Abstract
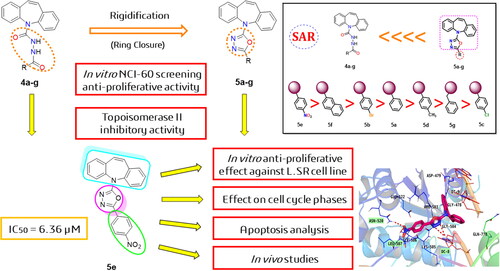
In this research, two novel series of dibenzo[b,f]azepines (14 candidates) were designed and synthesised based on the rigidification principle and following the reported doxorubicin’s pharmacophoric features. The anti-proliferative activity was evaluated at the NCI against a panel of 60 cancer cell lines. Further, the promising candidates (5a–g) were evaluated for their ability to inhibit topoisomerase II, where 5e was noticed to be the most active congener. Moreover, its cytotoxicity was evaluated against leukaemia SR cells. Also, 5e arrested the cell cycle at the G1 phase and increased the apoptosis ratio by 37.34%. Furthermore, in vivo studies of 5e showed the inhibition of tumour proliferation and the decrease in its volume. Histopathology and liver enzymes were examined as well. Besides, molecular docking, physicochemical, and pharmacokinetic properties were carried out. Finally, a SAR study was discussed to open the gate for further optimisation of the most promising candidate (5e).
Two novel series of dibenzo[b,f]azepines were designed and synthesised based on the rigidification principle in drug design.
The anti-proliferative activity was evaluated at the NCI against a panel of 60 cancer cell lines.
5e was the most active anti-topo II congener (IC50 = 6.36 ± 0.36 µM).
5e was evaluated against leukaemia SR cells and its cytotoxic effect was confirmed (IC50 = 13.05 ± 0.62 µM).
In vivo studies of 5e significantly inhibited tumour proliferation by 62.7% and decreased tumour volume to 30.1 mm3 compared to doxorubicin treatment.
Highlights
Introduction
Among the leading causes of death, cancer remains one of the most severe health problems worldwideCitation1,Citation2. It starts when abnormal cells of the body grow uncontrollably in any organ or tissue and then extend outside their normal boundaries to invade adjacent portions of the body and/or spread to more distant organs in metastasisCitation3,Citation4. Globally, the total count of cancer death is one in six deaths (9.6 million) in 2018Citation5. However, prostate, lung, stomach, colorectal, and liver cancers are the most common in men, while, breast, colorectal, cervical, and thyroid ones are common among womenCitation6. The strategy of finding novel chemotherapeutic anticancer agents with improved potency, selectivity, and minimised toxicity is still the target of a large group of researchersCitation7,Citation8. Increasing the understanding of the apoptotic pathway enabled scientists to develop more effective anticancer small molecules.
Multitarget therapies for complicated diseases, including cancer, have gained scientists’ interest over the last few decadesCitation2,Citation9,Citation10. Although cancer treatment faces many challenges, the current chemotherapeutics drugs can effectively prolong patient lives or even completely cure the diseaseCitation11. However, in the hope of maximising their effects, using a combination of more than one drug may be a well-established approachCitation12–14. In addition, using a drug that can target two different pathways of cancer development may potentiate the activity and minimise drug resistanceCitation12. The advantages of the last approach over the former one encouraged us to move towards the design of a multitarget compound.
Among the different chemotherapeutic agent classes still, the DNA-interfering drugs are of great importance either through direct binding to the DNA itself or the enzymes needed for normal DNA functions leading ultimately to cell deathCitation15,Citation16. DNA intercalation is a mechanism that gained much attention owing to its hopeful anticancer activity. These agents are distinguished by their ability to intercalate between two base pairs of a DNA molecule, a process that causes structural changes in the DNA molecule that hinders DNA replicationCitation17. However, A well-defined structural study of several FDA-approved DNA intercalators revealed that the intercalation process is stabilised by the presence of different hydrophobic interactions between the compound’s planner system, chromophore, and the DNA base pairsCitation18,Citation19.
On the reverse side, DNA topoisomerases (topo) are nuclear enzymes that change the topological state of the DNACitation20,Citation21. DNA replication as well as transcription requires the DNA helix to unwind in a process that causes helical tension in the rest of the DNA moleculeCitation21,Citation22. The role of topoisomerase is to free this tension upon the formation of either a single-stranded break (topo I) or a double-stranded break (topoisomerase II) in the DNA double helixCitation23. Thus, human topoisomerase II has been considered a promising target for many anticancer agents to control different tumour types.
Forced by the former collected data and to continue our previous workCitation16,Citation18,Citation24–26, we decided to design a multitarget scaffold that may act as both a topoisomerase II inhibitor and DNA intercalator aiming to find new drugs with maximum activity and minimum resistance and side effects. Investigation of drugs that act as DNA intercalators and topoisomerase II inhibitors () showed that the main part that contributes to their action is a planner system that intercalates between the DNA base pairsCitation27. In addition, a basic centre linked to the planner system gives the compound the advantage of being protonated at the physiological pH and ionically interacts with the receptor’s phosphate anionic centreCitation28,Citation29.
Figure 1. A group of previously reported drugs as topoisomerase II inhibitors and DNA intercalators, bear the same doxorubicin’s pharmacophoric features.

Deep research in the literature clarified that some heteroaromatic systems bearing a dibenzo[b,f]azepine system were proved to be effective topoisomerase II inhibitors and DNA intercalatorsCitation30. In addition, the oxadiazole moiety showed a noticed topoisomerase II inhibitoryCitation31. Based on these findings, we herein planned to build a new scaffold linking the two mentioned moieties in addition to a groove-binding moiety to obtain potent anticancer candidates.
The rationale for work design
Our lab teamwork started to design and then synthesise two novel series of dibenzo[b,f]azepine derivatives in the hope of finding new promising anticancer agents. This was done based on the reported doxorubicin’s pharmacophoric features, an FDA-approved topoisomerase II inhibitor, and DNA intercalator anticancer drug, .
It is worth mentioning that doxorubicin and the other known topoisomerase II inhibitors and DNA intercalators shared three typical features, including, (a) a polyaromatic planner skeleton, chromophore, to be sandwiched between the base pairs of DNA, (b) an easily protonated cationic species to interact with the receptor’s sugar-phosphate moiety and, (c) a side chain to occupy the receptor’s minor grooveCitation16.
Moreover, guided by the rigidification principle in drug design, which results in increasing the new compounds’ rigidity to improve their binding, increasing their selectivity, and decreasing their side effectsCitation32. Herein, we synthesised the open analogues of N′-benzoyl-5H-dibenzo[b,f]azepine-5-carbohydrazide candidates (4a–g) followed by their chemical conversion into the corresponding closed analogues of 2-(5H-dibenzo[b,f]azepin-5-yl)-5-phenyl-1,3,4-oxadiazole derivatives (5a–g), . This was done to study the effect of decreasing the flexibility of compounds (4a–g) on the anticancer activity by comparing it to those of the newly designed rigid candidates (5a–g).
Figure 2. Schematic representation of main pharmacophoric features of the dibenzo[b,f]azepine derivatives (5a–g) as topoisomerase II inhibitors and DNA intercalators (like doxorubicin) produced by the rigidification of candidates (4a–g) using the ring closure principle.
