) m/z 493 (M++H), Yield 621 mg (66.3%), m.p. > 250°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-cyclohexylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (36). Rf0.4 (5% MeOH/DCM); 1HNMR (CDCl3) δ 10.76 (d, 1H, J = 8.1 Hz), 9.11 (s, 1H), 8.74 (d, 1H, J = 8.3 Hz), 7.46–7.23 (m, 6H), 6.57 (d, 1H, J = 8.4 Hz), 5.65 (dd, 1H, J = 2.6, 8.1 Hz), 5.20 (s, 2H), 4.50–4.48 (m, 1H), 4.16 (d, 1H, J = 4.9 Hz), 3.77–3.70 (m, 1H), 2.55 (t, 1H, J = 2.2 Hz), 1.84–1.81 (m, 1H), 1.55–1.51 (m, 2H), 1.37–0.88 (m, 7H); MS (ES+) m/z 507 (M++H), Yield 598 mg (62.1%), m.p. > 250°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-hydroxy-1-phenyl-2-phenylcarbamoyl-ethyl)-amide (37). Rf0.4 (5% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.45 (d, 1H, J = 8.8 Hz), 9.65 (s, 1H), 9.02 (s, 1H), 8.69 (d, 1H, J = 8.3 Hz), 7.70 (d, 1H, J = 8.3 Hz), 7.60 (d, 2H, J = 7.8 Hz), 7.42–7.20 (m, 7H), 7.02–6.97 (m, 1H), 6.38 (d, 1H, J = 5.5 Hz), 5.63 (d, 1H, J = 8.1 Hz), 5.26 (s, 2H), 4.38 (d, 1H, J = 3.8 Hz), 3.52 (s, 1H); MS (ES+) m/z 501 (M++H), Yield 402 mg (42.2%), m.p. > 250°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-(4-fluoro-phenylcarbamoyl)-2-hydroxy-1-phenyl-ethyl]-amide (38). Rf0.7 (7% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.46 (d, 1H, J = 9.0 Hz), 9.80 (bs, 1H), 9.04 (s, 1H), 8.71 (d, 1H, J = 8.1 Hz), 7.73–7.64 (m, 3H), 7.40-7.25 (m, 5H), 7.09–7.07 (m, 2H), 6.41 (bs, 1H), 5.65–5.63 (m, 1H), 5.28 (s, 2H), 4.39 (bs, 1H), 3.54 (s, 1H); MS (ES+) m/z 519 (M++H), Yield 208 mg (21.1%), m.p. > 250°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-2-(4-methoxy-phenylcarbamoyl)-1-phenyl-ethyl]-amide (39). Rf0.4 (10% MeOH/DCM); 1HNMR (CDCl3) δ 10.81 (d, 1H, J = 7.9 Hz), 9.08 (s, 1H), 8.70 (d, 1H, J = 8.3 Hz), 8.57 (s, 1H), 7.49–7.20 (m, 8H), 6.75 (d, 2H, J = 8.7 Hz), 5.77 (d, 1H, J = 7.9 Hz), 5.17 (s, 2H), 4.77 (d, 1H, J = 5.2 Hz), 4.67 (s, 1H), 3.73 (s, 3H), 2.54 (s, 1H); MS (ES+) 531 (M++H), Yield 446 mg (44.2%), m.p. > 250°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-benzylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (40). Rf0.5 (7% MeOH/DCM); 1HNMR (CDCl3) δ 10.77 (d, 1H, J = 8.4 Hz), 9.03 (s, 1H), 8.68 (d, 1H, J = 8.3 Hz), 7.46–6.98 (m, 12H), 5.73 (dd, 1H, J = 2.4, 8.4 Hz), 5.20 (s, 2H), 4.61–4.52 (m, 2H), 4.40 (d, 1H, J = 5.3 Hz), 4.28–4.21 (m, 1H), 2.57 (t, 1H, J = 2.4 Hz); MS (ES+) m/z 515 (M++H), Yield 566 mg (57.8%), m.p. 140-142°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(pyridin-2-ylcarbamoyl)-ethyl]-amide (41). Rf0.4 (5% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.50 (d, 1H, J = 9.0 Hz), 9.68 (s, 1H), 9.02 (s, 1H), 8.71 (d, 1H, J = 8.2 Hz), 8.24 (d, 1H, J = 4.3 Hz), 8.05 (d, 1H, J = 8.3 Hz), 7.78–7.69 (m, 2H), 7.43–7.21 (m, 5H), 7.10–7.06 (m, 1H), 6.57 (d, 1H, J = 5.5 Hz), 5.67 (d, 1H, J = 9.0 Hz), 5.26 (s, 2H), 4.48 (d, 1H, J = 5.5 Hz), 3.52 (s, 1H); MS (ES+) m/z 502 (M++H), Yield 281 mg (29.4%), m.p. > 250°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(pyridin-3-ylcarbamoyl)-ethyl]-amide (42). Rf0.4 (7% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.49 (d, 1H, J = 8.9 Hz), 10.0 (s, 1H), 9.04 (s, 1H), 8.78 (bs 1H), 8.70 (d, 1H, J = 8.3 Hz), 8.21 (d, 1H, J = 4.0 Hz), 8.06–8.03 (m, 1H), 7.72 (d, 1H, J = 8.3Hz), 7.43–7.25 (m, 6H), 6.48 (d, 1H, J = 5.7 Hz), 5.65 (dd, 1H, J = 1.5, 8.9 Hz), 5.27 (s, 2H), 4.45–4.42 (m, 1H), 3.53 (t, 1H, J = 2.3 Hz); MS (ES+) m/z 502 (M++H), Yield 381 mg (39.9%), m.p. > 250°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(thiazol-2-ylcarbamoyl)-ethyl]-amide (43). Rf0.4 (7% MeOH/DCM); 1HNMR (CDCl3) δ 10.76 (d, 1H, J = 8.2 Hz), 10.48 (bs, 1H), 9.1 (s, 1H), 8.67 (d, 1H, J = 8.3 Hz), 7.50–7.22 (m, 7H), 6.89 (d, 1H, J = 3.5 Hz), 5.99 (bs, 1H), 5.83–5.80 (m, 1H), 5.24–5.11 (m, 2H), 4.78 (s, 1H), 2.55 (t, 1H, J = 2.3 Hz); MS (ES+) m/z 508 (M++H), Yield 295 mg (30.6%), m.p. > 250°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-hydroxy-3-oxo-1-phenyl-3-piperidin-1-yl-propyl)-amide (44). Rf0.7 (7% MeOH/DCM); 1HNMR (CDCl3) δ 10.56 (d, 1H, J = 9.0 Hz), 9.07 (s, 1H), 8.74 (d, 1H, J = 8.3 Hz), 7.53–7.27 (m, 6H), 5.54 (d, 1H, J = 9.0 Hz), 5.29–5.10 (m, 2H), 4.74–4.72 (m, 1H), 4.47 (d, 1H, J = 6.4 Hz), 3.60–3.49 (m, 4H), 2.53 (t, 1H, J = 2.5 Hz), 1.71–1.57 (m, 6H); MS (ES+) m/z 493 (M++H), Yield 381 mg (40.6%), m.p. 180-182°C.
7-Chloro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-hydroxy-3-morpholin-4-yl-3-oxo-1-phenyl-propyl)-amide (45). Rf0.6 (5% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.37 (d, 1H, J = 8.4 Hz), 9.06 (s, 1H), 8.71 (d, 1H, J = 8.3 Hz), 7.69 (d, 1H, J = 8.3 Hz), 7.41–7.24 (m, 5H), 5.42 (d, 1H, J = 8.4 Hz), 5.30 (s, 2H), 5.20 (d, 1H, J = 6.2 Hz), 4.76 (s, 1H), 3.50–3.40 (m, 9H); MS (ES+) m/z 495 (M++H), Yield 276 mg (29.3%), m.p. > 250°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-cyclopropylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (46). Rf0.5 (10% MeOH/DCM); 1HNMR (CDCl3) δ 10.62 (d, 1H, J = 8.0 Hz), 9.12 (s, 1H), 8.51 (d, 1H, J = 7.2 Hz), 7.44–7.26 (m, 5H), 6.79 (bs, 1H), 5.65–5.62 (m, 1H), 5.21 (s, 2H), 4.49–4.43 (m, 1H), 3.85 (bs, 1H), 2.69–2.57 (m, 2H), 0.74–0.65 (m, 2H), 0.48–0.33 (m, 2H); MS (ES+) m/z 483 (M++H), Yield 328 mg (38.2%), m.p. > 250°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-cyclopentylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (47). Rf0.4 (10% MeOH/DCM); 1HNMR (CDCl3) δ 10.62 (d, 1H, J = 8.1 Hz), 9.04 (s, 1H), 8.43 (d, 1H, J = 7.2 Hz), 7.38–7.35 (m, 2H), 7.27–7.15 (m, 3H), 6.57 (d, 1H, J = 7.6 Hz), 5.58 (d, 1H, J = 6.0 Hz), 5.13 (s, 2H), 4.41 (s, 1H), 4.14–4.07 (m, 1H), 4.02 (d, 1H, J = 4.8 Hz), 2.49 (s, 1H), 1.65–1.6 (m, 1H), 1.55–1.26 (m, 4H), 1.18–1.10 (m, 3H); MS (ES+) m/z 511 (M++H), Yield 456 mg (50.2%), m.p. 