ABSTRACT
It has been reported that incorporation of fire retardants into home furnishings and electronics increases the toxicity of smoke produced during combustion in house fires. Studies have been limited to exercises in analytical chemistry but the biological effects of emissions, particularly regarding chronic toxicity, have not been investigated. The combustion of furnishings with and without chemical flame retardants (FR) regarding (1) ignition resistance and fire progression, (2) chemical composition of smoke (analytical chemistry), and (3) toxicity was compared. Data demonstrated that flame retarded furnishings slowed the generation of toxic levels of acutely toxic gases. The potential chronic toxicity of smoke was assessed using the ToxTracker® assay. Smoke samples from rooms with less flame retarded furnishings exhibited a lesser response in this assay than smoke samples from rooms with flame retarded furnishings. Chemicals associated with activation of the aryl hydrocarbon receptor (AHR), namely benzo[b]fluoranthene, benzo[a]anthracene, benzo[a]pyrene, chrysene, and indeno[1,2,3-cd]pyrene, were not found in smoke from more flame retarded furnished rooms, but were present only in smoke from rooms with less flame retarded furnishings. In conclusion, smoke resulting from combustion of flame retarded furnishings did not increase indicators of potential chronic toxicity hazards relative to non-flame retarded furnishings.
Introduction
The measurement and assessment of the toxicity attributed to smoke generated from combustion is an important topic. Other than well mixed and well-ventilated fires, such as those in gas furnaces or on gas stoves, most fires involve chemically complex fuels and limited levels of oxygen resulting from incomplete combustion. This occurs both with residential fires and wildland fires and produces a wide variety of hazardous chemicals (McNamee et al. Citation2020; Wakefield Citation2010).
This paper is a companion paper to Blais, Carpenter, and Fernandez (Citation2020), who recently reported on the fire science aspects, such as heat release and smoke development as well as the presence of toxic chemicals in smoke collected from combustion experiments conducted in rooms with furnishings from either the United Kingdom (UK), France (FRA), or the United States (US). In this paper, some of these fire-related findings are cited from Blais, Carpenter, and Fernandez (Citation2020), but our current aim was to present our observations in the context of toxicology. Although the relevance of some of the fire-related data will be briefly highlighted with respect to acute toxicity, this paper primarily addresses the use of short-term bioassays to assess the impact of flame retardants (FRs) on the potential chronic toxicity of smoke produced from the combustion of room furnishings.
A detailed discussion of fire science is beyond the scope of this paper, however, some basic processes merit discussion. The generation of smoke from a fire is a dynamic process that changes during the fire as a function of lack of oxygen and temperature causing pyrolysis (Guillaume et al. Citation2014; NFPA Citation2003). Following ignition, the oxygen concentration in the burn is generally unchanged, the smoke is generally white and contains relatively few products of incomplete combustion (PICs). As the fire grows, oxygen is depleted and an increase in higher molecular weight PICs, such as polycyclic aromatic hydrocarbons (PAHs) is detected, producing dark smoke (NFPA Citation2003). FRs (1) slow the fire growth, and (2) reduce compartment temperature and pyrolysis resulting in less oxygen used with less total dark smoke and total PICs produced. This results in reduced production of flammable gases, such as carbon monoxide and hydrogen and thus diminished acute toxicity. The same is true for PAHs and polyhalogenated dioxins and furans that are not formed at appreciable levels until oxygen is consumed therefore limiting their production in flame retarded environments (Blais, Carpenter, and Fernandez Citation2020; NFPA Citation2003).
In the absence of FRs more total fuel and oxygen are consumed more quickly, and the rate of PICs production grows rapidly. The dark/ mostly black smoke is usually generated just prior to or during flashover of the room when super-heated approximately 1,100 degrees Fahrenheit (0F). PICs exit the room in which the fire is occurring Flashover is defined as “the sudden involvement of a room or an area in flames from floor to ceiling caused by thermal radiation feedback” (Flatley Citation2005). Thermal radiation feedback is “the energy of the fire being radiated back to the contents of the room from the walls, floor, and ceiling.” The result is that the room’s contents reach their ignition temperature, leading to immediate and simultaneous ignition, the flashover. At this point, the fire is fully developed and is at peak intensity (Flatley Citation2005).