![Figure 2. Schematic representation of main pharmacophoric features of the dibenzo[b,f]azepine derivatives (5a–g) as topoisomerase II inhibitors and DNA intercalators (like doxorubicin) produced by the rigidification of candidates (4a–g) using the ring closure principle.](/cms/asset/64c3e498-7a25-4d71-9bbf-d3401865a03c/ienz_a_2157825_f0002_c.jpg)
Furthermore, the former criteria of DNA intercalators and topoisomerase II inhibitors were, herein, achieved via applying the cross-hybridization strategy. Thus, the dibenzo[b,f]azepine scaffold was chosen to play the role of the planner polyaromatic system while, an oxadiazole moiety linked to different moieties including, phenyl, 4-bromophenyl, 4-chlorophenyl, p-tolyl, 4-nitrophenyl, naphthalenyl, and benzyl were introduced to act as a cationic centre and groove binding moieties, respectively, and Scheme 1.
Materials and methods
Chemistry
General
The general chemistry protocol was applied as described in the supplementary information (SI 1). Moreover, the spectral data of compounds (4a–g and 5a–g), (IR, 1H NMR, 13C NMR, and Mass spectroscopy) are depicted in the supporting information (SI 2).
General procedure for the synthesis of 5H-dibenzo[b,f]azepine (2)
Carbamazepine (CBZ, 12 g) was suspended in water (600 ml) then NaOH (32 g, 20 mol equiv.) was added. The mixture was then heated under reflux. The reaction was followed by TLC until no more start then filtered while hot to afford 9.4 g (95%) of Compound 2 as an orange solid. The melting point of Compound 2 was 203 °C–205 °C similar to that recorded in literatureCitation33.
General procedure for the synthesis of 5H-dibenzo[b,f]azepine-5-carbohydrazide (3)
Compound 2 (9.5 g) was suspended in toluene (300 ml) followed by the addition of phosgene (50 ml, 12.5% w/v in toluene) and triethylamine (7.5 ml, 5 g). The mixture was stirred at room temperature for 24 h. Then, excess hydrazine hydrate (100 ml) was added to the mixture and stirred for 1 h then cooled and the product (6.0 g, 48%) was filtered off to produce a white solid of Compound 3 with a melting point of 192 °C–194 °CCitation34.
General procedure for the synthesis of N′-benzoyl-5H-dibenzo[b,f]azepine-5-carbohydrazide derivatives (4a–g)
To a stirred solution of compound 3 (0.75 g, 3 mmol) in chloroform, appropriate acid chloride (3 mmol) was added followed by triethylamine (0.3 g, 0.42 ml, 3 mmol). The reaction mixture was stirred at room temperature for 18 h and then washed with NaHCO3 (10%, 20 ml) and water. The organic layer was then separated, dried with Na2SO4, and evaporated under vacuum to afford crude products 4a–g, which were further purified by crystallisation from ethanol.
N′-Benzoyl-5H-dibenzo[b,f]azepine-5-carbohydrazide (4a)
Yield (0.9 g, 90%), white solid, m.p. 222 °C–224 °C. 1H NMR (400 MHz, DMSO-D6) δ 10.08 (s, 1H), 7.82–7.76 (m, 2H), 7.73 (s, 1H), 7.53–7.38 (m, 9H), 7.33 (ddd, J = 8.2, 5.8, 2.6 Hz, 2H), 7.00 (s, 2H); 13C NMR (101 MHz, DMSO-D6) δ 166.36, 155.92, 140.06, 135.29, 133.26, 132.11, 130.91, 129.99, 129.77, 129.42, 129.09, 128.89, 127.98, 127.84; ESI–-MS (m/z, %): 354 ([M-H]–, 100%); 355 ([M]–, 28%).
N′-(4-Bromobenzoyl)-5H-dibenzo[b,f]azepine-5-carbohydrazide (4b)
Yield (0.86 g, 66%), white solid, m.p. 237 °C–239 °C. IR (KBr, cm−1): 3375 (NH), 3259 (NH), 1658 (broad, C = O); 1H NMR (400 MHz, DMSO-D6) δ 10.16 (s, 1H), 7.77 (s, 1H), 7.72 (d, J = 8.6 Hz, 2H), 7.64 (d, J = 8.6 Hz, 2H), 7.49–7.39 (m, 6H), 7.33 (ddd, J = 8.2, 5.4, 3.1 Hz, 2H), 6.99 (s, 2H); 13C NMR (101 MHz, DMSO-D6) δ 165.50, 155.84, 139.99, 135.26, 132.00, 130.89, 129.93, 129.76, 129.40, 128.00, 125.94; ESI-MS (m/z, %): 432 (79Br), ([M-H]–, 100%) 433 (79Br) ([M]–, 26%) , 434 (81Br) ([M-H]–, 100%), 435 (81Br) ([M]–, 26%).
N′-(4-Chlorobenzoyl)-5H-dibenzo[b,f]azepine-5-carbohydrazide (4c)
Yield (0.8 g, 70%), white solid, m.p. 229 °C–231 °C. 1H NMR (400 MHz, DMSO-D6) δ 10.16 (s, 1H), 8.28 (s, 1H), 7.79 (t, J = 7.7 Hz, 2H), 7.50 (d, J = 8.6 Hz, 2H), 7.47–7.39 (m, 6H), 7.33 (ddd, J = 8.2, 5.5, 2.9 Hz, 2H), 6.99 (s, 2H); 13C NMR (101 MHz, DMSO-D6) δ 165.37, 155.86, 140.01, 136.99, 135.27, 132.01, 130.89, 129.99, 129.76, 129.40, 129.06, 128.00, 79.72; ESI-MS (m/z, %): 388 ([M-H]–, 100%); 389 ([M]–, 30%), 390 ([M + H]–, 34%).
N′-(4-Methylbenzoyl)-5H-dibenzo[b,f]azepine-5-carbohydrazide (4d)
Yield (0.62 g, 60%), white solid, m.p. 151 °C–153 °C. 1H NMR (400 MHz, DMSO-D6) δ 10.00 (s, 1H), 7.69 (d, J = 8.2 Hz, 3H), 7.44 (ddd, J = 9.0, 5.1, 0.9 Hz, 6H), 7.36–7.29 (m, 2H), 7.22 (d, J = 8.0 Hz, 2H), 6.99 (s, 2H), 2.29 (s, 3H). 13C NMR (101 MHz, DMSO-D6) δ 166.23, 155.95, 142.04, 140.06, 135.28, 130.90, 130.44, 129.98, 129.75, 129.41, 127.97, 127.86, 21.50. ESI-MS (m/z, %): 368 ([M-H]–, 100%); 369 ([M]–, 30%).
N′-(4-Nitrobenzoyl)-5H-dibenzo[b,f]azepine-5-carbohydrazide (4e)
Yield (0.9 g, 81%), yellow solid, m.p. 241 °C–243 °C. IR (KBr, cm−1): 3402 (NH), 3251 (NH), 1697 (C = O), 1658 (C = O); 1H NMR (400 MHz, DMSO-D6) δ 10.40 (s, 1H), 8.27 (d, J = 9.0 Hz, 2H), 7.99 (d, J = 9.0 Hz, 2H), 7.90 (s, 1H), 7.49–7.40 (m, 6H), 7.38–7.29 (m, 2H), 7.00 (s, 2H); 13C NMR (101 MHz, DMSO-D6) δ 164.87, 155.74, 149.77, 141.98, 139.95, 138.96, 135.27, 130.90, 130.02, 129.78, 129.40, 129.24, 128.04, 124.20; ESI-MS (m/z, %): 399 ([M-H]–, 100%); 400 ([M]–, 30%), 401 ([M + H]–, 10%).
