ABSTRACT
Quaternary ammonium compounds (QACs) or quats are a large class of antimicrobial chemicals used in households and institutions as sanitizers and disinfectants. These chemicals are utilized as food processing sanitizers, algicides, in the process of water treatment, and preservatives in cosmetics. The aim of this study was to determine an Adverse Outcome Pathway (AOP) whereby two widely used QACs, alkyl dimethyl benzyl ammonium chloride (ADBAC) and didecyl dimethyl ammonium chloride (DDAC), may result in respiratory tract and gastrointestinal tract effects. When inhaled or ingested, these QACs are incorporated into the epithelial cell membrane at the point of contact. With sufficient dosage, the epithelial membrane is disrupted, reducing its fluidity, and releasing cellular contents. Further, ADBAC and DDAC might disrupt mitochondrial functions leading to decreased ATP production. Both events might lead to cell death, either attributed to direct lysis, necrosis, or apoptosis. Pro-inflammatory mediators are recruited to the tissue, inducing inflammation, edema, and excess mucus production. The primary tissue-level adverse outcome is epithelial degeneration and dysplasia. Most important, no apparent metabolism or distribution is involved in QAC action. Based upon this knowledge, it is suggested to replace default Uncertainty Factors for risk assessments with a set of Data Derived Extrapolation Factors.
Introduction
Quaternary ammonium compounds (QACs), also known as quats, are a large class of chemicals used in a variety of consumer and industrial products with unique attributes that make them an important public health tool, particularly due to their antimicrobial properties (Ogilvie et al. Citation2021; Zubris et al. Citation2017). QACs have a long history of safe use and are routinely reviewed by regulatory authorities for safety in the marketplace (Biomonitoring California Citation2020; ECHA Citation2015a; ECHA. Citation2015b). There are several QAC sub-classes, and sometimes these sub-classes have very different functions such as herbicidal QACs versus non-herbicidal QACs (Biomonitoring California Citation2020). The aim of this study was to focus on two main antimicrobial sub-classes: alkyl dimethyl benzyl ammonium chloride (ADBAC)-type QACs which includes the commonly used benzalkonium chloride and didecyl dimethyl ammonium chloride (DDAC)-type QACs. ADBAC and DDAC are groups of active ingredients registered with the U.S. Environmental Protection Agency and other global agencies for antimicrobial products.Footnote1 QAC antimicrobial products include household and institutional sanitizers and disinfectants, food processing sanitizers, algicides, water treatment systems, and preservatives in cosmetics (Bureš Citation2019; Choi et al. Citation2018; DeLeo et al. Citation2020; Piola and Grandison Citation2017; Zubris et al. Citation2017). The toxicology of these compounds has been reviewed extensively (ECHA Citation2015a, Citation2021; ECHA. Citation2015b; EPA Citation2017a, Citation2017b; Luz et al. Citation2020). In general, QACs are poorly absorbed following ingestion or dermal application and do not produce systemic toxicity, but rather cause point of contact effects. This is supported by the ECHA (Citation2020) review of the ADBAC and DDAC database which reached the same conclusion:
“According to the available studies on toxicokinetics and metabolism as well as to the toxicity study package, no systemic effects in the absence of local effects were observed in any of those studies. Therefore, only a local risk assessment was considered necessary for the use of alkyl (C12-16) dimethylbenzyl ammonium chloride. To this aim, the local risk assessment has been performed applying the read-across principles from data presented for didecyldimethylammonium chloride (DDAC), a structurally related quaternary ammonium compound. (ECHA Citation2020)”.
The aim of this investigation was two-fold. First, to review and apply scientific data to outline an Adverse Outcome Pathway (AOP) whereby ADBAC and DDAC may initiate respiratory tract and gastrointestinal tract effects. Second, using the AOP and associated knowledge, to apply scientific and regulatory guidance to generate data derived extrapolation factors (DDEFs) for human health risk assessment of ADBAC and DDAC following inhalation and oral route exposure. DDEF is the term used by the U.S. Environmental Protection Agency (EPA Citation2014) and is similar in concept to the World Health Organization’s (WHO) “chemical-specific adjustment factors” (CSAFs) (WHO Citation2005) and the EU REACH program’s use of “informed adjustment factor” and “substance-specific adjustment factors” as defined by the European Center for Ecotoxicology and Toxicology of Chemicals (ECETOC) (ECETOC Citation2010). The development of DDEFs considers existing regulatory guidance on DDEFs from the EPA, the National Academy of Sciences (NAS), and EU and chemical-specific data on DDAC and ADBAC.
Individuals may be exposed to QACs orally via indirect food contact or from incidental hand-to-mouth transfer from treated surfaces (Hagi-Pavli et al. Citation2014; Luz et al. Citation2020). Inhalation of QACs might occur from aerosol formation of breathable particle sizes, household sprays or from QACs adhered to dusts as QACs are not volatile (Clausen et al. Citation2020; Johnson Citation2017; Kwon et al. Citation2019). Exposures from typical consumer product use are well below those expected to produce adverse effects (Osimitz and Droege Citation2021). Thus, it is necessary to understand that there is a threshold of QAC exposure, depending on the exposure scenario, below which there is no safety concern. Safe use instructions are provided on product labels. However, at higher exposures, direct-acting irritation might occur, and the biological pathways by which that occurs are described in this paper.
Methods
Mode of Action (MOA) and Adverse Outcome Pathway (AOP)
The Mode of Action (MOA) of a chemical or chemical class is defined as a series of biologically plausible events that lead to an effect. The World Health Organization’s International Programme on Chemical Safety (WHO IPCS) developed a framework to organize and present chemical-specific MOA information (Boobis et al. Citation2008; Meek et al. Citation2014; Seed et al. Citation2005; Sonich-Mullin et al. Citation2001). This led to development of the AOP analysis (OECD Citation2017, Citation2018) based upon the concept that toxicity results from the chemical reaching a critical target and triggering a molecular initiating event (MIE). Given sufficient dose this is followed by downstream key events (KE) leading to an adverse outcome. An understanding of the AOP may be used to develop DDEFs to replace default uncertainty factors (UF) for risk assessment (EPA Citation2014). These DDEFs address interspecies variability, intraspecies variability, and varying durations of exposure (short, intermediate, and long term).
The AOP scheme developed in this investigation follows principles outlined by the OECD:
“An AOP describes a sequence of events commencing with initial interaction(s) of a stressor with a biomolecule within an organism that initiates a perturbation in its biology such as MIE, which progresses through a dependent series of intermediate KEs and culminate in an adverse outcome (AO) considered relevant to risk assessment or regulatory decision-making. AOPs are typically represented sequentially, moving from one KE to another. In this respect, AOPs define a series of measurable biological changes that may be expected to occur if the perturbation is sufficiently severe in terms of potency, duration or frequency to drive the pathway all the way to the AO. Importantly, AOPs do not describe every detail of the biology but instead focus on describing critical steps or checkpoints along the path to adversity, which are both measurable and have potential predictive value. (OECD Citation2018)”.
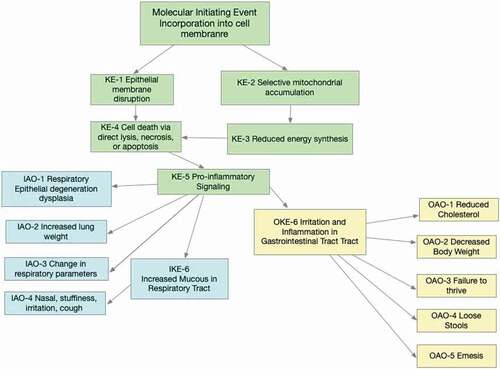
An AOP is presented for the adverse effects resulting from inhalation and oral exposure to QACs (). Both oral and inhalation routes of exposure share the same MIE and several of the initial KE. Once inflammation and pro-inflammatory signaling is underway (KE-5), the AOPs diverge, and the AO become route-specific.
Molecular Initiating Event – (MIE) (inhalation and oral routes) – Incorporation of QAC in Cell Membranes
Numerous studies examined how disinfectants, including QACs, kill or inhibit unwanted microbes (Gilbert and Moore Citation2005; Wessels and Ingmer Citation2013). The MIE for QAC results from the QAC long alkyl chains’ ability to “permeate into the membrane and disrupt its physical and biochemical properties” of surface cells such as epithelial cells of the eye, skin, lung or stomach (Wessels and Ingmer Citation2013). The charged nitrogen ‘head’ of the QAC remains at the membrane surface and the hydrophobic carbon chains are in the membrane bilayer. Eventually the increased concentration leads to decreased fluidity of the membrane and formation of holes in the membrane that enable the cell contents to leak out, which may further lead to cell lysis, repair or death.
Key events (KE) – inhalation and oral routes of exposure
For each KE below, biological changes are presented that occur and the scientific evidence supporting those events. Please refer to , as some KEs are relevant for both inhalation and oral exposures while others are specific to one route of exposure.