General categories of FRs include (Döring, Greiner, and Goedderz Citation2021):
◦ Metal hydroxides and mineral fillers
◦ Halogenated flame retardants
◦ Phosphorus-containing flame retardants
◦ Organic radical-forming agents and nitrogen-, oxygen-, and antimony-containing synergists
◦ Synergistic flame retardant system
Fire smoke is a complex mixture of substances and varies depending upon the fuel being burned, the combustion temperature, and ventilation conditions (Austin et al. Citation2001; Fabian et al. Citation2014; Fent et al. Citation2018). Peeters et al. (Citation2021) placed the chemicals generated into 4 groups based upon their biological effects: (1) asphyxiants, such as carbon monoxide, carbon dioxide, and hydrogen cyanide), (2) irritants, such as ammonia, hydrogen chloride, nitrogen oxides, phenol, and sulfur dioxide, (3) allergens, such as isocyanates, and (4) carcinogens/chronic toxics including PAHs, polychlorinated biphenyls, dioxins, furans and certain heavy metals. The asphyxiants, irritants, and allergens pose immediate, acute hazards, whereas the carcinogen/chronic toxics exhibit the greatest potential adverse health impact following repeated exposure over a long period of time, such as might be experienced by a career firefighter.
Residential structure fires are of particular interest as concern exists for both residents of homes in which structure fires occur and for firefighters who attempt to extinguish the fires (Purser and McAllister Citation2016). Given the likely exposure of residents and firefighters to smoke from residential structure fires the role of FRs in affecting the toxicity of the smoke has received attention.
Consideration of acute toxicity is of vital concern to occupants of a house on fire because the levels of these toxic gases and the speed by which they develop are factors that affect the ability of people to escape and survive safely. Numerous studies investigated the generation of toxic gases including carbon monoxide (CO), hydrogen cyanide (HCN) nitrogen oxides (NOx), and sulfur dioxide (SO2), produced during fires. Several reports suggest that FRs enhance the toxicity of smoke by generating acid gases, such as hydrogen chloride (HCl) and hydrogen fluoride (HF) and that PICs are increased (de Boer and Stapleton Citation2019; McKenna et al. Citation2018; Stec and Hull Citation2011). It is noteworthy that these claims have been challenged by fire scientists (Blais Citation2019; Hirschler Citation2019). Differences in opinions on this matter often result from the depiction of smoke per unit of mass without regard to the total mass combusted. FRs may elevate production of PICs per gram of materials burned. However, overall, FRs reduce both the total mass consumed and total smoke produced, indicating that less total PICs are generated. FRs prevent a large fire by both preventing ignition and burning of the materials containing the FRs.
Blais and Carpenter (Citation2015) reported results from chamber-based experiments that measured gas/vapor emissions from combustion of flexible polyurethane foams (FPUFs) with and without FRs. FPUFs were covered with FR and non-FR fabrics to simulate cushions. Both FR and non-FR FPUFs showed comparable smoke toxicity, as evidenced by measuring nine standard toxic gases including CO, carbon dioxide, NOx, SO2, carbonyl sulfide, hydrogen halides, HCN, aldehydes, and hydrocarbons) cited in ASTM E800 (ASTM International Citation2020). As well as dioxins and furans (ASTM International Citation2020). The quantity of polyhalogenated dioxins and furans generated from combustion of these materials was less than would have been produced by burning an equivalent mass of wood. The use of a barrier material with a non-FR FPUF resulted in the most toxic smoke.
Most toxicity-related research conducted by fire scientists has focused on measurement of acutely toxic gases such as those mentioned above. ISO standard methods for bench-scale assessment of fire effuent toxicity include the steady-state tube furnace (ISO TS 19700), smoke density chamber (ISO 5659–2), and cone calorimeter (ISO 5660–1) tests (ISO Citation2015a, Citation2016b, Citation2017). Several investigators reviewed various lab approaches in detail (Guillaume Citation2021; Peeters et al. Citation2021; Purser Citation2016). Toxicity of the numerous chemicals detected was assumed to be additive. Toxicity is expressed as a fraction of the lethal concentration for 50% of the population for a 30-min acute inhalation exposure (LC50). Summing these individual contributions yields a fractional effective dose (FED) for lethality (ISO Citation2015b). Amon, McNamee, and Blomqvist (Citation2014). Evaluated the existing models and measurement methods and summarized methods to develop toxicity and ecotoxicity data from different scale experiments. Another way to characterize the toxicity of fires in buildings is estimation of the time at which occupants experience compromised tenability (ISO Citation2012). Tenability is defined as the ability of humans to perform cognitive and motor-skill functions at an acceptable level when in a fire environment. (Guillaume et al. Citation2014).
