Abstract
Tyrosinase is a multi-copper enzyme which is widely distributed in different organisms and plays an important role in the melanogenesis and enzymatic browning. Therefore, its inhibitors can be attractive in cosmetics and medicinal industries as depigmentation agents and also in food and agriculture industries as antibrowning compounds. For this purpose, many natural, semi-synthetic and synthetic inhibitors have been developed by different screening methods to date. This review has focused on the tyrosinase inhibitors discovered from all sources and biochemically characterised in the last four decades.
Introduction
Browning of fruits, fungi and vegetables and hyperpigmentation in human skin are two common undesirable phenomena. Tyrosinase is the main enzyme recognised as responsible for this enzymatic browning and melanogenesis in mammalsCitation1,Citation2. This encouraged researchers and scientists to focus on the identification, isolation, synthesis and characterisation of new potent tyrosinase inhibitors for various application in the foodCitation3, cosmeticsCitation4 and medicinal industries. However, very few inhibitors are qualified for clinical use and skin-whitening agents. Moreover, as the clinical and industrial demands for tyrosinase inhibitors increase, in vitro assays and improved screening techniques are also undergoing rapid development for in vitro high-throughput screening tyrosinase inhibitors and putative skin-whitening agentsCitation5. In other words, sensitive and correct assay methods for screening and development of effective tyrosinase inhibitors are of great importance. For this purpose, several spectrophotometricCitation6–10, chromatographicCitation11–17, electrophoreticCitation18–22, radiometricCitation23,Citation24 and electrochemicalCitation25–27 assays have been applied and developed by researchers so far. Recently, a novel fluorescent biosensorCitation28 and tyrosinase-based thin-layer chromatography-autography have been suggested for tyrosinase inhibitor screeningCitation29.
Additionally, further improvements of in vitro detection methods for rapidly screening tyrosinase inhibitors may be achieved through using virtual screeningCitation30 and construction of quantitative structure–activity relationship (QSAR) models of inhibitorsCitation31,Citation32. Thus, a combination of bioinformatics simulation and biological in vitro analysis will be useful to understand the functional mechanisms of the tested compoundsCitation9,Citation21,Citation27,Citation33–48. Lately, Gao et al. have performed a virtual screening from Traditional Chinese medicine (TCM) and predicted tyrosinase inhibition by 3 D QSAR pharmacophore modelsCitation49. For more information about successful utilisation of computational tools like QSAR-based and ligand-based virtual screening, a review published by Khan in 2012 organised and summarised novel and potent inhibitors of the enzymeCitation50. Furthermore, with regard to tyrosinase inhibition importance, several other reviews have presented the organisation of tyrosinase inhibitors from natural, semi- and full synthetic sourcesCitation1,51–62.
The present review also focuses on the tyrosinase inhibitors discovered from all sources, including synthetic compounds, extracts and active ingredients of natural products, virtual screening and structure-based molecular docking studies published in the last four decades. We hope that the knowledge offered in this review serves as an updated comprehensive database contributing to the development of new safe and efficient anti-tyrosinase agents for the prevention of browning in plant-derived foods, seafood and hyperpigmentation treatments.
The role of tyrosinase in the melanin biosynthesis
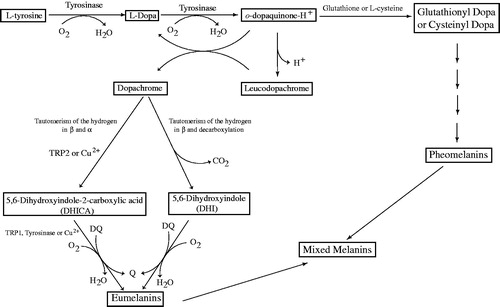
Melanins, the main pigment primarily responsible in the skin, hair and eyes pigmentation of human, are produced by melanocytes through melanogenesis. Melanogenesis and skin pigmentation are the most important photoprotective factor in response to ultraviolet radiation damaging from the sun and skin photo-carcinogenesis. The abnormal loss of melanin and depigmentation can be a serious facial esthetic and dermatological problem among humanCitation63. On the contrary, the increased melanin synthesis and accumulation of these pigments occur in many types of skin disorders, including Acanthosis nigricans, Cervical Poikiloderma, melasma, Periorbital hyperpigmentation, Lentigines, neuro-degeneration associated with Parkinson’s disease and skin cancer riskCitation64–66. Although melanogenesis is a complicated process represented by numerous enzymatic and chemical reactions, the enzymes such as tyrosinase and other tyrosinase-related proteins (TYRP1 and TYRP2) have a critical role in melanin synthesis. Tyrosinase is a multifunctional copper-containing metalloenzyme with dinuclear copper ions, which plays as a rate-limiting enzyme in the synthesis of melanin ()Citation52,Citation67. Also, tyrosinase constitutes the primary cause for undesired browning of fruits and vegetables as well as diseases resulting from overproduction of melanin. Therefore, controlling the activity of enzyme by tyrosinase inhibitors is an essential endeavor for treating hypopigmentary disorders of mammals and enzymatic browning of fruits and fungi. To date, numerous effective inhibitors are identified and developed for using in the medical and cosmetic products, as well as food bioprocessing and agricultural industries and environmental industries. However, in medicine, tyrosinase inhibitors are a class of important clinical antimelanoma drugs but only a few compounds are known to serve as effective and safe tyrosinase inhibitors.
Figure 1 Scheme of the biosynthetic pathway of eumelanins and pheomelanins. The activities of tyrosinase are indicated in the scheme. Moreover, the enzyme can oxidize DHICA to its o-quinone directly, or it can oxidize DHICA and DHI indirectly via the formation of o-dopaquinone. TRP2 (dopachrome tautomerase) or Cu2+ can participate in the evolution of dopachrome to DHICA. The oxidation of DHICA can be catalyzed by TRP1, (DHICA oxidase), tyrosinase or Cu2+. When glutathione or L-cysteine attack o-dopaquinone, glutathione-dopa or cysteinyl-dopa adducts are formed and these later evolve to pheomelanins Citation67.
Mushroom tyrosinase properties
Tyrosinases have been isolated and purified from different sources such as some plants, animals and microorganisms. Although many of them (such as human) have been sequenced, only few of them have been characterised. Recently, a novel tyrosinase produced by Sahara soil actinobacteria have been isolated and biochemically charactrised with the aim to identify novel enzymes with exclusive features for biotechnological applicationsCitation68–80. However, among different sources of tyrosinase, mushroom tyrosinase from Agaricus bisporus is a major and cheap source of tyrosinase with high similarity and homology compared to human tyrosinaseCitation78. Because of these good properties, the structural, functional and biochemical characteristics of mushroom tyrosinase have been studied extensively as a model system for screening of tyrosinase inhibitors and melanogenic studies, enzyme-catalysed reactions and enzyme-inhibitor structural studies so farCitation78,Citation8Citation1–90. Tyrosinase from Agaricus bisporus is a 120 kDa tetramer with two different subunits, heavy and lightCitation91, which was the first isolated by Bourquelot and BertrandCitation92 in 1895. It has three domains and two copper binding sites which bind to six histidine residues and interact with molecular oxygen in the tyrosinase active site. Also, a disulfide linkage stabilise its structureCitation93. Recently, a 50 kDa tyrosinase isoform from Agaricus bisporus (H-subunit) have been purified with a high specific tyrosinase activity of more than 38,000 U/mgCitation94.
Reaction mechanism
Tyrosinase (EC 1.14.18.1) has two activities in its catalytic cycle, see Citation95,Citation96, a monophenolase activity where it hydroxylates monophenols (e.g l-tyrosine) to o-diphenols (e.g. l-dopa) and a diphenolase activity where tyrosinase oxidises o-diphenols to o-quinones (o-dopaquinone). At the same time of these enzymatic reactions, there are different chemical reactions coupled where two molecules of o-dopaquinone react their-selves generating an o-diphenol molecule (L-dopa) and a dopachrome molecule.
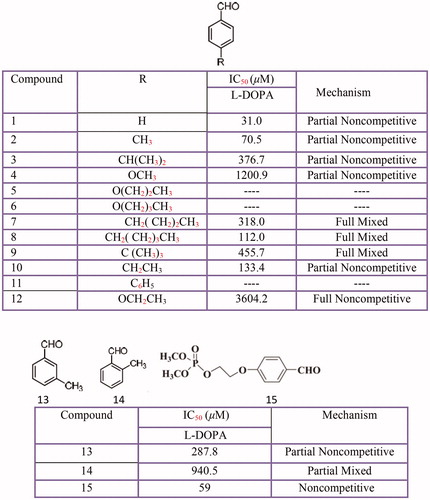
Figure 2 Monophenolase and diphenolase activities of Tyrosinase. EmM, met‐tyrosinase/monophenol complex; M, monophenol; D, o-diphenol; Em, met‐tyrosinase; EmD, met‐tyrosinase/o‐diphenol complex; Ed, deoxy‐tyrosinase; O2, molecular oxygen; Eox, oxy‐tyrosinase; EoxD, oxy‐tyrosinase/o‐diphenol complex; EoxM, oxy‐tyrosinase/monophenol complex; Q, o-quinone; Cr, Dopachrome.
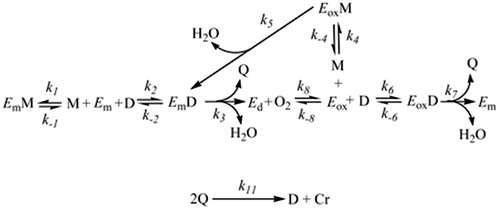
Diphenolase activity can be independently studied, when tyrosinase reacts with an o-diphenol (see ). The form met-tyrosinase (Em) binds the o-diphenol (D) originating the complex EmD. This complex oxidises the o-diphenols transforming it to o-quinone and the enzyme is converted into the form deoxy-tyrosinase (Ed). Ed has a very big affinity for the molecular oxygen originating the form oxy-tyrosinase (Eox), which binds another o-diphenol molecule and originating the complex EoxD. After that, the o-diphenol is oxidised again to o-quinone and the form Em is formed again completing the catalytic cycle. However, after these enzymatic reactions, two o-quinone molecules (e.g. o-dopaquinone) react generating dopachrome and regenerating a molecule of o-diphenol.
As mentioned before, we can independently study the diphenolase activity. However, it is not applicable for the monophenolase activity, see , because the chemical reactions of diphenolase activity have to occur at the same time of monophenolase activity. Tyrosinase shows the monophenolase activity with a lag period. This period is the time that the enzyme requires to accumulate a quantity of o-diphenol in reaction medium and is proportional to the quantity of monophenol used. shows the new complexes appeared in the monophenolase activity: EoxM (oxy-tyrosinase bound to monophenol) and EmM (met-tyrosinase bound to monophenols). EoxM is active and is transformed into EmD, which is an intermediate of the catalytic cycleCitation95. o-Quinones formed by these two oxidation cycle spontaneously react with each other to form oligomersCitation97.
Tyrosinase inhibition
Due to the critical role of tyrosinase in the melanogenesis and browning process, several investigations have been reported for the identification of tyrosinase inhibitor from both natural (fungi, bacteria, plants) and synthetic sources so far. General speaking, tyrosinase inhibitors are examined in the presence of a monophenolic substrate such as tyrosine or a diphenolic substrate such as l-dopa, and activity is assessed based on dopachrome formation.
Inhibition mechanism
Among different types of compounds such as specific tyrosinase inactivators and inhibitors, o-dopaquinone scavengers, alternative enzyme substrates, nonspecific enzyme inactivators and denaturants, only specific tyrosinase inactivators and reversible inhibitors actually bind to the enzyme as true inhibitors and really inhibit its activity:
Specific tyrosinase inactivators. They are called suicide inactivators or mechanism-based inhibitors. This group of compounds can be considered very interested from a pharmacological point of view, in hyperpigmentation processes ()Citation98.
Figure 3 Detail of the structural mechanism proposed to explain the suicide inactivation of tyrosinase during its action on o‐diphenols. Em, met‐tyrosinase; Eox, oxy‐tyrosinase; EoxD, oxy‐tyrosinase/o‐diphenol complex; (Eox‐D)1, oxy‐tyrosinase/o‐diphenol complex axially bound to a Cu atom; (Eox‐D)2, oxy‐tyrosinase/o‐diphenol complex axially bound to the two Cu atoms; (Eox‐D)3, oxy‐tyrosinase/o‐diphenol complex axially bound to one Cu atom and the deprotonated hydroxyl group of C‐3; Ei, inactive form of tyrosinase. A general view of this scheme is shown in Ref Citation98.
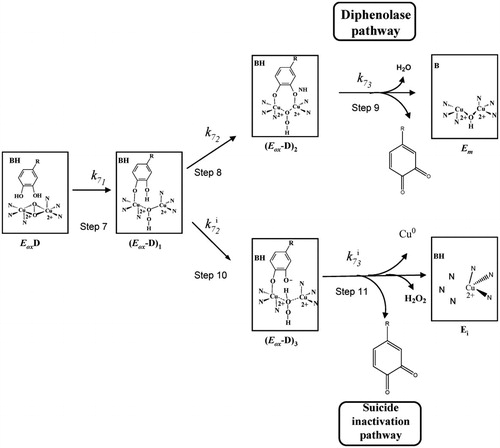
To explain the suicide inactivation of tyrosinase, mainly two mechanisms have been proposedCitation98,Citation99. Accordingly, Haghbeen et al. have suggested that the conformational changes, triggered by the substrate then mediated by the solvent molecules, in the tertiary and quaternary structures of tyrosinase, might be the real reason for the suicide inactivationCitation100. On the other hand, however, based on reports, it was found that acetylation of tyrosine residues with N-acetylimidazole protects mushroom tyrosinase from the suicide inactivation in the presence of its catecholic substrate, 4-[(4-methylbenzo) azo]-1,2-benzenediol without any major impact on the secondary structure of enzymeCitation101.
The studies about the kinetics of suicide inactivation of tyrosinase have been carried out with several o-diphenolic substratesCitation102, ascorbic acidCitation103, l- and d-dopaCitation104 and with different aminophenols and o-diaminesCitation105. The authors have established that the suicide inactivation could occur after the transference of a proton to the peroxide group on the active site of oxy-tyrosinaseCitation98,Citation106, also it has been proposed that the monophenols do not inactivate the enzymeCitation107,Citation108.
The chemical structure of the different substrates is diverse, but the process always requires a step of oxidation/reduction: o-diphenolsCitation102,Citation104, ascorbic acidCitation103, aminophenols and o-diaminesCitation105, hydroxyhydroquinoneCitation109, tetrahydrobiopterinesCitation110, tetrahydrofolic acidCitation111 and NADHCitation112.
Generally, the mode of inhibition by “true inhibitors” is one of these four types: competitive, uncompetitive, mixed type (competitive/uncompetitive), and noncompetitive. A competitive inhibitor can bind to a free enzyme and prevents substrate binding to the enzyme active site. Regarding the property that tyrosinase is a metalloenzyme, copper chelators such as many aromatic acids, phenolic and poly-phenolic compounds, a few non-aromatic compounds, can inhibit tyrosinase competitively by mimicking the substrate of tyrosinaseCitation52,Citation60. Recently, it was found that d-tyrosine negatively regulates melanin synthesis by inhibiting tyrosinase activity, competitivelyCitation113. In addition, l-tyrosine has been shown as an inhibitorCitation114.
In contrast, an uncompetitive inhibitor can bind only to the enzyme-substrate complex and a mixed (competitive and uncompetitive mixed) inhibitor can bind to both forms of free enzyme and enzyme-substrate complex. Finally, noncompetitive inhibitors bind to a free enzyme and an enzyme–substrate complex with the same equilibrium constantCitation115. Non-competitive and mixed-inhibition are frequent modes observed in the kinetics studies on mushroom tyrosinase activities. Phthalic acid and cinnamic acid hydroxypyridinone derivativesCitation116 are two examples of mixed type inhibitors of mono-phenolase activityCitation117. Also, some compounds such as phthalic acidCitation46 and terephthalic acidCitation118, D-(−)-arabinoseCitation119, brazileinCitation120, thymol analogsCitation121 were demonstrated as mixed-type effector examples of di-phenolase activity. Furthermore, other compounds such as bi-pyridine derivativesCitation122, two thiadiazole derivativesCitation44 barbarinCitation123, chlorocinnamic acidsCitation124, propanoic acidCitation125, some N-(mono- or dihydroxybenzyl)-N-nitrosohydroxylaminesCitation126 and p-alkylbenzaldehydesCitation127 inhibited catecholase activity of mushroom tyrosinase uncompetitively. Some derivatives of thiazoles are examples for noncompetitive tyrosinase inhibitionCitation128.
In addition to determining the inhibition mechanism, inhibitory strength which is expressed as the IC50 value (the concentration of inhibitor at which 50% of your target is inhibited) should be calculated in the enzyme kinetics studies and inhibitor screening to compare the inhibitory strength of an inhibitor with others. However, the IC50 values may be incomparable due to the varied assay conditions (different substrate concentrations, incubation time, and different sources of tyrosinase) but a positive control can be used for this purposeCitation52. Although, some researchers have not calculated IC50 and have not applied a positive control in their studies but, fortunately, in most studies conducted for screening new tyrosinase inhibitors, the popular whitening agents, such as kojic acid, arbutin or hydroquinone, were used as a positive controlCitation129 at the same time. However, among different types of mushroom tyrosinase inhibitors, some inhibitors such as hydroquinoneCitation49 arbutin, kojic acidCitation15,Citation49 , azelaic acid, l-ascorbic acid, ellagic acid and tranexamic acid have been reported as skin-whitening agents in the cosmetic industry but there are a few reports failed to confirm their effect as an agent to lighten skin in clinical trials despite the safety of this compoundCitation5. Recently, Mann et al., have compared the inhibitory effects of hydroquinone, arbutin and kojic acid by human tyrosinase and mushroom tyrosinase. They have found hydroquinone and arbutin and kojic acid (IC50 > 500 µmol/L) weekly inhibits human tyrosinase. In contrast, a resorcinyl-thiazole derivative, thiamidol, is a most potent inhibitor of human tyrosinase (IC50 of 1.1 µmol/L) but inhibits mushroom tyrosinase weakly (IC50 = 108 µmol/L)Citation130. Also, deoxyarbutin, a novel reversible tyrosinase inhibitor with effective in vivo skin lightening potency, have been reported due to its increased skin penetration and binding affinity to human tyrosinaseCitation131. In another research, Sugimoto et al. have investigated a comparison of inhibitory effects of alpha-arbutin and arbutin with human tyrosinase and they have found α-arbutin is stronger than arbutinCitation132.
Natural tyrosinase inhibitor sources
Natural sources including plants, bacteria and fungi have recently become of increasing interest for their antityrosinase activity by producing bioactive compounds. A number of researchers prefer to identify inhibitors from natural sources due to their less toxicity and better bioavalibility, especially for food, cosmetic and medicinal applications.
Plants
It is well known that phenolic compounds are the largest group of phytochemicals found in plants, which are mainly the factors responsible for the activities in plant extractsCitation52. Tyrosinase inhibitory activity of many plant extracts was carried out to find new sources of anti-tyrosinase compounds. For example, anti-tyrosinase activities of the following plants have been reported by various researchers: Asphodelus microcarpusCitation133, Morus nigra LCitation134, Greyia radlkoferi SzyszylCitation45, Limonium tetragonumCitation135, Arctostaphylos uva-ursiCitation136, Pleurotus ferulaeCitation137, Agastache rugosa Kuntze fermented with Lactobacillus rhamnosus and Lactobacillus paracaseiCitation138, Artemisia aucheri BoissCitation139, Cassia toraCitation140, S. brevibracteata subspCitation141, Rhodiola crenulata, Alpinia officinarum Hance and Zanthoxylum bungeanum MaximCitation142, Mangifera indicaCitation143, Podocarpus falcatusCitation144, Momordica charantiaCitation142, Cymbopogon citratesCitation145, Greyia flanaganii (IC50 = 32.62 µg/ml)Citation146, Vitis vinifera Leaf extracts (IC50 = 3.84 mg/mL)Citation147 and Inula britannica L.Citation146. Also, tyrosinase inhibitory activity of 91 native plants from central Argentina was carried out by Chiari et al.Citation138,Citation147. Their results approved the inhibitory activity of these extracts against tyrosinase: Achyrocline satureioides, Artemisia verlotiorum, Cotoneaster glaucophylla, Dalea elegans, Flourensia campestris, Jodina rhombifolia, Kageneckia lanceolata, Lepechinia floribunda, Lepe-chinia meyenii, Lithrea molleoides, Porlieria microphylla, Pterocaulon alopecuroides, Ruprechtia apetala, Senna aphylla, Sida rhombifolia, Solanum argentinum, Tagetes minuta, and Thalictrum decipiens. Besides, plants from the Moraceae family including genera Morus species, Artocarpus, Maclura (Cudrania), Broussonetia, Milicia (Chlorophora), and Ficus have shown in vitro tyrosinase inhibitionCitation148. Also, ethanolic and methanolic extracts of some other plants such as Ardisia elliptica ThunbCitation149, Phyllanthus acidus (L.) Skeels, Rhinacanthus nasutus L. Kurz (IC50 value of 271.50 µg/ml), Arbutus andrachne L. (IC50 = 1 mg/mL)Citation150, Withania somnifera L. Dunal and Solanum nigrum L. berriesCitation151, Pulmonaria officinalis and Centarium umbellatumCitation152 and Camel’s foot creeper leaves (Bauhinia vahlii)Citation153 significantly inhibited tyrosinase activity, too. Quispe et al. have screened tyrosinase inhibitory properties of Peruvian medicinal plants. Among these plant extracts, Hypericum laricifolium Juss, Taraxacum officinale F.H.Wigg. (IC50 value of 290.4 µg/ml), and Muehlenbeckia vulcanica Meisn (IC50 value of 280.1 µg/ml) showed the greatest anti-tyrosinase activityCitation154. Furthermore, tyrosinase inhibitory activity of mangrove plants in MicronesiaCitation155, Korean indigenous plantsCitation156, plants from Brazilian CerradoCitation157, five traditional medicinal plants from IranCitation158, ethanol extracts from medicinal and edible plants cultivated in OkinawaCitation159, seashore plantsCitation160, some tropical plantsCitation161 and Bangladeshi indigenous medicinal plantsCitation162, have been investigated by various researchers. Bonesi et al. have reported recent trends in the discovery of tyrosinase inhibitors from plant sourcesCitation163.
