Abstract
Kukoamine A (KukA) is a spermine (SPM) conjugate with dihydrocaffeic acid (DHCA), with interesting biological activities. The four possible regioisomers of KukA, as well as a series of KukA analogs incorporating changes in either the SPM or the DHCA structural units, were evaluated for their antioxidant activity and their inhibitory activity on soybean lipoxygenase (LOX) and lipid peroxidation. The reducing properties of the compounds were evaluated using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging assay and found to be in the range 5–97.5%. KukA significantly inhibits LOX with IC50 9.5 μM. All tested analogs inhibited lipid peroxidation in the range of 11–100%. The most potent compounds KukA and its analog 3, in which the DHCA units had been replaced by O,O9-dimethylcaffeic acid units, were studied for their anti-inflammatory activity in vivo on rat paw edema induced by carrageenan and found to be of comparable activity to indomethacin. The results of the biological tests are discussed in terms of structural characteristics.
Introduction
Caffeic acid (CA), dihydrocaffeic acid (DHCA), and their analogs are potent natural antioxidants with multiple mechanisms involving free radical scavenging, metal ion chelation, and inhibitory actions on specific enzymes that induce free radical and lipid hydroperoxide formationCitation1,Citation2. Therefore, their antioxidative actions could prevent oxidative rancidity in foods and oxidative damage in vivo, relating to diseases such as cancer, diabetes, and cardiovascular, Alzheimer’s, and Parkinson’s diseases.
Spermine (SPM) is a natural antioxidant, which is found in all living organisms. Research shows that SPM has an important function in those areas of the body that are exposed to a high level of oxygen usage. This pertains to the skin, the brain, sperm cells, and the lungs in particular. Spermine’s role is that of protecting cells against free radicals and of their destructive effectsCitation3.
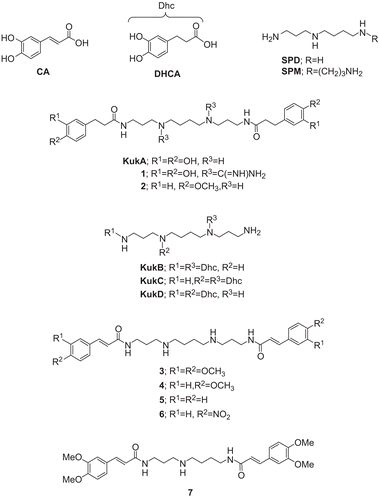
Today, there is increased interest in the combination of two pharmacophores on the same scaffold. This procedure leads to hybrid molecules or conjugates, which are usually more effective than their individual components. Kukoamines A (KukA) and B (KukB) () are SPM alkaloids, actually SPM conjugates with DHCA, which have been isolated from the medicinal plant Lycium chinenseCitation4,Citation5. More recently, alkaloids of the kukoamine type, e.g. KukA, were identified in a range of solanaceous species, including potatoCitation6. KukA shows hypotensive activityCitation4, and is a potent and selective inhibitor of trypanothione reductase (TryR)Citation7, a crucial enzyme for the survival of pathogenic trypanosomatid parasites from oxidative stress.
There is an increasing interest in antioxidants, particularly in those intended to prevent the presumed deleterious effects of free radicals in the human body, and to prevent the deterioration of fats and other constituents of foodstuffs. In both cases, there is a preference for antioxidants from natural rather than from synthetic sources. In an effort to identify the structural characteristics of the kukoamine alkaloids responsible for their antioxidant activity, we have synthesized all four possible regioisomers of kukoamine A, namely KukA–D ()Citation8, as well as a series of KukA analogsCitation9,Citation10, examples of which (compounds 1–7) are presented in . We now considered it of interest to examine the possible lipoxygenase (LOX) activity of KukA and its analogs as presented in as well as their potential to inhibit lipid peroxidation of biological membranes and to act as anti- inflammatory agents. Through these compounds, we could hopefully determine the effect of the following structural elements: (a) the site of attachment of the DHCA units on the polyamine skeleton (KukA–D), (b) the replacement of the phenolic hydroxyl functions by H, OMe, or NO2 groups (analogs 2–6), (c) the replacement of the DHCA units by caffeic acid (CA) units (e.g. analogs 3–6), (d) the use of polyamines with shorter chains, e.g. spermidine (SPD) as exemplified with analog 7, and (e) the replacement of the secondary amino functions in SPM with the strongly basic and better binding guanidyl functions (analog 1), on the biological activity.