Chronic toxicity, including, but not limited to carcinogenicity, refers to effects resulting from “repeated exposure by the oral, dermal, or inhalation route for more than approximately 10% of the life span in humans (more than approximately 90 days to 2 years in typically used laboratory animal species)” (EPA Citation2021). Chronic toxicity of smoke is a concern primarily for firefighters who may be repeatedly exposed to smoke and soot intermittently throughout their careers in a variety of scenarios, such as residential fire, commercial fire, or vehicle fire (Daniels et al. Citation2015, Citation2014; Fabian et al. Citation2014; IARC Citation2010). Various investigators documented the presence of PAHs, many of which are human carcinogens, and halogenated dioxins and furans on gear worn by firefighters and inside their suits. Multiple publications estimated the potential exposure of firefighters to such residues that included exposure estimates from biomonitoring data from firefighters following fire suppression activities (Bolstad-Johnson et al. Citation2000; Brandt-Rauf et al. Citation1988; Evans and Fent Citation2015; Fent et al. Citation2014; Fent and Evans Citation2011; Keir et al. Citation2020; Kirk and Logan Citation2015).
Toxicologists usually assess chronic toxicity utilizing repeated, long-term studies, typically using rodents, most appropriately following OECD guidelines and Good Laboratory Practices (GLP). Following exposures ranging from more than 90 days (subchronic) to lifetime (18 and 24 months for mice and rats, respectively), animals are necropsied and tissues and organs are collected and processed for histopathological examination by a toxicologic pathologist. The cost and time involved in these efforts are significant and generally permit the study of only a relatively small number of discrete chemical entities. Seldom are such studies performed on complex mixtures. At present, we are not aware of any long-term lab exposure studies with smoke or smoke extracts from residential fires or from the combustion of household furnishings. Discussions regarding potential chronic toxicity are usually based upon the presence of various chemicals known individually to pose chronic toxicity hazards. The utility of such approach is limited as it does not consider the interaction of these chemicals with complex biological systems, nor does it take into account potential antagonism, additivity, or potentiation that may exist when various chemicals are present together during an exposure. The aim of this study was to examine testing the whole smoke extract mixture in in vitro bioassays.
Materials and methods
Combustion experiments
The details of the experimental set-up and sampling were previously described by Blais, Carpenter, and Fernandez (Citation2020). Briefly, this study used three replicates of identical rooms for each country tested (France, United Kingdom, United States). All furniture was of the same design and purchased from IKEA in each respective country. France and the United States rely on smolder only furniture flammability standards while the United Kingdom has a more stringent standard that relies on a smolder and open flame ignition test, thus requiring the use of more flame retarded materials than the United States or France.Footnote1 Because the furnishings employed in this investigation were purchased from retail stores, it is not possible to determine precise chemical identity of the FRs used. Elemental analysis was performed on couch, chair, and television components for the purpose of finding, which FRs were most likely present. Phosphorus-containing FR appeared to be in each of the television sets. Chlorine, bromine, and antimony (a synergist) were detected to various degrees in the furnishings (Blais, Carpenter, and Fernandez Citation2020).
Each test room contained a three-cushion couch, chair, and flat panel television of identical models/manufacturers purchased in the respective countries. In addition, each room was fitted with identical furnishings (obtained from a U.S. Walmart) including a coffee table, end table, curtain, and bookcase, as well as 12 kg of locally sourced books. All rooms were meticulously set up to ensure comparability of results. The combustion rooms had interior dimensions of 2.44 m wide by 3.66 m deep and 2.44 m high with interior surfaces lined with fire-resistant Type X gypsum board and a door opening (0.8 m ± 0.01 m wide and 2 m ± 0.01 m high) in the center of one of the 2.44 m walls (roughly corresponding to ISO 9705 (ISO Citation2016a)). A hood and exhaust duct system collected combustion products and designed to not disturb the fire-induced flow in the doorway. That is, all combustion products leaving the room through the doorway during a test were collected.