KE-1-epithelial membrane disruption
Description of Event. Gilbert and Moore (Citation2005) described the progression of membrane disruption by QAC molecules as these compounds accumulate in the membrane. The charged head group of the QAC remains at the membrane surface and the carbon-chain tails align with the phospholipids. Eventually, the increased chemical concentration leads to diminished membrane fluidity and void formation, which may further result in initiation of cell lysis and repair or death mechanisms. Interaction with cell membranes can differ among QACs. DDAC has demonstrated high-affinity binding with significant initial uptake, whereas ADBAC appears to be adsorbed concentration-dependently along the lines of the Langmuir adsorption equilibrium model (Wessels and Ingmer Citation2013).
Supporting Data. Bleb formation (i.e., bulges) on the surface of cell membranes or membrane systems were found to occur at QAC concentrations > 50 mg/ml (Yoshimatsu and Hiyama Citation2007). Leakage of proteins from bacteria, or leakage of tracer dyes from liposomes both indicated that membrane integrity is altered with exposure to ADBAC or DDAC concentrations of approximately 3 mg/ml or higher (Yoshimatsu and Hiyama Citation2007). Cellular autolysis is initiated upon a breakdown in RNA and membranes, in direct relationship with potassium depletion and leakage of proteins and β-galactosidase. The overall protein level and lactate dehydrogenase (LDH) activity measured in the bronchiolar-alveolar lavage fluid (BAL) may reflect disruption of the epithelial barrier and vascular permeability due to direct damage of airway and alveolar epithelial cells (Ohnuma et al. Citation2011). QAC exposure promotes what appears to be a biphasic response to cell injury with the initial effect attributed to the actual interaction of QAC with the cell membrane and, a greater effect resulting later because of the upregulated inflammatory response (Berg, Lie, and Steinsvåg Citation1994; Ohnuma et al. Citation2011).
Experiments demonstrating epithelial membrane disruption were generally conducted in either bacterial cell cultures found in gut flora (Gilbert and Moore Citation2005) via intra-tracheal instillation (Ohnuma et al. Citation2011) or other surface epithelial cells (Walters et al. Citation2012). These data demonstrated the surfactant effect of QAC on relevant epithelial cells, which are consistent with microscopic observations of epithelium irritation of the GI tract in dogs administered subchronic oral doses of DDAC (Cox and Bailey Citation1975) or rats treated with subchronic inhalation doses of DDAC (Kim, Lee, and Lim Citation2017). KE-1was also detected in cultured human respiratory mucosa (Berg, Henriksen, and Steinsvåg Citation1995) and in bacteria found in human gut flora (Wessels and Ingmer Citation2013).
KE-2-selective mitochondrial accumulation
Description of Event. Lipophilic cations including QACs were reported to selectively accumulate in mitochondria (Inácio et al. Citation2013; Liberman et al. Citation1969; Ross et al. Citation2005). This selective accumulation is driven by a membrane potential-dependent mechanism as shown by Bragadin and Dell’Antone (Citation1996). It was postulated that the negative charge of the mitochondrial matrix coupled with the QAC positive charge and ability to pass through biological membranes enables compounds to accumulate in mitochondria several 100-fold above intracellular levels (Murphy and Smith Citation2007). Because mitochondria are absent in prokaryotic cells such as bacteria, the discussion of this KE and subsequent downstream events and effects are limited to mammalian cells.
Supporting Data. Inácio et al. (Citation2013) demonstrated that mitochondrial membrane function was disrupted in Madin-Darby canine kidney (MDCK) II cells at concentrations below which produced cell lysis. QACs require time to build up concentrations in the mitochondria sufficient to initiate effects such as cytokine release measured 3 hr after treatment (Inácio et al. Citation2013). In several studies KE-2 follows a dose-response relationship in that increased QAC or other cationic surfactant result in elevated presence in the mitochondria and/or loss of mitochondrial function as measured by barrier function, ATP production, and loss of membrane potential (Bragadin and Dell’Antone Citation1996; Inácio et al. Citation2013; Ross et al. Citation2005).
Numerous models in both animal and cellular systems such as, isolated mitochondria spanning multiple mammalian species including rodent, human and dog and methods to determine mitochondrial function demonstrated results consistent with proposed mechanism. Inácio et al. (Citation2011) investigated nonionic, zwitterionic, anionic, and cationic surfactants and found that cationic surfactants were the only group in which cytotoxicity occurred at concentrations too low to provoke cell lysis (Inácio et al. Citation2011). Data suggest that the positive charge is central to enhanced cytotoxicity that differentiates cationic surfactants from other surfactants cytotoxic through a less specific surface-active action.
Kwon et al. (Citation2014) noted effects for KE-2 in human cell models. Although substantial differences are known between cell membrane structure and mitochondria between species, the lipid bilayer (permeable to QAC) and negatively charged mitochondria are consistent between humans and the experimental models used.
KE-3-reduced energy synthesis
Description of Event. Accumulation of QAC in the mitochondria of epithelial cells results in decreased ATP production, which leads to apoptotic or necrotic cell death (). QACs inhibit mitochondrial complex I (Datta et al. Citation2017; Inácio et al. Citation2013) that reduces NADH and translocates 4 protons from the mitochondrial matrix to the intermembrane space, which is a primary driver of the electrochemical gradient.
Supporting Data. ATP synthase requires the electrochemical proton gradient to function. Inhibition of complex I might result in collapse of mitochondrial membrane potential observed by Datta et al. (Citation2017), Inácio et al. (Citation2013), and Bragadin and Dell’Antone (Citation1996). Bragadin and Dell’Antone (Citation1996) proposed that the loss in mitochondrial membrane potential was mediated by increased membrane permeability as evidenced by calcium and potassium ion leakage. However, arrested complex I loss of membrane potential, due to loss of proton translocation, might also lead to ion release from losing the electrochemical gradient. Data suggest that complex I inhibition is likely the culprit for reducedATP production, but mitochondrial membrane disruption is a plausible alternative or complimentary mechanism that might induce similar effects on energy production. Changes in mitochondrial morphology observed by Inácio et al. (Citation2013) might result from membrane disruption, but observed fragmentation was also associated with apoptosis. Finally, Datta et al. (Citation2017) screened several compounds and demonstrated similar dose responses between cetylpyridinium chloride and ADBAC in reducing ATP production and oxygen consumption. Evidence indicates that the approach here to generalize cetylpyridinium chloride (Datta et al. Citation2017) and cetrimonium bromide (Bragadin and Dell’Antone Citation1996; Inácio et al. Citation2013) behavior to that of the ADBAC and DDAC is appropriate when discussing mitochondrial effects.
KE-3 consistently follows a dose-response relationship whereby QAC or other cationic surfactants result in decreased ATP production and threshold-based loss of membrane potential in a dose-dependent manner. Datta et al. (Citation2017) showed increased benzalkonium chloride concentrations produced diminished oxygen consumption and ATP production. It is important to note that there appears to be a threshold concentration where benzalkonium chloride does not significantly impact ATP production or mitochondrial oxygen consumption compared to negative control.
Numerous models animal and cellular such as isolated mitochondria spanning multiple mammalian species including rodent, human and canine and methods to assess mitochondrial functions show results consistent with the KE-3. QACs are capable of passing through intact cell membrane and accumulate in mitochondria in polarized epithelium in human cells. Assuming that QACs reach the target tissue such as the respiratory or GI epithelium at sufficient concentration, this effect on mitochondria is relevant to the observed QAC toxicity.
KE-4-cell death via apoptosis, direct lysis, or necrosis
Description of Event. It is well established that direct membrane lysis or decrease or depletion of cellular ATP produces cell death by apoptosis or necrosis (Eguchi, Shimizu, and Tsujimoto Citation1997; Inácio et al. Citation2011). Inácio et al. (Citation2013) demonstrated that QACs including ADBAC produce dose-related necrotic or apoptotic cell death depending upon the ATP/ADP ratio.
Supporting Data. Inácio et al. (Citation2011), using human cell lines, demonstrated that, at lower doses, pre-apoptotic signaling occurred whereas, high levels of QAC rapidly depleted ATP and necrotic cell death occurred. As concentrations at the cell approach the critical micelle concentration, the surface-active action of QAC predominates and results in cell lysis. Inácio et al. (Citation2011) also examined various lengths of cetrimonium bromide, cetylpyridinium bromide, and benzalkonium bromide and found that when the hydrocarbon chain lengths were comparable, the critical micelle concentration normalized relative toxicity scale was C12 benzalkonium bromide ≈ C12 cetylpyridinium bromide ≫ C12 cetrimonium bromide.
KE-5-pro-inflammatory signaling and inflammation
Most of the evidence to support this KE originates from studies of the responses of respiratory epithelium. Nonetheless, it is reasonable to assume qualitatively similar responses would also occur in gastrointestinal epithelium.
Description of Event. Pro-inflammatory signaling occurs following damage-induced loss of membrane integrity leading to potential enhanced vascular permeability and protein leakage and further propagation of the inflammatory signaling cascade. This is predominantly due to cytotoxicity that includes generation of reactive-oxygen species (ROS). Cell death via apoptosis might also lead to inflammation, but likely less extensive than via necrosis. Although proinflammatory components are released from necrotic cells, this proinflammatory signal is quenched over time as the “debris” is degraded and diluted (Rock and Kono Citation2008). Thus, low-level cellular injury results in limited inflammation, which is reversible and finite below a certain dose.
