Abstract
This paper reports the synthesis of O-methyl and O-ethyl NSAID hydroxamic acids, their antimicrobial activities, and their ability to inhibit urease and soybean lipoxygenase activities. Ibuprofen and fenoprofen hydroxamic acids with free hydroxy groups present the highest antimicrobial activity, while indomethacin and diclofenac analogs show significantly lower antimicrobial activity. Diclofenac hydroxamic acid 4e exerts the highest anti-urease activity. Indomethacin O-ethyl hydroxamic acid 3h and ibuprofen O-benzyl hydroxamic acid 4b exert significant inhibitory activities on soybean lipoxygenase. Fenoprofen and indomethacin O-ethyl hydroxamic acids 3b and 3h and diclofenac and indomethacin O-benzyl analogs 4g and 4i highly inhibit lipid peroxidation. The highest antioxidant activity was shown by fenoprofen derivative 3b.
| Abbreviations: | ||
| AAPH, | = | 2,2′-azobis(2-amidinopropane) dihydrochloride |
| DPPH, | = | 1,1-diphenyl-2-picrylhydrazyl radical |
| LO, | = | lipoxygenase |
| LP, | = | lipid peroxidation |
| MIC, | = | minimal inhibitory concentration |
| MMcC, | = | minimal microbicidal concentration |
| NDGA, | = | nordihydriguaiaretic acid |
| NFC, | = | norfloxacin |
| NSAID, | = | nonsteroidal anti-inflammatory drug |
| NST, | = | nystatin |
| OTC, | = | oxytetracycline hydrochloride |
| ROS, | = | reactive oxygen species |
Introduction
Hydroxamic acid derivatives of nonsteroidal anti-inflammatory drugs (NSAIDs) are efficient anti-inflammatory drugs, and some of them are registered internationallyCitation1–4. In general, they present lower acute toxicity and a favorable therapeutic index, and they are less damaging to the gastrointestinal tract, less irritating, and more permeable through the topical membrane than NSAIDs from which they are derivedCitation5,Citation6. In this article we report the synthesis of O-methyl and O-ethyl NSAID hydroxamic acids (derivatives of ibuprofen, fenoprofen, ketoprofen, diclofenac, and indomethacin) and their antimicrobial activity as well as their ability to inhibit soybean lipoxygenase. The suggested structural variations could affect both efficiency and their tolerability, partly due to differences in their physicochemical properties, which determine their distribution in the body and their ability to pass through and to enter the interior of the membranesCitation7,Citation8.
Hydroxamic acids are well known to form strong complexes with a variety of transition metals. This property has been exploited in the use of hydroxamates as inhibitors of several metalloenzymes. Since it is generally believed that lipoxygenase (LO) contains a catalytically important iron atom, this enzyme is a logical candidate for inhibition by hydroxamic acid derivativesCitation9. Hydroxamic acids have been reported as highly specific and potent inhibitors of bacterial, jack bean, and sword bean ureaseCitation10–12. Urease inhibitors have been regarded as targets for the treatment of ulcer, urolithiasis, pyelonephritis, ammonia and hepatic encephalopathy, hepatic coma, and urinary catheter encrustationCitation10. In the present study, the potential of NSAID hydroxamic acid derivatives to inhibit urease is reported as well.
Reactive oxygen species (ROS) are characteristic of aerobic organisms that can normally defend themselves against these highly reactive species using enzymes and naturally occurring antioxidants. ROS, like superoxide radical anion, hydrogen peroxide, and hydroxyl radical, are produced during the inflammation process by phagocytic leukocytes (e.g. neutrophils, monocytes, macrophages, eosinophils) that invade the tissue. Moreover, these reactive species are involved in the biosynthesis of prostaglandins and in the cycloxygenase and lipoxygenase mediated conversion of arachidonic acid into proinflammatory intermediatesCitation13,Citation14. The rates of ROS production are increased in most pathophysiological conditionsCitation15; therefore, it is evident that the treatment of various diseases could benefit from the use of drugs that combine antioxidant and anti-inflammatory activity. This has been already proven for a number of commercially available NSAIDs, which act either as inhibitors of free radical production or as radical scavengersCitation16–18. Compounds with antioxidant properties could be expected to offer protection in rheumatoid arthritis and inflammation and to lead to potentially effective drugsCitation15,Citation19–25. Thus, we tested the new derivatives with regard to their antioxidant ability and in comparison to well known antioxidant agents.
