Article title: Design, synthesis and biological evaluation of 2-((4-sulfamoylphenyl)amino)-pyrrolo[2,3-d]pyrimidine derivatives as CDK inhibitors
Authors: Bo Yang, Yanni Quan, Wu-li Zhao, Yingjie Ji, Xiaotang Yang, Jianrui Li, Yi Li, Xiujun Liu, Ying Wang, Yanping Li
Journal: Journal of Enzyme Inhibition and Medicinal Chemistry
Bibliometrics: Volume 38, Number 1
DOI: https://doi.org/10.1080/14756366.2023.2169282
The authors would like to point out that the wrong version of and has appeared in the published article. Right chemical structure of R2 group for compound 2g in has been replaced. Also, the missing is added. The corrected Table and Figure are presented in the below corrigendum, and does not alter the conclusion drawn from this work. The authors sincerely apologize for these errors during the proof confirmation.
Figure 3. The anti-proliferative activity of new synthetic compounds is mainly mediated by CDK9. (A) 2g inhibited the phosphorylation of Rb and RNA polymerase II, induced apoptosis through down-regulation of Mcl-1 and c-Myc protein. MIA PaCa-2 cells were incubated with or without 2g at indicated concentration for 6 h. (B) CDK9 was dose-dependently knockdown by its specific siRNA after 48 h treatment in MIA PaCa-2 cells. (C) Downregulation of CDK9 using siCDK9 (50 nM) decreased the sensitivity of MIA PaCa-2 cell to compound 2g (0.2 µM). The cells were incubated with 2g for 72 h. NS no significance; ***p < 0.001; ****p < 0.0001. Whole-cell lysates were subjected to immunoblotting. A representative protein band of three independent experiments is shown. (D) Representative illustration of the binding of synthetic compound to CDK9/cyclin T. Small molecules 1 (left column), 2g (middle column), and 2i (right column) were shown in stick representation with carbons colored yellow.
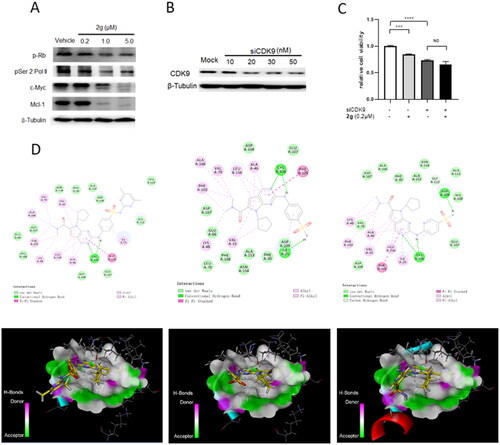
Table 1. Anti-proliferative activity of 2-((4-sulfamoylphenyl)amino)-pyrrolo[2,3-d]pyrimidine derivatives in pancreatic cancer MIA PaCa-2 cell culture.