Supporting Data. Reactive-oxygen species and inflammation signaling was reported after ischemia-reperfusion injury (Rock and Kono Citation2008). Generation of oxygen or nitrogen radicals lead to activation of antioxidant response elements (AREs) and pro-inflammatory gene transcription and expression including hemoxygenase-1,Footnote2 and various cytokines/chemokines.
DDAC administration to the respiratory tract cells in vivo resulted in loss of membrane integrity enabling enhanced vascular permeability and leakage of proteins (Ohnuma et al. Citation2011). This led to induction of oxidative damage with evidence of detectable hemoxygenase-1 and upregulation of cytokines (Ohnuma et al. Citation2011, Citation2010; Ohnuma-Koyama et al. Citation2013).
Inhalation Route Specific Key Event – KEinhalation-6 – Irritation and Inflammation in the Respiratory Tract
Description of Event. Following inhalation exposures to QACs, proinflammatory signaling induced by cell death gives rise to increased inflammatory cell count in bronchoalveolar lavage fluid, in particular polymorphonuclear cells and macrophages.
Supporting Data. In some studies, an increase in neutrophilic inflammation was observed, in a pattern simulating ozone exposure (Larsen, Verder, and Nielsen Citation2012). The recruitment of inflammatory mediators increased in degree and severity with dose. Based upon elevated numbers of macrophages and polymorphonuclear neutrophils in a dose-dependent manner (Larsen, Verder, and Nielsen Citation2012), data suggest that direct epithelial interaction with increasing concentrations of ADBAC and other QACs initiate pro-inflammatory signaling leading to inflammatory cell recruitment. Inhalation studies of ADBAC or DDAC in mice or rats showed significant elevation in inflammatory markers in bronchoalveolar lavage (BAL) fluid from short-term high doses (Larsen, Verder, and Nielsen Citation2012; Swiercz et al. Citation2013, Citation2008). Longer term studies (2-, 4- and 13-week studies) at lower doses produced no or small changes in BAL fluid proinflammatory markers (Kim, Lee, and Lim Citation2017; Lim and Chung Citation2014; Weinberg Citation2011). A dose-dependent, threshold-based response was demonstrated.
Inflammatory mediators that recruit leukocytes also result in vasodilation and increased vascular permeability (Scallan, Huxley, and Korthuis Citation2010). These, and other secondary effects including protein infiltration into tissue, resulting in accumulation of extracellular fluid. Several investigators noted that QAC-mediated tissue injury from inhalation or intratracheal/nasal administration also induced edematous changes (Cho et al. Citation2000; Kuboyama, Suzuki, and Hara Citation1997; Ohnuma-Koyama et al. Citation2013). Elevated protein production, likely the result of cellular influx and potential neutralization of lung surfactants, was measured in BAL fluid of BAC-exposed rats (Larsen, Verder, and Nielsen Citation2012). Given sufficient exposure, influx of fluid from capillary leak syndrome resulting from elevated protein levels and LDH activity release was measured in BAL fluid (Ohnuma et al. Citation2011). The temporal relationship between QAC exposure and inflammatory response in mice (Ohnuma et al. Citation2011) is biphasic with an initial effect attributed to epithelial degeneration and a greater effect resulting later because of cell membrane disruption and upregulated inflammatory response.
An alternative minor pathway is the cholinergic action QACs exert on muscarinic receptors in the peripheral and central neural receptors leading to histamine release in rodent mast cells in vitro (Miszkiel et al. Citation1988b). However, H1-histamine receptor antagonists induce only a minor decrease in histamine release, suggesting this is likely a minor, pathway (Miszkiel, Beasley, and Holgate Citation1988a; Miszkiel et al. Citation1988b).
Inhalation route specific key event – keinhalation-7-increased mucus
Description of Event. Nasal epithelial cells constantly produce mucus for protection and particulate transport. Mucociliary clearance aids in the removal of noxious substances that might get trapped in the mucus blanket (Stanley et al. Citation1985). A common response to inhaled irritants or allergens is increased mucus production (Cho et al. Citation2000).
Supporting Data. This irritant response accompanies clinical and histopathological observations in rats and human inhalation studies of ADBAC or DDAC. Inhalation studies of DDAC in rats for 28 days found mucus production upon histopathological investigation (Weinberg Citation2011). Nasal administration of BAC to rats produced congestion (Cho et al. Citation2000; Kuboyama, Suzuki, and Hara Citation1997). Nasal spray delivery of BAC in human volunteers induced mucosal swelling and discharge (Graf, Enerdal, and Hallén Citation1999; Riechelmann et al. Citation2004). Nasal congestion resulted from BAC-containing nasal spray in healthy volunteers but repeat applications over 10 days did not enhance symptoms (Storaas et al. Citation2000).
Inflammatory mediators and cytokines that contribute to edema and mucus production resulting from QAC insult may contribute to both downstream effects. It is reasonable to assume that QAC mediated inflammatory signaling might result in downstream mucus production consistent with the understanding of mucus secretion inducers such as inhaled irritants (Rogers Citation2007).
Oral Route Specific Key Event – KEoral-6 – Irritation and Inflammation in the Gastrointestinal Tract
Description of Event. Standard repeat-dose studies were conducted in mice, rats, rabbits and dogs via oral, dermal and inhalation routes along with developmental and reproductive toxicity evaluations (Luz et al. Citation2020). The results from these studies reveal a consistent pattern: local irritation rapidly followed by signs of generalized toxicity, as expected with the MOA of an irritant.
Supporting Data. Animal studies employing oral dosing resulted in inflammation of tissues lining the GI tract. Cox and Bailey (Citation1975) treated dogs with high doses of DDAC (50 mg/kg/day) in the diet for 90 days and reported histopathological observations of slight-to-mild mucosal congestion of the small intestine, and to a lesser extent the large intestine. Further Van Miller (Citation1988b) noted that Sprague-Dawley rats treated with 3000 ppm DDAC in the diet for 90 days initiated irritation of the gastric mucosa and/or gastrointestinal lesions; these lesions were not detected in animals given diets containing 1000 ppm.
Adverse outcomes – inhalation route
AOinhalation-1-epithelial degeneration, dysplasia
Description of Outcome. Animal inhalation studies demonstrated that QACs result in direct portal-of-entry tissue damage (Luz et al. Citation2020; Weinberg Citation2011).
Supporting Data. Micropipette administration of benzalkonium chloride to the nostrils of rats resulted in squamous metaplasia, degeneration of the trilaminar membrane, and mitochondrial swelling (Lebe et al. Citation2004). Cüreoğlu et al. (Citation2002) sprayed benzalkonium chloride into rabbit nostrils and noted mitochondrial effects and degenerative changes in the supportive and olfactory cells (Cüreoğlu et al. Citation2002). Cho et al. (Citation2000) performed micropipette administration of benzalkonium chloride to rats and found nasal lesions, including intraepithelial glandular formation, inflammatory cell infiltration, vascular hyperplasia, and edematous change (Cho et al. Citation2000). Kuboyama, Suzuki, and Hara (Citation1997) administered benzalkonium chloride nasally via micropipette to rats and noted edema, desquamation, and inflammation. Data demonstarted a dose response, including a no effect threshold where increased BAC concentration resulted in elevated epithelial desquamation. Ohnuma-Koyama et al. (Citation2013) showed that intratracheal instillation of DDAC to mice produced inflammation, inflammatory cell infiltration, edema, and cell death. Choi et al. (Citation2020) demonstrated that acute inhalation exposure to 20 mg/m3 f benzalkonium chloride was associated with deep breathing and nasal discharge as well as histopathological alterations in the nasal cavity and lungs of Fischer-344 rats postulated to be due to oxidative damage. Although no direct human data are available demonstrating this effect, it is biologically plausible. QACs are irritating and, other than accidental overexposure, it is unlikely that humans would be subjected to sufficiently high enough concentrations of QACs to elicit such damage.
It is clear from the studies above that sufficient necrotic or apoptotic cell death initiates a tissue level effect. Mitochondrial effects noted by Lebe et al. (Citation2004) and Cüreoğlu et al. (Citation2002) further support the link between mitochondrial dysfunction and ultimate epithelial degeneration. Overall, the portal-of-entry effects were relatively consistent in their dose response and composition: inflammatory cell infiltration, membrane disruption, mitochondrial degeneration, edema, and inflammation were commonly noted across most investigations.
AOinhalation-2-increased lung weight
Description of Outcome. Some inhalation toxicology studies in animals demonstrate that QACs might produce an increase in lung weights as determined at necropsy.
Supporting Data. Rats administered aerosols of ADBAC following short-term (< 7 days), high-dose inhalation exposures (Swiercz et al. Citation2013), or of DDAC (Kim, Lee, and Lim Citation2017; Lim and Chung Citation2014; Swiercz et al. Citation2013; Weinberg Citation2011) following lower-dose, longer-term exposures (14–90 days) resulted in increased lung weights. Acute inflammation and/or evidence of edema were observed accompanied by elevated lung weights. However, a longer duration of dosing did not result in a rise in lung weights which is consistent with an irritant response where lower doses are tolerated and do not progress. This is also observed with other point-of-contact irritant responses.