Materials and methods
Materials
All the chemicals used were of analytical grade and commercially available from Merck. 1,1-Diphenyl-2- picrylhydrazyl (DPPH) and nordihydroguairetic acid (NDGA) were purchased from Aldrich Chemical Co. (Milwaukee, WI, USA). Soybean lipoxygenase, linoleic acid sodium salt, and indomethacin were obtained from Sigma Chemical Co. (St.Louis, MO, USA) and carrageenan, type K, was commercially available. For the in vivo experiments, male and female Fischer-344 rats (180–240 g) were used.
KukA and B are known compoundsCitation4,Citation5 and KukC and D and KukA analogs 1–7 have already been describedCitation8–10. For the purpose of the present biological studies, all these compounds were prepared as follows. KukA was obtained as its corresponding dihydrochloride salt using the methodology presented in reference 10. KukB–D were obtained as their corresponding dihydrochloride salts according to the methods described in reference 8. KukA analog 1 was obtained as the corresponding bistrifluoroacetate salt according to the method described in reference 9. KukA analogs 3–6 were obtained as their corresponding bishydroxysuccinimidate salts according to the methodo-logy described in reference 10. Finally, the methodology described in reference 10 was also employed for the preparation of KukA analogs 2 and 7.
Physicochemical studies
Determination of lipophilicity as Clog P values
Since lipophilicity is a significant physicochemical property determining distribution, bioavailability, metabolic activity, and elimination, we tried to calculate theoretically the lipophilicity values of KukA and its analogs as Clog P values in n-octanol-buffer using the CLOGP Program of Biobyte Corp.Citation11.
Biological assays
In vitro assays
For the in vitro tests a Lambda 20 (PerkinElmer) UV–Vis double beam spectrophotometer was used. Each in vitro experiment was performed at least in triplicate and the standard deviation of absorbance was less than 10% of the mean.
In this investigation all compounds were studied in order to gain insight into their biological response. The KukA analogs and the parent molecules CA and DHCA were studied with regard to their antioxidant ability as well as their inhibition of soybean lipoxygenase.
Determination of reducing activity of the stable radical 1,1-diphenyl-picrylhydrazyl (DPPH)Citation12
To an ethanolic solution of DPPH (0.05 mM) in absolute ethanol an equal volume of compound dissolved in dimethyl sulfoxide (DMSO) was added. The mixture was shaken vigorously and allowed to stand for 20 min or 60 min; absorbance at 517 nm was determined spectrophotometrically and the percentage of reducing activity (RA %) was calculated. All tests were undertaken on three replicates and the results presented in were averaged.
Table 1. Interaction percent with DPPH (RA %), in vitro inhibition of soybean lipoxygenase (LOX) (IC50), inhibition percent of lipid peroxidation (AAPH %), and inhibition percent of carrageenan-induced rat paw edema (ICPE %) by KukA and analogs.
Soybean lipoxygenase inhibition study in vitroCitation12
The tested compounds dissolved in DMSO were incubated at room temperature with sodium linoleate (0.1 mL) and 0.2 mL of enzyme solution (1/9 × 10−4 w/v in saline) in Tris buffer pH 9. The conversion of sodium linoleate to 13-hydroperoxylinoleic acid at 234 nm was recorded and compared with the appropriate standard inhibitor.
Inhibition of linoleic acid lipid peroxidationCitation13
Production of conjugated diene hydroperoxide by oxidation of linoleic acid in an aqueous dispersion was monitored at 234 nm. 2,29-Azobis(2-amidinopropane) dihydrochloride (AAPH) was used as a free radical initiator. Ten microliters of the 16 mM linoleic acid dispersion was added to the UV cuvette containing 0.93 mL of 0.05 M phosphate buffer, pH 7.4, prethermostated at 37°C. The oxidation reaction was initiated at 37°C under air by the addition of 50 μL of 40 mM AAPH solution. Oxidation was carried out in the presence of aliquots (10 μL) of KukA and its analogs. In the assay without antioxidant, lipid oxidation was measured in the presence of the same level of DMSO. The rate of oxidation at 37°C was monitored by recording the increase in absorption at 234 nm caused by conjugated diene hydroperoxides.