N′-(2-Naphthoyl)-5H-dibenzo[b,f]azepine-5-carbohydrazide (4f)
Yield (0.82 g, 80%), white solid, m.p. 243 °C–245 °C. 1H NMR (400 MHz, DMSO-D6) δ 9.99 (s, 1H), 8.36–8.24 (m, 1H), 8.00–7.86 (m, 3H), 7.60–7.41 (m, 10H), 7.40–7.30 (m, 2H), 7.02 (s, 2H); 13C NMR (101 MHz, DMSO-D6) δ 168.59, 155.91, 140.08, 135.34, 133.77, 133.53, 130.95, 130.49, 130.47, 130.04, 129.80, 129.52, 128.56, 128.02, 127.13, 126.84, 126.23, 125.81, 125.47; ESI-MS (m/z, %): 404 ([M-H]–, 40%); 405 ([M]–, 26%).
N′-(2-Phenylacetyl)-5H-dibenzo[b,f]azepine-5-carbohydrazide (4g)
Yield (0.35 g, 30%), white solid, m.p. 134 °C–136 °C. IR (KBr, cm−1): 3379 (NH), 3290 (NH), 1666 (broad, C = O); 1H NMR (400 MHz, DMSO-D6) δ 9.73 (d, J = 1.5 Hz, 1H), 7.61 (d, J = 1.5 Hz, 1H), 7.40 (ddd, J = 4.8, 3.6, 1.1 Hz, 6H), 7.34–7.27 (m, 2H), 7.22 (d, J = 4.4 Hz, 4H), 7.15 (dd, J = 8.1, 5.3 Hz, 1H), 6.96 (s, 2H), 3.34 (s, 2H); 13C NMR (101 MHz, DMSO-D6) δ 169.82, 155.68, 140.02, 136.30, 135.26, 130.85, 129.94, 129.72, 129.60, 129.39, 128.61, 127.94, 126.87, 45.82; ESI-MS (m/z, %): 368 ([M-H]–, 100%); 369 ([M]–, 26%).
General procedure for synthesis of 2–(5H-dibenzo[b,f]azepin-5-yl)-5-phenyl-1,3,4-oxadiazole derivatives 5a–g
A solution of an appropriate derivative 4a–g in phosphorusoxychloride (POCl3, 5.0 ml) was refluxed for 30 min. The solution was then diluted carefully with water then NaHCO3 was added till being alkaline. The precipitate was filtered, washed with water, and recrystallized from ethanol to afford compounds 5a–g.
2-(5 h-Dibenzo[b,f]azepin-5-yl)-5-phenyl-1,3,4-oxadiazole (5a)
Yield (0.25 g, 65%), white solid, m.p. 209 °C–211 °C, 1H NMR (400 MHz, CHLOROFORM-D) δ 7.77–7.71 (m, 2H), 7.60 (d, J = 7.9 Hz, 2H), 7.53–7.45 (m, 2H), 7.37 (t, J = 6.1 Hz, 7H), 6.91 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 162.82, 159.38, 140.04, 134.60, 130.73, 130.58, 129.82, 129.77, 129.51, 129.11, 128.72, 128.22, 128.12, 127.87, 125.91, 124.31; APCI+-MS (m/z, %) 338 ([M + H]+ 100%).
2-(4-Bromophenyl)-5-(5H-dibenzo[b,f]azepin-5-yl)-1,3,4-oxadiazole (5b)
Yield (0.3 g, 70%), white solid, m.p. 185 °C–187 °C. 1H NMR (400 MHz, CHLOROFORM-D) δ 7.61–7.56 (m, 4H), 7.52–7.45 (m, 4H), 7.38 (dd, J = 5.9, 1.9 Hz, 4H), 6.91 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 162.96, 158.66, 139.91, 134.57, 132.01, 130.71, 129.85, 129.79, 128.18, 127.81, 127.30, 124.92, 123.27; APCI+-MS (m/z,%): 416 (79Br) ([M]+ 100%), 417 (79Br) ([M + H]+ 28%), 418 (81Br) ([M]+ 100%), 419 (81Br) ([M + H]+ 100%).
2-(4-Chlorophenyl)-5-(5H-dibenzo[b,f]azepin-5-yl)-1,3,4-oxadiazole (5c)
Yield (0.26 g, 68%), white solid, m.p. 174 °C–176 °C. 1H NMR (400 MHz, CHLOROFORM-D) δ 7.64 (s, 2H), 7.56 (s, 2H), 7.47 (d, J = 3.6 Hz, 2H), 7.42–7.25 (m, 6H), 7.00–6.82 (m, 2H); 13C NMR (101 MHz, CDCl3) δ 162.94, 158.58, 139.92, 136.59, 134.57, 130.71, 129.85, 129.80, 129.07, 128.18, 127.81, 127.14, 124.15, 122.82; APCI+-MS (m/z, %): 372 ([M + H]+ 100%).
2-(5 h-Dibenzo[b,f]azepin-5-yl)-5-(p-tolyl)-1,3,4-oxadiazole (5d)
Yield (0.22 g, 73%), white solid, m.p. 239 °C–241 °C. 1H NMR (400 MHz, CHLOROFORM-D) δ 7.61 (dd, J = 12.9, 8.1 Hz, 4H), 7.52–7.44 (m, 2H), 7.37 (d, J = 4.1 Hz, 4H), 7.15 (d, J = 8.0 Hz, 2H), 6.90 (s, 2H), 2.34 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 162.68, 159.58, 140.89, 140.18, 134.61, 130.75, 129.79, 129.73, 129.40, 128.03, 127.88, 125.89, 121.60, 21.55; APCI+-MS (m/z, %): 352 ([M + H]+ 100%).
2-(5 h-Dibenzo[b,f]azepin-5-yl)-5–(4-nitrophenyl)-1,3,4-oxadiazole (5e)
Yield (0.39 g, 92%), green solid, m.p. 146 °C–148 °C. IR (KBr, cm−1): 1604 (C = N), 1573 (C = N). 1H NMR (400 MHz, CHLOROFORM-D) δ 8.24–8.22 (m, 1H), 8.22–8.20 (m, 1H), 7.91–7.89 (m, 1H), 7.89–7.87 (m, 1H), 7.60 (s, 1H), 7.58 (s, 1H), 7.54–7.47 (m, 2H), 7.41 (dd, J = 1.9, 0.8 Hz, 2H), 7.40 (d, J = 0.9 Hz, 2H), 6.92 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 163.52, 157.68, 148.63, 139.49, 134.50, 130.68, 129.95, 129.91, 129.77, 128.44, 127.70, 126.48, 124.15; APCI+-MS (m/z, %): 383 ([M + H]+ 100%).
2-(5 h-Dibenzo[b,f]azepin-5-yl)-5-(naphthalen-2-yl)-1,3,4-oxadiazole (5f)
Yield (0.27 g, 68%), white solid, m.p. 201 °C–203 °C. 1H NMR (400 MHz, CHLOROFORM-D) δ 9.15 (d, J = 8.7 Hz, 1H), 7.91–7.81 (m, 2H), 7.75 (dd, J = 7.3, 1.2 Hz, 1H), 7.66 (d, J = 7.9 Hz, 2H), 7.60–7.46 (m, 4H), 7.39 (d, J = 4.3 Hz, 5H), 6.93 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 162.61, 159.39, 140.13, 134.64, 133.75, 131.44, 130.78, 129.86, 129.78, 128.46, 128.13, 127.92, 127.76, 126.93, 126.47, 124.73, 120.76; APCI+-MS (m/z, %): 388 ([M + H]+ 100%).