Each burn was started with the ignition source placed in the center of the middle seat cushion with the vertical surface of the ignition source in contact with the vertical back part of the middle seat cushion. The orientation of the ignition source (cribFootnote2) was similar in all tests. The only difference between samples designated with a ‘2’ or ‘3’ (e.g. US2, US3) was the size of the open flame ignition sources with the ‘2’ source approximately equal to 250 W, and the ‘3’ source approximately equal to 900 W (Blais, Carpenter, and Fernandez Citation2020). Isopropyl alcohol, 1.4 ml, was applied to the crib 30 sec prior to being placed on the couch and ignited with a propane lighter. Heat release and smoke generation data were collected. This video shows the response of the three furnished rooms attempted ignition: https://www.youtube.com/watch?v=wlnAAxOKYLg&feature=youtu.be.
Smoke sampling and chemical analysis
Sampling for volatile organic compounds (VOCs) was performed using EPA Test Method TO-15 (EPA Citation1999) designed to collect a wide range of toxic VOCs. The collection was performed using Summa® canisters through a 4 m, 150°C heated sample line. All VOC samples were collected via sampling probes made from SS316 stainless steel with 4.6 mm ID tubing. Probes were at 1.52 m in the center of the doorway. Samples were analyzed within 24 hr. Concentration is reported as picograms per microliter (pg/µl).
Sampling for semi-volatile organic compounds (SVOCs) and chlorinated and brominated dioxins and furans as well as samples destined for toxicology testing were collected through the same sampling lines as the VOCs using XAD resin solid sorbent collectors. EPA Test Method 8290a (EPA Citation2007) was employed for PAH/SVOC and EPA Test Method 23 (EPA Citation2017) was utilized for dioxins and furans. Continuous sampling with XAD trains was performed from ignition until extinguishment of the first test (#1) for each country’s configuration. Subsequent tests (#2, #3) for each country had the XAD sampling run from ignition to the appearance of black smoke. The collection was then switched to a second sampling train that collected from the appearance of black smoke until the end of the test. Technical difficulties prevented the analysis of the #1 burns for VOC and SVOC; however, data for chemicals were developed from both the light and dark smoke phases of the 2# and #3 burns. Lines were cleaned between tests and a sample blank was collected for each test. The filters were desorbed with dichloromethane, dried, and resuspended in dimethyl sulfoxide (DMSO). Aliquots of DMSO solutions were used in subsequent toxicity evaluations (presented below).
All analytical samples (VOCs, SVOCs, dioxins, and furans) were analyzed by the Analytical and Environmental Chemistry Laboratory of Southwest Research Institute, San Antonio, TX (EPA, ISO 17025, and NELAC certified with annual audits). Samples were measured in µg material captured on the cartridge and converted to concentration by using flow rate and time of collection. All samples had background sampling, trip blanks, and spike confirmation samples. In addition, samples exceeding the calibration curve were diluted and re-analyzed. Approximate uncertainties include: ±10% in the airflow measurement, ±25% for the analytical near the LOQ and ±10% at higher concentration above the first quartile of the concentration range. Temperature measurements ±0.1% below 100°C and ±1% above 100°C. Smoke opacity is ±1% below 100 and ±5% above 100. All methods are standard ASTM, EPA, or NFPA with well-established criteria and performance. Additional details of sampling and analysis are provided in Blais, Carpenter, and Fernandez (Citation2020).
Assessment of toxicity
Although the acute toxicity of these emissions was not a primary focus and no biological assessment of acute toxicity was performed, it is important to discuss the analytical results, especially for VOCs in the context of the growth and progression of the fire in each of the three rooms tested. VOCs levels found were compared with the appropriate health and safety guidelines. The levels of SVOCs, particularly categorized as human carcinogens (IARC Group 1) or likely human carcinogens (IARC 2A, 2B) were compared across the three furniture types (IARC Citation2022). Chronic toxicity of the smoke was assessed with both the ToxTracker® reporter assay and the aryl hydrocarbon receptor (AhR) activation assay.