Materials and methods
Chemistry
General experimental details
Melting points were determined on a Stuart melting point apparatus SMP3 (Stuart Barworld Scientific, UK) and were uncorrected. Infrared (IR) spectra were recorded on an FTIR PerkinElmer Paragon 500 spectrometer (PerkinElmer, UK). 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded on a BruAvanse DRX 500, DRX 300 (Bruker, Germany), operating at 300 and 75.5 MHz for the 1H and 13C nuclei, respectively. Samples were measured in dimethyl sulfoxide (DMSO)-δ6 solutions at 20°C in 5-mm NMR tubes. Chemical shifts (δ) in ppm were referred to tetramethylsilane (TMS). Coupling constants (J) in Hz were observed through three bonds. For thin layer chromatography (TLC), silica gel plates Kieselgel 60 F254 (Merck, Germany) and mixtures of cyclohexane/ethyl acetate/methanol (3:1:0.6) and chloroform/methanol (95:5) were used. Spots were visualized by short-wave UV light, iodine vapor, or Fe(III) chloride solution (w = 1%).
Benzotriazole, triphosgene, O-methylhydroxylamine hydrochloride, O-ethylhydroxylamine hydrochloride, and O-benzylhydroxylamine hydrochloride were purchased from Aldrich (USA), triethylamine from Sigma (USA), and hydroxylamine hydrochloride from Carlo Erba (Italy). Ibuprofen, fenoprofen, ketoprofen, diclofenac, and indomethacin were obtained as gift samples from PLIVA (Zagreb, Croatia), Belupo (Koprivnica, Croatia), and the University of Potchefstroom (South Africa). All solvents were of analytical grade purity and were dried prior to use.
NSAID benzotriazolides 2a–e were prepared following the method previously described by usCitation26,Citation27. N-hydroxy-2-(4-isobutylphenyl) propanamide (4a), N-benzyloxy-2-(4-isobutylphenyl) propanamide (4b), N-hydroxy-2-(3-phenoxyphenyl)propanamide (4c), N-benzyloxy-2-(3-phenoxyphenyl)propanamide (4d), 2- [(2,6-dichlorophenyl)amino]-N-hydroxybenzeneacetamide (4e), 2-[(2,6-dichlorophenyl)amino]-N-hydroxy-N-methylbenzeneacetamide (4f), N-benzyloxy-2-[(2,6-dichlorophenyl)amino]-benzeneacetamide (4g), 1-(4-chlorobenzoyl)-N-hydroxy-5-methoxy-2-methyl-1H-indole-3-acetamide (4h), and N-benzyloxy-1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indole-3-acetamide (4i) were prepared following the method previously described by usCitation28.
General method for the preparation of hydroxamic acid derivatives (3a–h)
A suspension of NSAID benzotriazolide 2a–e (0.0010 mol), appropriate hydroxylamine hydrochloride (0.0012 mol), triethylamine (TEA) (0.0050 mol), and sodium dithionite (10 mg) in toluene (10 mL) was stirred at room temperature for 6.5–48 h. Compounds 3a–f: the reaction mixture was extracted four times with water, and the organic layer was dried (Na2SO4), filtered, and evaporated. Compounds 3g and 3h: the synthesis was done with 1.3 equivalents of TEA (0.0013 mol). The reaction mixture was evaporated. The residue was dissolved in acetone/H2O and acidified with 5% HCl to pH 1. Acetone was evaporated under reduced pressure without heating. The precipitated product was filtered off and washed several times with water.
The following compounds were prepared: N-ethoxy-2-(4-isobutylphenyl)propanamide (3a), N-ethoxy-2-(3-phenoxyphenyl)propanamide (3b), N-ethoxy-2-(3-benzoylphenyl)propanamide (3c), N-benzyloxy-2-(3-benzoylphenyl)propanamide (3d), N-methoxy-2-[2-(2,6-dichlorophenyl)amino]benzene-acetamide (3e), N-ethoxy-2-[2-(2,6-dichlorophenyl)amino]benzeneacetamide (3f), 1-(4-chlorobenzoyl)-2-methyl-N-methoxy-5-methoxy-1H-indol-3-acetamide (3g), and N-ethoxy-1-(4-chlorobenzoyl)-2-methyl-5-methoxy-1H-indol-3-acetamide (3h).