AOinhalation-3-reduced tidal volume and other changes to respiratory parameters
Description of Outcome. Reduced tidal volume and other respiratory parameters were reported in rats and mice (Larsen, Verder, and Nielsen Citation2012; Yang et al. Citation2012) and humans with asthma (Miszkiel, Beasley, and Holgate Citation1988a; Miszkiel et al. Citation1988b) following short-term exposure to BAC.
Supporting Data. Irritant responses are well known to affect respiratory parameters (ECETOC Citation2003, Citation2010; Larsen, Verder, and Nielsen Citation2012). DDAC and ADBAC are well established direct-acting irritants, with adverse respiratory effects seen in humans and animals. Reduced tidal volume and other respiratory parameters were noted in rats and mice (Larsen, Verder, and Nielsen Citation2012; Yang et al. Citation2012) and humans with asthma (Miszkiel, Beasley, and Holgate Citation1988a; Miszkiel et al. Citation1988b) following short-term exposure to BAC. Dose-response relationships were reported in mice following short term exposure to BAC, and three other QACs (hexadecyl trimethyl ammonium bromide, hexadecyl pyridinium chloride, and dimethyl dioctadecyl ammonium bromide) (Larsen, Verder, and Nielsen Citation2012). Concentration-dependent increases in respiratory frequency were observed above 1 mg/m3. Concentration-dependent decreases in tidal volume were also observed, with the highest doses producing approximately 30% reduction from baseline. Markers of the sensory irritation response indicated that QACs initiated no activation of trigeminal nerve endings, suggesting that the respiratory responses are due to irritation. Changes in respiratory parameters in rats administered aerosols of DDAC found only effects at the highest dose given (Yang et al. Citation2012).
AOinhalatonl-4-nasal stuffiness, irritation, cough
Description of Outcome. Inhalation studies of DDAC in rats for 28 days noted mucus production upon histopathological investigation, with clinical signs of rales and yellow discharge reported at the highest dose (Weinberg Citation2011).
Supporting Data. BAC administered nasally to humans produced immediate irritation, followed later by swelling and stuffiness. Nasal administration of an ADBAC to rats produced dose-related sneezing and nasal rubbing and nasal sounds, indicating irritation and congestion (Cho et al. Citation2000; Kuboyama, Suzuki, and Hara Citation1997). The findings in animals are consistent with data in humans where nasal spray delivery of an ADBAC induced irritation, pain, mucosal swelling and stuffiness (Graf, Enerdal, and Hallén Citation1999; Riechelmann et al. Citation2004; Storaas et al. Citation2000). Reduced tidal volume in mice and bronchoconstriction in human subjects with asthma following higher QAC exposures were rapid, dose-related, and not observed in concurrent controls. These observed adverse outcomes are consistent with other direct-acting irritant chemicals. Dose-related and temporally related responses noted for DDAC and ABDAC follow postulated AOP. These responses are consistent for animals and humans.
Adverse outcomes – oral route
AOoral-1 reduced serum cholesterol levels
Description of Effect. High doses of QACs may produce a reduction in circulating serum cholesterol levels in animals.
Supporting Data. Evidence for an effect of QACs on serum cholesterol levels is extremely limited. Nonetheless, in the interest of completeness, it is being included as a possibility as reported by Hrubec et al. (Citation2021) who noted in humans that QACs in blood were associated with disruption of cholesterol synthesis, increased inflammation, and reduced mitochondrial function. Serum cholesterol was determined in chronic rat toxicity studies with ADBAC (Gill, Chun, and Wagner Citation1991) and DDAC (Gill, Hermansky, and Wagner Citation1991). No significant treatment related effects were noted. Similarly, a 1-year chronic toxicity study in dogs with DDAC showed no marked effects. The only treatment-related observation of changes in cholesterol were noted in a 1-year chronic toxicity study in dogs with ADBAC (Goldenthal Citation1994). A significant decrease in serum cholesterol was noted in females of the high dose group (1200 ppm, n = 4 animals). No other clinical chemistry or organ-specific changes were detected.
Changes in serum cholesterol may constitute effects secondary to reduced caloric intake and general stress. It is notable that approximately half of the cholesterol in the human body derives from biosynthesis de novo. The liver accounts for approximately 10% and the intestines for approximately 10% of the amount produced each day (The Medical Biochemistry Page Citation2021). The remainder is a result of ingestion and GI uptake of cholesterol contained in foods. Thus, it is reasonable that damage to the GI tract due to the irritating effects of QACs may exert a significant effect on GI biosynthesis and/or uptake of cholesterol into the body, thereby reducing serum cholesterol levels. The above notwithstanding, a 1-year chronic toxicity study in dogs exposed to ADBAC indicated no consistent relationship between body weight, body weight gain, or food consumption and serum cholesterol levels (Goldenthal Citation1994).
Note: In a dashed line was used to indicate a qualitatively possible direct relationship between selective mitochondrial accumulation (KE-1) and cholesterol levels. Recently, investigators examined the effect of QACs on various steps in cholesterol biosynthesis and associated gene expression (Herron et al. Citation2016; Seguin et al. Citation2019). An exploratory in vivo study reported the effect of QAC on lipid metabolism-related genes in the brains of neonatal mice exposed during in utero to 120 mg/kg QAC (Herron et al. Citation2019). Unfortunately, only a single dose level was studied, a level at which Melin et al. (Citation2014) reported clear evidence of significant toxicity consisting of “clinical signs initiating euthanasia included: partial closure of eyelids (squinting), reduced activity, ataxia, kyphosis, hypothermia, rapid breathing and dyspnea, and cyanosis” (Melin et al. Citation2014).
These doses are far higher than the mean daily U.S. exposure of the population. For example, 120 mg/kg/day is almost 4000-fold as high as the average daily U.S. exposure to ADBAC and DDAC combined (0.0303 mg/kg/day) (EPA Citation2017a, Citation2017b). Thus, although a possible link in our AOP was included, this extremely high dose is quantitatively irrelevant for safety assessment of QAC for humans.
Generalized Gastrointestinal Effects (AOoral-2 Failure to Thrive, AOoral-3 Loose Stools, AOoral-4 Emesis)
Description of Effects. The most common GI effects including decreased body weight, body weight gain, and food consumption were observed in dogs, Guinea pigs, mice and rats (Luz et al. Citation2020). EmesisFootnote3 was also noted following dosing in a 1-year chronic dog study (Schulze Citation1991).
Supporting Data. Gastrointestinal effects are observed after repeated dosing. A longer duration of dosing did not result in greater effects. This is consistent with an irritant response where lower doses are tolerated and do not progress upon repeated exposure. Decreased body weight, failure to thrive, loose stools, and emesis (in dogs) are consistent observations from oral toxicity studies.
Results
Assessment of the postulated aop for QACs
Many KEs are common to both inhalation and oral exposures (). Following oral exposure, QACs might affect GI tract cells directly or produce GI irritation. This leads to loss of appetite, emesis, loose stools and, with long-term high doses, decreased body weight and failure to thrive. The postulated AOP for the inhalation route is well supported by in vitro data of lung and nasal epithelial cells, and in vivo studies in animals following inhalation or nasal delivery. Studies of QAC compounds in human cells and human volunteers provide a strong, clear and consistent evidence base. Sufficient dose-response, temporal data, consistent and plausible information are available in animals and humans to substantiate the KEs depicted in the AOP scheme. For oral exposure, an extensive database including short-term and long-term oral toxicity studies in mice, rats, rabbits, and dogs consistently demonstrate that toxicity is limited to effects at the point of contact (Luz et al. Citation2020). Studies in which histopathology was performed presented no marked evidence of cellular toxicity in distant tissues. Changes in body weight were noted in some investigations, but these were attributed to effects of irritation and inflammation at the point of contact and reduced food consumption. Further, QACs are poorly absorbed following ingestion or dermal application and do not lead to systemic toxicity.
Uncertainties, inconsistencies, and data gaps
Evidence of ADBAC- or DDAC-induced cell death arises from in vitro studies using human and animal cells and from histopathology evidence from in vivo investigations. Although direct evidence of cell death in humans is not available, sufficient information from human and animal cell studies in vitro and animal in vivo studies show that similar inflammatory processes occur in animals and humans.
Alternate AOP for oral route of exposure – alteration of gut microflora
In sub-chronic and chronic dietary studies, QACs irritate the GI tract, but speculation is that QACs may also alter the microflora because of their potent antimicrobial properties and thus produce secondary effects of weight loss and a general decline in health via this mechanism (Cox and Bailey Citation1975; Miller Citation1988b; Osheroff Citation1990; Van Miller Citation1988a). Although no definitive data were located for this alternative MOA, it is plausible and cannot be excluded as a significant contributor to GI irritation.
Alternate AOP for inhalation route of exposure – fibrosis
Ohnuma-Koyama et al. (Citation2013) used RNA expression analysis and immunohistochemical staining of lung cells to demonstrate that fibrotic markers were elevated from inter-tracheal exposure to DDAC. These investigators concluded that DDAC may exhibit the potential to induce fibrotic pathways but noted that longer-term dosing studies were needed to determine if this hypothesis is accurate. However, longer-term (4- and 13-week) inhalation studies using DDAC detected no fibrotic lesions (Kim, Lee, and Lim Citation2017; Weinberg Citation2011).