2-Benzyl-5-(5H-dibenzo[b,f]azepin-5-yl)-1,3,4-oxadiazole (5g)
Yield (0.2 g, 60%), white solid, m.p. 189 °C–191 °C. 1H NMR (400 MHz, CHLOROFORM-D) δ 7.46 (d, J = 7.9 Hz, 2H), 7.44–7.38 (m, 2H), 7.33 (dd, J = 4.6, 1.0 Hz, 4H), 7.26 (ddd, J = 6.7, 5.3, 2.6 Hz, 3H), 7.19 (dd, J = 7.8, 1.6 Hz, 2H), 6.87 (s, 2H), 3.96 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 163.25, 159.55, 140.13, 134.55, 134.41, 130.70, 129.73, 129.66, 128.70, 128.66, 127.97, 127.81, 127.20, 31.78; APCI+-MS (m/z, %): 352 ([M + H]+ 100%).
Biological activity
In vitro NCI-60 one-dose screening anti-proliferative activity
Anti-proliferative effects of the counterparts 4a–g and 5a–g were screened against 60 human cancer cell lines, following the Developmental Therapeutics Program protocol, National Cancer InstituteCitation35.
Topoisomerase II inhibitory assay
All the promising anticancer closed analogues (5a–g) were then investigated to measure the topoisomerase II inhibitory effect using the reported procedure by Patra et al.Citation36 and doxorubicin as a reference drug.
Anti-proliferative effect against SR cell line
The anti-proliferative effect of the 5e counterpart against the SR cell line was estimated using the MTT assay protocolCitation37.
Effect on cell cycle phases
This test was performed using propidium iodide (PI) staining following the detailed procedure in the supporting information (SI 3)Citation38.
Apoptosis analysis
The apoptotic analysis was performed according to the detailed description in the supporting information (SI 4)Citation39.
In vivo studies
The full methodology of tumour induction, treatments, and blood measurements was carried out according to the described details in the supporting information (SI 5). Moreover, the experimental protocol was approved by the Research Ethics Committee at Suez Canal University (Approval number REC139/2022, Chemistry Department, Faculty of Science, Suez Canal University).
In silico studies
Molecular modelling
Discovery studio software 4.1Citation40 was used to carry out molecular docking studies at the Misr University for Science and Technology. The selected poses of the most promising candidates were visualised using PyMOL program softwareCitation41. This study was achieved to examine the binding interactions of the target entities against topoisomerase II complexed with DNA by using EVP and doxorubicin as reference standards.
The tested candidates’ preparation
The ChemDraw Professional 16.0 was used to sketch the target compounds. Then, the target compounds, doxorubicin, and EVP were inserted into a single database and exposed to force field energy minimisationCitation42,Citation43.
Topoisomerase II-DNA complex preparation
The X-ray structure of human topoisomerase II complexed with DNA was extracted from the protein data bank (ID: 3QX3). The downloaded complex was prepared by the deletion of water residues followed by 3 D protonation and fixation of the missed amino acidsCitation44.
Docking into topoisomerase II-DNA target
Docking procedures were achieved and the proper binding pose was selected. This was carried out using Discovery studio software 4.1 protocolsCitation40. The docking poses were accomplished according to their binding energies. Briefly, the best binding pose was selected according to some criteria like having the crucial previously mentioned binding amino acids.
Physicochemical and pharmacokinetic properties
The physicochemical and pharmacokinetic parameters were evaluated using SwissADME (http://swissadme.ch/index.php), a free online web toolCitation45,Citation46.
Results and discussion
Chemistry
Iminostilbene 2 was synthesised with a very good yield (95%) from CBZ, for the first time, via basic hydrolysis using aqueous NaOH. Then, carbohydrazide 3 was prepared following the reported proceduresCitation34,Citation47. Indeed, carbohydrazide 3 could be prepared from CBZ or compound 2 via diverse chemical pathways. Hydrazinolysis of CBZ would provide compound 3 in one step; however, it did not work. Moreover, the reaction of 2 with alkylchloroformates followed by hydrazinolysis led to compound 3. Also, the reaction of 2 with phosgene followed by the addition of hydrazine to the produced carbonyl chloride will afford compound 3 in a “two-step one-pot” reaction instead of two isolated reactions in the former method (Scheme 1). The latter method has been adopted to prepare compound 3 with a good yield. Further reaction of 3 with different acid chlorides at room temperature in chloroform and the presence of triethylamine afforded compounds (4a–g).
Compounds 4a–g have been characterised by NMR and Mass spectroscopy as described in the experimental section and supporting information. In 13C-NMR, two signals corresponding to carbonyl carbon have been observed at δC 164–169 and δc 153–157 ppm. Also, all aromatic carbons were observed at δC 100–150 ppm, and the aliphatic methyl of 4d and methylene of 4g were detected at δC 21.5 and 45 ppm, respectively. The 1H-NMR showed only aromatic protons for all compounds except compound 4d, which displayed methyl signal at δH 2.29 ppm, and compound 4g, where a very characteristic triplet resulted from overlapped doublet of doublet signals of the prochiral methylene group was noted at δH 1.15 ppm. The most characteristic signal of compounds 4a–g is the amidic NH singlet signal at δH 10.0 ppm, while the other amidic NH was observed at δH 7.0–8.0 ppm, very close to aromatic protons. The mass spectra of compounds 4a–g were performed using negative mode ESI and showed the molecular ion signal as a minor peak, but the [M-H]– was present in all spectra as the base peak.
Compounds 5a–g (1,3,4-oxadiazole derivatives) have been accomplished through intramolecular cyclodehydration of compounds 4a–g. Heating in solvents like pyridine, or DMF is a method of Cyclodehydration. Moreover, cyclisation is performed in presence of catalysts such as SOCl2, P2O5, 1-ethyl-3–(3-dimethylaminopropyl)carbodiimide (EDC), POCl3, H2SO4, triphenylphospine, Burgess reagent, or triflic anhydrideCitation48. In this study, POCl3 was used as a solvent and cyclo-dehydrating agent. Besides, the addition of water to the hot reaction (very slowly) to hydrolyse POCl3 was a very important step in getting filterable powder.
Spectral confirmation of compounds 5a–g has been performed where the NH bands have disappeared from 1H-NMR. In 13C-NMR two imine signals of the oxadiazole ring were revealed at δ 157–164 ppm. The mass spectra of compounds 5a–g have been performed in positive APCI and showed the molecular ion peak as the base peak for all compounds.
Biological activity
In vitro NCI-60 one-dose screening anti-proliferative activity
The anti-tumour activity of the fourteen synthesised congeners was investigated at the NCI against a panel of 60 cancer cell lines (NCI-60 Cell One-Dose Screen). The tested dibenzo[b,f]azepine derivatives were screened at a single dose level (10 μM), .
Table 1. Growth inhibition % of the tested compounds (4a–g and 5a–g) against different 60 cell lines (NCI-60).
Notably, the open analogues of dibenzo[b,f]azepine carbohydrazide derivatives (4a–g) showed very weak anticancer activities towards the tested 60 cancer cell lines. However, the corresponding closed analogues of dibenzo[b,f]azepin oxadiazole derivatives (5a–g) achieved remarkable anticancer activities.
Collectively, the most responsive cell line among the tested cells was leukaemia cancer cell lines, in particular, the SR cell line. The growth inhibition GI % of the tested congeners 5b, 5c, 5d, 5e, 5f, and 5 g against SR cells were 72.97%, 67.50%, 60.59%, 30.12%, 58.44%, and 17.55%, respectively.
Topoisomerase II inhibitory activity
Aiming to confirm the suggested mechanism, the synthesised closed analogues of dibenzo[b,f]azepin oxadiazole congeners (5a–g) with the highest anti-tumour activities were all examined for their ability to inhibit topoisomerase II in vitro. Therefore, following the procedure reported by Patra et al., the topoisomerase II activity was evaluatedCitation36. Doxorubicin, an FDA-approved topoisomerase II inhibitor, was co-assayed as a reference. The IC50 values of the tested congeners and doxorubicin were recorded and represented in .