N-hydroxy-2-(3-benzylphenyl)propanamide (3i) After three vacuum/H2 cycles to remove air from the reaction flask, the suspension of 3d (0.0010 mol) and 10% Pd/C (50 mg) in methanol (20 mL) was hydrogenated at ambient pressure and room temperature for 2.3 h. The reaction mixture was filtered, and the filtrate evaporated under reduced pressure.
Biological evaluation
General experimental details
Microbial species (Bacillus subtilis ATCC6633, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Kocuria rhizophila ATCC 9341, Escherichia coli ATCC 10536, Salmonella enterica subsp. enterica ATCC 13076, Pseudomonas aeruginosa ATCC 27853, and Candida albicans ATCC 10231) used in the study were obtained from the American Type Culture Collection, LGC Promochem (UK). Trypticasa soy agar and Müller–Hinton agar were purchased from Merck (Germany), Sabouraud 2% (m/V)-glucose agar from BBL (Germany), oxytetracycline hydrochloride (OTC) and nystatin (NST) from Pliva (Croatia), and norfloxacin (NFC) from Krka (Slovenia).
Urease (5 U mg−1 derived from jack bean) was obtained from Merck. Phenol red was bought from Kemika (Croatia) and used as a solution (1 g L−1 phenol red in 96% ethanol). The control buffer of pH 7.70 was prepared by diluting a mixture of 1 M KH2PO4 (0.1 mL) and 1 M Na2HPO4 (0.7 mL) with H2O to 80 mL. Buffer of pH 6.70 was prepared by diluting a mixture of 1 M KH2PO4 (1 mL) and 1 M Na2HPO4 (1 mL) with H2O to 100 mL. The buffered urea solution of pH 6.70 was obtained by dissolving urea (3 g) in a buffer of pH 6.70 (100 mL).
DPPH, AAPH, NDGA, sodium linoleate, soybean lipoxygenase, caffeic acid, and trolox were purchaced from Aldrich-Sigma (USA).
Each experiment in vitro was performed at least in triplicate and the standard deviation of absorbance was less than 10% of the mean.
Antimicrobial activity
Before testing, the microbial strains were removed from the culture collection in nutrient agar, and subcultured twice on trypticasa soy agar for bacterial species, and on Sabouraud 2% (m/V)-glucose agar for yeast species during 24 h at 37°C. All inoculums from microbial culture used for antimicrobial susceptibility testing were from freshly 24 h-prepared culture in physiological saline. Müller–Hinton agar was used as nutritional medium for zones of inhibition detections. OTC, NST, and NFC were used as a control of susceptibility of microbial cultures by hole-plate diffusion method. Stock solutions of antibiotics were prepared in buffer solutions according to European Pharmacopoeia guidelinesCitation29. At the highest concentration of min. 99%, solvent (DMSO) did not show antimicrobial activity.
Zones of inhibition were determined in vitro using the hole-plate diffusion method according to European Pharmacopoeia guidelinesCitation29. A solution of the tested substance in DMSO (50 μL) was dropped in a hole, and incubated first for 1 h at +4°C, then 18 h at 37°C. DMSO was used as a control. For determination of the minimal inhibitory (MIC) and the minimal microbicidal concentrations (MMcC), the microdilution broth method in 96-well microtiter plates (TTG, Switzerland) using Clinical and Laboratory Standards Institute M7-A4 guidelines and Müller–Hinton broth was employedCitation30. The inoculum was prepared with physiological saline to obtain approximately 0.5 McFarland units density of the microbial cells. MIC and MMcC endpoints were determined after the incubation period (24 h), and after the subcultivation of all dilutions to the surface of the Müller–Hinton agar. After 24 h of incubation, MIC endpoints were determined as the lowest concentration causing growth of ≤20% of the control level, and MMcC as the lowest concentration with no visual growth.