ToxTracker® is a mouse embryonic stem (mES) cell-based assay that monitors activation of specific cellular signaling pathways for detection of the biological reactivity of compounds (Hendriks et al. Citation2016). In contrast to the cancer-derived cell lines commonly used for in vitro genotoxicity testing, stem cells are genetically stable and proficient in all cellular pathways required for accurate detection of potentially carcinogenic properties of compounds. Extensive whole-genome transcription profiling led to identification of a panel of biomarker genes that are preferentially activated upon exposure to different classes of carcinogens and toxicants. Green fluorescent protein (GFP) mES reporter cell lines were developed to enable assessment of the activation status of these biomarker genes. The mES reporter cell lines discriminate between induction of DNA damage, oxidative stress, protein damage, and general cellular stress. Specifically:
DNA damage – genotoxicity – Detected by the Bscl2-GFP reporter activated by promutagenic DNA lesions and DNA replication stress and the Rtkn-GFP reporter associated with DNA double-strand breaks.
Oxidative stress – The Srxn1-GFP and Blvrb-GFP reporters indicate activation of the Nrf2 and Hmox1 antioxidant responses.
DNA damage and oxidative stress – The Btg2-GFP reporter is activated as part of a p53-mediated response.
Unfolded Protein Response (UPR) – The DDIT3-GFP reporter is directly associated with the unfolded protein response.
Prior to chemical testing, a dose range finding study was performed using wild-type mES cells (strain B4418). Wild type mES cells were exposed to 20 different concentrations of the test substances with a maximal concentration of 1%. Cytotoxicity was estimated by cell count after a 24 hr exposure using a flow cytometer and is expressed as % viable cells after 24 hr exposure compared to vehicle controls. From this dosa range finding, five concentrations were selected. If little or no cytotoxicity was observed, the maximal concentration of 1% was utilized for all test samples.
For the assay, six independent mES reporter cell lines are seeded in gelatin-coated 96-well cell culture plates in 200 μl mES cell medium (50,000 cells per well). Twenty-four hr after seeding the cells in the 96-well plates, medium is aspirated and fresh mES cell medium containing 10% fetal calf serum and diluted chemicals is added to the cells. For each tested compound, five concentrations are tested in 2-fold dilutions. Induction of the GFP reporters is determined after 24 hr exposure using a flow cytometer. Only GFP expression in intact single cells was determined. Mean GFP fluorescence was measured and used to calculate GFP reporter induction compared to a vehicle control treatment. Positive control treatments with cisplatin (DNA damage), diethyl maleate (oxidative stress), tunicamycin (unfolded protein response), and aflatoxin B1 (metabolic activation of progenotoxins by S9) were included in all experiments. Solvent concentration was the same in all wells and never exceeded 1% DMSO.
Many compounds such as PAHs are not directly reactive but become genotoxic following metabolic activation to form electrophiles and detoxification reactions in the liver, kidneys, and lung (Nebert and Dalton Citation2006). The major enzymes involved in metabolic activation of pro-genotoxins, such as cytochrome P450s and epoxide hydrolyases, are minimally or not expressed in mES reporter cell lines used as the basis for this ToxTracker® assay (Hendriks et al. Citation2016). Thus, whether increased metabolic capability via addition of S-9 rat liver extract during treatment of mES with the test articles elicited genotoxic properties was determined. For samples that showed strong auto-fluorescence, the GFP fluorescence observed in exposed wild-type cells was subtracted from the signal measured in the reporter cell lines. Exposures to control compounds were included in each test to determine the technical performance and reproducibility of the ToxTracker® assay. Quantitative data analysis was conducted using ToxPlot© (Hendriks et al. Citation2016).