Table 2. Topoisomerase II activity of the tested candidates (5a–g) against doxorubicin.
An initial overview revealed that the synthesised members possessed potent to moderate topoisomerase II inhibiting activities with IC50 ranging from 6.36 ± 0.36 to 31.76 ± 1.78 µM. Obviously, compound 5e was noticed to be the most active among the tested new members, as it inhibited topoisomerase II in an IC50 value of 6.36 ± 0.36 µM which was quite close to that of doxorubicin (IC50 = 3.44 ± 0.19 µM). Furthermore, 5b and 5f members exhibited efficient topoisomerase II inhibitory activities with IC50 of 10.24 ± 0.58 and 9.561 ± 0.53 µM, respectively, .
As seen in , compounds 5e and 5f with the promising IC50 values exhibited the largest cell inhibition of 89, and 88% inhibition at the concentration of 100 µM.
Figure 3. Topoisomerase II inhibitory activity of the tested compounds (5a–g) against doxorubicin. (A) Percentage of topoisomerase II inhibition at concentration (100 µM) of the tested compounds; (B) DNA-fragmentation gel images of the most active compounds (5e and 5f) at various concentrations (100–1.3 µg/ml).

Anti-proliferative effect against SR cancer cell line
Depending on the previous results, the cytotoxic activity of the designed candidate 5e was then evaluated against leukaemia SR cells. Doxorubicin was also evaluated as a reference drug. The obtained results confirmed the cytotoxic effect of the examined member 5e with an IC50 value of 13.05 ± 0.62 µM against the aforementioned cell line.
Following, the cytotoxic effect of compound 5e against a normal cell line, PSC-800–011, was also assigned to evaluate the selectivity of the compound towards the cancer cell lines. Results revealed the weak effect of compound 5e against the normal cell line. However, the selectivity index (SI) of compound 5e was also calculated to confirm its selectivity. It was known that any sample that has an SI value higher than 3 will be considered to have high selectivity. Thus, the results demonstrated in revealed the high selectivity of the tested member.
Table 3. The cytotoxicity results of compound 5e against SR and normal cell lines.
Effect on cell cycle phases
The topoisomerase II inhibitory evaluation, besides the cytotoxicity results of the new compounds, encouraged us to assess the effect of 5e (the most promising candidate) on cell cycle progression in SR cells. Notably, the 5e effects on the cell cycle, besides the %age of the cellular population in each phase are summarised in .
Figure 4. Analysis of cell cycle for 5e-treated SR cells. *P < 0.05 compared to control by GraphPad prism using unpaired t-test.

Observing the obtained results revealed that member 5e arrested cell cycle progression at the G1 phase as it caused a significant increase of the cell levels to 61.29% versus 52.84% accumulation of the control cells. It was also disclosed that member 5e caused an increase in the S phase % of the examined cells to 36.86% instead of 34.18% in the untreated cells. The former results confirmed the potential of 5e to arrest SR cell progression at the G1 phase effectively. Cell cycle histograms are supported in the supporting information (Figure SI 1).
Apoptosis analysis
Annexin-V staining assay was applied to determine the apoptosis induced by compound 5e. Thus, compound 5e was added to SR cells with a concentration equivalent to its IC50 value of 13.05 ± 0.62 µM. According to the results represented in , compound 5e increased the apoptosis ratio by 37.34% compared to the untreated cells. In detail, 13.61% and 24.33% for the early phase and late phase of apoptosis, respectively, with respect to the control (0.44%, and 0.16%, respectively). Annexin V/PI staining histograms are supported in the supporting information (Figure SI 2).
In vivo studies
Validating the anti-tumour activity of 5e against Solid Ehrlich carcinoma (SEC) proliferation, tumour mass increased during the experimental period to 226 mg in the solid mass. shows that upon treatment both 5e and doxorubicin had a significant anti-tumour effect, with a decrease in the solid tumour mass to 96.3 mg and 82.3 mg, respectively. Regarding tumour inhibition ratio, compound 5e treatment significantly recorded a 62.7% compared to 65.8% (doxorubicin treatment). Moreover, compound 5e decreased the tumour volume to 30.1 mm3 compared to 80.6 mm3 in the SEC-control, while doxorubicin treatment decreased tumour volume to 27.5 mm3.
Figure 6. Solid tumour growth was inhibited in SEC-bearing mice by administration of compound 5e (6 mg/Kg B.W., IP) (SEC model). (A) The tumour mass of different treated groups (morphological representation); “normal control, SEC-group, SEC+ 5e, and SEC + Dox.” (B) Anti-tumour activity of tumour mass, volume, and TIR% in different treated groups. (C) Comparative morphological examinations of the tumour tissues tested groups. “Values are expressed as Mean ± SEM values of mice in each group (n = 7).” Values denoted by sign **(P ≤ 0.001) were found to be statistically different from the SEC control when compared with an unpaired t-test in GraphPad Prism. (H&E stain, magnification ×400).

Following the tumour reduction, histopathological examinations () of tumour tissues in normal mice with uniform skeletal muscle bundles (Black arrow) with underlying fatty tissue (Red arrow). SEC group showing tumour (Black arrow) is formed of solid groups and irregular glandular structures, composed of malignant epithelial cells, with cellular and nuclear pleomorphism, nuclear hyperchromasia, and increased nucleo-cytoplasmic ratio. There are areas of necrosis (Red arrow). The SEC-treated group showed part of the tumour tissue (about 60%) (Black arrow) was necrotic (Black arrows). There are islands of viable tumour cells (Red arrows).
ALT and AST (liver enzymes) dramatically elevated to 65.9 and 71 U/L, respectively, due to hepatocellular injury after tumour inoculation. After 5e-treatment, both liver enzymes significantly lowered to 39.8 and 35.6 U/L, respectively, compared to doxorubicin treatment which decreased the ALT and AST levels to 34.8 and 35.6 U/L, . These findings demonstrated a significant enhancement of hepatocellular functions.
Figure 7. (A): ALT and AST in different SEC-treated groups. “Values are expressed as Mean ± SD of independent trials (n = 7),” “*(P ≤ 0.05) significantly different between SEC control and normal control, while #(P ≤ 0.05) is significantly different between treated groups compared to SEC control.” (B) Histopathological examinations of liver tissues in different treated groups of SEC-bearing mice. (H&E stain, magnification ×400). Normal mice with uniform hepatocytes, and portal tract with uniform portal tract (Black arrows). SEC group show portal tract expansion with chronic inflammatory cells (Black arrows), area of lytic necrosis (Arrowhead), and hydropic degeneration of hepatocytes (Red arrows). Treated 5e group show portal tract inflammation (Black arrow), with spillage of inflammatory cells into the limiting plate (Red arrowhead), foci of lytic necrosis, and mild lobular inflammation (Black arrowheads). Hepatocytes show mild hydropic degeneration (Red arrows).

Interestingly, histological investigations of liver sections also indicated considerable improvements, correlating with the improvement in liver enzymes, .
Our results follow previous studiesCitation49,Citation50, illustrating the anticancer activity of tested compounds through tumour inhibition, boosting liver enzyme activities, and repairing histopathological damage.
In silico examinations
Molecular modelling
Discovery studio software 4.1Citation40 was used to carry out a docking study via the selection of the X-ray crystallography of the human topoisomerase II-DNA complex from the PDB database (code: 3QX3). The selected poses of the most promising candidates were visualised using PyMOL program softwareCitation41.