Antiurease activity
The antiurease activity was determined by a modified colorimetric timing methodCitation31,Citation32. Phenol red solution (25 μL) was added to DMSO (50 μL) diluted with the control buffer (175 μL), referred to as C solution, and to sample solution (tested compound in DMSO; 50 μL) diluted with the buffered solution of pH 6.70 (175 μL), referred to as S solution. After the addition of the urease solution (460 units in 25 mL H2O; 250 μL), both C and S solutions were pre-incubated at 30°C for 30 min. The C solution was diluted with the control buffer (2.5 mL) and absorbance of the resulting solution was measured at 560 nm. The S solution was diluted with buffered urea solution of pH 6.70 (2.5 mL). A time interval for the absorbance of the S solution to reach the measured absorbance of the C solution was measured at 560 nm by using a stop-watch. Each sample was measured in triplicate.
Interaction with 1,1-diphenyl-picrylhydrazyl (DPPH) activityCitation33
To a solution of DPPH (0.05 mM) in absolute ethanol an equal volume of 0.1 or 0.05 mM ethanolic solution of the tested compound was added. After 20 and 60 min the absorbance was recorded at 517 nm and compared with the appropriate standard NDGA. Ethanol was used as a control.
Soybean lipoxygenase inhibition activityCitation33
DMSO solution of the tested compound was incubated with sodium linoleate (0.1 mM) and 0.2 mL of soybean lipoxygenase solution (1/9 × 10−4 w/v in saline) at room temperature. The conversion of sodium linoleate to 13-hydroperoxylinoleic acid was recorded at 234 nm and compared with the standard inhibitor caffeic acid, according to the procedure previously reported.
Inhibition of linoleic acid lipid peroxidationCitation34
Oxidation of linoleic acid to conjugated diene hydroperoxide in an aqueous dispersion was monitored at 234 nm. AAPH was used as a free radical initiator. Ten microliters of the 16 mM linoleic acid dispersion was added to the UV cuvette containing 0.93 mL of 0.05 M phosphate buffer, pH 7.4, prethermostated at 37°C. The oxidation reaction was initiated at 37°C under air by the addition of 50 μL of 40 mM AAPH solution. Oxidation was carried out in the presence of the compound (10 μL, final concentration 0.1 mM). In the assay with no antioxidant, lipid oxidation was measured in the presence of the same level of DMSO. The rate of oxidation was monitored at 37°C by recording the increase of absorption at 234 nm caused by conjugated diene hydroperoxide. The results were compared to the standard inhibitor trolox.
Determination of lipophilicity as Clog P
Lipophilicity was theoretically calculated as Clog P value in n-octanol-buffer using the CLOGP Program of Biobyte Corp.Citation35.
Results and discussion
Chemistry
NSAID hydroxamic acid derivatives 3a–h and 4a–i were prepared from NSAID benzotriazolides 2a–e (NSAIDs: ibuprofen, fenoprofen, ketoprofen, diclofenac, and indomethacin) and corresponding hydroxyl-amine (hydroxylamine, N-methylhydroxylamine, O-benzylhydroxylamine, O-methylhydroxylamine, and O-ethylhydroxylamine) following our published procedureCitation28. Product 3i was obtained by catalytic hydrogenation of ketoprofen O-benzylhydroxamic acid (3d). depicts the general method for the conversion of NSAIDs to hydroxamic acid derivatives. Compounds from series 3 are new compounds (except 3e and 3g) and their structures were deduced from analysis of their IR and 1H and 13C NMR spectra and confirmed by elemental analysis ( and ). Compounds 4a–i are known substances and their synthesis has been previously described by us or other authors.
Table 1. Reaction conditions and analytical data for compounds 3a–i.
Table 2. Spectroscopic data and atom enumeration for compounds 3a–i.
O-alkyl substituted NSAID hydroxamic acid derivatives 3a–h were tested for antimicrobial activity and for their ability to inhibit urease, lipoxygenase, and lipid peroxidation. Antioxidant activity was screened by 1,1-diphenyl-2-picrylhydrazyl radical (DPPH) test. Their activity was compared with that of NSAID hydroxamic acids 4a–i and 3i with free and O-benzyl substituted hydroxy groups.