214–216°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-cyclohexylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (48). Rf 0.4 (5% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.34 (d, 1H, J = 8.8 Hz), 9.07 (s, 1H), 8.65 (d, 1H, J = 7.7 Hz), 7.37–7.20 (m, 6H), 6.06 (d, 1H, J = 5.4 Hz), 5.48 (d, 1H, J = 8.8 Hz), 5.31 (d, 2H, J = 2.0 Hz), 4.16 (dd, 1H, J = 2.0, 5.4 Hz), 3.56–3.40 (m, 2H), 1.60–1.45 (m, 5H), 1.22–0.99 (m, 5H); MS (ES+) m/z 525 (M++H), Yield 325 mg (34.8%), m.p. 215–217°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-hydroxy-1-phenyl-2-phenylcarbamoyl-ethyl)-amide (49). Rf0.5 (10% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.42 (d, 1H, J = 8.8 Hz), 9.63 (s, 1H), 9.06 (s, 1H), 8.64 (d, 1H, J = 7.8 Hz), 7.61 (d, 2H, J = 7.8 Hz), 7.44–7.23 (m, 7H), 7.04–7.02 (m, 1H), 6.39 (d, 1H, J = 5.2 Hz), 5.65 (d, 1H, J = 8.8 Hz), 5.29 (s, 2H), 4.40 (s, 1H), 3.54 (s, 1H); MS (ES+) m/z 519 (M++H), Yield 301 mg (32.6%), m.p. > 250°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-(4-fluoro-phenylcarbamoyl)-2-hydroxy-1-phenyl-ethyl]-amide (50). Rf0.5 (7% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.42 (d, 1H, J = 8.5 Hz), 9.78 (s, 1H), 9.06 (s, 1H), 8.63 (m, 1H), 7.66–7.63 (m, 2H), 7.42–7.24 (m, 5H), 7.11–7.05 (m, 2H), 6.41 (d, 1H, J = 5.4 Hz), 5.65–5.63 (m, 1H), 5.29 (s, 2H), 4.40 (s, 1H), 3.55 (s, 1H); MS (ES+) m/z 537 (M++H), Yield 275 mg (28.8%), m.p. > 250°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-(3-chloro-4-fluoro-phenylcarbamoyl)-2-hydroxy-1-phenyl-ethyl]-amide (51). Rf0.7 (7% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.42 (d, 1H, J = 9.0 Hz), 9.97 (s, 1H), 9.05 (s, 1H), 8.62 (d, 1H, J = 7.7 Hz), 7.96–7.92 (m, 1H), 7.63–7.58 (m, 1H), 7.42–7.22 (m, 6H), 6.49 (d, 1H, J = 5.7 Hz), 5.65–5.62 (m, 1H), 5.29–5.28 (m, 2H), 4.40 (dd, 1H, J = 2.2, 5.7 Hz), 3.54 (t, 1H, J = 2.3 Hz); MS (ES+) m/z 571 (M++H), Yield 270 mg (26.6%), m.p. 202-204°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-benzylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (52). Rf0.3 (10% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.41 (d, 1H, J = 8.6 Hz), 9.09 (s, 1H), 8.57 (d, 1H, J = 7.8 Hz), 8.32 (s, 1H), 7.50–7.24 (m, 5H), 7.05–6.93 (m, 5H), 6.24 (s, 1H), 5.60–5.58 (m, 1H), 5.30 (s, 2H), 4.44–4.39 (m, 1H), 4.26 (s, 1H), 4.08–4.05 (m, 1H), 3.54 (s, 1H); MS (ES+) m/z 533 (M++H), Yield 312 mg (32.9%), m.p.236–238°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(pyridin-2-ylcarbamoyl)-ethyl]-amide (53). Rf0.5 (10% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.46 (d, 1H, J = 9.0 Hz), 9.67 (s, 1H), 9.07 (s, 1H), 8.67 (d, 1H, J = 7.8 Hz), 8.25 (d, 1H, J = 3.7 Hz), 8.04 (d, 1H, J = 8.3 Hz), 7.79–7.38 (m, 1H), 7.43–7.22 (m, 5H), 7.11–7.07 (m, 1H), 6.60 (d, 1H, J = 5.6 Hz), 5.68–5.65 (m, 1H), 5.28–5.27 (m, 2H), 4.48 (dd, 1H, J = 1.9, 5.6 Hz), 3.54 (t, 1H, J = 2.3 Hz); MS (ES+) m/z 542 (M++H), Yield 225 mg (23.3%), m.p. > 