Fungi and bacteria
Fungi from different genera such as Aspergillus sp.Citation164, Trichoderma sp.Citation165, Paecilomyces sp.Citation166, Phellinus linteusCitation167, Daedalea dickinsiiCitation168, Dictyophora indusiataCitation169 along with a liquid culture of Neolentinus lepideusCitation170 have been reported as a source of novel tyrosinase inhibitor by producing bioactive compounds. Also, there have been several reports on tyrosinase inhibitors from some marine fungi species such as Myrothecium sp. isolated from algaeCitation171 and Pestalotiopsis sp. Z233Citation172. Also, there are several reports on tyrosinase inhibition by bacterial species and their metabolites. Among them, Streptomyces sp., such as S. hiroshimensis TI-C3 isolated from soilCitation173, an actinobacterium named Streptomyces swartbergensis sp. Nov.Citation174 and Streptomyces roseolilacinus NBRC 12815Citation175 are potential bacterial sources of tyrosine inhibitors. Moreover, some tyrosinase inhibitors have been reported from a gram-negative marine bacterium Thalassotalea sp. Pp2-459Citation176 and a toxic strain of the cyanobacterium, Oscillatoria agardhiiCitation177. Interestingly, some probiotics such as Lactobacillus sp.Citation178 which are used in the fermentation process have been investigated as natural tyrosinase inhibitor sources. Based on the studies, it has been confirmed that the physiological activities of fermented extracts are considerably higher than those of unfermented extracts and their cytotoxic activity is lower as compared to unfermented extractsCitation179. Recently, tyrosinase inhibitory four different lactic acid bacteria (LAB) strains isolated from dairy cow feces have been proved by Ji et al.Citation180.
Finally, in an updated review by Fernandes from reported findings, tyrosinase inhibitors produced by microorganisms have been summarisedCitation61. This review shows that diverse tyrosinase inhibitors isolated from plant sources and fungi are mostly phenolic compounds, steroids, and alkaloids structurally comparable with each other. In contrast, tyrosinase inhibitors from bacteria comprise a smaller group of alkaloids, macrolides, and polyphenols, which competitively inhibit the enzymeCitation61.
Inhibitors from natural, semisynthetic and synthetic sources
Simple phenols
Phenolic compounds which are characterised by having at least one aromatic ring and one (or more) hydroxyl group can be classified based on the number and arrangement of their carbon atoms. These compounds are commonly found to be conjugated to sugars and organic acids. Phenolics range from simple to large and complex tannins and derived polyphenols due to their molecular-weight and number of aromatic-ringsCitation180.
The simple phenols such as hydroquinoneCitation181,Citation182 and its derivativesCitation183,Citation184, deoxyarbutinCitation185,Citation186 and its derivativesCitation187, 4–(6-Hydroxy-2-naphthyl)-1,3-bezendiol, resorcinol (or resorcin)Citation188 and 4-n-butylresorcinolCitation189, vanillinCitation190 and its derivativesCitation191,Citation192 have been reported in the scientific literature as possible phenolic inhibitors of the tyrosinase (). Chen et al. have found the alkylhydroquinone 10'(Z)-heptadecenylhydroquinone, isolated from the sap of the lacquer tree Rhus succedanea, can inhibit the activity of tyrosinase and suppress melanin production in animal cells. The IC50 of this compound (37 µM) is less than hydroquinone (70 µM) as a known inhibitor of tyrosinase. They have suggested that the potent inhibitory effect of this derivative on tyrosinase activity is likely due to its heptadecenyl chain, which facilitates the oxidation of the hydroquinone ringCitation183,Citation184.
Isotachioside, a methoxy-hydroquinone-1-O-beta-d-glucopyranoside isolated from Isotachis japonica and Protea neriifolia and its glycoside derivatives (glucoside, xyloside, cellobioside, and maltoside) are categorised as analogs of arbutin. However, isotachioside and arbutin could not be determined as potent inhibitor. But, glucoside, xyloside, cellobioside and maltoside derivatives, missing methyl and benzoyl groups, acted as tyrosinase inhibitors with IC50s of 417, 852, 623 and 657 µM, respectively. Among these novel inhibitors, glucoside derivative (IC50 = 417 µM) was the most potent, indicating that the structural combination of resorcinol and glucose was significant for inducing the inhibitory effectCitation193.
Hydroquinone and some of its known derivatives, including α and β-arbutin, are described as both a tyrosinase inhibitor and a substrateCitation194,Citation195. Deoxyarbutin and its second-generation derivatives have been proposed as promising agents to ameliorate hyperpigmented lesions or lighten skin due to less toxicity at their effective inhibitory doseCitation185,Citation186.
Monophenolic compounds such as l-tyrosine, l-α-methyl-tyrosine and tyramine are substrates of tyrosinase. o-Quinone evolves in the medium of reaction accumulating o-diphenol and this accumulation provokes that met-tyrosinase (Em) is transformed into oxy-tyrosinase (Eox), which is the active form of the tyrosinase for monophenols and o-diphenols. Therefore, tyrosinase is active with monophenols such as: umbelliferoneCitation196, hydroquinoneCitation197,Citation198 p-hydroxybenzyl alcoholCitation199, 4-hexylresorcinolCitation200, oxyresveratrolCitation201, 4-n-butylresorcinolCitation202, resorcinolsCitation203, α and β-arbutinCitation195 and p-coumaric acidCitation204,Citation205 when we add the following reagents to medium of reaction: hydrogen peroxide (transforms Em to Eox), an o-diphenol or a reducing agent such as ascorbic acid transforming Em to Ed which, with molecular oxygen, is transformed into Eox. A particular case is deoxyarbutin, which acts as a substrate of tyrosinase even if any reagent is not added to the medium of reactionCitation206. Taking into consideration all the previous comments, several methods have been developed to discriminate between true inhibitors and alternative substrates of the enzymeCitation98,Citation207.
Polyphenols
Plants produce a large diverse class of polyphenols including phenolic acids, flavonoids, stillbenes and lignansCitation208,Citation209. A large number of these compounds have been reported as a weak or potent inhibitor of tyrosinase from naturalCitation210–215 and syntheticCitation216–219 sources.
Flavonoids
Among polyphenolic compounds, some of the flavonoid derivatives mostly found in herbal plants, fruits and synthetic sources have been raveled to be the potent inhibitors of tyrosinaseCitation133,Citation211,Citation220Citation225. There is a significant correlation between the inhibitory potency of flavonoids on mushroom tyrosinase and melanin synthesis in melanocytesCitation226. In searching effective tyrosinase inhibitors from natural products, many flavonoid compounds have been isolated and evaluated for their inhibitory activity on mushroom tyrosinase from different natural sources such as Trifolium nigrescens Subsp. PetrisaviCitation227, mung bean (Vigna radiatae L.)Citation228, calamondin peelCitation229, Morus yunnanensisCitation230, Bhagwa and Arakta cultivarCitation231, Tibouchina semidecandra LCitation232, Maackia faurieCitation232, Pleurotus ostreatusCitation233, Potentilla bifurcaCitation234, Alpinia officinarumCitation235, roots of Morus lhouCitation236, Garcinia subellipticaCitation160, Artocapus altilisCitation190, Myrsine africanaCitation237, Pulsatilla cernuaCitation238, Salvia miltiorrhiza-Carthamus tinctorius (Danshen-Honghua, DH) herbal pairCitation239 and other various medicinal plantsCitation240.
Generally, major flavonoids () are classified into several main classes: flavones, flavonols, isoflavones, flavanones, flavanoles and anthocyanidins. Minor flavonoids included: dihydroflavones, flavan-3,4-diols, coumarins, chalcones, dihydrochalcones and auronesCitation241. Also, prenylated and vinylated flavonoids, such as flavonoid Glycosides, are other subclasses of flavonoids. Some flavonoid glycosides such as myricetin 3-galactoside and quercetin 3-O-β-galactopyronaside from Limonium tetragonumCitation133 and 3',5'-di-C-β glucopyranosylphloretin from unripe calamondin peel (IC50 = 0.87 mg/ml)Citation229, have been investigated for their inhibitory activities on tyrosinase. Moreover, the inhibitory activities of some other prenylated and vinylated flavonoids, such as kuwanon C, papyriflavonol A, sanggenon D and sophoflavescenol, and sanggenon D (IC50 = 7.3 µM) against tyrosinase, have been approved by Lee et al.Citation242. However, according to their findings, the prenylation with isoprenyl group or the vinylation of some flavonoid molecules does not enhance their tyrosinase inhibitory activityCitation242. Interestingly, it has even demonstrated that deglycosylation of some flavonoid glycosides by far-infrared irradiation can be improved tyrosinase inhibitory activityCitation243. In a survey from reported findings (2008–2013), Orhan et al. reviewed many examples of tyrosinase inhibitors with flavonoid structureCitation220. In the following, some tyrosinase inhibitors from various flavonoid classes have been mentioned and discussed.
Flavones and dihydroflavones
The most common flavones are luteolin, apigenin, baicalein, chrysin and their glycosides (e.g. apigetrin, vitexin, and baicalin)Citation209. Furthermore, nobiletin and tangeretin are the polymethoxylated flavonesCitation244. Nguyen et al. have investigated the presence of apigenin and nobiletin from the methanolic extract of the heartwood of Artocapus altilis with 11 other phenolic compounds for their inhibitory activities on tyrosinaseCitation190. In another research, Shang et al. have found a derivative of flavone, namely 7,8,4´-trihydroxyflavone which inhibits diphenolase activity of tyrosinase with an IC50 value of 10.31 ± 0.41 µM and a noncompetitive manner with a Ki of 9.50 ± 0.40 µM. The quenching anlaysis of tyrosinase by this compound showed a static mechanism and a single binding site with a binding constant of 7.50 ± 1.20 × 104 M−1 at 298 K. Based on the thermodynamics parameters, the binding process involved hydrogen bonds and van der Waals forces. Also, docking simulation illustrated hydrogen bonds between this compound and the residues His244 and Met280 of active siteCitation245.
In addition, several hydroxyflavones including baicalein, 6-hydroxyapigenin, 6-hydroxygalangin and 6-hydroxy-kaempferolCitation246 and tricin (5,7,4′-trihydroxy-3′,5′-dimethoxyflavone)Citation247 have been demonstrated as inhibitors of diphenolase activity of tyrosinase. The mechanism of inhibition by baicalein (IC50 = 0.11 mM) indicated a mix-type (Ki of 0.17 mM, α = 0.56). A single binding site with a binding constant of 2.78 × 105 M − 1 was obtained from the quenching fluorescence analysis for this compound. Thermodynamic parameters suggested spontaneous binding through hydrogen bonding and van der Waals forces. Furthermore, circular dichroism spectra indicated a reduction in the content of α-helix from 32.67% to 29.00% due to this binding. Docking simulations also indicated that baicalein mainly bound tyrosinase via its Met280 residueCitation248. While, tricin was found as a noncompetitive inhibitor of tyrosinase with good efficacy compared to its control. Based on circular dichroism spectra, the interactions between tricin and tyrosinase did not change the secondary structure. Fluorescence quenching revealed that the interaction of tricin with residues in the hydrophobic pocket of tyrosinase is stabilised by hydrophobic interactions and hydrogen bonding. Also, docking results implied that the stereospecific effects of tricin on substrates or products and flexible conformation alterations of tyrosinase produced by weak interactions between tricin and this enzyme are the possible inhibitory mechanisms of this compoundCitation247.
Another flavone named morusone from the twigs of Morus alba L. (IC50 = 290.00 ± 7.90 µM)Citation249, a new bioflavone 4''',5,5″,7,7″-pentahydroxy-3',3'''-dimethoxy-3-O-β-d-glucosyl-3″,4'-O-biflavone from Trifolium nigrescens Subsp. PetrisaviCitation227, along with apigenin, flavone glucoside vitexin (IC50 = 6.3 mg/ml) and a C-glycosylflavone isovitexin (IC50 = 5.6 mg/ml) from Vigna radiatae L. extracts exhibited significant tyrosinase inhibition activitiesCitation228. Also, inhibitory effects of five flavones including mormin (IC50 = 0.088 mM), cyclomorusin (IC50 = 0.092 mM), morusin (IC50 = 0.250 mM), kuwanon C (IC50 = 0.135 mM) and norartocarpetin (IC50 = 1.2 µM) isolated from the stem barks of Morus lhou (S.) Koidz, have been investigated by Ryu et al. The mechanism of inhibition indicated that mormin, cyclomorusin, kuwanon C and norartocarpetin inhibited tyrosinase competitivelyCitation250.
Flavonoles
Myricetin, kaempferol, quercetin, morin, isorhamnetin, galangin and their glycosides (e.g. rutin, quercitrin, and astragalin) are the predominant flavonols most commonly found as O-glycosidesCitation209. So far, several flavonols such as kaempferol from Hypericum laricifolium JussCitation154 and Crocus sativus L.Citation251, quercetin from Olea europaea L.Citation252, quercetin-4'-O-beta-d-glucoside from Potentilla bifurcaCitation253, quercetin-3-O-(6-O-malonyl)-β-d-glucopyranoside and kaempferol-3-O-(6-O-malonyl)-β-d-glucopyranoside from mulberry leavesCitation253, galangin from Alpinia officinarumCitation235, morinCitation254 and (±) 2,3-cis-dihydromorin (IC50 = 31.1 µM), 2,3-trans-dihydromorin (IC50 = 21.1 µM) from Cudrania cochinchinensisCitation255, were identified as tyrosinase inhibitors.
Based on kinetics studies, morin reversibly inhibited tyrosinase through a multi-phase kinetic process and bind to tyrosinase at a single binding site mainly by hydrogen bonds and van der Waals forces. It inhibited tyrosinase reversibly in a competitive manner with Ki = 4.03 ± 0.26 mM and the binding of morin to tyrosinase-induced rearrangement and conformational changes of the enzymeCitation254. Furthermore, it was reported that three flavonols including galanginCitation235, kaempferolCitation251 and quercetin inhibit the oxidation of L-DOPA catalysed by mushroom tyrosinase and presumably this inhibitory activity comes from their copper chelating ability. While their corresponding flavones, chrysin, apigenin and luteolin, are not identified as copper chelator, Kubo et al. believed that the chelation mechanism by flavonols may be attributed to the free 3-hydroxyl groupCitation251. Interestingly, quercetin behaves as a cofactor and does not inhibit monophenolase activity. In contrast, galangin inhibits monophenolase activity and does not act as a cofactor, and kaempferol neither acts as a cofactor nor inhibits monophenolase activity. However, inhibiting of diphenolase activity by chelating copper in the enzyme is the common feature of these three flavonolsCitation160.
Recently, 8-prenylkaempferol as a competitive tyrosinase inhibitor along with Kushenol A (noncompetitive) isolated from Sophora flavescensCitation256, have been investigated with IC50 values less than 10 µM. Finally, based on the literature review, many flavonol inhibitors are usually competitive inhibitors due to the 3-hydroxy-4-keto moiety of the flavonol structure, which chelates the copper in the active site Citation251. Also, among all these compounds, quercetin-4'-O-beta-d-glucoside with a IC50 value of 1.9 µM is revealed stronger tyrosinase inhibition than their positive control, kojic acidCitation236. While the other flavonol inhibitors listed above are very weak inhibitors and have little potential as skin whitening or food antibrowning.
Isoflavones
Isoflavones such as daidzein, genistein, glycitein, formononetin, and their glycosides (e.g. genistin, daidzin) mostly are detected in the medicinal herbsCitation209. Park et al. have investigated tyrosinase inhibition activities of some natural o-dihydroxyisoflavone derivatives with variable hydroxyl substituent at the aromatic ring of isoflavone isolated from five-year-old Korean fermented soybean paste. They have demonstrated that two derivatives 7,8,4'-trihydroxyisoflavone and 7,3',4'-trihydroxyisoflavone inhibit tyrosinase by IC50 value of 11.21 ± 0.8 µM and 5.23 ± 0.6 µM, respectively, whereas very low inhibition activity was obtained for 6,7,4'-trihydroxyisoflavone, daidzein, glycitein and genisteinCitation257. Also, 6,7,4'-trihydroxyisoflavone was identified as a potent competitive inhibitor of monophenolase activity of tyrosinase by Chang et al., with an IC50 value of 9.2 µM, which is six times potent than kojic acidCitation258. But, its analogs, glycitein, daidzein, and genistein showed little anti-tyrosinase activity. Therefore, they have suggested that C-6 and C-7 hydroxyl groups of the isoflavone skeleton might play an important role in the tyrosinase inhibitory activity. Furthermore, two other isoflavone metabolites, 7,8,4'-trihydroxyisoflavone and 5,7,8,4'-tetrahydroxyisoflavone isolated from soygerm koji, were investigated by Chang et al.Citation259. These compounds inhibited both monophenolase and diphenolase activities with an irreversible inhibition manner. Interestingly, by using HPLC analysis and kinetic studies, they have found that 7,8,4'-trihydroxyisoflavone and 5,7,8,4'-tetrahydroxyisoflavone are potent suicide substrates of mushroom tyrosinase. It may be concluded that the hydroxyl groups at both the C7 and C8 positions could completely change the inhibitory mechanism of the isoflavones from the reversible competitive to the irreversible suicide formCitation52.
Recently, a noncompetitive inhibitor, glabridin (IC50 = 0.43 µM), isolated from the root of Glycyrrhiza glabra Linn, has exhibited excellent inhibitory effects on tyrosinase. The quenching analysis of tyrosinase by glabridin showed a static mechanismCitation260. Notably, a drug delivery system by using glabridin microsponge-loaded gel as a new approach for hyperpigmentation disorders have been proposed by Deshmukh et al.Citation261. In another research, Jirawattanapong et al. have identified a synthetic glabridin, 3'',4''-dihydroglabridin, with higher activity than glabridin (IC50 = 11.40 µM) against tyrosinase. They have suggested the more effective interaction with the enzyme may be due to more conformational flexibly of this compound that has occurred by the 4-substituted resorcinol skeleton and the lacking of double bond between carbon atom 3'' and 4'' in its structureCitation262. Also, Nerya et al. have reported that another isoflavone, glabrene, in the licorice extract can inhibit both monophenolase and diphenolase tyrosinase activitiesCitation263. In the study reported by Heo et al., two new isoflavones desmodianone H and uncinanone B have been identified as novel tyrosinase inhibitors. However, uncinanone B has higher anti-tyrosinase rate than desmodianone HCitation264. Glyasperin C from Glycyrrhiza glabra is another kind of isoflavone identified as tyrosinase inhibitorCitation265. Furthermore, some other isoflavones, formononetin, genistein, daidzein, texasin, tectorigenin, odoratin and mirkoin isolated from the stems of Maackia fauriei, have been investigated by Kim et al. for their tyrosinase inhibition activity. Based on their results, among these falvonoids, mirkoin (IC50 = 5 µM) revealed stronger tyrosinase inhibition than the positive control, kojic acid and inhibited tyrosinase reversibly in a competitive modeCitation232. Recently, two isoflavonoids lupinalbin (IC50 = 39.7 ± 1.5 µM), and 2′-hydroxygenistein-7-O-gentibioside (IC50 = 50.0 ± 3.7 µM) from Apios americana were identified as competitive inhibitors, with Ki values of 10.3 ± 0.8 µM and 44.2 ± 1.7 µM, respectivelyCitation266.
Flavanones
Flavanones such as naringenin, hesperetin, eriodictyol and their glycosides (e.g. naringin, hesperidin, and liquiritin) and flavanonols (taxifolin) are mainly found in citrus fruits and the medicinal herbsCitation209. A copper chelator flavanone named hesperetin inhibits tyrosinase reversibly and competitively. Based on the ANS-binding fluorescence analysis, hesperetin disrupted of tyrosinase structure by hydrophobic interactions. In addition, hesperetin chelates a copper ion coordinating with 3 histidine residues (HIS61, HIS85, and HIS259) within the active site pocket of the enzyme due to docking simulation resultsCitation267. In another study, Chiari et al. have illustrated tyrosinase inhibitory activity of a 6-isoprenoid-substituted flavanone isolated from Dalea elegansCitation268. Also, Steppogenin is a natural flavanone with a strong tyrosinase inhibitory activity (IC50 = 0.98 ± 0.01 µM), from Morus alba LCitation249. Recently, a new isoprenylated sanggenon-type flavanone, nigrasin K, along with some other analogs including sanggenon M, C and O, chalcomoracin, sorocein H and kuwanon J isolated from the twigs of Morus nigra have been identified as potent tyrosinase inhibitors by Hu et al.Citation269. Among these natural inhibitors, sanggenon D revealed stronger tyrosinase inhibition than the positive control, kojic acid or arbutin.