Antimicrobial activity
The results of the hole-plate diffusion method showed that only NSAID hydroxamic acids with free and O-ethyl substituted hydroxy groups possessed noticeable antimicrobial activity at concentration 29.6 mg mL−1 (data presented in ). The other compounds showed no inhibition zones of growth, and were considered inactive in the concentration used, probably due to their poor solubility in Müller–Hinton agar (precipitation of the product; data not shown). The ibuprofen and fenoprofen derivatives 4a and 4c showed a broad spectrum of antimicrobial activity against gram-positive and gram-negative bacterial species tested. Compounds 4e and 4h (diclofenac and indomethacin hydroxamic acids) showed noticeable bactericidal activity only against E. faecalis, while compound 3i showed activity against S. aureus, E. coli, S. enterica subsp. enterica, and P. aeruginosa. O-ethyl NSAID-hydroxamic acids 3a–c were active against B. subtilis, while 3a showed noticeable activity against K. rhizophila and P. aeruginosa. Only compound 3c exhibited antifungal activity against C. albicans.
Table 3. Antimicrobial activity of NSAID hydroxamic acid derivatives 3 and 4 determined by hole-plate diffusion method.
The minimal inhibitory (MIC) and the minimal microbicidal concentrations (MMcC) were determined by the microdilution broth method (). Compound 4a showed the same MIC/MMcC value as 4c against B. subtilis (0.116 and 0.232 mg mL−1, respectively) and S. enterica subsp. enterica species. On the other hand, 4a showed lower MIC/MMcC values against E. coli, while 4c was more active against S. aureus, E. faecalis, K. rhizophila, and P. aeruginosa. Product 3i showed only weak activity against S. aureus, while 3a and 3b showed activity against K. rhizophila. In general, antimicrobial activity of the tested compounds was weak.
Table 4. Minimal inhibitory (MIC) and minimal microbicidal concentrations (MMcC) of NSAID hydroxamic acid derivatives 3 and 4 determined by microdilution broth methoda.
Antiurease activity
Antiurease activities of NSAID hydroxamic acids 3 and 4 against jack bean urease were determined by the modified colorimetric timing method described by Van Slyke and ArchibaldCitation31 and by Quan et al.Citation32. Although there is a significant difference in inhibition of H. pylori urease and jack bean ureaseCitation12, jack bean urease was used in our research because H. pylori urease is rather difficult to obtain. The percentage of inhibition (%) for each sample was calculated according to the following equation:
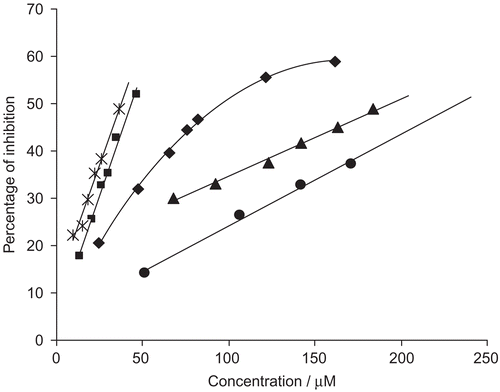
where t is a time interval (s) measured at each molar sample concentration, and t0 is a time interval (s) measured at zero molar sample concentration. The following IC50 values (concentration of the compound given in μM required to inhibit urease activity by 50%) were obtained: 3i (193.8), 4a (97.4), 4c (43.2), 4e (37.4), and 4h (233.3), respectively. All other compounds were inactive. The results showed that only NSAID hydroxamic acids bearing a free hydroxy group inhibited urease activity. The highest inhibition was observed for diclofenac derivative 4e, while its N-methyl and O-alkyl derivatives were inactive. Dose-dependent inhibition of jack bean urease is presented in .
Antioxidant activity
The interaction of the examined compounds with the stable free radical DPPH was studied. Interaction with DPPH indicates radical scavenging ability in an iron-free system. In general, it seems that compounds interact with DPPH in a concentration dependent manner. Interactions were monitored after 20 and 60 min at two concentrations of the compound (0.05 and 0.1 mM). The results are presented in . No significant changes are observed with time with the exception of analogs 3a, 3d, 3f, 3i, 4b, 4d. Perusal of percentage values at 0.05 mM shows that compounds 3b and 3i are very potent (77 and 58%). Compounds 3b and 3i are the most active in the 0.1 mM assay followed by 3d > 3a > 4g > 3h > 4b. No role for the lipophilicity of the whole molecules is defined. On the contrary, the lipophilic contribution π of substituents seems to be important. Thus, lower π values are correlated with higher reducing abilities at 0.1 mM, e.g. 3a (76% ) > 4b (63%) (π –CH2CH3 = 1.02, π –C6H5CH2 = 2.01), 3b (86%) > 4d (63%) (π –CH2CH3 = 1.02, π –C6H5CH2 = 2.01).