250°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(pyridin-3-ylcarbamoyl)-ethyl]-amide (54). Rf0.5 (10% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.42 (d, 1H, J = 8.6 Hz), 9.98 (s, 1H), 9.05 (s, 1H), 8.78 (bs, 1H), 8.62 (d, 1H, J = 7.7 Hz), 8.21–8.22 (m, 1H), 8.05–8.02 (m, 1H), 7.42–7.24 (m, 6H), 6.48 (d, 1H, J = 5.3 Hz), 5.64 (d, 1H, J = 8.6 Hz), 5.27 (s, 2H), 4.42 (d, 1H, J = 5.3 Hz), 3.54 (s, 1H); MS (ES+) m/z 520 (M++H), Yield 298 mg (30.9%), m.p. > 250°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(pyridin-4-ylcarbamoyl)-ethyl]-amide (55). Rf0.6 (10% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.41 (d, 1H, J = 9.0 Hz), 10.10 (s, 1H), 9.04 (s, 1H), 8.62 (d, 1H, J = 7.7 Hz), 8.36 (d, 2H, J = 5.7 Hz), 7.67 (d, 2H, J = 6.2 Hz), 7.42–7.21 (m, 5H), 6.48 (d, 1H, J = 5.7 Hz), 5.66–5.63 (m, 1H), 5.27 (d, 2H, J = 1.8 Hz), 4.43 (dd, 1H, J = 2.1, 5.7 Hz), 3.54 (s, 1H); MS (ES+) m/z 520 (M++H), Yield 237 mg (25.6%), m.p. > 250°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(thiazol-2-ylcarbamoyl)-ethyl]-amide (56). Rf0.5 (10% MeOH/DCM); 1HNMR (CDCl3) δ 10.61 (d, 1H, J = 8.4 Hz), 9.03 (s, 1H), 8.38 (d, 1H, J = 7.2 Hz), 7.41–7.15 (m, 5H), 7.01 (d, 1H, J = 3.5 Hz), 6.80 (d, 1H, J = 3.5 Hz), 6.27 (bs, 1H), 5.82–5.79 (m, 1H), 5.22-5.19 (m, 2H), 4.67 (s, 1H), 2.49–2.48 (m, 1H); MS (ES+) m/z 526 (M++H), Yield 318 mg (34.0%), m.p. > 250°C.
7-Chloro-6-fluoro-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-hydroxy-3-oxo-1-phenyl-3-piperidin-1-yl-propyl)-amide (57). Rf0.7 (10% MeOH/DCM); 1HNMR (CDCl3) δ 10.47 (d, 1H, J = 9.0 Hz), 9.06 (s, 1H), 8.50 (d, 1H, J = 7.2 Hz), 7.51–7.24 (m, 5H), 5.54–5.51 (m, 1H), 5.24–5.08 (m, 2H), 4.73–4.72 (m, 1H), 4.48 (d, 1H, J = 6.0 Hz), 3.61–3.49 (m, 4H), 2.55 (s, 1H), 1.98–1.99 (m, 1H), 1.78–1.44 (m, 5H); MS (ES+) m/z 511 (M++H), Yield 377 mg (41.5%), m.p. 217-219°C.
7-Methyl-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid [2-hydroxy-1-phenyl-2-(pyridin-2-ylcarbamoyl)-ethyl]-amide (58). Rf0.3 (5% MeOH/DCM); 1HNMR (DMSO-d6) δ 10.62 (d, 1H, J = 9.0 Hz), 9.67 (s, 1H), 8.97 (s, 1H), 8.59 (d, 1H, J = 8.1 Hz), 8.24 (d, 1H, J = 4.3 Hz), 8.04 (d, 1H, J = 8.2 Hz), 7.78–7.73 (m, 1H), 7.51 (d, 1H, J = 8.1 Hz), 7.43–7.21 (m, 5H), 7.09–7.05 (m, 1H), 6.56 (d, 1H, J = 5.6 Hz), 5.67–5.64 (m, 1H), 5.33 (s, 2H), 4.48–4.47 (m, 1H), 3.48–3.47 (m, 1H), 2.65 (s, 3H); MS (ES+) m/z 504 (M++H), Yield 457 mg (43.9%), m.p. > 250°C.
2-Hydroxy-3-[(4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8]naphthyridine-3-carbonyl)-amino]-3-phenyl-propionic acid ethyl ester (59). Rf0.6 (7% MeOH/DCM); 1HNMR (CDCl3) δ 10.65 (d, 1H, J = 8.6 Hz), 9.08 (s, 1H), 8.77–8.74 (m, 2H), 7.43–7.19 (m, 6H), 5.66–5.63 (m, 1H), 5.19 (dd, 2H, J = 2.1, 7.4 Hz), 4.47 (d, 1H, J = 2.1 Hz), 4.23-4.16 (m, 2H), 3.44 (d, 1H, J = 5.2 Hz), 2.44 (s, 1H), 1.23 (t, 3H, J = 7.1 Hz); MS (ES+) m/z 420 (M++H), Yield 427 mg (46.4%), m.p. > 250°C.