Flavanoles and flavan-3,4-diols
Flavan-3-ols are the most complex subclass of flavonoids ranging from the simple monomers (+)-catechin and its isomer (−)-epicatechin to the oligomeric and polymeric proanthocyanidins, which are also known as condensed tannins. Flavanols, such as catechin, epicatechin, epi-gallocatechin, epicatechin gallate (ECG), epigallocatechin gallate (EGCG) and proanthocyanidins are widespread in the medicinal herbs and higher plantsCitation231,Citation270. Alphitonia neocaledonica (Rhamnaceae) is an endemic tree of New Caledonia, which has been identified as an anti-tyrosinase source due to the presence of tannins and gallocatechinCitation228. Moreover, a catechin compound isolated from the ethanol extract of Distylium racemosum branches, with IC50 value of 30.2 µg/mL, showed higher tyrosinase inhibition activity than arbutin as a positive controlCitation271. Also, a proanthocyanidins from Clausena lansium demonstrated potent mushroom tyrosinase inhibition in a mixed competitive manner and illustrated strong inhibition of the melanogenic activity of B16 cells. The IC50 values for the monophenolase and diphenolase activities were 23.6 ± 1.2 and 7.0 ± 0.2 µg/mL, respectively. Furthermore, from the inhibition mechanism of this compound, it can be concluded that a chelation between the hydroxyl group on the B ring of the proanthocyanidins and dicopper ions of the enzyme has been occurredCitation39.
Another investigation revealed that procyanidin-type proanthocyanidins, purified from cherimoya (Annona squamosa) pericarp could powerfully inhibit the activities of monophenolase and diphenolase of tyrosinase, competitivelyCitation272. In addition, Kim et al. have demonstrated that (+)-catechin-aldehyde polycondensates inhibit the l-tyrosine hydroxylation and L-DOPA oxidation by chelation to the active site of tyrosinaseCitation273. Recently, another tyrosinase inhibitor from this class, condensed tannins (mixtures of procyanidins, propelargonidins, prodelphinidins) and their acyl derivatives (galloyl and p-hydroxybenzoate) from Longan Bark indicated the reversible and mixed (competitive is dominant) inhibition of tyrosinaseCitation274.
Anthocyanidins
Anthocyanins, including anthocyanidins (e.g. cyanidin, delphinidin, malvidin, peonidin, pelargonidin, etc.) and their glycosides, are widely distributed in the medicinal herbsCitation217. It seems that there is a significant relationship between anthocyanin content with anti-human and anti-mushroom tyrosinase activitiesCitation275.
Curcuminoids
Two phenolic compounds, namely curcumin and desmethoxycurcumin have been isolated from the methanolic extract of the heartwood of Artocapus altilis and showed more potent tyrosinase inhibitory activities than the positive control kojic acidCitation190. Also, a curcumin included in Chouji and Yakuchi extracts inhibited the enzyme competitivelyCitation192. In addition, some synthetic curcumin derivative compoundsCitation217,Citation276 and its analogs possessing m-diphenols and o-diphenols have been investigated as potent inhibitors of mushroom tyrosinaseCitation216. Based on the results, 4-hydroxyl groups in curcumin analogs containing 4-hydroxyl-substituted phenolic rings with C-2/C-4- or C-3/C-4-dihydroxyl-substituted diphenolic rings make them more active than kojic acidCitation217.
Coumarins
In search of tyrosinase inhibitors, the inhibitory effects of several coumarin derivatives ()Citation277–279 such as 3-aryl and 3-heteroarylcoumarinsCitation280, esculetinCitation281, coumarinolignoid 8'-epi-cleomiscosinCitation282, umbelliferone and their analogsCitation283, phenyl coumarinsCitation284, hydroxycoumarinsCitation285,Citation286, thiophosphonic acid diamides, diazaphosphinanes coumarin derivativesCitation287, cardol-coumarin derivativesCitation288 and coumarin-resveratrol hybridsCitation289, were evaluated on tyrosinase activity.
Figure 6 Inhibitory effects of the coumarins derivatives against mushroom tyrosinase activity: 3-aryl and 3-heteroarylcoumarins (1–10, 23–24), 3-hydroxycoumarin (11), 4-hydroxycoumarin (12), 6-hydroxycoumarin (13), 7-hydroxycoumarin (14), umbelliferone analogs (15–16), Esculetin (17) umbelliferone (18), 3-phenyl coumarins with bromo substituent (19), thiophosphonic acid diamides (20–22).
Interestingly, among hydroxycoumarins, the 3-hydroxycoumarinCitation286 and 7-hydroxycoumarin showed potent activity for the tyrosinase inhibitionCitation278, while the 4-hydroxycoumarin is not an inhibitorCitation286. Also, 2-(1-(coumarin-3-yl)-ethylidene) hydrazinecarbothioamide and 2-(1-(6-chlorocoumarin-3-yl) ethylidene)-hydrazinecarbothioamide demonstrated an irreversible inhibition of tyrosinaseCitation277. Recently, in the screening of natural products for the development of cosmetic ingredients, two major compounds, trans-N-coumaroyltyramine (IC50 = 40.6 µM) and cis-N-coumaroyltyramine (IC50 = 36.4 µM) from Humulus japonicus showed potent tyrosinase inhibitionCitation290.
Chalcones and dihydrochalcones
Chalcones (butein, phloretin, sappan-chalcone, carthamin, etc.), or 1,3-diphenyl-2-propen-1-ones, are one of the most important classes of flavonoids. Chalcone-containing plants have been used for a long time in traditional medicineCitation209. Based on the reports, some natural and synthetic chalcones and their derivatives are identified as new potent depigmentation agents and tyrosinase inhibitors (. So far, natural chalcones isoliquiritigenin (2′,4′,4-trihydroxychalcone) and glabrene from licorice rootsCitation283, 2,4,2',4'-hydroxycalcone and three of its analogs with 3'-substituted resorcinol moieties from Morus australis (19–22)Citation291, 2,4,2',4'-tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone from Morus nigraCitation292, vulpinoideol B from Carex vulpinoidea seedsCitation293, dihydrochalcones from Flemingia philippinensisCitation210, 2,4,2′,4′-tetrahydroxychalcone (IC50 = 0.07 ± 0.02 µM) and morachalcone A (IC50 = 0.08 ± 0.02 µM) from Morus alba L.Citation249 and bavachinin from Psoralea corylifoliaCitation21 have been presented as tyrosinase inhibitors.
Figure 7 Tyrosinase Inhibition Activity of chalcone derivatives inhibitors: Oxindole-based chalcone (1–8), chalcones isolated from Morus australis (9–12) azachalcones (13–14), oxime based chalcone series (15,16) 2,3-dihydro-1H-inden-1-one chalcone-like derivatives (17,18), Dihydrochalcones from Flemingia philippinensis (19–21). chalcone (22).
Also, tyrosinase inhibitory effects of several synthetic chalcones and their derivatives were evaluated by various researchers. Oxindole-based chalconesCitation294, 1-(2-cyclohexylmethoxy-6-hydroxy-phenyl)-3-(4-hydroxymethyl-phenyl) propenone derivativeCitation295, isoxazole chalcone derivativesCitation296, some azachalcones and their oximesCitation297,Citation298, 2,4,2',4'-tetrahydroxychalcone and its two derivatives (1,3,5-tris-(2,4-dihydroxy-phenyl) pentane-1,5-dione and 7,2',4'-trihydroxyflavanone)Citation299, 2',4',6'-trihydroxychalconesCitation300, naphthyl chalconesCitation301 and chalcone thiosemicarbazide derivativesCitation302 have been identified as a new class of tyrosinase inhibitors. Interestingly, the most important factors in the efficacy of a chalcone are the location of the hydroxyl groups on both aromatic rings and the number of these hydroxyls and the presence of a catechol moiety don't correlate with increasing tyrosinase inhibition potencyCitation303.
Aurones
Okombi et al. have identified Z-benzylidenebenzofuran-3(2H)-one and analogs as human tyrosinase inhibitors. However, they found that aurones are weak inhibitors, but their derivatives with two or three hydroxyl groups preferably at 4,6 and 4' positions make them significant tyrosinase inhibitors. For example, the most potent aurone, 4,6,4'-trihydroxyaurone induces 75% inhibition at 0.1 mM concentration and is highly effective compared to kojic acidCitation304. In addition to synthetic compounds, several natural compounds such as (2'R)-2',3'-dihydro-2'-(1-hydroxy-1-methylethyl)-2,6'-bibenzofuran-6,4'-diolCitation305 and 2-arylbenzofurans isolated from Morus notabilisCitation306 and Morus yunnanensisCitation230, benzofuran flavonoids such as mulberrofuran G (MG) and albanol B (AB) isolated from Morus spCitation307 and macrourins E isolated from Morus macroura (IC50 = 0.39 µM) are potent tyrosinase inhibitors among auronesCitation308.
Phenolic acids
Phenolic acids are divided into hydroxybenzoates and hydoxycinnamates. The most common hydroxycinnamates are p-coumaric, caffeic and ferulic acids. So far, p-hydroxybenzoic acid, chlorogenic acid (the ester of caffeic acid), vanilic acid (4-hydroxy-3-methoxybenzoic acid) and protocatechuic acid (a dihydroxybenzoic acid) from Hypericum laricifolium JussCitation154, protocatechualdehyde (IC50 = 0.40 µg/mL) from Phellinus linteusCitation175, benzoic acid propyl gallateCitation309, orsellinic acid (2,4-dihydroxy-6-methylbenzoic acid) and orsellinates (2,4-dihydroxy-6-methyl benzoates)Citation310, p-coumaric acid from ginseng leavesCitation311, m-coumaric acidCitation312, p-coumarateCitation313 and its derivatives from leaves of Breynia officinalisCitation184 caffeic acid and its n-nonyl esterCitation314, ferulic acid from Spiranthes sinensisCitation224, 4-Hydroxy cinnamic acidCitation315, synthetic hydroxycinnamoyl phenylalanyl/prolyl hydroxamic acid derivativesCitation316, and seven hydroxycinnamoyl derivatives in green coffee beansCitation317 have been investigated for their tyrosinase inhibition activity. Among these, propyl gallate is a reversible and mixed-type inhibitor on diphenolase activity of tyrosinase with KIS = 2.135 mM and Ki = 0.661 mM309. Furthermore, n-butyl, iso-propyl, sec-butyl, n-pentyl, n-hexyl and n-octyl orsellinates (uncompetitive, with an inhibition constant of 0.99 mM) behaved as inhibitors at 0.50 mM, whereas methyl, ethyl, n-propyl, tert-butyl, and n-cetyl orsellinates acted as tyrosinase activators. Thus, tyrosinase inhibition increased with chain elongation, suggesting that the enzyme site can accept an eight-carbon alkyl chainCitation310.
In addition to these compounds, 3-phenylbenzoic acid (3-PBA) was revealed to be the most potent inhibitor against monophenolase (noncompetitive, IC50 = 6.97 µM) and diphenolase (mixed type inhibition, IC50 = 36.3 µM) activity of mushroom tyrosinase. Also, Oyama et al. have found that some modification such as esterification can abrogate this inhibitory activity of tyrosinaseCitation318.
Stillbenes
Resveratrol is the most common stilbene. Several stillbenes derivatives from natural and synthetic sources () have been investigated for their tyrosinase inhibition activity including: resveratrol from Morus albaCitation319, Pleurotus ferulaeCitation135, vitis viniferae caulisCitation320, Carignan grape juiceCitation321 Artocarpus gomezianusCitation322 and Streptomyces avermitilis MA4680Citation323 and also, its derivatives from Dipterocarpaceae plantsCitation324 and synthetic sourcesCitation325, oxyresveratrolCitation326 from Morus australisCitation327, Morus alba L (IC50 = 0.10 ± 0.01 µM)Citation249 and Cudrania cochinchinensis (IC50 = 2.33 µM)Citation255, azo-resveratrol and its derivatives such as (E)-2-((2,4-dihydroxyphenyl)diazenyl) phenyl 4 methylbenzenesulfonateCitation328 and azo-oxyresveratrolCitation329, trans-resveratrol from Streptomyces avermitilis MA4680 Citation313, a resveratrol dimer named gnetin C, from melinjo (Gnetum gnemon)Citation330. Also, several hydroxystillbene compounds from synthetic and semisynthetic sourcesCitation331,Citation332 and from the extract of Veratrum patulumCitation333, along with synthetic glycosides of resveratrol, pterostilbene, and pinostilbeneCitation334, synthetic trans-stilbene derivativesCitation335, azastilbene analogsCitation336, a newly synthesised stillbene 5-(6-hydroxy-2-naphthyl)-1,2,3-benzenetriolCitation337, coumarin-resveratrol hybridsCitation290, synthetic polyphenolic deoxybenzoinsCitation218, hydroxy substituted 2-phenyl-naphthalenesCitation338 and 4-(6-hydroxy-2-naphthyl)-1,3-bezendiolCitation339 have been studied for their inhibition activity against tyrosinase. However, based on the enzymatic assays, resveratrol did not inhibit the diphenolase activity of tyrosinase, but L-tyrosine oxidation by tyrosinase was suppressed in presence of 100 µM resveratrol. Interestingly, after the 30 min of preincubation of tyrosinase and resveratrol, both monophenolase and diphenolase activities of tyrosinase were significantly suppressed. Furthermore, this effect was reduced with the addition of l-cysteine, which indicated suicide inhibition mechanism of resveratrolCitation340. Also, oxyresveratrolCitation201 is identified as a tyrosinase substrate like hydroquinone, arbutin, caffeic acid and some other inhibitors. In addition to these studies on resveratrol, Fachinetti et al., have demonstrated that the incorporation of resveratrol into nanostructured lipid carriers allowed an enhanced tyrosinase inhibitory activityCitation341.
Lignans
Lignans are complex and diverse structures, which are formed from three primary precursors. So far, lignans and lignan glycosides isolated from exocarp of Castanea henryiCitation342, Marrubium velutinum and Marrubium cylleneumCitation343, Pinellia ternateCitation344 and Crataegus pinnatifidaCitation345 have been evaluated for their tyrosinase inhibitory potentials. However, these compounds mostly displayed a moderate mushroom tyrosinase inhibitory activity.
Terpenoid derivatives
Carvacrol is a monoterpenoid phenol. To date, some carvacrol derivativesCitation346 from synthetic sources, bakuchiol, a terpene phenol from Psoralea corylifoliaCitation21, iridoid glucosides (another type of monoterpenoids) from Wulfenia carinthiaca JacqCitation347 and two new bis-iridoids, namely 7-O-caffeoyl-sylvestroside I and 7-O-(p-coumaroyl)-sylvestroside I isolated from Scabiosa stellataCitation348 have been investigated for their anti-tyrosinase activities. Among these terpenoid derivatives, Cheng et al. have demonstrated that bakuchiol is a potent inhibitor by applying capillary electrophoresis with reliable online immobilised enzyme microreactorCitation21. Also, carvacrol derivatives such as 2-[2-methyl-5-(propan-2-yl)phenoxy]-2-oxoethyl(2E)-3–(2,4-dihydroxyphenyl)prop-2-enoate showed excellent tyrosinase inhibitory activity by a noncompetitive manner with Ki value 0.05 µM and IC50 = 0.0167 µMCitation349.
Quinone derivatives
The quinones are a class of small molecules that are mostly derived from aromatic compounds such as benzene or naphthalene. Among these compounds, Aloin, an anthraquinone-C-glycoside from Aloe vera Citation349, anthraquinones from Polygonum cuspidatumCitation350 and tanshinone IIA (IC50 = 1214 µM) have been verified as tyrosinase inhibitorsCitation239.
Phenyl derivatives
Several biphenyl derivativesCitation351 () such as 4,4'-dihydroxybiphenylCitation352, biphenyl ester derivativesCitation340, biphenyl construction from flavan-3-ol substratesCitation353, hydroxylated biphenylsCitation26, functionalised bis-biphenyl substituted thiazolidinonesCitation36, phenylbenzoic acid derivativesCitation354, phenylethylamide and phenylmethylamide derivativesCitation355, hydroxy substituted 2-phenyl-naphthalenesCitation318, 4-hydroxyphenyl beta-d-oligoxylosidesCitation356, benzenethiol or phenylthiolCitation357, 2-((1Z)-(2–(2,4-dinitrophenyl)hydrazin-1-ylidene)methyl) phenolCitation358 and 4-[(4-hydroxyphenyl)azo]-benzenesulfonamideCitation359, have been identified as tyrosinase inhibitors.
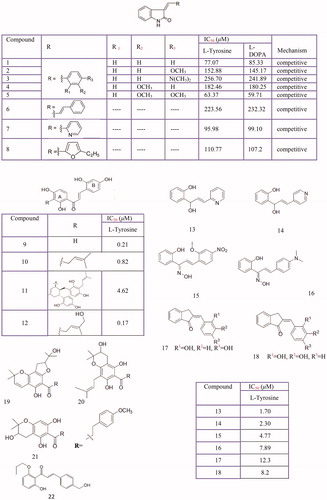
Pyridine, Piperidine, pyridinones and hydroxypyridinone derivatives
Some hydroxypyridinone derivativesCitation360, 3-hydroxypyridine-4-one derivativesCitation361 hydroxypyridinone-L-phenylalanineCitation362 and pyridinonesCitation363 have been characterised for their antityrosinase activity (). Among these inhibitors, one mixed-type inhibitor from hydroxypyridinone-l-phenylalanine conjugates named ((S)-(5-(benzyloxy)-1-octyl-4-oxo-1,4-dihydropyridin-2-yl) methyl 2-amino-3-phenylpropanoate) showed potent inhibitory effect with IC50 values of 12.6 and 4.0 µM for monophenolase and diphenolase activities, respectivelyCitation362.
Thiosemicarbazones, Thiosemicarbazide and other Thio derivatives
Several kinds of thiosemicarbazone derivativesCitation38,Citation34,Citation364–376 has been investigated as possible tyrosinase inhibitors (). Furthermore, some benzaldehyde derivatives of thiosemicarbazone such as chlorobenzaldehyde thiosemicarbazonesCitation363, p-hydroxy and p-methoxy benzaldehyde thiosemicarbazoneCitation362 along with p-methoxybenzaldehyde thiosemicarbazone and 4-dimethylaminobenzaldehyde-thiosemicarbazone and 4-dimethylaminobenzaldehyde-N-phenyl-thiosemicarbazoneCitation377 were evaluated for their inhibitory activities on mushroom tyrosinase.
Figure 11 Inhibitory effects of some thiosemicarbazone derivatives on the tyrosinase monophenolase activity.
Based on the findings, the appropriate functionalisation of thiosemicarbazone may be improved the inhibitory activity of these inhibitors. Dong et al. believe that the sterically bulky group at the C-4 position of the thiophene ring contributes to this activity. For example, the 4-functionalisation thiophene-2-carbaldehyde thiosemicarbazone with a methoxyacetyl groupCitation368 or introducing benzene ring to the 4-functionalised ester groupCitation367 enhanced inhibitory activity of thiophene-2-carbaldehyde thiosemicarbazone. However, 5-functionalisation decreased its inhibitory activity. Also, Soares et al., have demonstrated thiosemicarbazones Thio-1, Thio-2, Thio-3 and Thio-4 substituted with oxygenate moieties, displayed better inhibitory activity (IC50 0.42, 0.35, 0.36 and 0.44 mM, respectively) than Thio-5, Thio-6, Thio-7 and Thio-8Citation34.
In addition to thiosemicarbazone derivatives, thiosemicarbazide and its derivativesCitation378–381, 5-benzylidene(thio)barbiturate-beta-d-glycosidesCitation382, n-alkylCitation383, p-phenylene-bis, phenylCitation384, benzyl, p-xylidine-bis and p-pyridine dithiocarbamate sodium saltsCitation385, diethyldithiocarbamate, phenylthiourea3Citation86 and other thiourea derivatives () such as methimazole, thiouracil, methylthiouracil, propylthiouracil, ambazone, and thioacetazoneCitation387 have been identified as tyrosinase inhibitors.
Figure 12 Thiourea derivatives (1–14), methimazole (15), carbimazole (16), thiouracil (17), methylthiouracil (18), propylthiouracil (19), 6–(3-chlorophenylurenyl) saccharin (20), 6–(3-iodophenylthiourenyl) saccharin (21), 4,5,6,7-tetrahydro- 2-[[(phenylamino)thioxomethyl]amino]-benzo[b]thiophene-3-carboxylic acid derivatives (22–25), 2–(1,3,4-thiadiazol-2-yl) thio acetic acid derivatives (26–29).