Table 5. Interaction with DPPH, in vitro inhibition of soybean lipoxygenase (LO), lipid peroxidation (LP), and theoretically calculated Clog P values.
Inhibition of linoleic acid lipid peroxidation
Azo compounds generating free radicals through spontaneous thermal decomposition are useful for free radical production studies in vitro. The water soluble azo compound 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) has been extensively used as a clean and controllable source of thermally produced alkylperoxyl free radicals. In our studies AAPH was used as a free radical initiator to follow oxidative changes of linoleic acid to conjugated diene hydroperoxide. The results indicate that compounds 4i, 4g, 3h, and 3b are excellent inhibitors of lipid peroxidation ().
Soybean lipoxygenase inhibition
Compounds were further evaluated for inhibition of soybean lipoxygenase (LO) by the UV absorbance based enzyme assayCitation36. Lipoxygenases oxidize certain fatty acids at specific positions to hydroperoxides, precursors of leukotrienes, which contain a conjugated triene structure. It is known that soybean lipoxygenase, which converts linoleic to 13-hydroperoxylinoleic acid, is inhibited by NSAIDs and the previously described NSAID hydroxamic acids 3e and 3g4 in a qualitatively similar way to that of the rat mast cell lipoxygenase, and may be used in a reliable screen for such activity. Perusal of percentage inhibition values or IC50 values shows that compound 3h is the most active (IC50 = 82 μM) within the set, followed by compounds 4b and 4d ().
Most of the LO inhibitors are antioxidants or free radical scavengers, since lipoxygenation occurs via a carbon-centered radical. Although lipophilicity is referred to as an important physicochemical property for LO inhibitorsCitation37–39, all the above tested derivatives do not follow this concept with the exception of compound 4b, with a very high lipophilicity value (7.03). Our results indicate that lipophilicity of the molecules increases the biological response substantially. For compounds 3h, 3c, 4b, and 4g the LO IC50 values proceed in parallel to the percentage inhibitory values of lipid peroxidation. That means that their LO inhibitory activity is supported by lipid peroxidation inhibition.
Conclusions
The NSAID hydroxamic acids prepared in this study showed significant biological activity. In general, antimicrobial activity of the tested compounds was weak. Only NSAID hydroxamic acids with free and O-ethyl substituted hydroxy groups possessed noticeable antimicrobial activity, but at rather high concentrations. The reducing abilities of the tested compounds and their ability to scavenge free radicals were determined using the stable radical DPPH at 0.05 and 0.1 mM concentrations after 20–60 min. The results ranged from 8 to 77% and 20 to 93%, respectively. The highest activity was shown by compound 3b. Inhibitory activities against soybean lipoxygenase were measured as well. The in vitro tests at several concentrations showed that O-ethyl and O-benzyl hydroxamic acids were very potent inhibitors of soybean lipoxygenase and lipid peroxidation. These compounds exerted much higher inhibition on lipoxygenase compared to caffeic acid, and better inhibitory activity of lipid peroxidation compared to trolox. These results indicate that their lipophilicity increased their biological response substantially. Based on these findings they could be considered as potential antioxidant/anti-inflammatory drugs.
Acknowledgements
Support for this study by the Ministry of Science, Education and Sports of the Republic of Croatia (Project No. 006- 0000000-3216) as well as the National Employment and Development Agency (grant 14V09809) is gratefully acknowledged. The authors would like to thank Professor Tihana žanić-Grubišić for help with antiurease activity testing and Dr. C. Hansch and Biobyte Corp., 201 West 4th Str., Suite 204, Claremont, CA 91711, USA for free access to the C-QSAR program.
Declaration of interest: The authors report no conflicts of interest.
References
- Kleemann EA, Engel J, Kutscher B, Reichert D. Pharmaceutical Substances: Syntheses, Patents, Applications. Stuttgart: Thieme, 2001.