The ability of the same test articles to activate human AhR was determined using a human AhR reporter assay system with a commercial reporter cell line (Indigo Biosciences) in which luciferase is expressed from an AhR-dependent transgene in an AhR activation system. (3E)-6-Bromo-3-[3-(hydroxyamino)indol-2-ylidene]-1-methylindol-2-one (MeBio) was the positive control. The binding of AhR is an essential step in a pathway that includes the translocation of the ligand-receptor complex into the nucleus of the cell, dimerization of the AhR with the AhR nuclear translocator (ARNT) protein, and binding of the ligand:AhR:ARNT complex to the corresponding DNA recognition sequence. The result is transcription and translation of expression of multiple genes, most noteworthy for cytochrome P4501A1 (CYP1A1). All dioxin-like compounds are assumed to act through this AhR signal transduction pathway (Birnbaum Citation1994; Safe Citation1990). Among the toxicological effects associated with AhR activation are chronic adverse health effects, including cancer, cardiovascular disease, chronic kidney disease, immunotoxicity, and reproductive disorders (Larsson et al. Citation2018; Zhao et al. Citation2019). The AhR signaling pathway also cross talks with the estrogen receptor pathway suggesting that AhR-activating compounds may induce estrogen-like endocrine effects (Larsson et al. Citation2018). Besides screening chemicals for potential toxicity, Larsson et al. (Citation2018) noted that the AhR assay is used to measure levels of PAHs in samples of sediments and water, blood plasma, sewage sludge, and food.
Results
Smoke generation data
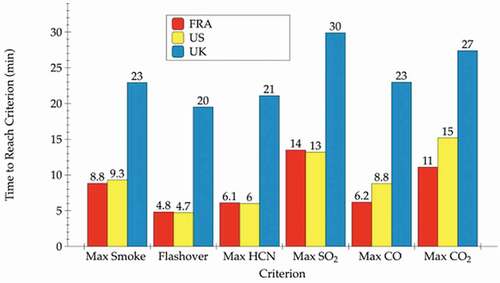
The average total smoke production (n = 3)Footnote3 of the FRA and US room configurations was comparable (74,775 m2, RSD 5.7% and 72,951 m2, RSD 15.6%, respectively), whereas the UK room configuration produced less total smoke (34,330 m2, RSD 30.8%). Thus, the most fire-resistant configuration, the UK room, produced approximately half of the total smoke compared to US or FRA room configuration, which were nearly identical (Blais, Carpenter, and Fernandez Citation2020). Most important in stark contrast to FRA and US rooms, the UK room, containing the most flame retarded furnishings, and required 20–30 min to reach the same level of smoke (). A similar pattern was found for time to flashover, which occurred prior to production of maximal smoke levels (Blais, Carpenter, and Fernandez Citation2020).
Note: Due to the rapid transition from light to dark smoke, sample FRA3 light was contaminated with black smoke during collection for VOC analysis and, thus, data are not reported. Line blockage occurred during sample collection for US3 dark for SVOC analysis and similarly data are not reported.
Acute toxicity
Detailed analytical data were previuosly published by Blais, Carpenter, and Fernandez (Citation2020). A summary and brief interpretation are below.
Levels exceeding the Immediately Dangerous to Life and Health (IDLH) thresholds (National Institute for Occupational Safety and Health Citation2019) were detected for several VOCs (Blais, Carpenter, and Fernandez Citation2020). Regarding HCN (IDLH = 50 ppm), for example, the FRA and US configurations produced 1,230 ppm and 1,600 ppm, respectively, at 6 min. The UK configuration produced the lowest peak concentration of collected HCN in any of the three-room configurations (1,030 ppm) but nonetheless this is still a lethal level. For all practical purposes, acute toxicity of the emissions from combustion of flame-retarded furnishings and non-flame-retarder furnishings are equivalent. Most notable, this toxic level was not detected until 21 min into the burn of the flame-retarded furnishings. Thus, data illustrate a primary benefit of FR as these compounds delay combustion thereby allowing more time for residents of dwellings to escape ().
Potential chronic toxicity of smoke
and show levels of VOCs that have IARC carcinogenic hazard classifications of IARC 1 (Carcinogenic to Humans), 2A (Probably Carcinogenic to Humans), and 2B (Possibly Carcinogenic to Humans) (IARC Citation2022), and National Toxicology Program (NTP) carcinogen classifications of “Known to be a human carcinogen” (K) and “Reasonably anticipated to be a human carcinogen” (R) (NTP Citation2021).