![Figure 12 Thiourea derivatives (1–14), methimazole (15), carbimazole (16), thiouracil (17), methylthiouracil (18), propylthiouracil (19), 6–(3-chlorophenylurenyl) saccharin (20), 6–(3-iodophenylthiourenyl) saccharin (21), 4,5,6,7-tetrahydro- 2-[[(phenylamino)thioxomethyl]amino]-benzo[b]thiophene-3-carboxylic acid derivatives (22–25), 2–(1,3,4-thiadiazol-2-yl) thio acetic acid derivatives (26–29).](/cms/asset/09c42d96-1d69-43f6-b5e2-460bcff8b112/ienz_a_1545767_f0012_c.jpg)
Azole and thiazolidine derivatives
So far, several azole derivatives () have been studied for their tyrosinase inhibitory activityCitation388. The discovered new types of inhibitors included DL-3(5-benzazolyl) alanines and alpha-methyldopa analogsCitation389, aryl pyrazolesCitation390, heterocyclic hybrids based on pyrazole and thiazolidinone scaffoldsCitation391, 3,5-diaryl-4,5-dihydro-1HCitation392 and 3,5-diaryl pyrazole derivativesCitation393, pyrazolo[4,3-e][1,2,4]triazine sulfonamides and sildenafilCitation394–396, 1,3-oxazine-tetrazoleCitation397, indole-spliced thiadiazoleCitation398, benzimidazole-1,2,3-triazole hybridsCitation399, 1,2,3-triazole-linked coumarinopyrazole conjugatesCitation400, isoxazolone derivativesCitation401 5(4H)-oxazolone derivativeCitation402, imidazolium ionic liquidsCitation403, thiazolyl resorcinolsCitation404 have demonstrated the inhibitory effect on tyrosinase. Furthermore, some thiazolidine derivatives have been evaluated for their tyrosinase inhibitory activity including azo-hydrazone tautomeric dyes substituted by thiazolidinone moietyCitation405, (Z)-5-(2,4-dihydroxybenzylidene) thiazolidine-2,4-dioneCitation406, 5-(substituted benzylidene) thiazolidine-2,4-dione derivativesCitation407, (2RS,4R)-2-(2,4-dihydroxyphenyl)thiazolidine-4-carboxylic acidCitation408, 2-(substituted phenyl) thiazolidine-4-carboxylic acid derivativesCitation409 and (Z)-5-(3-hydroxy-4-methoxybenzylidene)-2-iminothiazolidin-4-oneCitation410.
Kojic acid analogs
Kojic acid is a well-known tyrosinase inhibitor. When DL-DOPA, norepinephrine and dopamine are oxidised by tyrosinase, Kojic acid inhibits effectively the rate of formation of pigmented product(s) and of oxygen uptakeCitation411. Furthermore, several of its derivatives have demonstrated a potent tyrosinase inhibitory activityCitation361,Citation412–418. Noh et al. have modified kojic acid with amino acids and screened their tyrosinase inhibitory activity. Among them, kojic acid-phenylalanine amide showed a strong noncompetitive inhibitionCitation417. Interestingly, some kojic acid derivatives despite their depigmenting activities did not display tyrosinase inhibitory activitiyCitation419.
Recently, Xie et al. have reported a kojic acid analog namely 5-phenyl-3-[5-hydroxy-4-pyrone-2-yl-methylmercapto]-4-(2,4-dihydroxylbenzylamino)-1,2,4-triazol as a potent competitive tyrosinase inhibitor with an IC50 value of 1.35 ± 2.15 µMCitation412. Tyrosinase inhibitory activity of some kojic acid derivatives is shown in .
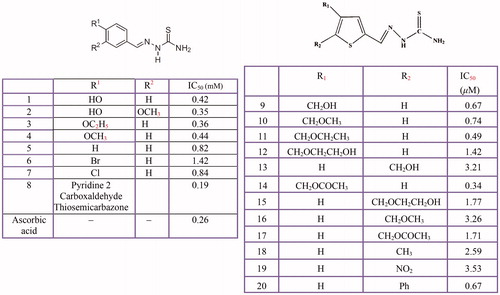
Benzaldehyde derivatives
BenzaldehydeCitation420 and its derivativesCitation421, hydroxy- or methoxy-substituted benzaldoximes and benzaldehyde-O-alkyloximesCitation422, piperonal or 4-(methylenedioxy) benzaldehyde mesoionic derivativesCitation423, 4-hydroxybenzaldehyde derivativesCitation424, anisaldehydeCitation425 have been investigated for their inhibitory activities against tyrosinase ().
Among these derivatives, 3,4-dihydroxybenzaldehyde-O-ethyloxime (IC50 = 0.3 ± 0.1 µM) is of the same magnitude as one of the best tyrosinase known inhibitors tropolone (IC50 = 0.13 ± 0.08 µM)Citation422. However, in benzaldehyde derivatives, the presence of the aldehyde group and the terminal methoxy group in C4 was found to play an important role in its inhibitory effect. But, due to their lower activity levels or serious side effects, unfortunately, most 4-substituted benzaldehyde derivatives cannot be considered for practical useCitation421.
Carboxylic acids
Inhibitory effects of pyruvic acid, acrylic acid, propanoic acid, 2-oxo-butanoic acid, and 2-oxo-octanoic acidCitation124, (S)- and (R)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acidsCitation426 have been investigated on tyrosinase activity.
Based on the findings investigated by Gheibi et al., aliphatic carboxylic acids have dual effects on the monophenolase and diphenolase activities of mushroom tyrosinase. They have found that optimal diphenolase activity of tyrosinase takes place in the presence of n-alkyl acids (pyruvic acid, acrylic acid, propanoic acid, 2-oxo-butanoic acid, and 2-oxo-octanoic acid). While, the monophenolase activity is inhibited by all types of n-alkyl acids. They have believed that there is a physical difference in the docking of mono- and o-diphenols to the tyrosinase active site. On the other hand, the binding of acids occurs through their carboxylate group with one copper ion of the binuclear site. So these carboxylic acid compounds completely block the monophenolase reaction, by preventing monophenol binding to the oxyform of the enzymeCitation124.
Xanthate derivative
The inhibitory effect of some synthesised xanthates including C3H7OCS2Na, C4H9OCS2Na, C5H11OCS2Na, C2H5OCS2Na, and C6H13OCS2Na have been examined for inhibition of both monophenolase and diphenolase activities of mushroom tyrosinase. Based on the reports, C3H7OCS2Na and C4H9OCS2Na showed a mixed inhibition pattern on monophenolase activity but C5H11OCS2Na and C6H13OCS2Na showed a competitive and C2H5OCS2Na showed uncompetitive inhibition pattern. For diphenolase activity, C3H7OCS2Na and C2H5OCS2Na showed mixed inhibition but C4H9OCS2Na and C5H11OCS2Na and C6H13OCS2Na showed competitive inhibitionCitation427. According to their results, it seems that the lengthening of the hydrophobic tail of the xanthates leads to a decrease of the Ki values for monophenolase inhibition and an increase of the Ki values for diphenolase inhibitionCitation428.
Other tyrosinase inhibitors
Except the inhibitors listed above, other compounds have also been registered for their tyrosinase inhibitory activity by different researchers such as: two Keggin-type polyoxometalates containing glycine as potent inorganic reversible inhibitorsCitation429, cadmium ions with an IC50 of 2.92 ± 0.16 mMCitation48 and rifampicin with an IC50 = 90 ± 0.6 µMCitation9 as reversible and noncompetitive inhibitors, ammonium tetrathiotungstateCitation430, amoxicillin (IC50 = 9.0 ± 1.8 mM)Citation431, mallotophilippen A and B Citation432 α-naphthol and β-naphtholCitation433, red koji extracts (IC50 of 5.57 mg/mL)Citation434 and alpha-hydrazinophloretic acidCitation435 as competitive inhibitors and rottlerin as a mixed inhibitorCitation432. Furthermore, n-alkyl sulfatesCitation436, sericin extracted from tasar silk fiber wasteCitation437, 2-hydroxy-3-methylcyclopent-2-enone (IC50 = 721.91 µg mL−1) isolated from ribose-histidine Maillard reaction productsCitation438, three natural compounds from safflowerCitation439 and mimosineCitation386 and ethylenediamineCitation440 are other kinds of tyrosinase inhibitors.
Synergistic effects of tyrosinase inhibitors
Synergistic strategy for tyrosinase inhibitors is a useful strategy for the improvement of their inhibitory activities. Based on the findings, the mixtures of glabridin:resveratrol, glabridin:oxyresveratrol, resveratrol:oxyresveratrol, phenylethylresorcinol:resveratrolCitation441, oxyresveratrol:dioscinCitation442, aloesin:arbutinCitation443, 4-methyl catechol: catecholCitation444, 3-(2,4-dihydroxyphenyl)propionic acid:l-ascorbic acidCitation445, dihydromyricetin:vitamin D3Citation37, linderanolide B combined with arbutin, 1-phenyl-2-thiourea or kojic acidCitation446, have shown synergistic effect on tyrosinase. These studies may provide a scientific strategy for screening effective tyrosinase inhibitors.
Conclusion
Due to the vital role of tyrosinase in the enzymatic browning of food and depigmentation disorders in humans, its inhibitors have been considered by researchers, extensively. As mentioned above, natural sources such as plants and microorganisms and their effective compounds have wonderful potential as organic anti-tyrosinase sources.
However, the majority of the compounds identified from natural sources were isolated from plants but, recently, microorganisms are considered as potential sources of tyrosinase inhibitors. It is interesting that despite the diversity of natural inhibitors, a large number of tyrosinase inhibitors are phenolic-based structures. Many researchers have designed appropriate scaffold inspired by the structure of natural compounds and developed novel synthetic inhibitors. In this paper, many natural, semi-synthetic and synthetic inhibitors have been summarised and the inhibitory effects of these compounds on the tyrosinase activity are discussed.
Based on the results, phenolic compounds (simple phenols and polyphenols) and their derivatives and several compounds including terpenoid, phenyl, pyridine, piperidine, pyridinone, hydroxypyridinone, thiosemicarbazone, thiosemicarbazide, azole, thiazolidine, kojic acid, benzaldehyde and xanthate derivatives were characterised as potent tyrosinase inhibitors. The appropriate functionalisation of these inhibitors such as C-6 and C-7 hydroxyl groups of the isoflavone skeleton, 4-functionalisation thiophene-2-carbaldehyde thiosemicarbazone with a methoxyacetyl group and the aldehyde group and methoxy group in C4 of benzaldehyde derivatives may be improved the inhibitory activity of these inhibitors. Furthermore, in cholcone derivatives, the location of the hydroxyl groups on both aromatic rings and the number of hydroxyls is an important factor in the efficacy of a chalcone. In contrast, some modifications such as the prenylation or the vinylation of some flavonoid molecules do not enhance their tyrosinase inhibitory activity while deglycosylation of some flavonoid glycosides by far-infrared irradiation can be improved tyrosinase inhibitory activity. Interestingly, among different inhibitors, some compounds, especially hydroquinone and its known derivatives (α and β-arbutin), are described as both a tyrosinase inhibitor and a substrate.
Actually, the main objective of this review is to provide a useful source of effective tyrosinase inhibitors. However, despite the existence of a wide range of tyrosinase inhibitors from natural and synthetic sources, only a few of them, in addition to being effective, are known as safe compounds. Therefore, it is recommended to examine the efficacy and safety of inhibitors by in vivo models, along with in vitro and docking experiments, especially for the application of such materials in food and medicinal products. Finally, we hope that the information provided in this study, which is the result of numerous researchers’ efforts, could serve as leads in the search for effective anti-tyrosinase agents from natural and synthetic sources with increased efficiency and safety in the food and cosmetics industries.
Disclosure statement
The authors report that they have no conflicts of interest.
Additional information
Funding
References
- Dembitsky VM, Kilimnik A. Anti-melanoma agents derived from fungal species. M J Pharma 2016;1:1–16.
- Maghsoudi S, Adibi H, Hamzeh M, et al. Kinetic of mushroom tyrosinase inhibition by benzaldehyde derivatives. J Rep Pharma Sci 2013;2:156–64.
- Halaouli S, Asther M, Kruus K, et al. Characterization of a new tyrosinase from Pycnoporus species with high potential for food technological applications. J Appl Microbiol 2005;98:332–43.
- Sahu RK, Roy A, Dwivedi J, Jha AK. Promotion and computation of inhibitory effect on tyrosinase activity of herbal cream by incorporating indigenous medicinal plants. Pak J Biol Sci 2014;17:146–50.
- Jeon SH, Jong-Uk HK, Kwang-Hoon K. Inhibitory effects on L-dopa oxidation of tyrosinase by skin-whitening agents. Bull Korean Chem Soc 2005;26:1135–7.
- Garcia-Molina F, Munoz JL, Varon R, et al. A review on spectrophotometric methods for measuring the monophenolase and diphenolase activities of tyrosinase. J Agric Food Chem 2007;55:9739–49.
- Ravani Ananda R, Nagaraja P. Quantification of 4-amino-5-hydroxynaphthalene-2,7-disulfonic acid mono sodium salt by oxidation with tyrosinase in the presence of 3-methyl-2-benzothiazolinone hydrazine. Chem Sci Rev Lett 2015;4:342–8.
- Winder AJ. A stopped spectrophotometric assay for the dopa oxidase activity of tyrosinase. J Biochem Biophys Methods 1994;28:173–83.
- Chai WM, Lin MZ, Song FJ, et al. Rifampicin as a novel tyrosinase inhibitor: inhibitory activity and mechanism. Int J Biol Macromol 2017;102:425–30.
- Lee SY, Baek N, Nam TG. Natural, semisynthetic and synthetic tyrosinase inhibitors . J Enzyme Inhib Med Chem 2016; 31:1–13.
- Zhou J, Tang Q, Wu T, Cheng Z. Improved TLC bioautographic assay for qualitative and quantitative estimation of tyrosinase inhibitors in natural products. Phytochem Anal 2017;28:115–24.
- García P, Ramallo IA, Furlan RLE. Reverse phase compatible TLC-bioautography for detection of tyrosinase inhibitors. Phytochem Anal 2017;28:101–5.
- García P, Furlan RL. Multiresponse optimisation applied to the development of a TLC autography for the detection of tyrosinase inhibitors. Phytochem Anal 2015;26:287–92.
- Wangthong S, Tonsiripakdee I, Monhaphol T, et al. Post TLC developing technique for tyrosinase inhibitor detection. Biomed Chromatogr 2007;21:94–100.
- Taibon JAA, Schwaiger S, Magnenat C, et al. Prevention of false-positive results: development of an HPTLC autographic assay for the detection of natural tyrosinase inhibitors. Planta Med 2015;81:1198–204.
- Misra BB, Dey S. TLC-bioautographic evaluation of in vitro anti-tyrosinase and anti-cholinesterase potentials of sandalwood oil. Nat Prod Commun 2013;8:253–6.
- Kamagaju L, Morandini R, Bizuru E, et al. Tyrosinase modulation by five Rwandese herbal medicines traditionally used for skin treatment. J Ethnopharmacol 2013;146:824–34.
- Liu DM, Yang JL, Ha W, et al. Kinetics and inhibition study of tyrosinase by pressure mediated microanalysis. Anal Biochem 2017;525:54–9.
- Tang L, Zhang W, Zhao H, Chen Z. Tyrosinase inhibitor screening in traditional Chinese medicines by electrophoretically mediated microanalysis. J Sep Sci 2015;38:2887–92.
- Jiang TF, Liang TT, Wang YH, et al. Immobilized capillary tyrosinase microreactor for inhibitor screening in natural extracts by capillary electrophoresis. J Pharm Biomed Anal 2013;84:36–40.
- Cheng M, Chen Z. Screening of tyrosinase inhibitors by capillary electrophoresis with immobilized enzyme microreactor and molecular docking. Electrophoresis 2017;38:486–93.
- Sun BB, Qi L, Mu XY, et al. A Chiral ligand exchange CE system for monitoring inhibitory effect of kojic acid on tyrosinase. Talanta 2013;116:1121–5.
- Winder AJ, Harris H. New assays for the tyrosine hydroxylase and dopa oxidase activities of tyrosinase. Eur J Biochem 1991;198:317–26.
- Chen YM, Chavin W. Radiometric assay of tyrosinase and theoretical considerations of melanin formation. Anal Biochem 1965;13:234–58.
- Vandeput M, Patris S, Silva H, et al. Application of a tyrosinase microreactor – detector in a flow injection configuration for the determination of affinity and dynamics of inhibitor binding. Sens Actuators B Chem 2017;248:385–94.
- Ruzza P, Serra PA, Davide Fabbri D, et al. Hydroxylated biphenyls as tyrosinase inhibitor: a spectrophotometric and electrochemical study. Eur J Med Chem 2017;126:1034–8.
- Tang H, Cui F, Li H, et al. Understanding the inhibitory mechanism of tea polyphenols against tyrosinase using fluorescence spectroscopy, cyclic voltammetry, oximetry, and molecular simulations. RSC Adv 2018;8:8310–8.
- Liu Z, Liu S. A novel fluorescent biosensor for adrenaline detection and tyrosinase inhibitor screening. Anal Bioanal Chem 2018;410:4145–52.
- Hsu KD, Chan YH, Chen HJ, et al. Tyrosinase-based TLC autography for anti-melanogenic drug screening. Sci Rep 2018;8:401.
- Bagherzadeh K, Shirgahi Talari F, Sharifi A, et al. A new insight into mushroom tyrosinase inhibitors: docking, pharmacophore-based virtual screening, and molecular modeling studies. J Biomol Struct Dyn 2015;33:487–501.
- Tang H, Cui F, Liu L, Li Y. Predictive models for tyrosinase inhibitors: challenges from heterogeneous activity data determined by different experimental protocols. Comput Biol Chem 2018;73:79–84.
- Li Q, Yang H, Mo J, et al. Identification by shape-based virtual screening and evaluation of new tyrosinase inhibitors. Peer J 2018;6:e4206.
- Suthar SK, Bansal S, Narkhede N, et al. Design, synthesis and biological evaluation of oxindole-based chalcones as small-molecule inhibitors of melanogenic tyrosinase. Chem Pharm Bull 2017;65:833–9.
- Soares MA, Almeida MA, Marins-Goulart C, et al. Thiosemicarbazones as inhibitors of tyrosinase enzyme. Bioorg Med Chem Lett 2017;27:3546–50.
- Gou L, Lee J, Hao H, et al. The effect of oxaloacetic acid on tyrosinase activity and structure:Integration of inhibition kinetics with docking simulation. Int J Biol Macromol 2017;101:59–66.
- Mutahir S, Khan MA, Khan IU, et al. Organocatalyzed and mechanochemical solvent-free synthesis of novel and functionalized bis-biphenyl substituted thiazolidinones as potent tyrosinase inhibitors: SAR and molecular modeling studies. Eur J Med Chem 2017;134:406–14.
- Fan M, Zhang G, Pan J, Gong D. An inhibition mechanism of dihydromyricetin on tyrosinase and the joint effects of vitamins B6, D3 or E. Food Funct 2017;8:2601–10.
- Liu J, Li M, Yu Y, Cao S. Novel inhibitors of tyrosinase produced by the 4-substitution of TCT (pi). Int J Biol Macromol 2017;103:1096–106.
- Chai WM, Lin MZ, Feng HL, et al. Proanthocyanidins purified from fruit pericarp of Clausena lansium (Lour.) Skeels as efficient tyrosinase inhibitors: structure evaluation, inhibitory activity and molecular mechanism. Food Funct 2017;8:1043–51.
- Kwong HC, Chidan Kumar CS, Mah SH, et al. Novel biphenyl ester derivatives as tyrosinase inhibitors: synthesis, crystallographic, spectral analysis and molecular docking studies. PLoS one 2017;12:e0170117.
- Garcia-Jimenez A, Teruel-Puche JA, Ortiz-Ruiz CV, , et al. Study of the inhibition of 3-/4-aminoacetophenones on tyrosinase. Reac Kinet Mech Cat 2017;120:1–13.
- Cui Y, Hu YH, Yu F, et al. Inhibition kinetics and molecular simulation of p-substituted cinnamic acid derivatives on tyrosinase. Int J Biol Macromol 2017;95:1289–97.
- Ferro S, De Luca L, Germano MP, et al. Chemical exploration of 4-(4-fluorobenzyl)piperidine fragment for the development of new tyrosinase inhibitors. Eur J Med Chem 2017;125:992–1001.
- Tang J, Liu J, Wu F. Molecular docking studies and biological evaluation of 1,3,4-thiadiazole derivatives bearing schiff base moieties as tyrosinase inhibitors. Bioorg Chem 2016;69:29–36.
- Lall N, Mogapi E, de Canha MN, et al. Insights into tyrosinase inhibition by compounds isolated from Greyia radlkoferi Szyszyl using biological activity, molecular docking and gene expression analysis. Bioorg Med Chem 2016;24:5953–9.
- Hassani S, Haghbeen K, Fazli M. Non-specific binding sites help to explain mixed inhibition in mushroom tyrosinase activities. Eur J Med Chem 2016;122:138–48.
- Wang R, Chai WM, Yang Q, et al. (4-Fluorophenyl)-quinazolin-4(3H)-one as a novel tyrosinase inhibitor: Synthesis, inhibitory activity, and mechanism. Bioorg Med Chem 2016;24:4620–5.
- Yue LM, Lee J, Lü ZR, et al. Effect of Cd2+ on tyrosinase: integration of inhibition kinetics with computational simulation. Int J Biol Macromol 2017; 94:836–44.
- Gao H. Predicting tyrosinase inhibition by 3D QSAR pharmacophore models and designing potential tyrosinase inhibitors from traditional Chinese medicine database. Phytomedicine 2018;38:145–57.
- Khan MT. Novel tyrosinase inhibitors from natural resources – their computational studies. Curr Med Chem 2012;19:2262–72.
- Chan CF, Huang CC, Lee MY, Lin YS. Fermented broth in tyrosinase- and melanogenesis inhibition. Molecules 2014;19:13122–35.
- Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci 2009;10:2440–75.
- Chang TS. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials 2012;5:1661–85.
- Chen CY, Lin LC, Yang WF, et al. An updated organic classification of tyrosinase inhibitors on melanin biosynthesis. Curr Org Chem 2015;19:4–18.