Validation was performed to assure the accuracy of the Discovery studio program. This was accomplished by the re-docking of EVP (co-crystallized inhibitor) within the topoisomerase II-DNA pocket, .
Molecular docking was applied for the synthesised derivatives to illustrate the binding modes and interactions against the topoisomerase II-DNA complex. Both EVP and doxorubicin were used as reference standards. The key binding sites of the topoisomerase II-DNA target comprise the amino acids; ASP479, GLN778, ARG503, and MET782, and the nucleobases; CYT8, ADE12, CYT11, GUA13, and THY9Citation16.
Analysing the docking results of the examined candidates on the active site of topoisomerase II-DNA showed that most of the synthesised derivatives achieved good interactions. The docking scores of the closed analogues of dibenzo[b,f]azepin oxadiazole derivatives (5a–g) were found to be better than those of the dibenzo[b,f]azepine carbohydrazide open analogues (4a–g). The binding scores of all newly synthesised and examined compounds (4a–g and 5a–g) are described in the supporting information (Table SI 1).
Doxorubicin, as a reference standard, showed good binding interaction and bound some key amino acids and nucleobases of the target receptor revealing its potentiality as a topoisomerase II inhibitor and DNA intercalatorCitation16. The amino group of doxorubicin (at C4 of the pyran ring) showed the formation of an H-bond with DG10. Additionally, the carbonyl group linked to the tetracene nucleus at C9 of doxorubicin is bound to DC9 via an H-bond. Moreover, the oxygen atom and hydroxyl group linked to C7 and C6 of the tetracene nucleus, respectively, formed H-bond with DT9. Besides, the oxygen atom of the methoxy group linked to C4 of the tetracene nucleus of doxorubicin was involved in H-bonds with ARG503, which is a crucial amino acid. The aromatic ring formed pi–alkyl interaction with ARG503 and pi–pi interaction with the second aromatic ring. Furthermore, doxorubicin-induced van der Waal interactions with GLY478, LEU502, LYS456, ASP479, GLN778, ALA779, and MET782, .
Additionally, the co-crystallized EVP is bound with some key amino acids and nucleobases of the target receptor. The hydroxyl group (at C17 of the aromatic ring) formed two H-bond with ASP 479. Oxygen atom attached to pyranodioxin moiety formed H-bond with DC8. Also, a hydrogen atom of pyranodioxin moiety formed H-bond with DT8. Additionally, a methoxy group formed H-bond with ARG503. Also, methoxy group pyranodioxin formed pi-alkyl interaction with MET 782. Both aromatic rings formed pi-alkyl interaction with Arg 503. The methyl group of pyranodioxin nucleus formed a pi–alkyl bond with MET 782. Furthermore, it induced a van der Waal bond with GLU477, GLN778, and PRO819, .
Compound 5e was the most potent compound; it exhibited the best interaction against the topoisomerase II-DNA complex. oxadiazole moiety induced H-bond interaction through nitrogen atom with DC8 and pi-pi stacked interaction. One aromatic group attached to azepine formed a pi–pi bond with ARG503 and the other one formed a pi–pi interaction with DT9. Also, the oxygen atom of the nitro group formed two H-bonds with LEU507 and ASN520 as well as an attractive charge with GLU522. Furthermore, the compound induced van der Waal interaction with GLN778, GLY504, ASP479, DG10, ILE506, and LYS505 .
Moreover, the planner system of congeners 5b and 5f was also inserted between the DNA base pairs forming several pi–pi bonds. In addition, the oxadiazole moiety of compound 5b formed an H-bond with DT9 (), while compound 5f was stabilised in the active site via the formation of two H-bonds with DT9 and DT12 ().
Moreover, the 2D and 3D binding modes of the examined candidates 4a–g and 5a–g are represented in the supporting information (Table SI 2).
Briefly, the docking study revealed that series 5a–g induced promising binding interactions on the active pocket of the topoisomerase II-DNA receptor and had the highest binding scores (Table SI 1), which may be due to cyclisation which confers certain rigidity and selectivity to the target receptor. Additionally, oxadiazole moiety exhibited good fitting to the receptor.
Physicochemical and pharmacokinetic properties
Simulation studies provide a reliable approach to investigating the molecule’s ability to act as a drug. Pharmacodynamic and pharmacokinetic properties were investigated online through the SWISSADME toolCitation46. Fortuitously, our newly synthesised derivatives obeyed Lipinski’s rule, illustrating their ability to be an orally-active drug with consideration of bioavailability.
Concerning the ADME properties, all the newly synthesised target derivatives showed high GIT absorption. Therefore, these members may be considered to be orally active due to their low lipophilicity. Furthermore, most of the synthesised compounds can penetrate the blood–brain barrier (BBB); so these candidates are expected to be used for CNS tumours. Fortunately, most of the synthesised derivatives are negative substrates for P-glycoprotein (Pgp-) transporter and so are not exposed to its efflux action. Moreover, our target entities could be considered lead candidates for further optimizations in the future. Last but not the least; they exhibited zero alerts in the pan-assay interference structure (PAINS). The calculated pharmacokinetics and physicochemical parameters of the most active compounds (5a–g) are illustrated in and , respectively. Also, the pharmacokinetics and physicochemical parameters of compounds 4a–g are represented in supporting information (Tables SI 3 and SI 4, respectively).
Table 4. Physicochemical parameters of the synthesised compounds 5a–g.
Table 5. Pharmacokinetic and ADMET parameters of the synthesised candidates 5a–g.
Structure–activity relationship
A deep study of the results obtained from the biological testing as well as the docking simulation provided us with the following arrangement of the designed compounds as effective topoisomerase II inhibitors and DNA intercalators, :
Figure 13. SAR for the newly synthesised derivatives 4a–g and 5a–g as topoisomerase II inhibitors and DNA intercalators.

It is worth mentioning that the dibenzo[b,f]azepin oxadiazole derivatives (5a–g) showed superior anticancer activities and docking scores compared to those of dibenzo[b,f]azepine carbohydrazide derivatives (4a–g).
Therefore, regarding the above, we can say that the rigidification of (4a–g) compounds through their ring closure to produce the most promising anticancer candidates (5a–g) improved greatly their binding affinity and selectivity as well.
The superior topoisomerase II inhibitory effect, besides the cytotoxic activity, was achieved upon linking the oxadiazole part with a nitrophenyl moiety (5e). However, the binding pattern of compound 5e into the active pocket of the enzyme strongly supported its high biological activity.
Except for the nitrophenyl-containing compound 5e, the bicyclic groove binding bearing member 5f possessed a greater topoisomerase II inhibiting effect than that of the monocyclic groove binding bearing members.
Comparing the halide-containing members revealed that the analogue with bromophenyl moiety 5b showed a high biological activity than the chlorophenyl moiety bearing one 5c.
The unsubstituted phenyl ring of the groove-binding side chain 5a increased the compound’s activity over the methyl-substituted phenyl 5d or the benzyl side chain as well 5g.