Discussion
Implications for risk assessment
Regulatory agencies have generally regarded the various apparently systemic effects following oral dosing as being secondary to effects on the GI tract. For example, ECHA says with respect to ADBAC:
“The results from the studies reveal a pattern of response (local irritation/corrosion followed by reduced food intake and reduction in body weight and body weight gain) that is consistent with the mode of action of a corrosive substance. Therefore, the systemic effects observed in these studies are regarded as secondary to the local irritation/corrosion caused by the test substance and as a result no adverse systemic effects were identified and no systemic risk characterization is required. (ECHA Citation2015a)”
Similarly for DDAC,
“As with acute exposure, DDAC repeated intake results in death in rodents at a concentration that affects the gastrointestinal mucosa or microflora or in unspecific toxicity with the major effects likely associated to the concentration dependent cytotoxicity, irritation and corrosivity at the site of contact. Indeed, the effects on which the NOEL [sic] derivation could have been based, independently on the species tested, were the reduction in body weight and body weight gain, consistent with decreased food consumption (US ISC; EQC). It was concluded that all effects could be attributed to local gastrointestinal irritation/ corrosion and consequent reduced food intake without observing any primary systemic effect. Therefore, the derivation of a NOAEL for systemic effects was deemed inappropriate. (ECHA. Citation2015b)”
The same principles apply to inhalation exposures. Hence, this analysis focuses on risk assessment for local effects.
Derivation of data derived extrapolation factors (ddefs) for local effects
In risk assessment practice, UFs are applied to the toxicity point of departure (PoD) (e.g., No Observed Adverse Effect Level – NOAEL) to account for variabilities and uncertainties in determining a health-protective toxicity benchmark. UFs for differences and intraspecies variability consist of two components: (1) a toxicokinetic component (variability in dose) and (2) a toxicodynamic component (variability in the response). The UFs for toxicokinetics encompass differences in metabolism and distribution of the chemical, whereas the UFs for toxicodynamics encompass the range of tissue responses/sensitivities to a given dose. Using the extensive database available for the QACs and guidance from regulatory agencies, DDEFs were determined to account for interspecies extrapolation, intraspecies extrapolation, and study duration.
Interspecies extrapolation
Various agencies and authoritative bodies including the European Center for Ecotoxicology and Toxicology of Chemicals (ECETOC), the U.S. Environmental Protection Agency (EPA), and the U.S. National Academy of Sciences’ National Research Council (NRC) have considered the MOAs and AOPs of chemicals causing point of contact toxicity (ECETOC Citation2003;, Citation2010; EPA Citation2014; NRC Citation2001; WHO Citation2005). EU regulators stated that sufficient evidence exists that humans are less responsive to irritants than animals. Examples of precedents in which DDEFs were used for specific direct acting chemicals include:
ECETOC (ECETOC Citation2003) – For chemicals, such as irritants producing direct local effects, they recommended UF = 1X for both toxicokinetic and pharmacodynamic differences.
NRC (NRC Citation2001) – Their Standard Operating Procedures for development of Acute Exposure Guideline Levels (AEGLs) stated that when the scientific evidence indicates that the mechanism or mode of action is not expected to differ among species, such as with chemicals causing local effects, an interspecies AF of 3 is generally used.
EPA (EPA Citation2017a, Citation2017b) – As part of their assessment of mineral acids, they stated that “ … the TD component of the UF can be set to 1, if the mechanism or mode of action, such as direct-acting irritation or alkylation, and is not expected to differ significantly between species.” Similarly for toxicokinetics they recommend a 1X UF for extrapolating from primate (source of mineral acid data) to humans.
EPA (EPA Citation2021) – In their draft risk assessment for chlorothalonil, a direct acting irritant with toxicity occurring at the point of contact in the respiratory tract, they concluded that the absorption, distribution, metabolism, and excretion characteristics are not likely to have a significant effect on the response among the human population. As such, the toxicokinetic component of the interspecies extrapolation UF was reduced to 1X.
The available data and information from computer-derived models of the respiratory tract in humans and rodents indicate that local effects of water soluble gases and vapors observed in the rat nasal cavity when extrapolated to the human situation are likely to over-estimate the effects in humans by factors of at least 2–4 (ECETOC Citation2010). Considering this, it is conceivable that a DDEF = 1 is appropriate for inhalation of QACs.
For oral exposures to QACs, the ECHA registration dossier for benzyl-C12-14-alkyldimethylammonium chlorides that underwent comprehensive ECHA review states that considerable species-related anatomical differences are known in the stomach and remaining GI tract and that this might lead to species-related differences in response. On this basis an interspecies UF = 2X is recommended (ECHA Citation2021). Evidence indicates that a DDEF = 1 to 3 is appropriate for assessment of oral intake of QACs, whereas a value of 3 may be most appropriate given the limited data available on the alteration of microflora.
Intraspecies extrapolation
Since no reproductive or developmental toxicity was seen in guideline studies on ADBAC and DDAC, the EPA determined that no additional safety factor for juveniles (also known as the FQPA factor) is required (EPA Citation2017a, Citation2017b).
Further, because no toxicokinetics, i.e., distribution and metabolism (often sources of inter-individual variability) are involved in QAC adverse outcomes in either the GI or respiratory tract, and variability in response may be narrow in DDAC and ADBAC studies, an inter-individual DDEF of 3 is appropriate for both routes per existing guidance (EPA Citation2017a,b; NRC Citation2001). Similarly, ECETOC (Citation2010) used a DDEF of 3 for workers and 5 for general population. The NRC states that “In those cases in which the mode or mechanism of action is such that the response elicited by exposure to the chemical by different subpopulations is unlikely to differ, an intraspecies UF of 3-fold is generally used. Typically, this response involves a direct-acting mechanism of toxicity in which metabolic or physiologic differences are unlikely to play a major role.” (NRC Citation2001). Similar guidance is provided elsewhere (EPA Citation2017a, b; ECETOC Citation2010; NRC Citation2001).
Study duration
Our analysis confirms that, regardless of exposure route, QACs are point-of-contact toxicants that disrupt cell membranes that may lead to irritation or cell lysis. Consistent with this is the reversibility of irritation and downstream effects from repeated low oral or inhalation exposures of ADBAC and DDAC. The adverse effects of QACs do not involve in situ or systemic metabolism. Inflammatory processes are balanced with anti-inflammatory processes and mechanism of cell repair (Wilson and Wynn Citation2009). Thus, at low doses, epithelial cells of either the respiratory or GI tract tolerate repeated low QAC exposures without measurable effect. Consequently, short-term and long-term exposures to small doses of QACs exert similar effects and display similar NOAEL values. Given the reversibility of the inhalation-induced KE and AO (Kim, Lee, and Lim Citation2017; Ohnuma et al. Citation2011; Weinberg Citation2011), the reversibility of other irritants (ECETOC Citation2010), and balance of inflammatory and anti-inflammatory processes in the lung (Wilson and Wynn Citation2009), no UF is needed to extrapolate from shorter to longer term studies. This is consistent with the observations of Shusterman, Matovinovic, and Salmon (Citation2006) that human respiratory irritation does not follow Haber’s law. Data on human inhalation exposures to direct acting irritants were dependent upon concentration but minimally on duration of exposure for initiating sensory irritation.
Summary
Based upon the above discussion, shows the scientifically supported DDEFs that are appropriate for QACs. A detailed presentation regarding the application of these DDEFs to the determination of Derived No-effect Levels (DNEL) or Reference Doses/Reference Concentrations (RfD/RfC) is beyond the scope of this study. However, a DNEL or RfD/RfC may be derived by dividing the PoD value (e.g., No Observed Adverse Effect Concentration (NOAEC) from a hazard identification study) by the total DDEF. A recent publication illustrates the use of these values to characterize risk to QACs via dermal, inhalation, and oral routes of exposure (Osimitz and Droege Citation2021).
Table 1. DDEFs for QACs
Conclusions
The overall database employed to develop our AOP for ADBAC and DDAC as representative QACs is strong. In vitro studies provide evidence on the molecular initiating events, where many in vivo animal studies are available to characterize the key events leading to AO. When inhaled or ingested, the QACs are incorporated into the epithelial cell membrane at the point of contact. With sufficient dose, the epithelial membrane is disrupted, reducing its fluidity, and releasing cell contents. Further, QACs might disrupt mitochondria of epithelia leading to reduced ATP production. Both events may lead to cell death, either from direct lysis, necrosis, or apoptosis. Pro-inflammatory mediators are recruited to the tissue, resulting in inflammation, edema and enhanced mucus production. The primary tissue-level AO is epithelial degeneration and dysplasia. Most important, no metabolism or distribution is involved in QAC action.
The application of such chemical-specific data for chemicals expressing local, point-of-contact effects has been extensively considered by regulatory agencies worldwide (EPA Citation2017a, Citation2017b, Citation2017c, Citation2017d; NRC Citation2001; NRC. Citation2010a; NRC Citation2010b, Citation2016; WHO Citation2005). In the context of these assessments, a set of DDEFs is proposed to replace default UFs for risk assessments on QACs. Such DDEFs not only use the knowledge gained from the many studies available, but are also protective of public health, while enabling continued use of these important beneficial antimicrobials.
Disclosure statement
This work was funded by the Quat Residue Group (QRG), under the auspices of Ignite Solutions (associated with the Household and Commercial Products Association). The two authors do occasional paid scientific analysis for the QRG.