- Hassan Khan MT. Molecular design of tyrosinase inhibitors: a critical review of promising novel inhibitors from synthetic origins. Pure Appl Chem 2007;79:2277–95.
- Likhitwitayawuid K. Stilbenes with tyrosinase inhibitory activity. Curr Sci 2008;94:44–52.
- Lin JW, Chiang HM, Lin YC, Wen KC. Natural products with skin – whitening effects. J Food Drug Anal 2008;16:1–10.
- Loizzo MR, Tundis R, Menichini F. Natural and synthetic tyrosinase inhibitors as antibrowning agents: an update. Compr Rev Food Sci Food Saf 2012;11:378–98.
- Parvez S, Kang M, Chung HS, Bae H. Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phytother Res 2007;21:805–16.
- Fernandes MS, Kerkar S. Microorganisms as a source of tyrosinase inhibitors: a review. Ann Microbiol 2017;67:343–58.
- Pillaiyar T, Manickam M, Namasivayam V. Skin whitening agents: medicinal chemistry perspective of tyrosinase inhibitors. J Enzyme Inhib Med Chem 2017;32:403–25.
- Wu B. Tyrosinase inhibitors from terrestrial and marine resources. Curr Top Med Chem 2014;14:1425–49.
- Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol 2008;84:539–49.
- Cestari TF, Dantas LP, Boza JC. Acquired hyperpigmentations. An Bras Dermatol 2014;89:11–25.
- Nouveau S, Agrawal D, Kohli M, et al. Skin hyperpigmentation in Indian population: insights and best practice. Indian J Dermatol 2016;61:487–95.
- Dorga S, Sarangal R. Pigmentary disorders: an insight. Pigment Int 2014;1:5–7.
- Garcia-Jimenez A, Teruel-Puche JA, Garcia-Ruiz PA, et al. Action of 2,2',4,4'-tetrahydroxybenzophenone in the biosynthesis pathway of melanin. Int J Biol Macromol 2017;98:622–9.
- Bull AT, Carter BLA. The isolation of tyrosinase from Aspergillus nidulans, its kinetic and molecular properties and some consideration of its activity in vivo. J Gen Microbiol 1973;75:61–73.
- Silva S. C d, Wisniewski C, Luccas PO, Magalhães CSD. Enzyme from banana (Musa sp.) extraction procedures for sensitive adrenaline biosensor construction. Am J Analyt Chem 2013;04: 293–300.
- Dolashki A, Voelter W, Gushterova A, et al. Isolation and characterization of novel tyrosinase from Laceyella sacchari. Protein Pept Lett 2012;19:538–43.,
- Haghbeen K, Rastegar j. F, Karkhaneh AA, Shareefi Borojerdi SH. Purification of tyrosinase from edible mushroom. Iran J Biotechnol 2004;2:189–94.
- Sambasiva Rao KRS, Tripathy NK, Srinivasa Rao D, Prakasham RS. Production, characterization, catalytic and inhibitory activities of tyrosinase. Res J Biotech 2013;8:187–99.
- Vieira NCS, Ferreira RA, Valquiria da CR, et al. Self-assembled films containing crude extract of avocado as a source of tyrosinase for monophenol detection. Mater Sci Eng C 2013;33:3899–902.
- Yamauchi K, Mitsunaga T, Batubara I. Isolation, identification and tyrosinase inhibitory activities of the extractives from Allamanda cathartica. Nat Res 2011;2:167–72.
- Yuan H, Ke-wu L, Dong Y, et al. Some properties of potato tyrosinase, chemical research and application. J Chem Res App 2005;1:22–7.
- Zh Y, Wu F. Catalytic properties of tyrosinase from potato and edible fungi. Biotechnology 2006;5:344–8.
- Harir M, Bellahcene M, Baratto MC, et al. Isolation and characterization of a novel tyrosinase produced by Sahara soil actinobacteria and immobilization on nylon nanofiber membranes. J Biotechnol 2018;265:54–64.
- Vanitha M, Soundhari C. Isolation and characterisation of mushroom tyrosinase and screening of herbal extracts for anti-tyrosinase activity. Int J ChemTech Research 2017;10:1156–67.
- Zekiri F, Molitor C, G.Mauracher SG, et al. Purification and characterization of tyrosinase from walnut leaves (Juglans regia). Phytochemistry 2014;101:5–15.
- Gasparetti C, Biochemical and structural characterisation of the copper containing oxidoreductases catechol oxidase, tyrosinase, and laccase from ascomycete fungi. Espoo: VTT Technical Research Centre of Finland; 2012.
- Boekelheide K, Graham DG, Mize PD, Jeffs PW. The metabolic pathway catalyzed by the tyrosinase of Agaricus bisporus. J Biol Chem 1980;255:4766–71.
- Ioniţă E, Stănciuc N, Aprodu I, et al. pH-induced structural changes of tyrosinase from Agaricus bisporus using fluorescence and in silico methods. J Sci Food Agric 2014;94:2338–44.
- Ioniţă E, Aprodu I, Stănciuc N, et al. Advances in structure-function relationships of tyrosinase from Agaricus bisporus – investigation on heat-induced conformational changes. Food Chem 2014;156:129–36.
- Khan IA, Ali R. Antigenicity, catalytic activity and conformation of Agaricus bisporus tyrosinase: interaction of conformation-directed antibodies with the native and irradiated enzyme. J Biochem 1986;99:445–52.
- Zhou L, Liu W, Zou L, et al. Aggregation and conformational change of mushroom (Agaricus bisporus) polyphenoloxidase subjected to thermal treatment. Food Chem 2017;214:423–31.
- Gheibi N, Saboury AA, Haghbeen K, Moosavi Movahedi AA. The effect of some osmolytes on the activity and stability of mushroom tyrosinase. J Biosci 2006;31:355–62.
- Narin R, Cresswell W, J Narin J. Mushroom tyrosinase: a model system to combine experimental investigation of enzyme-catalyzed reactions, data handling using R, and enzyme-inhibitor structural studies. Biochem Mol Biol Educ 2015;43:370–6.
- Della Longa S, Ascone I, Bianconi A, et al. The dinuclear copper site structure of Agaricus bisporus tyrosinase in solution probed by X-ray absorption spectroscopy. J Biol Chem 1996;271:21025–30.
- Ismaya WT, Tandrasasmita OM, Sundari S, et al. The light subunit of mushroom Agaricus bisporus tyrosinase: its biological characteristics and implications. Int J Biol Macromol 2017;102:308–14.
- Ismaya WT, Rozeboom HJ, Weijn A, et al. Crystal structure of Agaricus bisporus mushroom tyrosinase: identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011;50:5477–86.
- Strothkamp KG, Jolley RL, Mason HS. Quaternary structure of mushroom tyrosinase. Biochem Biophys Res Commun 1976;70:519–24.
- Bourquelot E, Bertrand A. Le beluissement et le noircissement des champignons. Comp Rend Soc Biol 1895;2:582–4.
- van Gelder CW, Flurkey WH, Wichers HJ. Sequence and structural features of plant and fungal tyrosinases. Phytochemistry 1997;45:1309–23.
- Lopez-Tejedor D, Palomo JM. Efficient purification of a highly active H-subunit of tyrosinase from Agaricus bisporus. Protein Expr Purif 2018;145:64–70.
- Sanchez-Ferrer A, Rodriguez-Lopez JN, Garcia-Canovas F, Garcia-Carmona F. Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta 1995;1247:1–11.
- Ortiz-Ruiz CV, Maria-Solano MA, Garcia-Molina Mdel M, et al. Kinetic characterization of substrate-analogous inhibitors of tyrosinase. IUBMB Life 2015;67:757–67.
- Dec J, Bollag JM. Effect of various factors on dehalogenation of chlorinated phenols and anilines during oxidative coupling. Environ Sci Technol 1995;29:657–63.
- Munoz-Munoz JL, Garcia-Molina F, Varon R, et al. Suicide inactivation of the diphenolase and monophenolase activities of tyrosinase. IUBMB Life 2010;62:539–47.
- Land EJ, Ramsden CA, Riley PA. The mechanism of suicide-inactivation of tyrosinase: a substrate structure investigation. Tohoku J Exp Med 2007;212:341–8.
- Haghbeen K, Saboury AA, Karbassi F. Substrate share in the suicide inactivation of mushroom tyrosinase. Biochim Biophys Acta 2004;1675:139–46.
- Saboury AA, Karbassi F, Haghbeen K, et al. Stability, structural and suicide inactivation changes of mushroom tyrosinase after acetylation by n-acetylimidazole. Int J Biol Macromol 2004;34:257–62.
- Munoz-Munoz JL, Garcia-Molina F, Garcia-Ruiz PA, et al. Phenolic substrates and suicide inactivation of tyrosinase: kinetics and mechanism. Biochem J 2008;416:431–40.
- Munoz-Munoz JL, Garcia-Molina F, Garcia-Ruiz PA, et al. Stereospecific inactivation of tyrosinase by L- and D-ascorbic acid. Biochim Biophys Acta 2009;1794:244–53.
- Munoz-Munoz JL, Acosta-Motos JR, Garcia-Molina F, et al. Tyrosinase inactivation in its action on dopa. Biochim Biophys Acta 2010;1804:1467–75.
- Munoz-Munoz JL, Garcia-Molina F, Berna J, et al. Kinetic characterisation of o-aminophenols and aromatic o-diamines as suicide substrates of tyrosinase. Biochim Biophys Acta 2012;1824:647–55.
- Muñoz-Muñoz JL, Berna J, Garcia-Molina F, et al. Unravelling the suicide inactivation of tyrosinase: a discrimination between mechanisms. J Mol Catal B Enzym 2012;75:11–9.
- Munoz-Munoz JL, Garcia-Molina Mdel M, Garcia-Molina F, et al. Indirect inactivation of tyrosinase in its action on 4-tert-butylphenol. J Enzyme Inhib Med Chem 2014;29:344–52.
- Munoz-Munoz JL, Garcia-Molina F, Acosta-Motos JR, et al. Indirect inactivation of tyrosinase in its action on tyrosine. Acta Biochim Pol 2011;58:477–88.
- del Mar Garcia-Molina M, Munoz-Munoz JL, Berna J, et al. Catalysis and inactivation of tyrosinase in its action on hydroxyhydroquinone. IUBMB Life 2014;66:122–7.
- Munoz-Munoz JL, Garcia-Molina F, Arribas E, et al. Suicide inactivation of tyrosinase in its action on tetrahydropterines. J Enzyme Inhib Med Chem 2011;26:728–33.
- Garcia-Molina F, Munoz-Munoz JL, Martinez-Ortiz F, et al. Tetrahydrofolic acid is a potent suicide substrate of mushroom tyrosinase. J Agric Food Chem 2011;59:1383–91.
- Garcia-Molina F, Munoz-Munoz JL, Garcia-Molina M, et al. Melanogenesis inhibition due to NADH. Biosci Biotechnol Biochem 2010;74:1777–87.
- Park J, Jung H, Kim K, et al. D-tyrosine negatively regulates melanin synthesis by competitively inhibiting tyrosinase activity. Pigment Cell Melanoma Res 2018;31:374–83.
- Hassani S, Gharechaei B, Nikfard S, et al. New insight into the allosteric effect of l-tyrosine on mushroom tyrosinase during l-dopa production. Int J Biol Macromol 2018;114:821–9.
- Zhao DY, Zhang MX, Dong XW, et al. Design and synthesis of novel hydroxypyridinone derivatives as potential tyrosinase inhibitors. Bioorg Med Chem Lett 2016;26:3103–8.
- Yin SJ, Si YX, Qian GY. Inhibitory effect of phthalic acid on tyrosinase: the mixed-type inhibition and docking simulations. Enzyme Res 2011;2011:1. doi: 10.4061/2011/294724.
- Yin SJ, Si YX, Chen YF, et al. Mixed-type inhibition of tyrosinase from Agaricus bisporus by terephthalic acid: computational simulations and kinetics. Protein J 2011;30:273–80.
- Liu HJ, Ji S, Fan YQ, et al. The effect of D-(−)-arabinose on tyrosinase: an integrated study using computational simulation and inhibition kinetics. Enzyme Res 2012;2012:731427. doi: 10.1155/2012/731427.
- Hridya H, Amrita A, Sankari M, et al. Inhibitory effect of brazilein on tyrosinase and melanin synthesis: kinetics and in silico approach. Int J Biol Macromol 2015;81:228–34.
- Ashraf Z, Rafiq M, Seo SY, et al. Kinetic and in silico studies of novel hydroxy-based thymol analogues as inhibitors of mushroom tyrosinase. Eur J Med Chem 2015;98:203–11.
- Karbassi F, Saboury AA, Khan MT, et al. Mushroom tyrosinase inhibition by two potent uncompetitive inhibitors. J Enzyme Inhib Med Chem 2004;19:349–53.
- Seo B, Yun J, Lee S, et al. Barbarin as a new tyrosinase inhibitor from Barbarea orthocerus. Planta Med 1999;65:683–6.
- Hu YH, Liu X, Jia YL, et al. Inhibitory kinetics of chlorocinnamic acids on mushroom tyrosinase. J Biosci Bioeng 2014;117:142–6.
- Gheibi N, Saboury AA, Haghbeen K, et al. Dual effects of aliphatic carboxylic acids on cresolase and catecholase reactions of mushroom tyrosinase. J Enzyme Inhib Med Chem 2009;24:1076–81.
- Shiino M, Watanabe Y, Umezawa K. Synthesis and tyrosinase inhibitory activity of novel N-hydroxybenzyl-N-nitrosohydroxylamines. Bioorg Chem 2003;31:129–35.
- Chen QX, Song KK, Wang Q, Huang H. Inhibitory effects on mushroom tyrosinase by some alkylbenzaldehydes. J Enzyme Inhib Med Chem 2003;18:491–6.
- Saeed A, Mahesar PA, Channar PA, et al. Synthesis, molecular docking studies of coumarinyl-pyrazolinyl substituted thiazoles as non-competitive inhibitors of mushroom tyrosinase. Bioorg Chem 2017;74:187–96.
- Mann T, Gerwat W, Batzer J, et al. Inhibition of human tyrosinase requires molecular motifs distinctively different from mushroom tyrosinase. J Invest Dermatol 2018; 138:1601–8.
- Hamed SH, Sriwiriyanont P, deLong MA, et al. Comparative efficacy and safety of deoxyarbutin, a new tyrosinase-inhibiting agent. J Cosmet Sci 2006;57:291–308.
- Sugimoto K, Nishimura T, Nomura K, et al. Syntheses of arbutin-alpha-glycosides and a comparison of their inhibitory effects with those of alpha-arbutin and arbutin on human tyrosinase. Chem Pharm Bull (Tokyo) 2003;51:798–801.
- Di Petrillo A, Gonzalez-Paramas AM, Era B, et al. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement Altern Med 2016;16:453.
- Koyu H, Kazan A, Demir S, et al. Optimization of microwave assisted extraction of Morus nigra L. Fruits maximizing tyrosinase inhibitory activity with isolation of bioactive constituents. Food Chem 2018;248:183–91.
- Lee SG, Karadeniz F, Seo Y, Kong CS. Anti-melanogenic effects of flavonoid glycosides from Limonium tetragonum (thunb.) bullock via inhibition of tyrosinase and tyrosinase-related proteins. Molecules 2017;22:1480–90.
- Matsuo K, Kobayashi M, Takuno Y, et al. Anti-tyrosinase activity constituents of Arctostaphylos uva-ursi. Yakugaku Zasshi 1997;117:1028–32.
- Alam N, Yoon KN, Lee JS, et al. Consequence of the antioxidant activities and tyrosinase inhibitory effects of various extracts from the fruiting bodies of Pleurotus ferulae. Saudi J Biol Sci 2012;19:111–18.
- Kim NY, Kwon HS, Lee HY. Effect of inhibition on tyrosinase and melanogenesis of Agastache rugosa Kuntze by lactic acid bacteria fermentation. J Cosmet Dermatol 2017;16:407–15.
- Taherkhani M. Chemical constituents, total phenolic content, antimicrobial, antioxidant and radical scavenging properties, chelating ability, tyrosinase inhibition and in vitro cytotoxic effects of Artemisia aucheri herbs. Pharm Chem J 2017;50:736–45.
- Lee GY, Cho BO, Shin JY, et al. Tyrosinase inhibitory components from the seeds of Cassia tora. Arch Pharm Res 2018;41:490–6.
- Senol FS, Orhan I, Yilmaz G, et al. Acetylcholinesterase, butyrylcholinesterase, and tyrosinase inhibition studies and antioxidant activities of 33 Scutellaria L. taxa from Turkey. Food Chem Toxicol 2010;48:781–8.
- Ya W, Chun-Meng Z, Tao G, et al. Preliminary screening of 44 plant extracts for anti-tyrosinase and antioxidant activities. Pak J Pharm Sci 2015;28:1737–44.
- Nithitanakool S, Pithayanukul P, Bavovada R, Saparpakorn P. Molecular docking studies and anti-tyrosinase activity of Thai mango seed kernel extract. Molecules 2009;14:257–65.
- Abdillahi HS, Finnie JF, Van Staden J. Anti-inflammatory, antioxidant, anti-tyrosinase and phenolic contents of four Podocarpus species used in traditional medicine in South Africa. J Ethnopharmacol 2011;136:496–503.
- Saeio K, Yotsawimonwat S, Anuchapreeda S, Okonogi S. Development of microemulsion of a potent anti-tyrosinase essential oil of an edible plant. Drug Discov Ther 2011;5:246–52.
- Mapunya MB, Hussein AA, Rodriguez B, Lall N. Tyrosinase activity of Greyia flanaganii (Bolus) constituents. Phytomedicine 2011;18:1006–12.
- Lin YS, Chen HJ, Huang JP, et al. Kinetics of tyrosinase inhibitory activity using Vitis vinifera leaf extracts. Biomed Res Int 2017;2017:5232680. doi: 10.1155/2017/5232680.
- Huang MH, Tai HM, Wang BS, Chang LW. Inhibitory effects of water extract of Flos Inulae on mutation and tyrosinase. Food Chem 2013;139:1015–20.
- Chiari ME, Joray MB, Ruiz G, et al. Tyrosinase inhibitory activity of native plants from central argentina: isolation of an active principle from Lithrea molleoides. Food Chem 2010;120:10–4.
- Burlando B, Clericuzio M, Cornara L. Moraceae plants with tyrosinase inhibitory activity: A review. Mini Rev Med Chem 2017;17:108–21.
- Chatatikun M, Chiabchalard A. Thai plants with high antioxidant levels, free radical scavenging activity, anti-tyrosinase and anti-collagenase activity. BMC Complement Altern Med 2017;17:487.
- Issa RA, Afifi FU, Amro BI. Studying the anti-tyrosinase effect of Arbutus andrachne L. extracts. Int J Cosmet Sci 2008;30:271–6.
- Hameed A, Akhtar N. Comparative chemical investigation and evaluation of antioxidant and tyrosinase inhibitory effects of Withania somnifera (L.) Dunal and Solanum nigrum (L.) berries. Acta Pharm 2018;68:47–60.
- Neagu E, Radu GL, Albu C, Paun G. Antioxidant activity, acetylcholinesterase and tyrosinase inhibitory potential of Pulmonaria officinalis and Centarium umbellatum extracts. Saudi J Biol Sci 2018;25:578–85.
- Panda P, Dash P, Ghosh G. Chemometric profile, antioxidant and tyrosinase inhibitory activity of Camel's foot creeper leaves (Bauhinia vahlii). Nat Prod Res 2018;32:596–9.
- Quispe YN, Hwang SH, Wang Z, Lim SS. Screening of peruvian medicinal plants for tyrosinase inhibitory properties: identification of tyrosinase inhibitors in Hypericum laricifolium Juss. Molecules 2017;22:doi: 10.3390/molecules22030402.
- Suh SS, Hwang J, Park M, et al. Phenol content, antioxidant and tyrosinase inhibitory activity of mangrove plants in Micronesia. Asian Pac J Trop Med 2014;7:531–5.
- Hun Son K, Young Heo M. Inhibitory effects of Korean indigenous plants on tyrosinase and melanogenesis. J Cosmet Sci 2013;64:145–58.
- Souza PM, Elias ST, Simeoni LA, et al. Plants from Brazilian cerrado with potent tyrosinase inhibitory activity. PLoS One 2012;7:e48589.
- Khazaeli P, Goldoozian R, Sharififar F. An evaluation of extracts of five traditional medicinal plants from Iran on the inhibition of mushroom tyrosinase activity and scavenging of free radicals. Int J Cosmet Sci 2009;31:375–81.
- Masuda T, Fujita N, Odaka Y, et al. Tyrosinase inhibitory activity of ethanol extracts from medicinal and edible plants cultivated in Okinawa and identification of a water-soluble inhibitor from the leaves of Nandina domestica. Biosci Biotechnol Biochem 2007;71:2316–20.
- Masuda T, Yamashita D, Takeda Y, Yonemori S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci Biotechnol Biochem 2005;69:197–201.
- Baurin N, Arnoult E, Scior T, et al. Preliminary screening of some tropical plants for anti-tyrosinase activity. J Ethnopharmacol 2002;82:155–8.
- Kubo I, Yokokawa Y, Kinst-Hori I. Tyrosinase inhibitors from Bolivian medicinal plants. J Nat Prod 1995;58:739–43.
- Bonesi M, Xiao J, Tundis R, et al. Advances in the tyrosinase inhibitors from plant source. Curr Med Chem 2018;25:1. doi: 10.2174/0929867325666180522091311.