In vivo assays
Inhibition of carrageenan-induced edemaCitation12
Edema was induced in the right hind paw of Fischer-344 rats (150–200 g) by the intradermal injection of 0.1 mL 2% carrageenan in water. Both sexes were used. Female pregnant rats were excluded. Each group was composed of 6–15 animals. The animals, which had been bred in our laboratory, were housed under standard conditions and received a diet of commercial food pellets and water ad libitum during the maintenance but they were entirely fasted during the experimental period. Our studies were in accordance with recognized guidelines on animal experimentation. The tested compounds (0.01 mmol/kg body weight) were suspended in water, with a few drops of Tween 80, having been ground in a mortar before use, and then they were given intraperitoneally, simultaneously with the carrageenan injection. The rats were euthanized 3.5 h after carrageenin injection. The difference between the weights of the injected and uninjected paws was calculated for each animal. The change in paw weight was compared with that in control animals (in which edema was also induced by the intradermal injection of 0.1 mL 2% carrageenan in water) and expressed as a percent inhibition of the carrageenan-induced paw edema (ICPE %) (). Indomethacin was tested as a reference compound at 0.01 mmol//kg (47%).
The value of ICPE % is the mean from two different experiments with a standard error of the mean less than 10%.
Results and discussion
In this work, we evaluated in vitro and in vivo a series of KukA analogs that were expected to offer protection against inflammation and radical attack.
Antioxidants are defined as substances that even at low concentration significantly delay or prevent oxidation of easily oxidizable substrates. There has been increased interest in using antioxidants for medical purposes in recent years. It is well known that free radicals play an important role in the inflammatory processCitation14. Many non-steroidal anti-inflammatory drugs have been reported to act either as inhibitors of free radical production or as radical scavengersCitation15. Consequently, compounds with antioxidant properties could be expected to offer protection in rheumatoid arthritis and inflammation and lead to potentially effective drugs. Thus, we tested the KukA analogs with regard to their antioxidant ability and in comparison to well known antioxidant agents, e.g. nordihydroguaiaretic acid (NDGA), caffeic acid (CA), and trolox.
Antioxidant potential of KukA and analogs
For estimating the antioxidative potential of chemical components, different experimental approaches are usedCitation16. Most of them require a spectrophotometric measurement and a certain reaction time in order to obtain reproducible resultsCitation17. The use of DPPH for a radical scavenging measuring method is employed. DPPH is a stable free radical in a methanolic solution. In its oxidized form, the DPPH radical has an absorbance maximum centered at about 517 nmCitation18. The DPPH method is described as a simple, rapid, and convenient method independent of sample polarity for screening of many samples for radical scavenging activityCitation19. These advantages make the DPPH method interesting for testing our analogs.
The interaction of the examined compounds with the stable free radical DPPH is shown in . This interaction indicates their radical scavenging ability in an iron-free system. With the exception of KukC, SPM, SPD, 2, and 7, all the other compounds highly interacted with DPPH at 0.1 mM. The combinations of DHCA with SPM (molar ratio 2:1) and DHCA with SPD (molar ratio 2:1) presented low interaction values (12 and 7%, respectively). These results support the idea that the interaction activity is correlated with the overall structure of the synthesized conjugates. The interaction remains highly constant. No changes are observed after 60 min. It is evident that the presence of the phenolic hydroxyl groups and the amino groups of the polyamine skeleton are correlated with higher values. The replacement of the phenolic hydroxyl groups by H, OMe, or NO2 groups leads to lower values. The site of attachment of the DHCA units on the polyamine skeleton in KukA–D influences their reducing ability. Thus, KukA and B present very high interaction values which disappeared in KukC and decreased in KukD (). The replacement of the DHCA unit (analog 2) by the CA moiety (analog 4) increases the interaction value. Analog 7 with a shorter polyamine chain (SPD) is correlated with very low antioxidant activity compared to analog 3 incorporating the SPM chain. Finally, the replacement of the secondary amino groups by guanidyl groups in the polyamine chain does not seem to influence reducing ability.