Table 1. Levels of VOCs in light smoke.
Table 2. Levels of VOCs in dark smoke.
presents the levels of SVOCs detected in the UK, US, and FRA rooms. These compounds were only found in black smoke. Thus, results from light smoke are not presented. The IARC and NTP carcinogen classifications are shown when applicable. In addition, the ability of the individual SVOCs to bind and activate the AhR is also indicated in cases where such data are available (Amakura et al. Citation2016; Behnisch, Hosoe, and Sakai Citation2003; Larsson et al. Citation2018; Petkov et al. Citation2010; Zhao et al. Citation2019, Citation2010).
Table 3. Levels of SVOCs in Dark Smoke.
Eleven chlorinated dioxins and furans were detected during the various room burns, all of which were below the level of quantification (LOQs ranged from 0.5 to 2.5 pg/µl). Six brominated dioxins and furans were also detected. Similarly, all of the detections were below the level of quantitation (LOQs ranged from 0.10 to 0.75 pg/µl) except for the UK2 dark smoke sample, which contained 1,2,3,4,6,7,8-HpBDF at 16 pg/µl. The following brominated and chlorinated dioxins/furans were detected (in alphabetical order):
1,2,3,4,6,7,8-HpBDD
1,2,3,4,6,7,8-HpBDF
1,2,3,4,6,7,8-HpCDD
1,2,3,4,6,7,8-HpCDF
1,2,3,4,7,8-HxBDF
1,2,3,4,7,8,9-HpCDF
1,2,3,4,7,8/1,2,3,6,7,8-HxBDD
1,2,3,7,8-PeCDD
1,2,3,7,8,9-HxCDD
1,2,3,7,8,9-HxCDF
2,3,4,6,7,8-HxCDF
2,3,4,7,8-PeBDF
2,3,7,8-TCDD
2,3,7,8-TCDF
2,4,6,8-TBDF
OCDD
OCDF
Biological activity
ToxTracker® assay
demonstrates results of the ToxTracker® Assay. The validity of the ToxTracker assay was confirmed using exposure to the reference compounds specific for the pathways evaluated. The genotoxic compound cisplatin displayed induction of DNA damage response (Bscl2, Rtkn) and p53-mediated cellular stress (Btg2). Diethyl maleate (DEM) induced primarily oxidative stress-related reporters Srxn1 and Blvrb, tunicamycin produced unfolded/misfolded protein stress response (DDIT3). The positive control compound aflatoxin B1, which requires metabolic activation to become genotoxic, selectively initiated the Bscl2 and Rtkn reporters when tested in the presence of S9 liver extract. Generally, controls showed GFP induction levels compliant with historical data and demonstrated the functionality of the mES reporter cell lines.
Figure 2. Results from the ToxTracker® Reporter Assay.
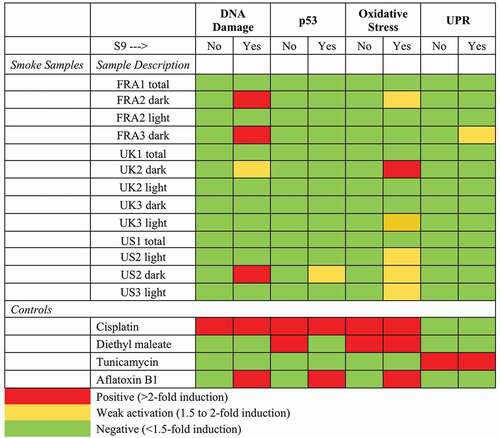
None of the samples that encompassed the entire duration of the burns (UK1 total, FRA1 total, US1 total) produced responses from any of the receptors. This may be attributed to the long sampling duration spanning light and dark phases of a burn which collected more mass than shorter light only or dark only samples, effectively diluting the content of biologically active components. Consequently, when tested, the response was below the level needed to produce a positive response.
Test samples FRA2 dark, FRA3 dark, and US2 dark activated more than 2-fold the Rtkn-GFP genotoxicity reporter. Activation of the Bscl2-GFP reporter is associated with DNA replication stress and induction of pro-mutagenic DNA adducts. Activation of the Rtkn-GFP ToxTracker® reporter is associated with enhanced number of DNA double-strand breaks and indicates induction of chromosome damage.