- Vasantha KY, Murugesh CS, Sattur AP. A tyrosinase inhibitor from Aspergillus niger. J Food Sci Technol 2014;51:2877–80.
- Tsuchiya T, Yamada K, Minoura K, et al. Purification and determination of the chemical structure of the tyrosinase inhibitor produced by Trichoderma viride strain H1-7 from a marine environment. Biol Pharm Bull 2008;31:1618–20.
- Lu R, Liu X, Gao S, et al. New tyrosinase inhibitors from Paecilomyces gunnii. J Agric Food Chem 2014;62:11917–23.
- Kang HS, Choi JH, Cho WK, et al. A sphingolipid and tyrosinase inhibitors from the fruiting body of Phellinus linteus. Arch Pharm Res 2004;27:742–50.
- Morimura K, Yamazaki C, Hattori Y, et al. A tyrosinase inhibitor, daedalin a, from mycelial culture of Daedalea dickinsii. Biosci Biotechnol Biochem 2007;71:2837–40.
- Sharma VK, Choi J, Sharma N, et al. In vitro anti-tyrosinase activity of 5-(hydroxymethyl)-2-furfural isolated from Dictyophora indusiata. Phytother Res 2004;18:841–4.
- Ishihara A, Ide Y, Bito T, et al. Novel tyrosinase inhibitors from liquid culture of Neolentinus lepideus. Biosci Biotechnol Biochem 2018;82:22–30.
- Li X, Kim MK, Lee U, et al. Myrothenones A and B, cyclopentenone derivatives with tyrosinase inhibitory activity from the marine-derived fungus Myrothecium sp. Chem Pharm Bull (Tokyo) 2005;53:453–5.
- Wu B, Wu X, Sun M, Li M. Two novel tyrosinase inhibitory sesquiterpenes induced by cucl2 from a marine-derived fungus Pestalotiopsis sp. Z233. Mar Drugs 2013;11:2713–21.
- Chang TS, Tseng M, Ding HY, Shou-Ku Tai S. Isolation and characterization of Streptomyces hiroshimensis strain TI-C3 with anti-tyrosinase activity. J Cosmet Sci 2008;59:33–40.
- le Roes-Hill M, Prins A, Meyers PR. Streptomyces swartbergensis sp. Nov., a novel tyrosinase and antibiotic producing actinobacterium. Antonie Van Leeuwenhoek 2018;111:589–600.
- Nakashima T, Anzai K, Kuwahara N, et al. Physicochemical characters of a tyrosinase inhibitor produced by Streptomyces roseolilacinus NBRC 12815. Biol Pharm Bull 2009;32:832–6.
- Deering RW, Chen J, Sun J, et al. N-acyl dehydrotyrosines, tyrosinase inhibitors from the marine bacterium Thalassotalea sp. PP2-459. J Nat Prod 2016;79:447–50.
- Sano T, Kaya K. Oscillapeptin G, a tyrosinase inhibitor from toxic Oscillatoria agardhii. J Nat Prod 1996;59:90–2.
- Ji K, Cho YS, Kim YT. Tyrosinase inhibitory and anti-oxidative effects of lactic acid bacteria isolated from dairy cow feces. Probiotics Antimicrob Proteins 2018;10:43–55.
- Wang GH, Chen CY, Tsai TH, et al. Evaluation of tyrosinase inhibitory and antioxidant activities of Angelica dahurica root extracts for four different probiotic bacteria fermentations. J Biosci Bioeng 2017;123:679–84.
- Crozier A, Jaganath IB, Clifford MN. Phenols, polyphenols and tannins: an overview. Plant Secondary Metabolites 2007;1–24. 10.1002/9780470988558.ch1.
- Sakuma K, Ogawa M, Sugibayashi K, et al. Relationship between tyrosinase inhibitory action and oxidation-reduction potential of cosmetic whitening ingredients and phenol derivatives. Arch Pharm Res 1999;22:335–9.
- Hashimoto A, Ichihashi M, Mishima Y. The mechanism of depigmentation by hydroquinone: a study on suppression and recovery processes of tyrosinase activity in the pigment cells in vivo and in vitro. Nihon Hifuka Gakkai Zasshi 1984;94:797–804.
- Chen YR, Y-Y R, Lin TY, et al. Identification of an alkylhydroquinone from Rhus succedanea as an inhibitor of tyrosinase and melanogenesis. J Agric Food Chem 2009;57:2200–5.
- Sasaki A, Yamano Y, Sugimoto S, et al. Phenolic compounds from the leaves of Breynia officinalis and their tyrosinase and melanogenesis inhibitory activities. J Nat Med 2018;72:381–9.
- Chawla S, deLong MA, Visscher MO, et al. Mechanism of tyrosinase inhibition by deoxyarbutin and its second-generation derivatives. Br J Dermatol 2008;159:1267–74.
- Boissy RE, Visscher M, DeLong MA. Deoxyarbutin: a novel reversible tyrosinase inhibitor with effective in vivo skin lightening potency. Exp Dermatol 2005;14:601–8.
- Chawla S, Kvalnes K, deLong MA, et al. Deoxyarbutin and its derivatives inhibit tyrosinase activity and melanin synthesis without inducing reactive oxygen species or apoptosis. J Drugs Dermatol 2012;11:e28–34.
- Tasaka K, Kamei C, Nakano S, et al. Effects of certain resorcinol derivatives on the tyrosinase activity and the growth of melanoma cells. Methods Find Exp Clin Pharmacol 1998;20:99–109.
- Kolbe L, Mann T, Gerwat W, et al. 4-n-Butylresorcinol, a highly effective tyrosinase inhibitor for the topical treatment of hyperpigmentation. J Eur Acad Dermatol Venereol 2013;27:19–23.
- Nguyen MH, Nguyen HX, Nguyen MT, Nguyen NT. Phenolic constituents from the heartwood of Artocapus altilis and their tyrosinase inhibitory activity. Nat Prod Commun 2012;7:185–6.
- Ashraf Z, Rafiq M, Seo SY, et al. Synthesis, kinetic mechanism and docking studies of vanillin derivatives as inhibitors of mushroom tyrosinase. Bioorg Med Chem 2015;23:5870–80.
- Shirota S, Miyazaki K, Aiyama R, et al. Tyrosinase inhibitors from crude drugs. Biol Pharm Bull 1994;17:266–9.
- Matsumoto T, Nakajima T, Iwadate T, Nihei KI. Chemical synthesis and tyrosinase-inhibitory activity of isotachioside and its related glycosides. Carbohydr Res 2018;465:22–8.
- Deri B, Kanteev M, Goldfeder M, et al. The unravelling of the complex pattern of tyrosinase inhibition. Sci Rep 2016;6:34993.
- Garcia-Jimenez A, Teruel-Puche JA, Berna J, et al. Action of tyrosinase on alpha and beta-arbutin: a kinetic study. PLoS One 2017;12:e0177330.
- Garcia-Molina MO, Munoz-Munoz JL, Garcia-Molina F, et al. Study of umbelliferone hydroxylation to esculetin catalyzed by polyphenol oxidase. Biol Pharm Bull 2013;36:1140–5.
- Maria Del Mar Garcia-Molina JB, Muñoz-Muñoz JL, García-Ruiz PA, Moreno MG, Martinez JR, Garcia-Canovas F. Action of tyrosinase on hydroquinone in the presence of catalytic amounts of o-diphenol. A kinetic study. React Kinet Mech Cat 2014;112:305–20.
- Garcia-Molina Mdel M, Munoz Munoz JL, Martinez-Ortiz F, et al. Tyrosinase-catalyzed hydroxylation of hydroquinone, a depigmenting agent, to hydroxyhydroquinone: a kinetic study. Bioorg Med Chem 2014;22:3360–9.
- Ortiz-Ruiz CV, Berna J, Garcia-Molina Mdel M, et al. Identification of p-hydroxybenzyl alcohol, tyrosol, phloretin and its derivate phloridzin as tyrosinase substrates. Bioorg Med Chem 2015;23:3738–46.
- Ortiz-Ruiz CV, Berna J, Rodriguez-Lopez JN, et al. Tyrosinase-catalyzed hydroxylation of 4-hexylresorcinol, an antibrowning and depigmenting agent: A kinetic study. J Agric Food Chem 2015;63:7032–40.
- Ortiz-Ruiz CV, Ballesta de Los Santos M, Berna J, et al. Kinetic characterization of oxyresveratrol as a tyrosinase substrate. IUBMB Life 2015;67:828–36.
- Garcia-Jimenez A, Teruel-Puche JA, Ortiz-Ruiz CV, et al. 4-n-Butylresorcinol, a depigmenting agent used in cosmetics, reacts with tyrosinase. IUBMB Life 2016;68:663–72.
- Garcia-Jimenez A, Teruel-Puche JA, Berna J, et al. Characterization of the action of tyrosinase on resorcinols. Bioorg Med Chem 2016;24:4434–43.
- Garcia-Jimenez A, Munoz-Munoz JL, Garcia-Molina F, et al. Spectrophotometric characterization of the action of tyrosinase on p-coumaric and caffeic acids: characteristics of o-caffeoquinone. J Agric Food Chem 2017;65:3378–86.
- Garcia-Jimenez A, Garcia-Molina F, Teruel-Puche JA, et al. Catalysis and inhibition of tyrosinase in the presence of cinnamic acid and some of its derivatives. Int J Biol Macromol 2018;119:548–54.
- Garcia-Jimenez A, Teruel-Puche JA, Garcia-Ruiz PA, et al. Structural and kinetic considerations on the catalysis of deoxyarbutin by tyrosinase. PLoS One 2017;12:e0187845.
- Ortiz-Ruiz CV, Garcia-Molina Mdel M, Serrano JT, et al. Discrimination between alternative substrates and inhibitors of tyrosinase. J Agric Food Chem 2015;63:2162–71.
- Lin D, Xiao M, Zhao J, et al. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016;21:1374. https://doi.org/10.3390/molecules21101374
- Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer 2010;62:1–20.
- Wang Y, Curtis-Long MJ, Lee BW, et al. Inhibition of tyrosinase activity by polyphenol compounds from Flemingia philippinensis roots. Bioorg Med Chem 2014;22:1115–20.
- Xue YL, Miyakawa T, Hayashi Y, et al. Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon, Diospyros kaki. J Agric Food Chem 2011;59:6011–7.
- Sirat HM, Rezali MF, Ujang Z. Isolation and identification of radical scavenging and tyrosinase inhibition of polyphenols from Tibouchina semidecandra L. J Agric Food Chem 2010;58:10404–9.
- Yoon NY, Eom TK, Kim MM, Kim SK. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J Agric Food Chem 2009;57:4124–9.
- Fujimoto A, Shingai Y, Nakamura M, et al. A novel ring-expanded product with enhanced tyrosinase inhibitory activity from classical Fe-catalyzed oxidation of rosmarinic acid, a potent antioxidative lamiaceae polyphenol. Bioorg Med Chem Lett 2010;20:7393–6.
- Solimine J, Garo E, Wedler J, et al. Tyrosinase inhibitory constituents from a polyphenol-enriched fraction of rose oil distillation wastewater. Fitoterapia 2016;108:13–9.
- Du ZY, Jiang YF, Tang ZK, et al. Antioxidation and tyrosinase inhibition of polyphenolic curcumin analogs. Biosci Biotechnol Biochem 2011;75:2351–8.
- Jiang Y, Du Z, Xue G, et al. Synthesis and biological evaluation of unsymmetrical curcumin analogues as tyrosinase inhibitors. Molecules 2013;18:3948–61.
- Ng LT, Ko HH, Lu TM. Potential antioxidants and tyrosinase inhibitors from synthetic polyphenolic deoxybenzoins. Bioorg Med Chem 2009;17:4360–6.
- Zheng ZP, Zhang YN, Zhang S, Chen J. One-pot green synthesis of 1,3,5-triarylpentane-1,5-dione and triarylmethane derivatives as a new class of tyrosinase inhibitors. Bioorg Med Chem Lett 2016;26:795–8.
- Orhan IE, Khan MT. Flavonoid derivatives as potent tyrosinase inhibitors – a survey of recent findings between 2008–2013. Curr Top Med Chem 2014;14:1486–93.
- Jegal J, Park SA, Chung K, et al. Tyrosinase inhibitory flavonoid from Juniperus communis fruits. Biosci Biotechnol Biochem 2016;80:2311–7.
- Muhammad D, Hubert J, Lalun N, et al. Isolation of flavonoids and triterpenoids from the fruits of Alphitonia neocaledonica and evaluation of their anti-oxidant, anti-tyrosinase and cytotoxic activities. Phytochem Anal 2015;26:137–44.
- Erdogan Orhan I, Senol FS, Aslan Erdem S, et al. Tyrosinase and cholinesterase inhibitory potential and flavonoid characterization of Viola odorata L. (Sweet Violet). Phytother Res 2015;29:1304–10.
- Liang CP, Chang CH, Liang CC, et al. In vitro antioxidant activities, free radical scavenging capacity, and tyrosinase inhibitory of flavonoid compounds and ferulic acid from Spiranthes sinensis (Pers.). AMES Mol 2014;19:4681–94.
- Badria FA, elGayyar MA. A new type of tyrosinase inhibitors from natural products as potential treatments for hyperpigmentation. Boll Chim Farm 2001;140:267–71.
- Promden W, Viriyabancha W, Monthakantirat O, et al. Correlation between the potency of flavonoids on mushroom tyrosinase inhibitory activity and melanin synthesis in melanocytes. Molecules 2018;23:1403. doi: 10.3390/molecules23061403.
- Demirkiran O, Sabudak T, Ozturk M, Topcu G. Antioxidant and tyrosinase inhibitory activities of flavonoids from Trifolium nigrescens Subsp. petrisavi. J Agric Food Chem 2013;61:12598–603.
- Yao Y, Cheng X, Wang L, et al. Mushroom tyrosinase inhibitors from mung bean (Vigna radiatae L.) extracts. Int J Food Sci Nutr 2012;63:358–61.
- Lou SN, Yu MW, Ho CT. Tyrosinase inhibitory components of immature calamondin peel. Food Chem 2012;135:1091–6.
- Hu X, Wu JW, Wang M, et al. 2-Arylbenzofuran, flavonoid, and tyrosinase inhibitory constituents of Morus yunnanensis. J Nat Prod 2012;75:82–7.
- Fawole OA, Makunga NP, Opara UL. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement Altern Med 2012;12:200.
- Kim JM, Ko RK, Jung DS, et al. Tyrosinase inhibitory constituents from the stems of Maackia fauriei. Phytother Res 2010;24:70–5.
- Alam N, Yoon KN, Lee KR, et al. Antioxidant activities and tyrosinase inhibitory effects of different extracts from Pleurotus ostreatus fruiting bodies. Mycobiology 2010;38:295–301.
- Piao X, Tian Y, Mi X, Cui J. tyrosinase inhibition of Potentilla bifurca. Zhongguo Zhong Yao Za Zhi 2009;34:1952–4.
- Lu YH, Lin T, Wang ZT, et al. Mechanism and inhibitory effect of galangin and its flavonoid mixture from Alpinia officinarum on mushroom tyrosinase and B16 murine melanoma cells. J Enzyme Inhib Med Chem 2007;22:433–8.
- Jeong SH, Ryu YB, Curtis-Long MJ, et al. Tyrosinase inhibitory polyphenols from roots of Morus lhou. J Agric Food Chem 2009;57:1195–203.
- Kishore N, Twilley D, Blom van Staden A, et al. Isolation of flavonoids and flavonoid glycosides from Myrsine africana and their inhibitory activities against mushroom tyrosinase. J Nat Prod 2018;81:49–56.
- Lee HS. Tyrosinase inhibitors of Pulsatilla cernua root-derived materials. J Agric Food Chem 2002;50:1400–3.
- Wang YL, Hu G, Zhang Q, et al. Screening and characterizing tyrosinase inhibitors from Salvia miltiorrhiza and Carthamus tinctorius by spectrum-effect relationship analysis and molecular docking. J Anal Methods Chem 2018;2018:1.
- Azizuddin, Khan AM, Choudhary MI. Tyrosinase inhibitory potential of natural products isolated from various medicinal plants. Nat Prod Res 2011;25:750–3.
- Falcone Ferreyra ML, Rius SP, Casati P. Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 2012;3:222.
- Lee NK, Son KH, Chang HW, et al. Prenylated flavonoids as tyrosinase inhibitors. Arch Pharm Res 2004;27:1132–5.
- Rho HS, Ahn SM, Lee BC, et al. Changes in flavonoid content and tyrosinase inhibitory activity in kenaf leaf extract after far-infrared treatment. Bioorg Med Chem Lett 2010;20:7534–6.
- Manthey JA, Cesar TB, Jackson E, Mertens-Talcott S. Pharmacokinetic study of nobiletin and tangeretin in rat serum by high-performance liquid chromatography-electrospray ionization-mass spectrometry. J Agric Food Chem 2011;59:145–51.
- Shang C, Zhang Y, You X, et al. The effect of 7,8,4-trihydroxyflavone on tyrosinase activity and conformation: spectroscopy and docking studies. Luminescence 2018;33:681–91.
- Gao H, Nishida J, Saito S, Kawabata J. Inhibitory effects of 5,6,7-trihydroxyflavones on tyrosinase. Molecules 2007;12:86–97.
- Mu Y, Li L, Hu SQ. Molecular inhibitory mechanism of tricin on tyrosinase. Spectrochim Acta A Mol Biomol Spectrosc 2013;107:235–40.
- Guo N, Wang C, Shang C, et al. Integrated study of the mechanism of tyrosinase inhibition by baicalein using kinetic, multispectroscopic and computational simulation analyses. Int J Biol Macromol 2018;118:57–68.
- Zhang L, Tao G, Chen J, Zheng ZP, Characterization of a new flavone and tyrosinase inhibition constituents from the Twigs of Morus alba L. Molecules 2016;21(9):1130. doi: 10.3390/molecules21091130
- Ryu YB, Ha TJ, Curtis-Long MJ, et al. Inhibitory effects on mushroom tyrosinase by flavones from the stem barks of Morus lhou (S.) Koidz. J Enzyme Inhib Med Chem 2008;23:922–30.
- Kubo I, Kinst-Hori I. Flavonols from saffron flower: tyrosinase inhibitory activity and inhibition mechanism. J Agric Food Chem 1999;47:4121–5.
- Omar SH, Scott CJ, Hamlin AS, Obied HK. Biophenols: enzymes (β-secretase, cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.)). Fitoterapia 2018;128:118–29.
- Yang Z, Zhang Y, Sun L, et al. An ultrafiltration high-performance liquid chromatography coupled with diode array detector and mass spectrometry approach for screening and characterising tyrosinase inhibitors from mulberry leaves. Anal Chim Acta 2012;719:87–95.
- Wang Y, Zhang G, Yan J, Gong D. Inhibitory effect of morin on tyrosinase: insights from spectroscopic and molecular docking studies. Food Chem 2014;163:226–33.
- Zheng ZP, Zhu Q, Fan CL, et al. Phenolic tyrosinase inhibitors from the stems of Cudrania cochinchinensis. Food Funct 2011;2:259–64.
- Kim JH, Cho IS, So YK, et al. Kushenol a and 8-prenylkaempferol, tyrosinase inhibitors, derived from Sophora flavescens. J Enzyme Inhib Med Chem 2018;33:1048–54.
- Park JS, Kim DH, Lee JK, et al. Natural ortho-dihydroxyisoflavone derivatives from aged Korean fermented soybean paste as potent tyrosinase and melanin formation inhibitors. Bioorg Med Chem Lett 2010;20:1162–4.
- Chang TS, Ding HY, Lin HC. Identifying 6,7,4'-trihydroxyisoflavone as a potent tyrosinase inhibitor. Biosci Biotechnol Biochem 2005;69:1999–2001.
- Chang TS. Two potent suicide substrates of mushroom tyrosinase: 7,8,4'-trihydroxyisoflavone and 5,7,8,4'-tetrahydroxyisoflavone. J Agric Food Chem 2007;55:2010–5.
- Chen J, Yu X, Huang Y. Inhibitory mechanisms of glabridin on tyrosinase. Spectrochim Acta A Mol Biomol Spectrosc 2016;168:111–7.
- Deshmukh K, Poddar SS. Tyrosinase inhibitor-loaded microsponge drug delivery system: new approach for hyperpigmentation disorders. J Microencapsul 2012;29:559–68.
- Jirawattanapong W, Saifah E, Patarapanich C. Synthesis of glabridin derivatives as tyrosinase inhibitors. Arch Pharm Res 2009;32:647–54.
- Nerya O, Vaya J, Musa R, et al. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J Agric Food Chem 2003;51:1201–7.
- Heo do Y, Kim YM, Lee J, et al. Desmodianone H and uncinanone B, potential tyrosinase inhibitors obtained from Lespedeza maximowiczii by using bioactivity-guided isolation. Biosci Biotechnol Biochem 2014;78:943–5.
- Kim HJ, Seo SH, Lee BG, Lee YS. Identification of tyrosinase inhibitors from Glycyrrhiza uralensis. Planta Med 2005;71:785–7.
- Kim JH, Kim HY, Kang SY, et al. Chemical constituents from Apios americana and their inhibitory activity on tyrosinase. Molecules 2018;23:232. doi: 10.3390/molecules23010232.
- Si YX, Wang ZJ, Park D, et al. Effect of hesperetin on tyrosinase: inhibition kinetics integrated computational simulation study. Int J Biol Macromol 2012;50:257–62.