It seems that hydrophilicity (low lipophilicity values) partly affects the reducing ability. In particular, KukA and B and analog 1 with the highest reducing ability are compounds characterized by low lipophilicity (according to their theoretical calculated Clog P values) whereas the analogs 3, 4, 5, and 6 with medium reducing ability (51–61%) are compounds characterized by higher lipophilicity.
LOX inhibition by KukA and analogs
Leukotrienes play an important role as mediators of a variety of inflammatory and allergic reactions and are derived from the biotransformation of arachidonic acid catalyzed by lipoxygenase (LOX). Lipoxygenases play a role in membrane lipid peroxidation by forming hydroperoxides in the lipid bilayerCitation20,Citation21. Inhibitors of LOX have attracted attention initially as potential agents for the treatment of inflammatory and allergic diseases, but their therapeutic potential has now been expanded to certain types of cancer and cardiovascular diseasesCitation22,Citation23.
Natural polyamines have been shown to inhibit dioxygenase activity of soybean lipoxygenase-1. The inhibitory power was dependent on the number of basic groups in the molecule, in the order SPM > SPD > cadaverine > putrescineCitation21. Both SPD and SPM acted as noncompetitive inhibitors of lipoxygenase-1 with respect to linoleic acid. The inhibitory power apparently correlated with the radical-trapping ability of the polyamines. SPD and SPM also inhibited the co-oxidase and peroxidase activities of lipoxygenase-1Citation21. In this context, we decided to further evaluate the synthesized KukA analogs for inhibition of soybean lipoxygenase (LOX) by the UV absorbance based enzyme assayCitation24 (). Most of the LOX inhibitors are antioxidants or free radical scavengersCitation25, since lipoxygenation occurs via a carbon-centered radical. Some studies suggest a relationship between LOX inhibition and the ability of the inhibitors to reduce Fe3+ at the active site to the catalytically inactive Fe2+. LOXs contain a “non-heme” iron per molecule in the enzyme active site as high-spin Fe2+ in the native state and high-spin Fe3+ in the activated state. Several LOX inhibitors are excellent ligands for Fe3+. This inhibition is related to their ability to reduce the iron species in the active site to the catalytically inactive ferrous formCitation25.
Furthermore, it has been shown that polyamines may inhibit lipid peroxidation by chelating iron, thus impairing the iron-mediated generation of free radicalsCitation26. Other lines of evidence suggest that the radical scavenging abilityCitation27 and the antioxidant powerCitation21 of polyamines might be important in the inhibition of lipid peroxidation. Perusal of the IC50 or percent inhibition values () shows that KukA is the most active (9.5 μM) within the set, followed by compounds 3 and 4. It is therefore apparent that the highest inhibitory activity is secured by SPM molecules bisacylated at their terminal amino functions by DHCA and to a lesser degree by CA analogs substituted by one or two methoxy groups. Although lipophilicity is referred toCitation28 as an important physicochemical property for LOX inhibitors, all the above tested derivatives do not follow this concept. Thus, KukA had an IC50 value of 9.5 μM (Clog P = −1.79) whereas analog 4 had an IC50 value of 72 μM (Clog P = 2.35). Under our experimental conditions and at 0.1 mM, SPM and SPD do not inhibit LOX. The combinations of DHCA with SPM (molar ratio 2:1) and DHCA with SPD (molar ratio 2:1) presented 5.4 and 30% inhibition, respectively.