Data Availability Statement
The authors state that there is no data set associated with the paper.Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Additional information
Funding
Notes
1. For conciseness these chemicals are referred to as ADBAC and DDAC, but, as mentioned above, each of these are groups of chemicals which have similar, but not identical, structures.
2. Heme oxygenase-1, also referred to as HO-1 or heat shock protein 90, is implicated in cytotoxic events and the inflammatory signaling response in alveolar epithelial cells (Rock and Kono Citation2008; Singal Citation2010).
3. Rodents do not have an emetic reflex, so emesis would not be expected in these species.
References
- Berg, O. H., R. N. Henriksen, and S. K. Steinsvåg. 1995. The effect of a benzalkonium chloride-containing nasal spray on human respiratory mucosa in vitro as a function of concentration and time of action. Pharmacol. Toxicol. 76 (4):245–49. doi:https://doi.org/10.1111/j.16000773.1995.tb00137.x.
- Berg, O. H., K. Lie, and S. K. Steinsvåg. 1994. The effect of decongestive nosedrops on human respiratory mucosa in vitro. Laryngoscope 104:1153–118.
- Biomonitoring California. 2020. Potential designated chemicals: Quaternary ammonium compounds. Materials for March 4, 2020 meeting of the Scientific Guidance Panel for Biomonitoring California, Accessed 8 January 2022. https://biomonitoring.ca.gov/downloads/potential-designated-chemicals-quaternary-ammonium-compounds .
- Boobis, A. R., J. E. Doe, B. Heinrich-Hirsch, M. E. Meek, S. Munn, M. Ruchirawat, J. Schlatter, J. Seed, and C. Vickers. 2008. IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit. Rev. Toxicol. 38 (2):87– 96. doi:https://doi.org/10.1080/10408440701749421.
- Bragadin, M., and P. Dell’Antone. 1996. Mitochondrial bioenergetics as affected by cationic detergents. Arch. Environ. Contam. Toxicol. 30 (2):280–84. doi:https://doi.org/10.1007/BF00215809.
- Bureš, F. 2019. Quaternary ammonium compounds: Simple in structure, complex in application. Top Curr Chem (Cham) 377 (3):14. doi:https://doi.org/10.1007/s41061-019-0239-2.
- Cho, J. H., Y. S. Kwun, H. S. Jang, J. M. Kang, Y. S. Won, and H. R. Yoon. 2000. Long-term use of preservatives on rat nasal respiratory mucosa: Effects of benzalkonium chloride and potassium sorbate. Laryngoscope 110 (2):312–17. doi:https://doi.org/10.1097/00005537-200002010-00025.
- Choi, H. Y., Y. H. Lee, C. H. Lim, Y. S. Kim, I. S. Lee, J. M. Jo, H. Y. Lee, H. G. Cha, H. J. Woo, and D. S. Seo. 2020. Assessment of respiratory and systemic toxicity of Benzalkonium chloride following a 14-day inhalation study in rats. Part Fibre Toxicol 17 (1):5. doi:https://doi.org/10.1186/s12989-020-0339-8.
- Choi, S. M., T. H. Roh, D. S. Lim, S. Kacew, H. S. Kim, and B. M. Lee. 2018. Risk assessment of benzalkonium chloride in cosmetic products. J Toxicol Environ Health B 21 (1):8–23. doi:https://doi.org/10.1080/10937404.2017.1408552.
- Clausen, P. A., T. A. Mørck, A. C. Ø. Jensen, T. W. Schou, V. Kofoed-Sørensen, I. K. Koponen, M. Frederiksen, A. Detmer, M. Fink, A. W. Nørgaard, et al. 2020. Biocidal spray product exposure: Measured gas, particle, and surface concentrations compared with spray model simulations. J Occup Environ Hyg 17 (1):15–29. doi:https://doi.org/10.1080/15459624.2019.1689247.
- Cox, G. E., and D. Bailey. 1975. 90-Day feeding study in dogs with a quaternary ammonium sanitizer BARDAC-22. Food and Drug Research Laboratories, Inc., Waverly, NY, USA. ( unpublished). MRID No. 40262901.
- Cüreoğlu, S., M. Akkuş, U. Osma, M. Yaldiz, F. Oktay, B. Can, C. Güven, M. Tekin, and F. Meriç. 2002. The effect of benzalkonium chloride on rabbit nasal mucosa in vivo: an electron microscopy study. Eur Arch Otorhinolaryngol 259 (7):362–64. doi:https://doi.org/10.1007/s00405-002-0458-x.
- Datta, S., G. He, A. Tomilov, S. Sahdeo, M. S. Denison, and G. Cortopassi. 2017. In vitro evaluation of mitochondrial function and estrogen signaling in cell lines exposed to the antiseptic cetylpyridinium chloride. Environ. Health Perspect. 125 (8):087015. doi:https://doi.org/10.1289/EHP1404.
- DeLeo, P. C., C. Huynh, M. Pattanayek, K. C. Schmid, and N. Pechacek. 2020. Assessment of ecological hazards and environmental fate of disinfectant quaternary ammonium compounds. Ecotoxicol. Environ. Saf. 206:111116. doi:https://doi.org/10.1016/j.ecoenv.2020.111116.
- ECETOC. 2003. Derivation of assessment factors for human health risk assessment. Technical Report No. 86, -0773-6347-86. Brussels, Belgium.
- ECETOC. 2010. Guidance on assessment factors to derive a DNEL Technical Report No. 110. Brussels, Belgium.
- ECHA. 2015a. Assessment report alkyl (C12-16) dimethylbenzyl ammonium chloride product-type 8. Wood Preservative Accessed 8 May 2021. https://echa.europa.eu/documents/10162/b9030b10-c8af-211b-456a-4f4b11d509b7 .
- ECHA. 2015b. Assessment report didecyldimethylammonium chloride product-type 8 (wood preservative), Accessed 8 May 2021. http://dissemination.echa.europa.eu/Biocides/ActiveSubstances/0067-08/0067-08_Assessment_Report.pdf .
- ECHA. 2020. Biocidal products committee (BPC). Opinion on the application for approval of the active substance: Alkyl (C12-16) dimethylbenzyl ammonium chloride product type:3. ECHA/BPC/267/2020’, Accessed 18 January 2022.https://echa.europa.eu/documents/10162/ea459fdd-abcd-3e16-115d-28082f99a968
- ECHA. 2021. Registration dossier: Quaternary ammonium compounds, benzyl C12-C16 (even numbered)-alkyldimethyl chlorides Accessed 18 August 2021. https://echa.europa.eu/registration-dossier/-/registered-dossier/13152/7/1 .
- Eguchi, Y., S. Shimizu, and Y. Tsujimoto. 1997. Intracellular ATP levels determine cell death fate by apoptosis or necrosis. Cancer Res. 57 (10):1835–40.
- EPA. 2014. Guidance for applying quantitative data to develop data-derived extrapolation factors for interspecies and intraspecies extrapolation. EPA/100/R-14/002F, Accessed 8 August 2021. https://www.epa.gov/sites/default/files/2015-01/documents/ddef-final.pdf .
- EPA. 2017a. Alkyl dimethyl benzyl ammonium chloride (ADBAC) final work plan. registration review: initial docket case number 0350. EPA-HQ-OPP-2015-0737, Accessed 20 March 2021. https://www.regulations.gov/document/EPA-HQ-OPP-2015-0737-0004 .
- EPA. 2017b. Didecyl dimethyl ammonium chloride (DDAC) final work plan. Registration review: Initial docket case number 3003. EPA-HQ-OPP-2015-0740, Accessed 20 March 2021. https://www.regulations.gov/document/EPA-HQ-OPP-2015-0740-0004 .
- EPA. 2017c. Memorandum. Sulfuric acid – Reevaluation of the Point of Departure (POD) and Uncertainty Factors used for inhalation risk assessment. EPA-HQ-OPP-2008-0766-0028, Accessed 14 February 2022. https://www.regulations.gov/document/EPA-HQ-OPP-2008-0766-0028 .
- EPA. 2017d. Mineral acids proposed interim registration review decision. Case Number 4064: EPA-HQ-OPP-2008-0766.
- EPA. 2021. Chlorothalonil: Revised human health draft risk assessment for registration review. EPA-HQ-OPP-20110840-0080, Accessed 14 February 2022. https://www.regulations.gov/document/EPA-HQ-OPP-2011-0840-0080 .
- Gilbert, P., and L. E. Moore. 2005. Cationic antiseptics: Diversity of action under a common epithet. J. Appl. Microbiol. 99 (4):703–15. doi:https://doi.org/10.1111/j.1365-2672.2005.02664.x.
- Gill, M. W., J. S. Chun, and C. L. Wagner. 1991. Chronic dietary toxicity/oncogenicity study with didecyldimethylammonium chloride in rats. Union Carbide, Bushy Run Research Center, Export, PA, USA. Report No. 53-566 (unpublished). MRID No. 41965101.
- Gill, M. W., S. J. Hermansky, and C. L. Wagner. 1991. Chronic dietary oncogenicity study with didecyldimethylammonium chloride in mice. Union Carbide, Bushy Run Research Center, Export, PA, USA. Report No. 53-528 ( unpublished). MRID No. 41802301.