- Chiari ME, Vera DM, Palacios SM, Carpinella MC. Tyrosinase inhibitory activity of a 6-isoprenoid-substituted flavanone isolated from Dalea elegans. Bioorg Med Chem 2011;19:3474–82.
- Hu X, Yu MH, Yan GR, et al. Isoprenylated phenolic compounds with tyrosinase inhibition from Morus nigra. J Asian Nat Prod Res 2018;20:488–93.
- Kim SS, Hyun CG, Choi YH, Lee NH. Tyrosinase inhibitory activities of the compounds isolated from Neolitsea aciculata (Blume) Koidz. J Enzyme Inhib Med Chem 2013;28:685–9.
- Ko RK, Kim GO, Hyun CG, et al. Compounds with tyrosinase inhibition, elastase inhibition and DPPH radical scavenging activities from the branches of Distylium racemosum Sieb. et Zucc. Phytother Res 2011;25:1451–6.
- Chai WM, Lin MZ, Wang YX, et al. Inhibition of tyrosinase by cherimoya pericarp proanthocyanidins: structural characterization, inhibitory activity and mechanism. Food Res Int 2017;100:731–9.
- Kim YJ, Chung JE, Kurisawa M, et al. New tyrosinase inhibitors, (+)-catechin-aldehyde polycondensates. Biomacromolecules 2004;5:474–9.
- Chai WM, Huang Q, Lin MZ, et al. Condensed tannins from longan bark as inhibitor of tyrosinase: structure, activity, and mechanism. J Agric Food Chem 2018;66:908–17.
- Jhan JK, Chung YC, Chen GH, et al. Anthocyanin contents in the seed coat of black soya bean and their anti-human tyrosinase activity and antioxidative activity. Int J Cosmet Sci 2016;38:319–24.
- Bukhari SN, Jantan I, Unsal Tan O, et al. Biological activity and molecular docking studies of curcumin-related α,β-unsaturated carbonyl-based synthetic compounds as anticancer agents and mushroom tyrosinase inhibitors. J Agric Food Chem 2014;62:5538–47.
- Liu J, Wu F, Chen L, et al. Biological evaluation of coumarin derivatives as mushroom tyrosinase inhibitors. Food Chem 2012;135:2872–8.
- Le-Thi-Thu H, Casanola-Martin GM, Marrero-Ponce Y, et al. Novel coumarin-based tyrosinase inhibitors discovered by OECD principles-validated QSAR approach from an enlarged, balanced database. Mol Divers 2011;15:507–20.
- Hassan M, Ashraf Z, Abbas Q, et al. Exploration of novel human tyrosinase inhibitors by molecular modeling, docking and simulation studies. Interdiscip Sci 2018;10:68–80.
- Pintus F, Matos MJ, Vilar S, et al. New insights into highly potent tyrosinase inhibitors based on 3-heteroarylcoumarins: anti-melanogenesis and antioxidant activities, and computational molecular modeling studies. Bioorg Med Chem 2017;25:1687–95.
- Masamoto Y, Ando H, Murata Y, et al. Mushroom tyrosinase inhibitory activity of esculetin isolated from seeds of Euphorbia lathyris L. Biosci Biotechnol Biochem 2003;67:631–4.
- Ahmad VU, Ullah F, Hussain J, et al. Tyrosinase inhibitors from Rhododendron collettianum and their structure–activity relationship (SAR) studies. Chem Pharm Bull (Tokyo) 2004;52:1458–61.
- Ashraf Z, Rafiq M, Seo SY, et al. Design, synthesis and bioevaluation of novel umbelliferone analogues as potential mushroom tyrosinase inhibitors. J Enzyme Inhib Med Chem 2015;30:874–83.
- Matos MJ, Santana L, Uriarte E, et al. New halogenated phenylcoumarins as tyrosinase inhibitors. Bioorg Med Chem Lett 2011;21:3342–5.
- Ueno T, Fukami H, Ohkishi H, et al. Synthesis of 3,4-dihydro-3-amino-7-hydroxycoumarin from s-methyl-l-cysteine and resorcinol by crystalline-beta-tyrosinase. Biochim Biophys Acta 1970;206:476–9.
- Asthana S, Zucca P, Vargiu AV, et al. Structure–activity relationship study of hydroxycoumarins and mushroom tyrosinase. J Agric Food Chem 2015;63:7236–44.
- Gardelly M, Trimech B, Belkacem MA, et al. Synthesis of novel diazaphosphinanes coumarin derivatives with promoted cytotoxic and anti-tyrosinase activities. Bioorg Med Chem Lett 2016;26:2450–4.
- Tocco G, Fais A, Meli G, et al. PEG-immobilization of cardol and soluble polymer-supported synthesis of some cardol-coumarin derivatives: preliminary evaluation of their inhibitory activity on mushroom tyrosinase. Bioorg Med Chem Lett 2009;19:36–9.
- Fais A, Corda M, Era B, et al. Tyrosinase inhibitor activity of coumarin-resveratrol hybrids. Molecules 2009;14:2514–20.
- Yang HH, Oh KE, Jo YH, et al. Characterization of tyrosinase inhibitory constituents from the aerial parts of Humulus japonicus using LC-MS/MS coupled online assay. Bioorg Med Chem 2018;26:509–15.
- Takahashi M, Takara K, Toyozato T, Wada K. A novel bioactive chalcone of Morus australis inhibits tyrosinase activity and melanin biosynthesis in B16 melanoma cells. J Oleo Sci 2012;61:585–92.
- Zhang X, Hu X, Hou A, Wang H. Inhibitory effect of 2,4,2',4'-tetrahydroxy-3-(3-methyl-2-butenyl)-chalcone on tyrosinase activity and melanin biosynthesis. Biol Pharm Bull 2009;32:86–90.
- Niesen DB, Ma H, Yuan T, et al. Phenolic constituents of Carex vulpinoidea seeds and their tyrosinase inhibitory activities. Nat Prod Commun 2015;10:491–3.
- Suthar SK, Bansal S, Narkhede N, et al. Design, synthesis and biological evaluation of oxindole-based chalcones as small-molecule inhibitors of melanogenic tyrosinase. Chem Pharm Bull (Tokyo) 2017;65:833–9.
- Kim BH, Park KC, Park JH, et al. Inhibition of tyrosinase activity and melanin production by the chalcone derivative 1-(2-cyclohexylmethoxy-6-hydroxy-phenyl)-3-(4-hydroxymethyl-phenyl)-propenone. Biochem Biophys Res Commun 2016;480:648–54.
- Niu C, Yin L, Nie LF, et al. Synthesis and bioactivity of novel isoxazole chalcone derivatives on tyrosinase and melanin synthesis in murine B16 cells for the treatment of vitiligo. Bioorg Med Chem 2016;24:5440–8.
- Radhakrishnan SK, Shimmon RG, Conn C, Baker AT. Evaluation of novel chalcone oximes as inhibitors of tyrosinase and melanin formation in B16 cells. Arch Pharm (Weinheim) 2016;349:20–9.
- Radhakrishnan SK, Shimmon RG, Conn C, Baker AT. Inhibitory kinetics of azachalcones and their oximes on mushroom tyrosinase: a facile solid-state synthesis. Chem Biodivers 2016;13:531–8.
- Dong X, Zhang Y, He JL, et al. Preparation of tyrosinase inhibitors and antibrowning agents using green technology. Food Chem 2016;197:589–96.
- Jun N, Hong G, Jun K. Synthesis and evaluation of 2',4',6'-trihydroxychalcones as a new class of tyrosinase inhibitors. Bioorg Med Chem 2007;15:2396–402.
- Radhakrishnan S, Shimmon R, Conn C, Baker A. Integrated kinetic studies and computational analysis on naphthyl chalcones as mushroom tyrosinase inhibitors. Bioorg Med Chem Lett 2015;25:4085–91.
- Liu J, Chen C, Wu F, Zhao L. Microwave-assisted synthesis and tyrosinase inhibitory activity of chalcone derivatives. Chem Biol Drug Des 2013;82:39–47.
- Nerya O, Musa R, Khatib S, et al. Chalcones as potent tyrosinase inhibitors: the effect of hydroxyl positions and numbers. Phytochemistry 2004;65:1389–95.
- Okombi S, Rival D, Bonnet S, et al. Discovery of benzylidenebenzofuran-3(2H)-one (aurones) as inhibitors of tyrosinase derived from human melanocytes. J Med Chem 2006;49:329–33.
- Zhu JJ, Yan GR, Xu ZJ, et al. Inhibitory effects of (2'R)-2',3'-dihydro-2'-(1-hydroxy-1-methylethyl)-2,6'-bibenzofuran-6,4'-diol on mushroom tyrosinase and melanogenesis in B16-F10 melanoma cells. Phytother Res 2015;29:1040–5.
- Hu X, Wang M, Yan GR, et al. 2-Arylbenzofuran and tyrosinase inhibitory constituents of Morus notabilis. J Asian Nat Prod Res 2012;14:1103–8.
- Koirala P, Seong SH, Zhou Y, et al. Structure(−)activity relationship of the tyrosinase inhibitors kuwanon G, mulberrofuran G, and albanol B from Morus species: a kinetics and molecular docking study. Molecules 2018;23:1413. doi: 10.3390/molecules23061413.
- Wang Y, Xu L, Gao W, et al. Isoprenylated phenolic compounds from Morus macroura as potent tyrosinase inhibitors. Planta Med 2018;84:336–43.
- Lin YF, Hu YH, Lin HT, et al. Inhibitory effects of propyl gallate on tyrosinase and its application in controlling pericarp browning of harvested longan fruits. J Agric Food Chem 2013;61:2889–95.
- Lopes TIB, Coelho RG, Honda NK. Inhibition of mushroom tyrosinase activity by orsellinates. Chem Pharm Bull (Tokyo) 2018;66:61–4.
- Lim JY, Ishiguro K, Kubo I. Tyrosinase inhibitory p-coumaric acid from ginseng leaves. Phytother Res 1999;13:371–5.
- Cabanes J, Garcia-Carmona F, Garcia-Canovas F, et al. Kinetic study on the slow inhibition of epidermis tyrosinase by m-coumaric acid. Biochim Biophys Acta 1984;790:101–7.
- An SM, Koh JS, Boo YC. p-Coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytother Res 2010;24:1175–80.
- Garcia-Jimenez A, Teruel-Puche JA, Garcia-Ruiz PA, et al. Action of tyrosinase on caffeic acid and its n-nonyl ester. Catalysis and suicide inactivation. Int J Biol Macromol 2018;107:2650–9.
- Hu YH, Chen QX, Cui Y, et al. 4-Hydroxy cinnamic acid as mushroom preservation: anti-tyrosinase activity kinetics and application. Int J Biol Macromol 2016;86:489–95.
- Kwak SY, Yang JK, Choi HR, et al. Synthesis and dual biological effects of hydroxycinnamoyl phenylalanyl/prolyl hydroxamic acid derivatives as tyrosinase inhibitor and antioxidant. Bioorg Med Chem Lett 2013;23:1136–42.
- Iwai K, Kishimoto N, Kakino Y, et al. In vitro antioxidative effects and tyrosinase inhibitory activities of seven hydroxycinnamoyl derivatives in green coffee beans. J Agric Food Chem 2004;52:4893–8.
- Oyama T, Takahashi S, Yoshimori A, et al. Discovery of a new type of scaffold for the creation of novel tyrosinase inhibitors. Bioorg Med Chem 2016;24:4509–15.
- Bernard P, Berthon JY. Resveratrol: an original mechanism on tyrosinase inhibition. Int J Cosmet Sci 2000;22:219–26.
- Park J, Boo YC. Isolation of resveratrol from Vitis Viniferae caulis and its potent inhibition of human tyrosinase. Evid Based Complement Alternat Med 2013;2013:645257.
- Gilly R, Mara D, Oded S, Zohar K. Resveratrol and a novel tyrosinase in carignan grape juice. J Agric Food Chem 2001;49:1479–85.
- Likhitwitayawuid K, Sritularak B, De-Eknamkul W. Tyrosinase inhibitors from Artocarpus gomezianus. Planta Med 2000;66:275–7.
- Lee N, Kim EJ, Kim BG. Regioselective hydroxylation of trans-resveratrol via inhibition of tyrosinase from Streptomyces avermitilis MA4680. ACS Chem Biol 2012;7:1687–92.
- Ohguchi K, Tanaka T, Ito T, et al. Inhibitory effects of resveratrol derivatives from dipterocarpaceae plants on tyrosinase activity. Biosci Biotechnol Biochem 2003;67:1587–9.
- Franco DC, de Carvalho GS, Rocha PR, et al. Inhibitory effects of resveratrol analogs on mushroom tyrosinase activity. Molecules 2012;17:11816–25.
- Shin NH, Ryu SY, Choi EJ, et al. Oxyresveratrol as the potent inhibitor on DOPA oxidase activity of mushroom tyrosinase. Biochem Biophys Res Commun 1998;243:801–3.
- Zheng ZP, Tan HY, Wang M. Tyrosinase inhibition constituents from the roots of Morus australis. Fitoterapia 2012;83:1008–13.
- Bae SJ, Ha YM, Kim JA, et al. A novel synthesized tyrosinase inhibitor: (E)-2-((2,4-dihydroxyphenyl) diazenyl) phenyl 4-methylbenzenesulfonate as an azo-resveratrol analog. Biosci Biotechnol Biochem 2013;77:65–72.
- Song YM, Ha YM, Kim JA, et al. Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg Med Chem Lett 2012;22:7451–5.
- Yanagihara M, Yoshimatsu M, Inoue A, et al. Inhibitory effect of gnetin c, a resveratrol dimer from melinjo (Gnetum gnemon), on tyrosinase activity and melanin biosynthesis. Biol Pharm Bull 2012;35:993–6.
- Kim YM, Yun J, Lee CK, et al. Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J Biol Chem 2002;277:16340–4.
- Ohguchi K, Tanaka T, Kido T, et al. Effects of hydroxystilbene derivatives on tyrosinase activity. Biochem Biophys Res Commun 2003;307:861–3.
- Kim DH, Kim JH, Baek SH, et al. Enhancement of tyrosinase inhibition of the extract of Veratrum patulum using cellulase. Biotechnol Bioeng 2004;87:849–54.
- Uesugi D, Hamada H, Shimoda K, et al. Synthesis, oxygen radical absorbance capacity, and tyrosinase inhibitory activity of glycosides of resveratrol, pterostilbene, and pinostilbene. Biosci Biotechnol Biochem 2017;81:226–30.
- Ismail T, Shafi S, Srinivas J, et al. Synthesis and tyrosinase inhibition activity of trans-stilbene derivatives. Bioorg Chem 2016;64:97–102.
- Lima LL, Lima RM, da Silva AF, et al. Azastilbene analogs as tyrosinase inhibitors: new molecules with depigmenting potential. Scientific World Journal 2013;2013:274643. doi: 10.1155/2013/274643.
- Choi J, Bae SJ, Ha YM, et al. A newly synthesized, potent tyrosinase inhibitor: 5-(6-hydroxy-2-naphthyl)-1,2,3-benzenetriol. Bioorg Med Chem Lett 2010;20:4882–4.
- Song S, Lee H, Jin Y, et al. Syntheses of hydroxy substituted 2-phenyl-naphthalenes as inhibitors of tyrosinase. Bioorg Med Chem Lett 2007;17:461–4.
- Ha YM, Chung SW, Song S, et al. 4-(6-Hydroxy-2-naphthyl)-1,3-bezendiol: a potent, new tyrosinase inhibitor. Biol Pharm Bull 2007;30:1711–5.
- Satooka H, Kubo I. Resveratrol as a kcat type inhibitor for tyrosinase: potentiated melanogenesis inhibitor. Bioorg Med Chem 2012;20:1090–9.
- Fachinetti N, Rigon RB, Eloy JO, et al. Comparative study of glyceryl behenate or polyoxyethylene 40 stearate-based lipid carriers for trans-resveratrol delivery: development, characterization and evaluation of the in vitro tyrosinase inhibition. AAPS PharmSciTech 2018;19:1401–9.
- Wu B, Zhang X, Wu X. New lignan glucosides with tyrosinase inhibitory activities from exocarp of Castanea henryi. Carbohydr Res 2012;355:45–9.
- Karioti A, Protopappa A, Megoulas N, Skaltsa H. Identification of tyrosinase inhibitors from Marrubium velutinum and Marrubium cylleneum. Bioorg Med Chem 2007;15:2708–14.
- Wu YY, Huang XX, Wu J, et al. A new cyclolignan glycoside from the tubers of Pinellia ternata. J Asian Nat Prod Res 2015;17:1097–103.
- Huang XX, Liu QB, Wu J, et al. Antioxidant and tyrosinase inhibitory effects of neolignan glycosides from Crataegus pinnatifida seeds. Planta Med 2014;80:1732–8.
- Ashraf Z, Rafiq M, Nadeem H, et al. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLoS One 2017; 12:e0178069.
- Mutschlechner B, Rainer B, Schwaiger S, Stuppner H. Tyrosinase inhibitors from the aerial parts of Wulfenia carinthiaca Jacq. Chem Biodivers 2018;15:e1800014
- Lehbili M, Alabdul Magid A, Hubert J, et al. Two new bis-iridoids isolated from Scabiosa stellata and their antibacterial, antioxidant, anti-tyrosinase and cytotoxic activities. Fitoterapia 2018;125:41–8.
- Tan C, Zhu W, Lu Y. Aloin, cinnamic acid and sophorcarpidine are potent inhibitors of tyrosinase. Chin Med J (Engl) 2002;115:1859–62.
- Leu YL, Hwang TL, Hu JW, Fang JY. Anthraquinones from Polygonum cuspidatum as tyrosinase inhibitors for dermal use. Phytother Res 2008;22:552–6.
- Bao K, Dai Y, Zhu ZB, et al. Design and synthesis of biphenyl derivatives as mushroom tyrosinase inhibitors. Bioorg Med Chem 2010;18:6708–14.
- Kim YJ, No JK, Lee JH, Chung HY. 4,4'-Dihydroxybiphenyl as a new potent tyrosinase inhibitor. Biol Pharm Bull 2005;28:323–7.
- van Rensburg WJ, Ferreira D, Malan E, Steenkamp JA. Tyrosinase catalysed biphenyl construction from flavan-3-ol substrates. Phytochemistry 2000;53:285–92.
- Oyama T, Yoshimori A, Takahashi S, et al. Structural insight into the active site of mushroom tyrosinase using phenylbenzoic acid derivatives. Bioorg Med Chem Lett 2017;27:2868–72.
- Le Mellay-Hamon V, Criton M. Phenylethylamide and phenylmethylamide derivatives as new tyrosinase inhibitors. Biol Pharm Bull 2009;32:301–3.
- Chiku K, Dohi H, Saito A, et al. Enzymatic synthesis of 4-hydroxyphenyl beta-D-oligoxylosides and their notable tyrosinase inhibitory activity. Biosci Biotechnol Biochem 2009;73:1123–8.
- Saboury AA, Zolghadri S, Haghbeen K, Moosavi-Movahedi AA. The inhibitory effect of benzenethiol on the cresolase and catecholase activities of mushroom tyrosinase. J Enzyme Inhib Med Chem 2006;21:711–7.
- Alijanianzadeh M, Saboury AA, Ganjali MR, et al. Inhibition of mushroom tyrosinase by a newly synthesized ligand: inhibition kinetics and computational simulations. J Biomol Struct Dyn 2012;30:448–59.
- Shareefi Borojerdi S, Haghbeen K, Asghar Karkhane A, et al. Successful resonance Raman study of cresolase activity of mushroom tyrosinase. Biochem Biophys Res Commun 2004;314:925–30.
- Shao LL, Wang XL, Chen K, et al. Novel hydroxypyridinone derivatives containing an oxime ether moiety: synthesis, inhibition on mushroom tyrosinase and application in anti-browning of fresh-cut apples. Food Chem 2018;242:174–81.
- Saghaie L, Pourfarzam M, Fassihi A, Sartippour B. Synthesis and tyrosinase inhibitory properties of some novel derivatives of kojic acid. Res Pharm Sci 2013;8:233–42.
- Li DF, Hu PP, Liu MS, et al. Design and synthesis of hydroxypyridinone-l-phenylalanine conjugates as potential tyrosinase inhibitors. J Agric Food Chem 2013;61:6597–603.
- Hider RC, Lerch K. The inhibition of tyrosinase by pyridinones. Biochem J 1989;257:289–90.
- Dong H, Liu J, Liu X, et al. Molecular docking and QSAR analyses of aromatic heterocycle thiosemicarbazone analogues for finding novel tyrosinase inhibitors. Bioorg Chem 2017;75:106–17.
- Song S, You A, Chen Z, et al. Study on the design, synthesis and structure-activity relationships of new thiosemicarbazone compounds as tyrosinase inhibitors. Eur J Med Chem 2017;139:815–25.
- Xie J, Dong H, Yu Y, Cao S. Inhibitory effect of synthetic aromatic heterocycle thiosemicarbazone derivatives on mushroom tyrosinase: insights from fluorescence, (1)H-NMR titration and molecular docking studies. Food Chem 2016;190:709–16.
- Zhu TH, Cao SW, Yu YY. Synthesis, characterization and biological evaluation of paeonol thiosemicarbazone analogues as mushroom tyrosinase inhibitors. Int J Biol Macromol 2013;62:589–95.
- Xu J, Liu J, Zhu X, et al. Novel inhibitors of tyrosinase produced by the 4-substitution of TCT. Food Chem 2017;221:1530–8.