Inhibition of lipid peroxidation by KukA and analogs
In this investigation, all compounds were studied in order to identify their possible inhibitory activity on lipid peroxidation. Azo compounds, generating free radicals through spontaneous thermal decomposition, are useful for free radical production studies in vitro. The water soluble azo compound AAPH has been extensively used as a clean and controllable source of thermally produced alkylperoxyl free radicals. In our studies, AAPH was used as a free radical initiator to follow oxidative changes of linoleic acid to conjugated diene hydroperoxide. Compounds KukA, 3–5, and 7 showed excellent inhibition of lipid peroxidation. Thus, of the four kukoamine isomers, KukA is by far the most potent inhibitor of lipid peroxidation. On the other hand, conjugates of either SPM or SPD (analog 7) with CA analogs unsubstituted, or incorporating one or two electron donating groups (e.g. methoxy) in the ring, provided the most potent inhibitors (92–100% inhibition) in this set of compounds. SPM and SPD did not inhibit the lipid peroxidation under our experimental conditions. However, the combinations of DHCA with SPM (molar ratio 2:1) and DHCA with SPD (molar ratio 2:1) presented high inhibitory activity (72.4 and 88.5%, respectively). Lipophilicity is the main physicochemical parameter influencing their interaction.
In vivo anti-inflammatory activity of KukA and analog 3
KukA and analog 3 were selected to be examined in vivo using the functional model of carrageenin-induced rat paw edema, on the basis that these compounds both highly inhibited LOX and presented high in vitro antioxidant activities. Carrageenan-induced edema is a non-specific inflammation resulting from a complex of diverse mediatorsCitation29. As shown in , both compounds induced equipotent inhibition against rat paw edema, which is very close to the effect produced by the commonly used standard, namely indomethacin.
Conclusions
A series of KukA isomers and analogs were tested for their antioxidant activity.
The highest activity was shown by KukA, KukB, and KukA analog 1. KukA showed the best inhibitory activity on LOX followed by KukA analogs 3 and 4. These compounds showed much higher inhibition of LOX compared to caffeic acid and better inhibitory activity on lipid peroxidation compared to trolox. KukA and KukA analog 3 with the most promising biological profile also showed very good anti-inflammatory activity in vivo compared to indomethacin.
In general, the presence of the hydrophilic SPM skeleton, the increased conformational freedom in the DHCA moiety (replacement of the rigid trans-CH=CH in CA by the CH2CH2 function in DHCA), and the presence of free phenolic hydroxyl groups seem to increase substantially the antioxidant properties and the ability to inhibit LOX and lipid peroxidation as well as show strong anti-inflammatory activity. However, inhibition of lipid peroxidation seems not to be dependent on the length of the polyamine chain, the absence of free phenolic hydroxyl groups, and the degree of unsaturation of the CA side-chain.
Acknowledgements
We would like to thank Dr C. Hansch and Biobyte Corp., 201 West 4th Str., Suite 204, Claremont, CA 91711, USA for free access to the C-QSAR program.
A part of this research was presented at the 13th Panhellenic Symposium of Pharmacochemistry.
Declaration of interest: The authors report no conflicts of interest.
References
- Sugiura M, Naito Y, Yamaura Y, Fukaya C, Yokoyama K. Inhibitory activities and inhibition specificities of caffeic acid derivatives and related compounds toward 5-lipoxygenase. Chem Pharm Bull 1989;37:1039–43.
- Michaluart P, Masferrer JL, Carothers AM, Subbharamaiah K, Zweifel BS, Koboldt C, Mestre JR, Grunberger D, Sacks PG, Tanabe T, Dannenberg AJ. Inhibitory effects of caffeic acid phenethyl ester on the activity and expression of cyclooxygenase-2 in human oral epithelial cells and in a rat model of inflammation. Cancer Res 1999;59:2347–252.
- Farbiszewski R, Bielawska A, Szymanska M, Skrzydlewska E. Spermine partially normalizes in vivo antioxidant defense potential in certain brain regions in transiently hypoperfused rat Brain. Neur Res 1996;21:1497–503.
- Funayama S, Yoshida K, Konno C, Hikino H. Structure of kukoamine A, a hypotensive principle of Lycium chinense root barks. Tetrahedron Lett 1980;21:1355–6.
- Funayama S, Zhang GR, Nozoe S. Kukoamine B, a spermine alkaloid from Lycium chinense. Phytochemistry 1995;38:1529–31.
- Parr AJ, Mellon FA, Colquoun IJ, Davies HV. Dihydrocaffeoyl polyamines (kukoamine A and allies) in potato (Solanum tuberosum) tubers detected during metabolite profiling. J Agric Food Chem 2005;53:5461–6.