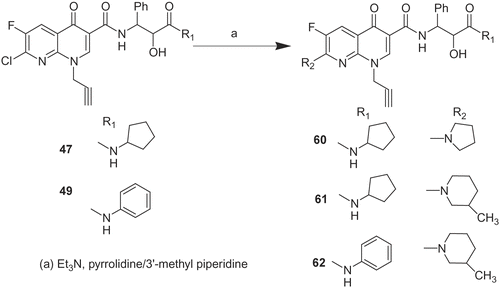
6-Fluoro-4-oxo-1-prop-2-ynyl-7-pyrrolidin-1-yl-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid (2-cyclopentylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (60). To a solution of 47 (500 mg, 0.9 mmol) and triethylamine (290 mg, 2.8 mmol) in acetonitrile (20 mL) was added pyrrolidine (0.10 g, 1.4 mmol). The resulting mixture was refluxed for 3h, concentrated, diluted with water and extracted in dichloromethane (50 mL). The organic layer was dried over sodium sulphate and concentrated to dryness to afford a crude product, which was chromatographed on silica gel (mesh size 100-200) column with 2% MeOH/DCM as eluent to afford compound 60 (0.41 g, 76.9%).
Rf0.4 (8% MeOH/DCM); 1HNMR (CDCl3) δ 11.09 (d, 1H, J = 8.1 Hz), 8.81 (s, 1H), 8.03 (d, 1H, J = 12.8 Hz), 7.47–7.22 (m, 5H), 6.71 (d, 1H, J = 7.8 Hz), 5.61 (dd, 1H, J = 2.6, 8.1 Hz), 5.05-5.04 (m, 2H), 4.50–4.49 (m, 1H), 4.19–4.16 (m, 1H), 3.81–3.80 (m, 4H), 2.45 (t, 1H, J = 2.4 Hz), 2.03-1.24 (m, 12H); MS (ES+) m/z 546 (M++H), Yield 408 mg (76.40%), m.p. > 250°C.
Compounds 61 and 62 were prepared in a similar way to compound 60.
6-Fluoro-7-(3-methyl-piperidin-1-yl)-4-oxo-1-prop-2-ynyl-1,4-dihydro-1,8] naphthyridine-3-carboxylic acid (2-cyclopentylcarbamoyl-2-hydroxy-1-phenyl-ethyl)-amide (61). Rf0.5 (8% MeOH/DCM); 1HNMR (CDCl3) δ 11.03 (d, 1H, J = 8.1 Hz), 8.82 (s, 1H), 8.06 (d, 1H, J = 13.7 Hz), 7.47–7.22 (m, 5H), 6.70 (d, 1H, J = 7.8 Hz), 5.62 (dd, 1H, J = 2.0, 7.8 Hz), 5.02 (s, 2H), 4.49–4.36 (m, 4H), 4.21–4.14 (m, 1H), 3.14–3.06 (m, 1H), 2.82–2.74 (m, 1H), 2.46–2.45 (m, 1H), 1.91–1.23 (m, 13H), 0.97 (d, 3H, J = 6.5 Hz); MS (ES+) m/z 574 (M++H), Yield 497 mg (88.6%), m.p. 177–179°C.
6-Fluoro-7-(3-methyl-piperidin-1-yl)-4-oxo-1-prop-2-ynyl-1,4-dihydro-[1,8] naphthyridine-3-carboxylic acid (2-hydroxy-1-phenyl-2-phenylcarbamoyl-ethyl)-amide (62). Rf0.5 (7% MeOH/DCM); 1HNMR (CDCl3) δ 11.15 (d, 1H, J = 7.5 Hz), 8.80–8.73 (m, 2H), 8.05 (d, 1H, J = 13.7 Hz), 7.55–7.49 (m, 4H), 7.34–7.04 (m, 6H), 5.72 (d, 1H, J = 3.9 Hz), 5.24 (s, 1H), 4.99 (s 2H), 4.69 (s, 1H), 4.45–4.35 (m, 2H), 3.14–3.05 (m, 1H), 2.82-2.74 (m, 1H), 2.44 (s, 1H), 1.92–1.70 (m, 3H), 1.26–1.24 (m, 2H), 0.96 (d, 3H, J = 6.2 Hz); MS (ES+) m/z 582 (M++H), Yield 472 mg (84.3%), m.p. > 250°C.