- Choi J, Choi KE, Park SJ, et al. Ensemble-based virtual screening led to the discovery of new classes of potent tyrosinase inhibitors. J Chem Inf Model 2016;56:354–67.
- Yi W, Cao R, Chen Z, et al. Rational design and synthesis of 4-o-substituted phenylmethylenethiosemicarbazones as novel tyrosinase inhibitors. Chem Pharm Bull (Tokyo) 2010;58:752–4.
- Yi W, Cao RH, Chen ZY, et al. Design, synthesis and biological evaluation of hydroxy- or methoxy-substituted phenylmethylenethiosemicarbazones as tyrosinase inhibitors. Chem Pharm Bull (Tokyo) 2009;57:1273–7.
- Chen LH, Hu YH, Song W, et al. Synthesis and antityrosinase mechanism of benzaldehyde thiosemicarbazones: novel tyrosinase inhibitors. J Agric Food Chem 2012;60:1542–7.
- Li ZC, Chen LH, Yu XJ, et al. Inhibition kinetics of chlorobenzaldehyde thiosemicarbazones on mushroom tyrosinase. J Agric Food Chem 2010;58:12537–40.
- El-Sadek MM, Hassan SY, Abdelwahab HE, Yacout GA. Synthesis of new 1,3,4-thiadiazole and 1,2,3,4-oxathiadiazole derivatives from carbohydrate precursors and study of their effect on tyrosinase enzyme. Molecules 2012;17:8378–96.
- Yi W, Dubois C, Yahiaoui S, et al. Refinement of arylthiosemicarbazone pharmacophore in inhibition of mushroom tyrosinase. Eur J Med Chem 2011;46:4330–5.
- Buitrago E, Vuillamy A, Boumendjel A, et al. Exploring the interaction of n/s compounds with a dicopper center: tyrosinase inhibition and model studies. Inorg Chem 2014;53:12848–58.
- Yang MH, Chen CM, Hu YH, et al. Inhibitory kinetics of DABT and DABPT as novel tyrosinase inhibitors. J Biosci Bioeng 2013;115:514–7.
- Liu J, Wu F, Chen C. Design and synthesis of aloe-emodin derivatives as potent anti-tyrosinase, antibacterial and anti-inflammatory agents. Bioorg Med Chem Lett 2015;25:5142–6.
- You A, Zhou J, Song S, et al. Structure-based modification of 3-/4-aminoacetophenones giving a profound change of activity on tyrosinase: from potent activators to highly efficient inhibitors. Eur J Med Chem 2015;93:255–62.
- Yi W, Cao R, Wen H, et al. Discovery of 4-functionalized phenyl-o-beta-d-glycosides as a new class of mushroom tyrosinase inhibitors. Bioorg Med Chem Lett 2009;19:6157–60.
- Liu J, Yi W, Wan Y, et al. 1-(1-Arylethylidene)thiosemicarbazide derivatives: a new class of tyrosinase inhibitors. Bioorg Med Chem 2008;16:1096–102.
- Yan Q, Cao R, Yi W, et al. Synthesis and evaluation of 5-benzylidene(thio)barbiturate-beta-d-glycosides as mushroom tyrosinase inhibitors. Bioorg Med Chem Lett 2009;19:4055–8.
- Gheibi N, Saboury AA, Mansuri-Torshizi H, et al. The inhibition effect of some n-alkyl dithiocarbamates on mushroom tyrosinase. J Enzyme Inhib Med Chem 2005;20:393–9.
- Amin E, Saboury AA, Mansouri-Torshizi H, et al. Evaluation of p-phenylene-bis and phenyl dithiocarbamate sodium salts as inhibitors of mushroom tyrosinase. Acta Biochim Pol 2010;57:277–83.
- Amin E, Saboury AA, Mansuri-Torshizi H, Moosavi-Movahedi AA. Potent inhibitory effects of benzyl and p-xylidine-bis dithiocarbamate sodium salts on activities of mushroom tyrosinase. J Enzyme Inhib Med Chem 2010;25:272–81.
- Zarivi O, Bonfigli A, Cesare P, et al. Truffle thio-flavours reversibly inhibit truffle tyrosinase. FEMS Microbiol Lett 2003;220:81–8.
- Choi J, Jee JG. Repositioning of thiourea-containing drugs as tyrosinase inhibitors. Int J Mol Sci 2015;16:28534–48.
- De B, Adhikari I, Nandy A, et al. In silico modelling of azole derivatives with tyrosinase inhibition ability: application of the models for activity prediction of new compounds. Comput Biol Chem 2018;74:105–14.
- Loriga M, Paglietti G, Sparatore F, et al. Synthesis of substituted DL-3(5-benzazolyl)alanines as dopa and alpha-methyldopa analogs and their effects on dopamine beta-hydroxylase, tyrosinase and diphenoloxidase. Farmaco 1992;47:439–48.
- Channar PA, Saeed A, Larik FA, et al. Synthesis of aryl pyrazole via suzuki coupling reaction, in vitro mushroom tyrosinase enzyme inhibition assay and in silico comparative molecular docking analysis with kojic acid. Bioorg Chem 2018;79:293–300.
- Gawande SS, Warangkar SC, Bandgar BP, Khobragade CN. Synthesis of new heterocyclic hybrids based on pyrazole and thiazolidinone scaffolds as potent inhibitors of tyrosinase. Bioorg Med Chem 2013;21:2772–7.
- Zhou Z, Zhuo J, Yan S, Ma L. Design and synthesis of 3,5-diaryl-4,5-dihydro-1H-pyrazoles as new tyrosinase inhibitors. Bioorg Med Chem 2013;21:2156–62.
- Bandgar BP, Totre JV, Gawande SS, et al. Synthesis of novel 3,5-diaryl pyrazole derivatives using combinatorial chemistry as inhibitors of tyrosinase as well as potent anticancer, anti-inflammatory agents. Bioorg Med Chem 2010;18:6149–55.
- Khan KM, Maharvi GM, Khan MT, et al. A facile and improved synthesis of sildenafil (viagra) analogs through solid support microwave irradiation possessing tyrosinase inhibitory potential, their conformational analysis and molecular dynamics simulation studies. Mol Divers 2005;9:15–26.
- Mojzych M, Tarasiuk P, Kotwica-Mojzych K, et al. Synthesis of chiral pyrazolo[4,3-e][1,2,4]triazine sulfonamides with tyrosinase and urease inhibitory activity. J Enzyme Inhib Med Chem 2017;32:99–105.
- Mojzych M, Dolashki A, Voelter W. Synthesis of pyrazolo[4,3-e][1,2,4]triazine sulfonamides, novel sildenafil analogs with tyrosinase inhibitory activity. Bioorg Med Chem 2014;22:6616–24.
- Qamar R, Saeed A, Larik FA, et al. Novel 1,3-oxazine-tetrazole hybrids as mushroom tyrosinase inhibitors and free radical scavengers: synthesis, kinetic mechanism and molecular docking studies. Chem Biol Drug Des 2018; doi: 10.1111/cbdd.13352.
- Nikalje APG, Gawhane P, Tiwari S, et al. Ultrasound promoted green synthesis, docking study of indole spliced thiadiazole, alpha-amino phosphonates as anticancer agents and anti-tyrosinase agents. Anticancer Agents Med Chem 2018;18:1. doi: 10.2174/1871520618666180417163226.
- Mahdavi M, Ashtari A, Khoshneviszadeh M, et al. Synthesis of new benzimidazole-1,2,3-triazole hybrids as tyrosinase inhibitors. Chem Biodivers 2018; 15:e1800120.
- Chekir S, Debbabi M, Regazzetti A, et al. Design, synthesis and biological evaluation of novel 1,2,3-triazole linked coumarinopyrazole conjugates as potent anticholinesterase, anti-5-lipoxygenase, anti-tyrosinase and anti-cancer agents. Bioorg Chem 2018;80:189–94.
- Kim SJ, Yang J, Lee S, et al. The tyrosinase inhibitory effects of isoxazolone derivatives with a (z)-beta-phenyl-alpha, beta-unsaturated carbonyl scaffold. Bioorg Med Chem 2018; doi: 10.1016/j.bmc.2018.05.047.
- Hamidian H, Tagizadeh R, Fozooni S, et al. Synthesis of novel azo compounds containing 5(4h)-oxazolone ring as potent tyrosinase inhibitors. Bioorg Med Chem 2013;21:2088–92.
- Heitz MP, Rupp JW. Determining mushroom tyrosinase inhibition by imidazolium ionic liquids: a spectroscopic and molecular docking study. Int J Biol Macromol 2018;107:1971–81.
- Mann T, Scherner C, Rohm KH, Kolbe L. Structure–activity relationships of thiazolyl resorcinols, potent and selective inhibitors of human tyrosinase. Int J Mol Sci 2018;19:690. doi: 10.3390/ijms19030690.
- Rezaei M, Mohammadi HT, Mahdavi A, et al. Evaluation of thiazolidinone derivatives as a new class of mushroom tyrosinase inhibitors. Int J Biol Macromol 2018;108:205–13.
- Kim SH, Ha YM, Moon KM, et al. Anti-melanogenic effect of (z)-5-(2,4-dihydroxybenzylidene) thiazolidine-2,4-dione, a novel tyrosinase inhibitor. Arch Pharm Res 2013;36:1189–97.
- Ha YM, Park YJ, Kim JA, et al. Design and synthesis of 5-(substituted benzylidene)thiazolidine-2,4-dione derivatives as novel tyrosinase inhibitors. Eur J Med Chem 2012;49:245–52.
- Han YK, Park YJ, Ha YM, et al. Characterization of a novel tyrosinase inhibitor, (2RS,4R)-2-(2,4-dihydroxyphenyl)thiazolidine-4-carboxylic acid (MHY384). Biochim Biophys Acta 2012;1820:542–9.
- Ha YM, Park YJ, Lee JY, et al. Design, synthesis and biological evaluation of 2-(substituted phenyl)thiazolidine-4-carboxylic acid derivatives as novel tyrosinase inhibitors. Biochimie 2012;94:533–40.
- Jung HJ, Lee MJ, Park YJ, et al. A novel synthetic compound, (z)-5-(3-hydroxy-4-methoxybenzylidene)-2-iminothiazolidin-4-one (mhy773) inhibits mushroom tyrosinase. Biosci Biotechnol Biochem 2018;82:759–67. doi: 10.1080/09168451.2018.1445518.
- Kahn V. Effect of kojic acid on the oxidation of DL-dopa, norepinephrine, and dopamine by mushroom tyrosinase. Pigment Cell Res 1995;8:234–40.
- Xie W, Zhang H, He J, et al. Synthesis and biological evaluation of novel hydroxybenzaldehyde-based kojic acid analogues as inhibitors of mushroom tyrosinase. Bioorg Med Chem Lett 2017;27:530–2.
- Chen MJ, Hung CC, Chen YR, et al. Novel synthetic kojic acid-methimazole derivatives inhibit mushroom tyrosinase and melanogenesis. J Biosci Bioeng 2016;122:666–72.
- Asadzadeh A, Sirous H, Pourfarzam M, et al. In vitro and in silico studies of the inhibitory effects of some novel kojic acid derivatives on tyrosinase enzyme. Iran J Basic Med Sci 2016;19:132–44.
- Xie W, Zhang J, Ma X, et al. Synthesis and biological evaluation of kojic acid derivatives containing 1,2,4-triazole as potent tyrosinase inhibitors. Chem Biol Drug Des 2015;86:1087–92.
- Lima CR, Silva JR, de Tassia Carvalho Cardoso E, et al. Combined kinetic studies and computational analysis on kojic acid analogous as tyrosinase inhibitors. Molecules 2014;19:9591–605.
- Noh JM, Kwak SY, Seo HS, et al. Kojic acid-amino acid conjugates as tyrosinase inhibitors. Bioorg Med Chem Lett 2009;19:5586–9.
- Lee YS, Park JH, Kim MH, et al. Synthesis of tyrosinase inhibitory kojic acid derivative. Arch Pharm (Weinheim) 2006;339:111–4.
- Cho JC, Rho HS, Joo YH, et al. Depigmenting activities of kojic acid derivatives without tyrosinase inhibitory activities. Bioorg Med Chem Lett 2012;22:4159–62.
- Nihei KI, Kubo I. Substituent effect of benzaldehydes on tyrosinase inhibition. Plant Physiol Biochem 2017;112:278–82.
- Rafiee M, Javaheri M. A theoretical study of benzaldehyde derivatives as tyrosinase inhibitors using ab initio calculated NQCC parameters. Mol Biol Res Commun 2015;4:151–9.
- Ley JP, Bertram HJ. Hydroxy- or methoxy-substituted benzaldoximes and benzaldehyde-o-alkyloximes as tyrosinase inhibitors. Bioorg Med Chem 2001;9:1879–85.
- Lopes ND, Chaves OA, de Oliveira MCC, et al. Novel piperonal 1,3,4-thiadiazolium-2-phenylamines mesoionic derivatives: synthesis, tyrosinase inhibition evaluation and HSA binding study. Int J Biol Macromol 2018;112:1062–72.
- Yi W, Cao R, Peng W, et al. Synthesis and biological evaluation of novel 4-hydroxybenzaldehyde derivatives as tyrosinase inhibitors. Eur J Med Chem 2010;45:639–46.
- Ha TJ, Tamura S, Kubo I. Effects of mushroom tyrosinase on anisaldehyde. J Agric Food Chem 2005;53:7024–8.
- Yu L. Inhibitory effects of (s)- and (r)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acids on tyrosinase activity. J Agric Food Chem 2003;51:2344–7.
- Alijanianzadeh M, Saboury AA, Mansuri-Torshizi H, et al. The inhibitory effect of some new synthesized xanthates on mushroom tyrosinase activities. J Enzyme Inhib Med Chem 2007;22:239–46.
- Saboury AA, Alijanianzadeh M, Mansoori-Torshizi H. The role of alkyl chain length in the inhibitory effect n-alkyl xanthates on mushroom tyrosinase activities. Acta Biochim Pol 2007;54:183–91.
- Xing R, Wang F, Zheng A, et al. Biological evaluation of two Keggin-type polyoxometalates containing glycine as mushroom tyrosinase inhibitors. Biotechnol Appl Biochem 2016;63:746–50.
- Park KH, Lee JR, Hahn HS, et al. Inhibitory effect of ammonium tetrathiotungstate on tyrosinase and its kinetic mechanism. Chem Pharm Bull (Tokyo) 2006;54:1266–70.
- Chen XX, Zhang J, Chai WM, et al. Reversible and competitive inhibitory kinetics of amoxicillin on mushroom tyrosinase. Int J Biol Macromol 2013;62:726–33.
- Hemachandran H, Jain F, Mohan S, et al. Glandular hair constituents of Mallotus philippinensis muell. Fruit act as tyrosinase inhibitors: insights from enzyme kinetics and simulation study. Int J Biol Macromol 2018;107:1675–82.
- Lin YF, Hu YH, Jia YL, et al. Inhibitory effects of naphthols on the activity of mushroom tyrosinase. Int J Biol Macromol 2012;51:32–6.
- Wu LC, Chen YC, Ho JA, Yang CS. Inhibitory effect of red koji extracts on mushroom tyrosinase. J Agric Food Chem 2003;51:4240–6.
- Fourche J, Jensen H, Neuzil E, Bellegarde B. [Alpha-hydrazinophloretic acid, competitive inhibitor of fungal tyrosinase]. CR Hebd Seances Acad Sci Ser D 1977;284:2163–6.
- Gheibi N, Saboury AA, Haghbeen K, Moosavi-Movahedi AA. Activity and structural changes of mushroom tyrosinase induced by n-alkyl sulfates. Colloids Surf B Biointerfaces 2005;45:104–7.
- Jena K, Pandey JP, Kumari R, et al. Tasar silk fiber waste sericin: new source for anti-elastase, anti-tyrosinase and anti-oxidant compounds. Int J Biol Macromol 2018;114:1102–8.
- Hwang SH, Wang Z, Suh HW, Lim SS. Antioxidant activity and inhibitory effects of 2-hydroxy-3-methylcyclopent-2-enone isolated from ribose-histidine maillard reaction products on aldose reductase and tyrosinase. Food Funct 2018;9:1790–9.
- Huang XX, Yan ZY, Liu S, et al. Investigation of chemical constituents of safflower and their tyrosinase inhibitory activity. J Asian Nat Prod Res 2018; 1–9. doi: 10.1080/10286020.2018.1430775.
- Alijanianzadeh M, Saboury AA, Ganjali MR, et al. The inhibitory effect of ethylenediamine on mushroom tyrosinase. Int J Biol Macromol 2012;50:573–7.
- Wang Y, Hao MM, Sun Y, et al. Synergistic promotion on tyrosinase inhibition by antioxidants. Molecules 2018;23:106. doi: 10.3390/molecules23010106.
- Liang C, Lim JH, Kim SH, Kim DS. Dioscin: a synergistic tyrosinase inhibitor from the roots of Smilax china. Food Chem 2012;134:1146–8.
- Jin YH, Lee SJ, Chung MH, et al. Aloesin and arbutin inhibit tyrosinase activity in a synergistic manner via a different action mechanism. Arch Pharm Res 1999;22:232–6.
- Schved F, Kahn V. Synergism exerted by 4-methyl catechol, catechol, and their respective quinones on the rate of DL-dopa oxidation by mushroom tyrosinase. Pigment Cell Res 1992;5:41–8.
- Chen X, Haniu A, Kashiwagi T, et al. The evaluation of the synergistic effect of 3-(2,4-dihydroxyphenyl)propionic acid and L-ascorbic acid on tyrosinase inhibition. Z Naturforsch C 2017;72:119–21.
- Hseu YC, Cheng KC, Lin YC, et al. Synergistic effects of linderanolide B combined with arbutin, PTU or kojic acid on tyrosinase inhibition. Curr Pharm Biotechnol 2015;16:1120–6.


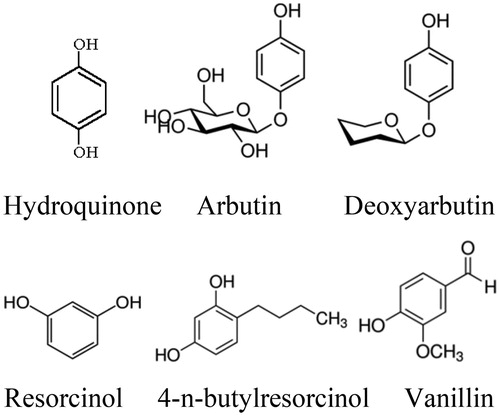
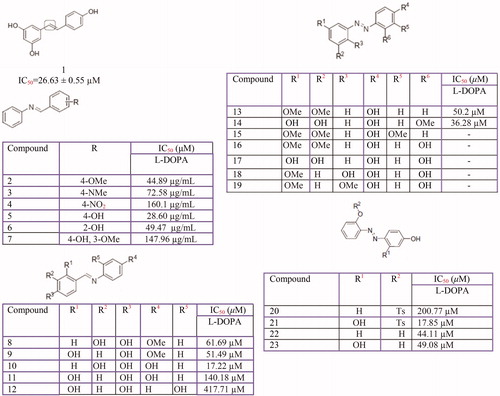
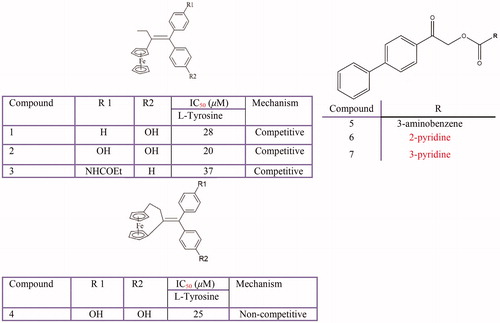
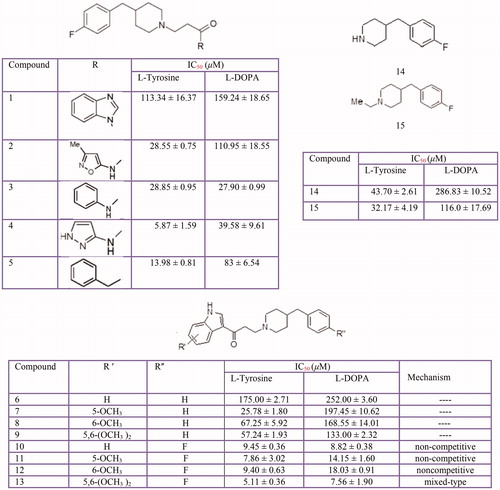
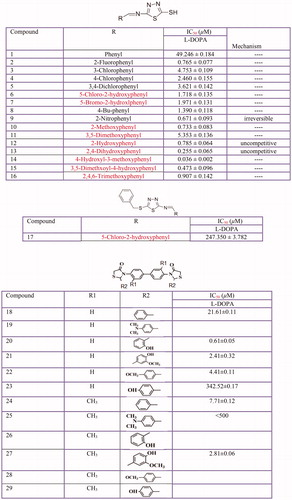
![Figure 14 Some kojic acid analogs: hydroxybenzaldehydebased kojic acid analogs (5-substituted-3-[5-hydroxy-4-pyrone-2-ylmethylmercapto]-4-arylmethylamino-1,2,4-triazole (1–10) and 5-substituted-3-[5-hydroxy-4-pyrone-2-yl-methylmercapto]-4-arylmethyleneamino-1,2,4-triazole (11–14).](/cms/asset/8420cdaf-df6f-4902-9ee1-38444229ad82/ienz_a_1545767_f0014_c.jpg)