- Ponasik JA, Strickland C, Faerman C, Savvides S, Karplus PA, Ganem B. Kukoamine A and other hydrophilic acylpolyamines: potent and selective inhibitors of Crithidia fasciculata trypanothione reductase. Biochem J 1995;311:371–5.
- Karigiannis G, Mamos P, Balayiannis G, Katsoulis I, Papaioannou D. Simple fragment synthesis of all four isomers of the spermine alkaloid kukoamine. Tetrahedron Lett 1998;39:5117–20.
- Athanassopoulos CM, Garnelis T, Pantazaka E, Papaioannou D. Efficient guanylation of Nα,Nω-difunctionalized polyamines at the secondary amino functions. Tetrahedron Lett 2004;45:8815–18.
- Garnelis T, Athanassopoulos CM, Papaioannou D, Eggleston IM, Fairlamb AH. Very short and efficient syntheses of the spermine alkaloid kukoamine A and analogs using isolable succinimidyl cinnamates. Chem Lett 2005;34:264–5.
- Biobyte Corp. C-QSAR Database. Claremont, CA: Biobyte Corp.
- Kontogiorgis C, Hadjipavlou-Litina D. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidants agents. J Enzyme Inhib Med Chem 2003;18:63–9.
- Liegois C, Lermusieau G, Colin S. Measuring antioxidant efficiency of wort, malt, and hops against the 2,2-azobis(2-amidinopropane) dihydrochloride-induced oxidation of an aqueous dispersion of linoleic acid. J Agric Food Chem 2000;48:1129–34.
- Flohe L, Beckman R, Giertz H, Loschen G. Oxygen-centered free radicals as mediators of inflammation. In: Sies H, ed. Oxidative Stress. London: Academic Press, 1985:403–35.
- Saldanha LA, Elias G, Gao MNA. Oxygen radical scavenging activity of phenylbutenones and their correlation with antiinflammatory activity. Arzneimittelforschung 1990;40:89–91.
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 2005;53:4290–303.
- Kulisic T, Radonic A, Katalinic V, Milos M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem 2004;85:633–40.
- Molyneux P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 2004;26:211–19.
- Koleva II, van Beek TA, Linssen JPH, de Groot A, Evstatieva LN. Screening of plant extracts for antioxidant activity: a comparative study on three testing methods. Phytochem Anal 2001;13:8–17.
- Kuhn H, Belkner J, Wiesner R, Brash AR. Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. J Biol Chem 1990;265:18351–61.
- Maccarrone M, Baroni A, Finazzi-Agro A. Natural polyamines inhibit soybean (Glycine max) lipoxygenase-1, but not the lipoxygenase-2 isozyme. Arch Biochem Biophys 1998;356:35–40.
- Shureiqi I, Lippman SM. Lipoxygenase modulation to reverse carcinogenesis. Cancer Res 2001;61:6307–12.
- Zhao L, Funk CD. Lipoxygenase pathways in atherogenesis. Trends Cardiovasc Med 2004;14:191–5.
- Taraborewala RB, Kauffman JM. Synthesis and structure-activity relationships of anti-inflammatory 9,10-dihydro-9-oxo-2-acridine-alkanoic acids and 4-(2-carboxyphenyl)aminobenzenealkanoic acids. J Pharm Sci 1990;79:173–8.
- Muller K. 5-Lipoxygenase and 12-lipoxygenase: attractive targets for the development of novel antipsoriatic drugs. Arch Pharm 1994;327:3–19.
- Tadolini B. Polyamine inhibition of lipoperoxidation. The influence of polyamines on iron oxidation in the presence of compounds mimicking phospholipid polar heads. Biochem J 1988; 249:33–6.
- Fujisawa S, Kadoma Y. Kinetic evaluation of polyamines as radical scavengers Anticancer Res 2005;25:965–9.
- Pontiki E, Hadjipavlou-Litina D. Lipoxygenase inhibitors: a comparative QSAR study review and evaluation of new QSARs. Med Res Rev 2008;28:39–117.
- Shen TY. Non steroidal anti-inflammatory agents. In: Wolf ME, ed. Burger’s Medicinal Chemistry. New York: John Wiley and Sons, 1980:1217–19.