Cytotoxicity
All the synthesized naphthyridine derivatives (22–62) were tested for in vitro cytotoxicity on eight tumors as well as on two non-tumorous cell lines and IC50 values were calculated in micro molar (μM). The human tumor cell lines used in the study are ovary (PA1), prostate (DU145), oral (KB), colon (SW620), breast (HBL100), lung (A-549), pancreas (MIAPaCa2) and leukemia (K562). All the 1,8-naphthyridine 22–62 and assay standard Doxorubicin HCl (data not shown) were also tested against normal mouse fibroblast (NIH3T3) and normal ovary (CHO) cell line to evaluate their cancer cell specificity (safety index). Derivatives of 1,8-naphthyridine 22-62 were screened for cytotoxic activity at the highest soluble concentration of 10 μM and on four lower concentrations on eight human tumor and two non-tumorous cell lines. Briefly, a three day MTT in vitro cytotoxicity assay was performed, which is based on the principle of uptake of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide), a tetrazolium salt, by the metabolically active cells where it is metabolized by active mitochondria into a blue colored formazan product that is read spectrophotometrically [Citation10]. MTT was dissolved in phosphate buffer saline with a pH of 7.4 to obtain an MTT concentration of 5 mg/mL; the resulting mixture was filtered through a 0.22-micron filter to sterilize and remove a small amount of insoluble residue. For each type of tumor and normal cell, 5000 to 10000 cells were seeded in a 96-well culture plate and incubated with various concentrations of 1,8-naphthyridine derivatives (22–62) in a CO2 incubator for 72 h. Control cells not treated with 1,8-naphthyridine-3-carboxamide derivatives (22–62) were similarly incubated. The assay was terminated after 72 h by adding 125 μg (25 μL) MTT to each well, then incubating for 3 h, and finally adding 50 μL of 10% SDS-0.01N HCl to each well to lyse the cells and dissolve formazan. After incubating for 1 h, the plate was read spectrophotometrically at 540 nm and the cytotoxicity percentage calculated using the following formula: Cytotoxicity percentage = (1-(X/R1)* 100, where X = (absorbance of treated sample at 540 nm)-(absorbance of blank at 540 nm), R1 = absorbance of control sample at 540 nm)-(absorbance of blank at 540 nm). The cytotoxicity data is summarized in and the compounds, which were inactive at 10 μM, are not listed.
Table 3. In vitro cytotoxicity of 1,8-Naphthyridine derivatives (22–62).
Anti-inflammatory activity
1,8-Naphthyridine-3-carboxamide derivatives (22–62) were also able to down regulate the levels of LPS stimulated TNF-α, IL-1β, IP-10 and MIP-1-α secreted by DCs and identified to have potential anti-inflammatory activity. The down regulation of cytokine and chemokine levels by >25% was considered as significant.
BMDC based ex-vivo septic shock assay to evaluate potential anti-inflammatory activity: Primary DC cultures were generated from femoral bone marrow of 8–12 weeks old C57BL/6 mice [Citation11]. Bone marrow progenitors were cultured in RPMI-1640 supplemented with 10% FBS (Hyclone) and rmGMCSF (20 ng/mL) at 37ºC, 5% CO2. Immature DCs were stimulated with lipopolysaccharide (LPS; 10 ng/mL,) and incubated with the naphthyridine carboxamide derivatives at various concentrations ranging from 0.001 to 10 μg/mL, preferably between 0.1 and 1 μg/mL for 24 h. The IL-1-β, TNF-α, MIP-1-α, and IP-10 secreted by the DCs were measured in culture supernatants by Enzyme Linked Immunosorbent Assays (R&D Systems Inc, MN, USA). Percentage change in cytokine/chemokine = {(B-A)/A*100, where B = concentration of cytokine/chemokine (pg/mL) secreted by LPS stimulated DCs when incubated with test molecule, A = concentration of cytokine/chemokine (pg/mL) secreted by LPS stimulated DCs alone. LPS treated DCs were used as positive control.
Results and discussion
1,8-Naphthyridine-3-carboxamide derivatives are divided into three categories based on the substitution pattern at C-6 and C-7 position, unsubstituted: compounds without any substitution at C-6 and C-7 position; monohalo substituted: C-7 chloro substituted; and dihalo substituted: C-6-fluoro-C-7-chloro substituted compounds.
In C-3’ cycloalkyl derived unsubstituted 1,8-naphthyridine derivatives (22–24) were inactive on different cancer cell lines. While, monohalo substituted derivatives (35 and 36) have resulted in improved cytotoxicity compared to unsubstituted derivatives (22–24). The C-3’ cyclohexyl substituted derivative 36 exhibited potent cytotoxicity on ovarian (PA-1) cancer cell line with IC50 of 1.19 μM and safety index of ∼7 against normal ovary (CHO) and ∼4 on NIH3T3 (normal fibroblast) cell lines. The dihalo-substituted 1,8-naphthyridine derivatives (47–48) showed potent to moderate cytotoxicity on ovarian and other cancer cell lines. The cyclopentyl-substituted derivative 47 has showed potent cytotoxicity with IC50 of 0.41, 0.77 and 1.5 μM on pancreas (MIAPaCa), leukemia (K-652) and lung (A549) cancer cell lines, respectively in this series. While, expansion of cycloalkyl ring from cyclopentyl (47) to cyclohexyl (48) leads to slight decrease in cytotoxicity.