- Muri EMF, Nieto MJ, Sindelar RD, Williamson JS. Hydroxamic acids as pharmacological agents. Curr Med Chem 2002;9:1631–53.
- Boznar B, Zmitek J, Kocjan D. 2-(4-Isobutylphenyl)propiohydroxamic acid. BE 830,825 1974 [Chem Abstr 1976;85:62817k]; EP 0 203 379 A1 1986 [Chem Abstr 1987;106:84185g ].
- Cetenko WA, Connor DT, Flynn DL, Sircar JC, (Warner-Lambert Company). Preparation of hydroxyindeneacetamide, and hydroxyindoleacetamide derivatives of selected nonsteroidal antiinflammatory acyl residues having cyclooxygenase, and 5-lipoxygenase inhibition activity. US 4,943,587 1990 [Chem Abstr 1990;112:216688j ].
- Orzalesi G, Selleri R, Caldini O, Volpato I, Innocenti F, Colome J, Sacristan A, Varez G. Ibuproxam and ibuprofen, a pharmacological comparison. Arzneimittelforschung 1977;27:1006–12.
- Sloan KB, Little R. Mannich-base hydroxamic acid prodrugs for the improved delivery of nonsteroidal antiinflammatory agents and a pharmaceutical composition containing them. EP 39051 A2 1981 [Chem Abstr 1982;96:104087g ].
- Brooks APM, Day RO. Variability in response to NSAIDs. Fact or fiction? N Engl J Med 1988;1716–25.
- Shanbhag VR, Crider AM, Gohkale R, Harpalani A, Dick RM. Ester and amide prodrugs of ibuprofen and naproxen: synthesis, anti-inflammatory activity, and gastrointestinal toxicity. J Pharm Sci 1992;81:149–54.
- Corey EJ, Cashmin JR, Kanther SS, Wright SW. Antioxidant and anti-inflammatory activity of aryl-acetic and hydroxamic acids as novel lipoxygenase inhibitors. J Am Chem Soc 1984;106:1503–5.
- Amtul Z, Rahman AU, Siddiqui RA, Choudhary MI. Chemistry and mechanism of urease inhibition. Curr Med Chem 2002;9:1323–48.
- Odake S, Morikava T, Tsuchiya M, Imamura L, Kobashi K. Inhibition of Helicobacter pylori urease activity by hydroxamic acid derivatives. Biol Pharm Bull 1994;17:1329–32.
- Muri EMF, Mishra H, Steinand SM, Williamson JS. Molecular modeling, synthesis and biological evaluation of heterocyclic hydroxamic acids designed as Helicobacter pylori urease inhibitors. Lett Drug Des Disc 2004;1:30–4.
- Garrido G, Gonzalez D, Delporte C, Backhouse N, Quintero G, Nunez-Selles AJ, Morales MA. Analgesic and antiinflammatory effects of Mangifera indica L. extract (Vimang). Phytother Res 2001;15:18–21.
- Weber V, Rubat C, Duroux E, Lartigue C, Madesclaire M, Coudert P. New 3- and 4-hydroxyfuranones as antioxidants and antiinflammatory agents. Bioorg Med Chem 2005;13:4552–64.
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine, 2nd edn. Oxford: Clarendon, 1989.
- Orhan H, Sahin G. In vitro effects of NSAIDs and paracetamol on oxidative stress-related parameters of human erythrocytes. Exp Toxicol Pathol 2001;53:133–40.
- Saldan LA, Elias G, Gao MNA. Oxygen radical scavenging activity of phenylbutenones and their correlation with anti-inflammatory activity. Arzneimittelforschung 1990;40:89–91.
- Kontogiorgis C, Litinas KE, Makri A, Nicolaides DN, Vronteli A, Hadjipavlou-Litina DJ, Pontiki E, Siohou A. Synthesis and biological evaluation of novel angular fused pyrrolocoumarins. J Enzyme Inhib Med Chem 2008;23:43–9.
- Burguete A, Pontiki E, Hadjipavlou-Litina D, Villar R, Vicente E, Solano B, Ancizu S, Pérez-Silanes S, Aldana I, Monge A. Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole analogs. Bioorg Med Chem Lett 2007;17:6439–43.