Conclusion
In the present work, fourteen new dibenzo[b,f]azepine derivatives were designed and synthesised in a continuation of our previous findings to discover potent anticancer small molecules. Herein, the most promising seven anticancer candidates (dibenzo[b,f]azepin oxadiazole derivatives (5a–g)) were designed and synthesised by molecular rigidification using the ring closure technique of another corresponding new seven open analogues (dibenzo[b,f]azepine carbohydrazide derivatives (4a–g)). The new counterparts were designed to achieve the basic requirements of the reported topoisomerase II inhibitors and DNA intercalators. The NCI-60 protocol was assigned to study the effect of the designed members on various cell lines. Results revealed the promising effect of the newly designed closed analogues (5a–g) on the leukaemia SR cell lines. Following, compounds 5a–g were examined for their potential to inhibit the topoisomerase II enzyme and doxorubicin was co-assayed as a reference drug. The obtained results confirmed the high efficacy of the designed members against the topoisomerase II enzyme. Notably, compound 5e was the most potent among the tested members compared to doxorubicin. It inhibited the enzyme with an IC50 of 6.362 ± 0.36 µM versus 3.445 ± 0.19 µM of the reference drug. Additionally, the cytotoxicity effect of the 5e member was evaluated against the SR cell line. Compound 5e exhibited a significant cytotoxic effect with an IC50 value of 13.05 ± 0.62 µM. Moreover, the tested member 5e inhibited the cell cycle in the G1 phase. Besides, compound 5e increased the apoptosis ratio by 37.34% more than the untreated control cells. Finally, SAR and docking studies clarified that the rigidification of compounds (4a–g) through their ring closure to produce the most promising anticancer candidates (5a–g) improved greatly their binding affinity and selectivity as well. Also, the superior topoisomerase II inhibitory effect besides the cytotoxic activity was achieved upon linking the oxadiazole part with a nitrophenyl moiety (5e).
Author contributions
Conceptualisation: Mohammed Farrag El-Behairy and Ahmed A. Al-Karmalawy; Data curation: Mohammed Farrag El-Behairy, Walaa Hamada Abd-Allah, Mohamed M. Khalifa, Mohamed S. Nafie, Mohamed A. Saleh, Wagdy M. Eldehna, and Ahmed A. Al-Karmalawy; Visualisation: Walaa Hamada Abd-Allah and Mohamed M. Khalifa; Methodology: Mohammed Farrag El-Behairy, Walaa Hamada Abd-Allah, Mohamed M. Khalifa, Mohamed S. Nafie, and Ahmed A. Al‐Karmalawy; Validation: Walaa Hamada Abd-Allah, Mohamed M. Khalifa, and Ahmed A. Al-Karmalawy; Supervision: Ahmed A. Al-Karmalawy; Writing – review and editing: Mohammed Farrag El-Behairy, Walaa Hamada Abd-Allah, Mohamed M. Khalifa, Mohamed S. Nafie, Mohamed A. Saleh, Wagdy M. Eldehna, and Ahmed A. Al‐Karmalawy. Finally, all authors revised and approved the final submitted version of the manuscript.
Supplemental Material
Download PDF (4.8 MB)Acknowledgement
The authors extend their appreciation to the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R25), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Martínez-Jiménez F, Muiños F, Sentís I, Deu-Pons J, Reyes-Salazar I, Arnedo-Pac C, Mularoni L, Pich O, Bonet J, Kranas H, et al. A compendium of mutational cancer driver genes. Nat Rev Cancer. 2020;20(10):555–572.
- Hammoud MM, Khattab M, Abdel-Motaal M, Van der Eycken J, Alnajjar R, Abulkhair H, Al‐Karmalawy AA. Synthesis, structural characterization, DFT calculations, molecular docking, and molecular dynamics simulations of a novel ferrocene derivative to unravel its potential antitumor activity. J Biomol Struct Dyn. 2022;1–18.
- Ezz Eldin RR, Al-Karmalawy AA, Alotaibi MH, Saleh MA. Quinoxaline derivatives as a promising scaffold for breast cancer treatment. New J Chem. 2022;46 (21):9975–9984.
- Hornberg JJ, Bruggeman FJ, Westerhoff HV, Lankelma J. Cancer: a systems biology disease. Biosystems. 2006;83 (2-3):81–90.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921.
- Avendaño C, Menendez JC. Medicinal chemistry of anticancer drugs. Amsterdam, Netherlands: Elsevier; 2015.
- Elshal M, Eid N, El-Sayed I, El-Sayed W, Al-Karmalawy AA. Concanavalin-A shows synergistic cytotoxicity with tamoxifen via inducing apoptosis in estrogen receptor-positive breast cancer: in vitro and molecular docking studies. Pharm Sci. 2021;28(1):76–85.
- Elmaaty AA, Darwish KM, Chrouda A, Boseila AA, Tantawy MA, Elhady SS, Shaik AB, Mustafa M, Al-Karmalawy AA. In silico and in vitro studies for benzimidazole anthelmintics repurposing as VEGFR-2 antagonists: novel mebendazole-loaded mixed micelles with enhanced dissolution and anticancer activity. ACS Omega. 2022;7(1):875–899.
- Ma C, Taghour MS, Belal A, Mehany AB, Mostafa N, Nabeeh A, Eissa IH, Al-Karmalawy AA. Design and synthesis of new quinoxaline derivatives as potential histone deacetylase inhibitors targeting hepatocellular carcinoma: in silico, in vitro, and SAR studies. Front Chem. 2021;9:725135.
- Read AF, Day T, Huijben S. The evolution of drug resistance and the curious orthodoxy of aggressive chemotherapy. Proc Natl Acad Sci USA. 2011;108(supplement_2):10871–10877.
- Mokhtari RB, Homayouni TS, Baluch N, Morgatskaya E, Kumar S, Das B, Yeger H. Combination therapy in combating cancer. Oncotarget. 2017;8(23):38022–38043.
- Anighoro A, Bajorath J, Rastelli G. Polypharmacology: challenges and opportunities in drug discovery: miniperspective. J Med Chem. 2014;57(19):7874–7887.
- Blagosklonny MV. Overcoming limitations of natural anticancer drugs by combining with artificial agents. Trends Pharmacol Sci. 2005;26(2):77–81.
- Goodsell D, Dickerson RE. Isohelical analysis of DNA groove-binding drugs. J Med Chem. 1986;29(5):727–733.
- Hammoud MM, Nageeb AS, Morsi MA, Gomaa EA, Elmaaty AA, Al-Karmalawy AA. Design, synthesis, biological evaluation, and SAR studies of novel cyclopentaquinoline derivatives as DNA intercalators, topoisomerase II inhibitors, and apoptotic inducers. New J Chem. 2022;46(23):11422–11436.
- El-Helby A-GA, Sakr H, Ayyad RR, Mahdy HA, Khalifa MM, Belal A, Rashed M, El-Sharkawy A, Metwaly AM, Elhendawy MA, et al. Design, synthesis, molecular modeling, in vivo studies and anticancer activity evaluation of new phthalazine derivatives as potential DNA intercalators and topoisomerase II inhibitors. Bioorg Chem. 2020;103:104233.
- Alesawy MS, Al‐Karmalawy AA, Elkaeed EB, Alswah M, Belal A, Taghour MS, Eissa IH. Design and discovery of new 1, 2, 4‐triazolo [4, 3‐c] quinazolines as potential DNA intercalators and topoisomerase II inhibitors. Arch Pharm. 2021;354(3):2000237.
- Wheate NJ, Brodie CR, Collins JG, Kemp S, Aldrich-Wright JR. DNA intercalators in cancer therapy: organic and inorganic drugs and their spectroscopic tools of analysis. Mini Rev Med Chem. 2007;7(6):627–648.
- Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009;9(5):327–337.
- Wang JC. Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol. 2002;3(6):430–440.
- Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70(1):369–413.
- Kellner U, Sehested M, Jensen PB, Gieseler F, Rudolph P. Culprit and victim–DNA topoisomerase II. Lancet Oncol. 2002;3(4):235–243.
- Attia EZ, Khalifa BA, Shaban GM, Amin MN, Akil L, Khadra I, Karmalawy AAA, Alnajjar R, Zaki MYW, Aly OM, et al. Potential topoisomerases inhibitors from Asergillus terreus using virtual screening. S Afr J Bot. 2022;149:632–645.