In C-3’ aryl substituted 1,8-naphthyridine derivatives (25–27), compound 25 has showed potent cytotoxicity with IC50 of 2.9 μM on prostate (DU-145) cancer cell line. While, the other aryl substituted derivatives with electron withdrawing or donating groups (26 and 27) were inactive. Benzyl substituted derivative 28 has shown no activity. However, in mono halo substituted 1,8-naphthyridine derivatives, compound 38 with electron withdrawing group has showed potent cytotoxicity on oral (KB) cancer cell line with IC50 of 2.6 μM. N’-Benzyl substituted derivative 40 has resulted in selective potent cytotoxicity on prostate (DU145) cancer cell line with IC50 of 1.6 μM. In dihalo substituted 1,8-naphthyridine derivatives (49–51), compound 49 has showed good activity on pancreas (MIAPaCa) and leukemia (K562) cancer cell lines. Benzyl substituted derivative 52 has shown moderate cytotoxicity on pancreas cancer cell line.
In C-3’ heteroaryl substituted 1,8-naphthyridine derivatives, amongst unsubstituted 1,8-naphthyridine derivatives (29–32), only compound 29 has showed potent cytotoxicity with IC50 of 0.41 and 1.4 μM on ovary (PA-1) and colon (SW620) cancer cell lines, respectively. Reversing the position of the nitrogen in pyridine ring from second position leads to complete loss of activity (30 and 31). In mono halo substituted 1,8-naphthyridine derivatives (41–43), improvement in cytotoxicity was observed in 3-amino pyridyl derivative (42) and thiazole (43) substituted derivatives but 2-amino pyridyl derivative (41) has showed very slight activity. In dihalo substituted 1,8-naphthyridine derivatives (53–55), compound 53 has showed broad spectrum of activity with IC50 < 4 μM on five cancer cell lines. While, compounds 54 and 55 have resulted in moderate cytotoxicity.
In C-3’-tertiary amine substituted 1,8-naphthyridine derivatives, unsubstituted compounds (33 and 34) were found to be inactive. Whereas, mono halo substituted derivatives piperidine (44) and morpholino (45) showed selective cytotoxicity on ovary (PA-1) cancer cell line. In dihalo substituted 1,8-naphthyridine derivative, compound 57 has shown potent activity on pancreas (MIAPaCa) cell line with IC50 1.78 μM and modest to low activities on other cell lines. Further we have studied that substitution of C-7 position with group having inductive effect like methyl (58) leads to the complete loss of activity and replacement of the C-3’ amide group by ester (59) linkage caused complete loss of activity.
As dihalo substituted 1,8-naphthyridine derivatives 47 and 49 have shown potent cytotoxicity, the C-7 chloro group of 47 and 49 was replaced with different secondary amine as shown in . But it leads to complete loss of activity (60–62). This indicates that C-7 halo group is essential for the activity.
The overall results indicated that halo substituted 1,8-naphthyridine derivatives with five and six membered cycloalkyl ring substituent have shown maximum cytotoxicity. Halo substituted compound 47 has shown IC50 of 0.41 and 0.77 μM on MIAPaCa and K-562 cancer cell lines, respectively. While, compound 36 has resulted in IC50 of 1.19 μM on PA-1 cancer cell line. However, one of the unsubstituted 1,8-naphthyridine 3’-heteroaryl derivative 29 has showed potent cytotoxicity with IC50 of 0.41 and 1.4 μM on ovary (PA-1) and colon (SW620) cancer cell lines, respectively. Replacement of C-7 halo group with secondary amine leads to loss of the activity.
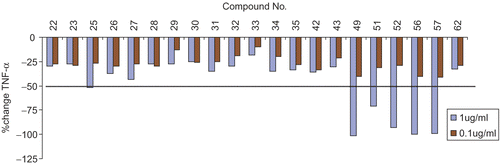
shows the down regulation of TNF-α (primary mediator of tissue damage and pain in inflammatory disorders) by selected 1,8-naphthyridine-3-carboxamide derivatives. Compounds 49, 51, 52, 56 and 57 exhibited a very high TNF-α inhibition at 1 μg/mL. demonstrates IC50 value for TNF-α inhibition by selected molecules screened at various concentrations ranging from 0.001 to 10 μg/mL.
Table 4. IC50 values for TNF-α modulation by selected 1,8-naphthyridine carboxamide derivatives. Molecules were subjected to screening over a multiple dose concentration range of 0.001 μg/mL to 10 μg /mL.