- Michaelidou A, Hadjipavlou-Litina D, Matsini I, Tsitsogianni E. Heterocyclic aryl(phenyl)acetic acid and aryl acetohydroxamic acids as antiinflammatory–antioxidant agents and inhibitors of lipoxygenase and serine proteases. Med Chem 2007;3:439–45.
- Molvi KI, Mansuri M, Sudarsanam V, Patel, MM, Andrabi SMA, Haque N. Synthesis, anti-inflammatory, analgesic and antioxidant activities of some tetrasubstituted thiophenes. J Enzyme Inhib Med Chem 2008;23:829–38.
- Maharvi GM, Ali S, Riaz N, Afza N, Malik A, Ashraf M, Iqbal L, Lateef M. Mild and efficient synthesis of new tetraketones as lipoxygenase inhibitors and antioxidants. J Enzyme Inhib Med Chem 2008;23:62–9.
- Suzen S, Gurkok G, Coban T. Novel N-acyl dehydroalanine derivatives as antioxidants: studies on rat liver lipid peroxidation levels and DPPH free radical scavenging activity. J Enzyme Inhib Med Chem 2006;21:179–85.
- Gacche N, Dhole NA, Kamble SG, Bandgar BP. In-vitro evaluation of selected chalcones for antioxidant activity. J Enzyme Inhib Med Chem 2007;23:28–31.
- Kontogiorgis CA, Savvoglou K, Hadjipavlou-Litina DJ. Antiinflammatory and antioxidant evaluation of novel coumarin derivatives. J Enzyme Inhib Med Chem 2006;21:21–9.
- Zorc B, Antolić S, Butula I. Macromolecular prodrugs. I. Synthesis of some non-steroidal anti-inflammatory drug esters. Acta Pharm 1993;43:127–33.
- Zorc B, Butula I. Macromolecular prodrugs. III. Esters of fenoprofen and probenecid. Acta Pharm 1994;44:103–8.
- Rajić Z, Butula I, Zorc B, Kraljević Pavelić S, Hock K, Pavelić K, Naesens L, De Clercq E, Balzarini J, Przyborowska M, Ossowski T, Mintas M. Cytostatic and antiviral activity evaluations of NSAID hydroxamic acids: a direct implication of JNK in ap optosis induction of HeLa cells. Eur J Med Chem 2009 (in press).
- European Pharmacopoeia, 5th edn. Strasbourg: Council of Europe, 2006.
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th edn. Approved Standard, NCCLS document M7-A4. Wayne, PA: National Committee for Clinical Laboratory Standards, 1997.
- Van Slyke DD, Archibald RM. Manometric, titrimetric and colorimetric methods for measurment of urease activity. J Biol Chem 1944;154:623–42.
- Quan HJ, Koyanagi J, Ohmori K, Uesato S, Tsuchido T, Saito S. Preparation of heterospirostanols and their pharmacological activities. Eur J Med Chem 2002;37:659–69.
- Pontiki E, Hadjipavlou-Litina D. Synthesis and pharmacochemical evaluation of novel aryl-acetic acid inhibitors of lipoxygenase, antioxidants, and anti-inflammatory agents. Bioorg Med Chem 2007;15:5819–27.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999;26:1231–7.
- Biobyte Corp. C-QSAR Database. Claremont, CA: Biobyte Corp.
- Taraborewala RB, Kauffman JM. Synthesis and structure-activity relationships of anti-inflammatory 9,10-dihydro-9-oxo-2-acridine-alkanoic acids and 4-(2-carboxyphenyl)aminobenzenealkanoic acids. J Pharm Sci 1990;79:173–8.
- Pontiki E, Hadjipavlou-Litina D. Quantitative-structure activity relationships on lipoxygenase inhibitors. Internet Electron J Mol Des 2002;1:134–41.
- Pontiki E, Hadjipavlou-Litina D. Review in quantitative structure activity relationships on lipoxygenase inhibitors. Mini Rev Med Chem 2003;3:487–99.
- Pontiki E, Hadjipavlou-Litina D. Quantitative structure activity relationships (QSARs) on lipoxygenase inhibitors. Curr Med Chem Anti-Inflammatory Anti-Allergy Agents 2004;3:139–56.