- Goldenthal, E. 1994. Evaluation of ADBAC in a one-year chronic dietary toxicity study in dogs. International Research and Development Corporation, Mattawan, MI, USA. Report No. 638-004 (unpublished). MRID No. 43221101.
- Graf, P., J. Enerdal, and H. Hallén. 1999. Ten days’ use of oxymetazoline nasal spray with or without benzalkonium chloride in patients with vasomotor rhinitis. Arch. Otolaryngol. Head Neck Surg. 125 (10):1128–32. doi:https://doi.org/10.1001/archotol.125.10.1128.
- Hagi-Pavli, E., D. M. Williams, J. L. Rowland, M. Thornhill, and A. T. Cruchley. 2014. Characterizing the immunological effects of oral healthcare ingredients using an in vitro reconstructed human epithelial model. Food Chem. Toxicol. 74:139–48. doi:https://doi.org/10.1016/j.fct.2014.09.007.
- Herron, J. M., K. M. Hines, H. Tomita, R. P. Seguin, J. Y. Cui, and L. Xu. 2019. Multi-omics investigation reveals benzalkonium chloride disinfectants alter sterol and lipid homeostasis in the mouse neonatal brain Toxicol Sci. 171:32–45.
- Herron, J., R. C. Reese, K. A. Tallman, R. Narayanaswamy, N. A. Porter, and L. Xu. 2016. Identification of environmental quaternary ammonium compounds as direct inhibitors of cholesterol biosynthesis. Toxicol. Sci. 151 (2):261–70. doi:https://doi.org/10.1093/toxsci/kfw041.
- Hrubec, T. C., R. P. Seguin, L. Xu, G. A. Cortopassi, S. Datta, A. L. Hanlon, A. J. Lozano, V. A. McDonald, C. A. Healy, T. C. Anderson, et al. 2021. Altered toxicological endpoints in humans from common quaternary ammonium compound disinfectant exposure. Toxicol Rep 8:646–56. doi:https://doi.org/10.1016/j.toxrep.2021.03.006.
- Inácio, Â. S., G. N. Costa, N. S. Domingues, M. S. Santos, A. J. M. Moreno, W. L. C. Vaz, and O. V. Vieira. 2013. Mitochondrial dysfunction is the focus of quaternary ammonium surfactant toxicity to mammalian epithelial cells. Antimicrob. Agents Chemother. 57 (6):2631–39. doi:https://doi.org/10.1128/AAC.02437-12.
- Inácio, Â. S., K. A. Mesquita, M. Baptista, J. Ramalho-Santos, W. L. Vaz, and O. V. Vieira. 2011. In vitro surfactant structure-toxicity relationships: Implications for surfactant use in sexually transmitted infection prophylaxis and contraception. PLoS One 6:e19850. doi:https://doi.org/10.1371/journal.pone.0019850.
- Johnson, N. F. 2017. Pulmonary toxicity of benzalkonium chloride. J Aerosol Med Pulm Drug Deliv 31 (1):1–17. doi:https://doi.org/10.1089/jamp.2017.1390.
- Kim, Y. S., S. B. Lee, and C. H. Lim. 2017. Effects of didecyldimethylammonium chloride (DDAC) on Sprague-Dawley rats after 13 weeks of inhalation exposure. Toxicol Res 33 (1):7–14. doi:https://doi.org/10.5487/TR.2017.33.1.007.
- Kuboyama, Y., K. Suzuki, and T. Hara. 1997. Nasal lesions induced by intranasal administration of benzaikonium chloride in rats. J Toxicol Sci 22 (2):153–60. doi:https://doi.org/10.2131/jts.22.2_153.
- Kwon, J. T., H. M. Kim, P. Kim, and K. Choi. 2014. Didecyldimethylammonium chloride induces oxidative stress and inhibits cell growth in lung epithelial cells. Mol Cell Toxicol 10 (1):41–45. doi:https://doi.org/10.1007/s13273-014-0005-z.
- Kwon, D., J. T. Kwon, Y. M. Lim, I. Shim, E. Kim, D. H. Lee, B. I. Yoon, P. Kim, and H. M. Kim. 2019. Inhalation toxicity of benzalkonium chloride and triethylene glycol mixture in rats. Toxicol. Appl. Pharmacol. 378:114609. doi:https://doi.org/10.1016/j.taap.2019.114609.
- Larsen, S. T., H. Verder, and G. D. Nielsen. 2012. Airway effects of inhaled quaternary ammonium compounds in mice. Basic Clin. Pharmacol. Toxicol. 110 (6):537–43. doi:https://doi.org/10.1111/j.1742-7843.2011.00851.x.
- Lebe, E., M. Baka, A. Yavaşoğlu, H. Aktuğ, U. Ateş, and Y. Uyanikgil. 2004. Effects of preservatives in nasal formulations on the mucosal integrity: An electron microscopic study. Pharmacology 72 (2):113–20. doi:https://doi.org/10.1159/000080183.
- Liberman, E. A., V. P. Topaly, L. M. Tsofina, A. A. Jasaitis, and V. P. Skulachev. 1969. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature 222 (5198):1076–80. doi:https://doi.org/10.1038/2221076a0.
- Lim, C. H., and Y. H. Chung. 2014. Effects of didecyldimethylammonium chloride on Sprague-Dawley rats after two weeks of inhalation exposure. Toxicol Res 30 (3):205–10. doi:https://doi.org/10.5487/TR.2014.30.3.205.
- Luz, A., P. DeLeo, N. Pechacek, and M. Freemantle. 2020. Human health hazard assessment of quaternary ammonium compounds: didecyl dimethyl ammonium chloride and alkyl (C12-C16) dimethyl benzyl ammonium chloride. Regul. Toxicol. Pharmacol. 116:104717. doi:https://doi.org/10.1016/j.yrtph.2020.104717.
- The Medical Biochemistry Page. 2021. Cholesterol: Synthesis, metabolism, and regulation, Accessed 7 October 2021.https://themedicalbiochemistrypage.org/cholesterol-synthesis-metabolism-and-regulation/
- Meek, M. E., A. Boobis, I. Cote, V. Dellarco, G. Fotakis, S. Munn, J. Seed, and C. Vickers. 2014. New developments in the evolution and application of the WHO/IPCS framework on mode of action/species concordance analysis. J. Appl. Toxicol. 34 (1):1–18. doi:https://doi.org/10.1002/jat.2949.
- Melin, V. E., H. Potineni, P. Hunt, J. Griswold, B. Siems, S. R. Werre, and T. C. Hrubec. 2014. Exposure to common quaternary ammonium disinfectants decreases fertility in mice. Reprod. Toxicol. 50:163–70.
- Miszkiel, K. A., R. Beasley, and S. T. Holgate. 1988. The influence of ipratropium bromide and sodium cromoglycate on benzalkonium chloride-induced bronchoconstriction in asthma. Br J Clin Pharmacol 26 (3):295–301. doi:https://doi.org/10.1111/j.1365-2125.1988.tb05280.x.
- Miszkiel, K. A., R. Beasley, P. Rafferty, and S. T. Holgate. 1988. The contribution of histamine release to bronchoconstriction provoked by inhaled benzalkonium chloride in asthma. Br J Clin Pharmacol 25 (2):157–63. doi:https://doi.org/10.1111/j.1365-2125.1988.tb03286.x.
- Murphy, M. P., and R. A. Smith. 2007. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 47 (1):629–56. doi:https://doi.org/10.1146/annurev.pharmtox.47.120505.105110.
- NRC. 2001. Standing operating procedures for developing acute exposure guideline levels for hazardous chemicals ’, The National Academies Press, Accessed 8 August 2021. https://doi.org/https://doi.org/10.17226/10122 .
- NRC. 2010a. Acute exposure guideline levels for selected airborne chemicals: Volume 8, The National Academies Press, Accessed 8 August 2021. https://doi.org/https://doi.org/10.17226/12770 .
- NRC. 2010b. Acute exposure guideline levels for selected airborne chemicals. Volume 9, The National Academies Press, Accessed 8 August 2021. https://doi.org/https://doi.org/10.17226/12978 .
- NRC. 2016. Acute exposure guideline levels for selected airborne chemicals: Volume 20, The National Academies Press, Accessed 8 August 2021. https://doi.org/https://doi.org/10.17226/23634 .
- OECD. 2017. OECD environment, health and safety publications. Series on testing and assessment. Draft revision of guidance document on developing and assessing adverse outcome pathways, Accessed 18 March 2021. http://www.oecd.org/env/ehs/testing/latestdocuments/Draft%20revised%20AOP%20Guidance%20Document_January%202017.pdf .
- OECD. 2018. Users’ handbook supplement to the guidance document for developing and assessing adverse outcome pathways. OECD Series on Adverse Outcome Pathways No. 1, OECD Puiblishing. Accessed 18 September 2021. https://doi.org/10.1787/5jlv1m9d1g32-en.
- Ogilvie, B. H., A. Solis-Leal, J. B. Lopez, B. D. Poole, R. A. Robison, and B. K. Berges. 2021. Alcohol-free hand sanitizer and other quaternary ammonium disinfectants quickly and effectively inactivate SARS-CoV-2. J. Hosp. Infect. 108:142–45. doi:https://doi.org/10.1016/j.jhin.2020.11.023.