- Khalifa MM, Al-Karmalawy AA, Elkaeed EB, Nafie MS, Tantawy MA, Eissa IH, Mahdy HA. Topo II inhibition and DNA intercalation by new phthalazine-based derivatives as potent anticancer agents: design, synthesis, anti-proliferative, docking, and in vivo studies. J Enzyme Inhib Med Chem. 2022;37(1):299–314.
- Ghanem A, Emara HA, Muawia S, Abd El Maksoud AI, Al-Karmalawy AA, Elshal MF. Tanshinone IIA synergistically enhances the antitumor activity of doxorubicin by interfering with the PI3K/AKT/mTOR pathway and inhibition of topoisomerase II: in vitro and molecular docking studies. New J Chem. 2020;44(40):17374–17381.
- Avendaño C, Menéndez J. Medicinal chemistry of anticancer drugs. Amsterdam, Netherlands: Elsevier; 2015. p. 133–195.
- Gallego J, Ortiz AR, de Pascual-Teresa B, Gago F. Structure–affinity relationships for the binding of actinomycin D to DNA. J Comput Aided Mol Des. 1997;11(2):114–128.
- Bailey SA, Graves DE, Rill R. Binding of actinomycin D to the T (G) nT motif of double-stranded DNA: determination of the guanine requirement in nonclassical, non-GpC binding sites. Biochemistry (Mosc). 1994;33(38):11493–11500.
- David-Cordonnier M-H, Hildebrand M-P, Baldeyrou B, Lansiaux A, Keuser C, Benzschawel K, Lemster T, Pindur U. Design, synthesis and biological evaluation of new oligopyrrole carboxamides linked with tricyclic DNA-intercalators as potential DNA ligands or topoisomerase inhibitors. Eur J Med Chem. 2007;42(6):752–771.
- Rao AS, Vardhan MV, Reddy NS, Reddy TS, Shaik SP, Bagul C, Kamal A. Synthesis and biological evaluation of imidazopyridinyl-1, 3, 4-oxadiazole conjugates as apoptosis inducers and topoisomerase IIα inhibitors. Bioorg Chem. 2016;69:7–19.
- Claveria-Gimeno R, Vega S, Abian O, Velazquez-Campoy A. A look at ligand binding thermodynamics in drug discovery. Expert Opin Drug Discov. 2017;12(4):363–377.
- Schindler W, Blattner H. Über Derivate des Iminodibenzyls Iminostilben-Derivate. HCA. 1961;44(3):753–762.
- Bhatt PV, Patel PM. Synthesis of 5H-dibenzo(b,f)azepine-5-carboxylic acid [3-chloro-2-(substitutedphenyl)-4-oxoazetidin-1-yl]amide from 5H-dibenzo(b,f) azepine-5-carbonyl chloride. Indian J Chem Sect B. 2005;44B:2082–2086.
- Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6(10):813–823.
- Patra N, De U, Kang J-A, Kim JM, Ahn MY, Lee J, Jung JH, Chung HY, Moon HR, Kim HS. A novel epoxypropoxy flavonoid derivative and topoisomerase II inhibitor, MHY336, induces apoptosis in prostate cancer cells. Eur J Pharmacol. 2011;658(2-3):98–107.
- Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89(2):271–277.
- Al-Warhi T, Abo-Ashour MF, Almahli H, Alotaibi OJ, Al-Sanea MM, Al-Ansary GH, Ahmed HY, Elaasser MM, Eldehna WM, Abdel-Aziz HA. Novel [(N-alkyl-3-indolylmethylene)hydrazono]oxindoles arrest cell cycle and induce cell apoptosis by inhibiting CDK2 and Bcl-2: synthesis, biological evaluation and in silico studies. J Enzyme Inhib Med Chem. 2020;35(1):1300–1309.
- Lo KK-W, Lee TK-M, Lau JS-Y, Poon W-L, Cheng S-H. Luminescent biological probes derived from ruthenium (II) estradiol polypyridine complexes. Inorg Chem. 2008;47(1):200–208.
- Accelrys Discovery Studio. Accelrys Discovery Studio Visualizer 2.1. San Diego, CA, USA: Accelrys; 2008.
- Yuan S, Chan HS, Hu Z. Using PyMOL as a platform for computational drug design. Wiley Interdiscip Rev Comput Mol Sci. 2017;7(2):e1298.
- Khattab M, Al-Karmalawy AA. Computational repurposing of benzimidazole anthelmintic drugs as potential colchicine binding site inhibitors. Future Med Chem. 2021;13(19):1623–1638.
- Mahmoud DB, Ismail WM, Moatasim Y, Kutkat O, ElMeshad AN, Ezzat SM, El Deeb KS, El-Fishawy AM, Gomaa MR, Kandeil A, et al. Delineating a potent antiviral activity of Cuphea ignea extract loaded nano-formulation against SARS-CoV-2: In silico and in vitro studies. J Drug Deliv Sci Technol. 2021;66:102845.
- Elebeedy D, Badawy I, Elmaaty AA, Saleh MM, Kandeil A, Ghanem A, Kutkat O, Alnajjar R, Abd El Maksoud AI, Al-Karmalawy AA. In vitro and computational insights revealing the potential inhibitory effect of Tanshinone IIA against influenza A virus. Comput Biol Med. 2022;141:105149.
- Hammouda MM, Elmaaty AA, Nafie MS, Abdel-Motaal M, Mohamed NS, Tantawy MA, Belal A, Alnajjar R, Eldehna WM, Al‐Karmalawy AA Design and synthesis of novel benzoazoninone derivatives as potential CBSIs and apoptotic inducers: In Vitro, in Vivo, molecular docking, molecular dynamics, and SAR studies. Bioorg Chem. 2022;127:105995.
- Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7(1):42717.
- Raoa GK, Kaur R, Pai PS. Synthesis and biological evaluation of dibenzo[b,f]azepine-5-carboxylic acid[1-(substituted-phenyl)-ethylidene]-hydrazides. Der Pharma Chemica. 2011;3(3):323–329.
- Augustine JK, Vairaperumal V, Narasimhan S, Alagarsamy P, Radhakrishnan A. Propylphosphonic anhydride (T3P®): an efficient reagent for the one-pot synthesis of 1,2,4-oxadiazoles, 1,3,4-oxadiazoles, and 1,3,4-thiadiazoles. Tetrahedron. 2009;65(48):9989–9996.
- ElZahabi HS, Nafie MS, Osman D, Elghazawy NH, Soliman DH, El-Helby AAH, Arafa RK. Design, synthesis and evaluation of new quinazolin-4-one derivatives as apoptotic enhancers and autophagy inhibitors with potent antitumor activity. Eur J Med Chem. 2021;222:113609.
- Boraei AT, Eltamany EH, Ali IA, Gebriel SM, Nafie MS. Synthesis of new substituted pyridine derivatives as potent anti-liver cancer agents through apoptosis induction: In vitro, in vivo, and in silico integrated approaches. Bioorg Chem. 2021;111:104877.

![Scheme 1. Synthesis of the novel open dibenzo[b,f]azepine analogues (4a–g) and their corresponding target closed ones (5a–g). (i) NaOH/H2O/Reflux, (ii) Phosgene/Toluene/NH2NH2/24 h/RT, (iii) Acid chloride/CHCl3/18 h/RT, and (iv) POCl3/30 min/Reflux.](/cms/asset/fdcfb2bf-30f7-45d2-83c2-c0f20d2b7967/ienz_a_2157825_sch0001_c.jpg)