Compounds showing high TNF-α down regulation 49, 51, 52 and 57 were also found to be potent inhibitors of IL-1-β secretion by LPS-stimulated DCs ().
Inhibition of MIP-1-α and IP-10 (pro-inflammatory chemokines) activity is suggestive of anti-inflammatory activity of 1,8-naphthyridine-3-carboxamide derivatives. Compounds 44, 45, 49, 50, 53 and 55 showed >50% down regulation of MIP-1-a in addition to TNF-a and IL-1-b inhibition (). Compounds 45, 48, 54, 55 and 56 have demonstrated high IP-10 inhibitory activity as shown in & .
Figure 3. IL-1-β modulation of selected molecules. Dotted line shows IC50 (concentration at which 50% inhibition occurs).
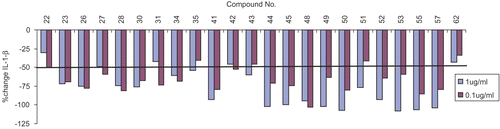
Figure 4. MIP-1-α modulation of selected molecules. Dotted line shows IC50 (concentration at which 50% inhibition occurs).
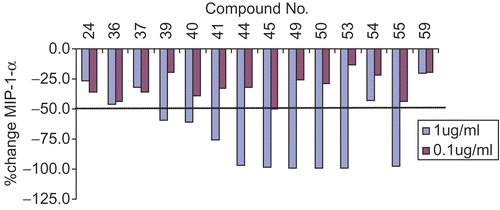
Figure 5. IP-10 modulation of selected molecules. Dotted line shows IC50 (concentration at which 50% inhibition occurs).
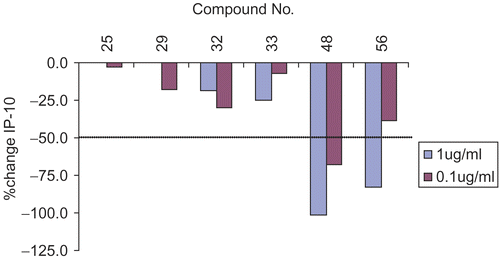
Table 5. Downregulation of IP-10 levels to 50% (IC50) of selected 1,8-naphthyridine derivatives. Molecules were subjected to screening over a multiple dose concentration range of 0.001 μg/mL to 10 μg/mL.
Compounds 45 and 55 were able to induce remarkable down regulation of TNF-α, IL-1-β, MIP-1-α and IP-10 activity and hence were found to be most active anti-inflammatory compounds among 1,8-naphthyridine-3-carboxamide derivatives.
Acknowledgment
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- FDA news Drug Pipeline Alert, March 21, (2006), 4, No. 56.
- Burden DA, Osheroff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta 1998; 1400:139–154.
- Tomita K, Tsuzuki Y, Shibamori K, Tashima M, Kajikawa F, Sato Y, Kashimoto S, Chiba K, Hino K. Synthesis and structure-activity relationships of novel 7-substituted 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acids as antitumor agents. Part 1. J Med Chem (2002); 45(25): 5564–5575.
- Tsuzuki Y, Tomita K, Shibamori K, Sato Y, Kashimoto S, Chiba K. Synthesis and structure-activity relationships of novel 7-substituted 1,4-dihydro-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acids as antitumor agents. Part 2. J Med Chem (2004); 47(8): 2097–2109.
- Kumar V, Mudgal, MM, Rani N, Jha A, Jaggi M, Singh AT, Sanna VK, Singh P, Sharma PK, Irchhaiya R, Burman AC. Synthesis of functionalized amino acid derivatives as new pharmacophores for designing anticancer agents. J Enz Inhib Med Chem (2008) -In press.
- Burden DA, Oshereroff N. Mechanism of action of eukaryotic topoisomerase II and drugs targeted to the enzyme. Biochim Biophys Acta (1998); 1400:139–154.
- Coussens LM, Werb Z. Inflammation and cancer. Nature (2002); 420:860–867.
- Grossi G, Di Braccio M, Roma G, Ballabeni V, Tognolini M, Barocelli E. 1,8-Naphthyridines v. novel N-substituted 5-amino-N,N-diethyl-9-isopropyl [1,2,4]triazolo[4,3-a] [1,8]naphthyridine-6-carboxamides, as potent anti-inflammatory and/or analgesic agents completely devoid of acute gastrolesivity. Eur J Med Chem (2005); 40: 155–165.
- Dianzani C, Collino M, Gallicchio M, Di Braccio M, Roma G, Fantozzi R. Effects of anti-inflammatory [1, 2, 4]triazolo[4, 3-a] [1, 8]naphthyridine derivatives on human stimulated PMN and endothelial cells: an in vitro study. J Inflamm (2006); 3: 4–14.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth (1983); 65: 55–63.
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Meth (1999); 223: 77–92.