- Ohnuma-Koyama, A., T. Yoshida, H. Tajima-Horiuchi, N. Takahashi, S. Yamaguchi, R. Ohtsuka, Y. Takeuchi-Kashimoto, M. Kuwahara, M. Takeda, N. Nakashima, et al. 2013. Didecyldimethylammonium chloride induces pulmonary fibrosis in association with TGF-β signaling in mice. Exp. Toxicol. Pathol. 65 (7–8):1003–09. doi:https://doi.org/10.1016/j.etp.2013.02.003.
- Ohnuma, A., T. Yoshida, H. Horiuchi, J. Fukumori, M. Tomita, S. Kojima, N. Takahashi, T. Fukuyama, K. Hayashi, S. Yamaguchi, et al. 2011. Altered pulmonary defense system in lung injury induced by didecyldimethylammonium chloride in mice. Inhal Toxicol 23 (8):476–85. doi:https://doi.org/10.3109/08958378.2011.584080.
- Ohnuma, A., T. Yoshida, H. Tajima, T. Fukuyama, K. Hayashi, S. Yamaguchi, R. Ohtsuka, J. Sasaki, J. Fukumori, M. Tomita, et al. 2010. Didecyldimethylammonium chloride induces pulmonary inflammation and fibrosis in mice. Exp. Toxicol. Pathol. 62 (6):643–51. doi:https://doi.org/10.1016/j.etp.2009.08.007.
- Osheroff, M. R. 1990. Subchronic oral toxicity study of didecyldimethylammonium chloride in dogs. Hazleton Washington Laboratories America, Inc., Vienna, VA, USA. Study No. 2545-100 (unpublished).
- Osimitz, T. G., and W. Droege. 2021. Quaternary ammonium compounds: Perspectives on benefits, hazards, and risk. Toxicol Res Appl 5. doi:https://doi.org/10.1177/23978473211049085.
- Piola, R., and C. Grandison. 2017. Assessments of quaternary ammonium compounds (QAC) for in-water treatment of mussel fouling in vessel internals and sea chests using an experimental seawater pipework system. Biofouling 33 (1):59–74. doi:https://doi.org/10.1080/08927014.2016.1261287.
- Riechelmann, H., T. Deutschle, A. Stuhlmiller, S. Gronau, and H. Bürner. 2004. Nasal toxicity of benzalkonium chloride. Am J Rhinol 18 (5):291–99. doi:https://doi.org/10.1177/194589240401800506.
- Rock, K. L., and H. Kono. 2008. The inflammatory response to cell death. Annu Rev Pathol 3 (1):99–126. doi:https://doi.org/10.1146/annurev.pathmechdis.3.121806.151456 .
- Rogers, D. F. 2007. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Resp Care 52:1134–49.
- Ross, M. F., G. F. Kelso, F. H. Blaikie, A. M. James, H. M. Cochemé, A. Filipovska, T. Da Ros, T. R. Hurd, R. A. Smith, and M. P. Murphy. 2005. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Moscow) 70 (2):222–30. doi:https://doi.org/10.1007/s10541-005-0104-5.
- Scallan, J., V. H. Huxley, and R. J. Korthuis. 2010. Integrated systems physiology: from molecule to function to disease. in, Capillary fluid exchange: regulation, functions, and pathology. San Rafael (CA)): Morgan & Claypool Life Sciences.
- Schulze, G. E. 1991. Chronic oral toxicity study of didecyldimethylammonium chloride in dogs. Hazleton Washington, Inc., Study No. 2545-102 (unpublished). MRID 41970401.
- Seed, J., E. W. Carney, R. A. Corley, K. M. Crofton, J. M. DeSesso, P. M. D. Foster, R. Kavlock, G. Kimmel, J. Klaunig, M. E. Meek, et al. 2005. Overview: Using mode of action and life stage information to evaluate the human relevance of animal toxicity data. Crit Rev Toxicol 35 (8–9):663–72. doi:https://doi.org/10.1080/10408440591007133.
- Seguin, R. P., J. M. Herron, V. A. Lopez, J. L. Dempsey, and L. Xu. 2019. Metabolism of benzalkonium chlorides by human hepatic cytochromes P450. Chem Res Toxicol 32 (12):2466–78. doi:https://doi.org/10.1021/acs.chemrestox.9b00293.
- Shusterman, D., E. Matovinovic, and A. Salmon. 2006. Does Haber’s law apply to human sensory irritation? Inhal Toxicol 18 (7):457–71. doi:https://doi.org/10.1080/08958370600602322.
- Singal, M. (2010). Nanoparticle induced inflammatory signaling in the lung: Mechanism of Aerosil200 particulate induced inflammatory gene expression in alveolar epithelial cells. VDM, Germany: VDM Verlag Dr. Müller GmbH & Co. KG, Saarbrücken.
- Sonich-Mullin, C., R. Fielder, J. Wiltse, K. Baetcke, J. Dempsey, P. Fenner-Crisp, D. Grant, M. Hartley, A. Knaap, D. Kroese, et al. 2001. IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol 34 (2):146–52. doi:https://doi.org/10.1006/rtph.2001.1493.
- Stanley, P. J., W. M. Griffin, R. Wilson, M. A. Greenstone, I. S. Mackay, and P. J. Cole. 1985. Effect of betamethasone and betamethasone with neomycin nasal drops on human nasal mucociliary clearance and ciliary beat frequency. Thorax 40 (8):607–12. doi:https://doi.org/10.1136/thx.40.8.607.
- Storaas, T., M. Andersson, C. G. Persson, S. K. K. Steinsv??g, G. Marko-Varga, and L. Greiff. 2000. Effects of benzalkonium chloride on innate immunity physiology of the human nasal mucosa in vivo. The Laryngoscope 110 (9):1543–47. doi:https://doi.org/10.1097/00005537-200009000-00025.
- Swiercz, R., T. Hałatek, J. Stetkiewicz, W. Wąsowicz, B. Kur, Z. Grzelińska, and W. Majcherek. 2013. Toxic effect in the lungs of rats after inhalation exposure to benzalkonium chloride. Int J Occup Med Environ Health 26 (4):647–56. doi:https://doi.org/10.2478/s13382-013-0137-8.
- Swiercz, R., T. Hałatek, W. Wasowicz, B. Kur, Z. Grzelińska, and W. Majcherek. 2008. Pulmonary irritation after inhalation exposure to benzalkonium chloride in rats. Int J Occup Med Environ Health 21 (2):157–63. doi:https://doi.org/10.2478/v10001-008-0020-1.
- Van Miller, J. P. 1988a. Ninety-day dietary subchronic oral toxicity study with didecyldimethylammonium chloride in rats. Union Carbide, Bushy Run Research Center, Export, PA, USA. Report No. 51-506 (unpublished). MRID No. 40966302.
- Van Miller, J. P. 1988b. Subchronic dietary dose range finding study with didecyldimethylammonium chloride in mice. Union Carbide, Bushy Run Research Center, Export, PA, USA. Report No. 51-507 (unpublished). MRID No. 40966301.
- Walters, R. M., G. Mao, E. T. Gunn, and S. Hornby. 2012. Cleansing formulations that respect skin barrier integrity. Dermatol Res Pract 2012:495917. doi:https://doi.org/10.1155/2012/495917.
- Weinberg, J. T. 2011. Maquat 4450-E: A 4-week aerosol inhalation toxicity study with a 2-week recovery period in sprague-dawley rats. WIL-782002, MRID No. 4866790. Laboratories, LLC. Ashland, OH, USA: WIL Research.
- Wessels, S., and H. Ingmer. 2013. Modes of action of three disinfectant active substances: A review. Regul Toxicol Pharmacol 67 (3):456–67. doi:https://doi.org/10.1016/j.yrtph.2013.09.006.
- WHO. 2005. Chemical-specific adjustment factors for interspecies differences and human variability: Guidance document for use of data in dose/concentration-response assessment. International Programme on Chemical Safety (IPCS), Harmonization Project Document No 2. Geneva: CH.
- Wilson, M. S., and T. A. Wynn. 2009. Pulmonary fibrosis: Pathogenesis, etiology and regulation. Mucosal Immunol 2 (2):103–21. doi:https://doi.org/10.1038/mi.2008.85.
- Yang, J. S., S. Y. Park, S.-B. Lee, J.-H. Han, M.-G. Kang, and S.-B. Choi. 2012. Research report disinfectant microparticle aerosol development and inhalation toxicity studies. Occupational Safety and Health Research Institute, Korea Occupational Safety and Health Agency (translated from Korean to English).
- Yoshimatsu, T., and K. Hiyama. 2007. Mechanism of the action of didecyldimethylammonium chloride (DDAC) against Escherichia coli and morphological changes of the cells. Biocontrol Science 12 (3):93–99. doi:https://doi.org/10.4265/bio.12.93.
- Zubris, D. L., K. P. Minbiole, and W. M. Wuest. 2017. Polymeric quaternary ammonium compounds: Versatile antimicrobial materials. Current Topics in Medicinal Chemistry 17 (3):305–18. doi:https://doi.org/10.2174/1568026616666160829155805.