With the exception of UK2 dark eliciting the oxidative stress reporters, none of the other burn samples produced a response in these receptors. No responses were noted in the absence of S-9 in any of the samples, suggesting that metabolic activation was required for some of the components of the samples to elicit a response. This is consistent with the response expected from products of combustion such as PAHs.
Aryl hydrocarbon hydroxylase
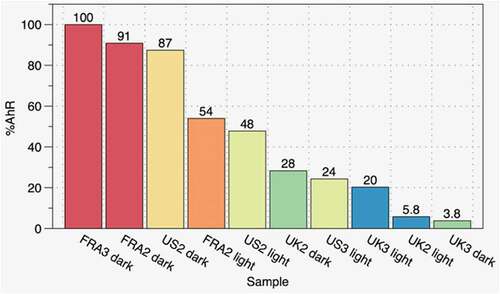
(2ʹZ,3ʹE)-6-Bromo-1-methylindirubin-3’-oxime (MeBio), the positive control, is a potent activator of the human AhR, with estimated best-fit EC50 values ranging from 6.6 − 17.9 nM. Reliable EC50 values could not be estimated for the test items from the dose–response curves as data did not plateau over the range of concentrations used in the assays. However, there was evidence for a clear monotonic dose–response curve in each case, which in combination with the evidence from one-way ANOVA tests, indicated that each of the test items exhibited the potential to activate the AhR, albeit with varying potencies. illustrates activation of the AhR (measured as induction of luciferase activity) in descending order expressed relative to the sample showing maximal activation (FRA3 dark). The samples that encompassed the entire duration of the burns (UK1 total, FRA1 total, US1 total) generally produced a lower response than corresponding sample from dark only smoke. The long sampling duration spanning the light and dark phases of a burn may have effectively diluted the biologically active components primarily contained in the dark-phase sample, such that these could not initiate a response in the test system, or only a diminished response.
Figure 3. Induction of Aryl Hydrocarbon Hydroxylase (AhR) as an Indicator of Possible Carcinogenicity and Chronic Toxicity - Maximum AhR.
Discussion
The FRA and US room configurations produced approximately 2-fold, the quantity of total smoke, as well as higher VOCs levels earlier in the combustion process than occurred in the UK-configured room. This is expected given that the UK has more stringent flammability standards than FRA and US, necessitating the use of greater amount of flame retarded materials than the US or FRA. All rooms produced concentrations of VOCs that exceeded IDLH levels at some point during the burns. However, it is crucial to note that the UK room furnishings provided a longer time for residents to escape a developing fire and reduced the potential exposure to high levels of several toxic gases compared to rooms with either FRA or US furnishings. Most individuals die from gases that originated from fires in rooms compared to those in which they were located. Inhabitants of the room burning would be overcome and go into immediate shock from exposure to high-temperature gases burning the upper airways. It is the migration of the toxic gases out of the room and their spread throughout the rest of the house that lead to inhabitants’ loss of consciousness and prevent their escape.
Dioxins including polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans are known to produce a wide spectrum of biological and toxicological effects, including immunotoxicity, carcinogenicity, and reproductive (Harris et al. Citation1973; Safe Citation1986). Eleven chlorinated dioxins and furans were detected during the various room burns, all of which were below the limit of quantitation (LOQ). Six brominated dioxins and furans were also detected but only one was above the LOQ. Given the sporadic nature of these findings and the levels, all of which were, with one exception, below the LOQ, one might conclude that, under the condition of this study, the measured chlorinated or brominated dioxins and furans were not toxicologically significant components of the smoke.
The SVOC data show that FRA and US furnished rooms consistently produced smoke containing more and higher levels of SVOCs than the UK room. Most notable, except for naphthalene, none of the detected SVOCs that are classified as IARC Category 1 or Category 2 (A or B) were identified in the UK room but were measured in either or both the US and FRA rooms. Naphthalene was measured at higher levels in US and FRA rooms compared to the UK room.
Analytical chemistry data demonstrated higher concentrations for several human carcinogens in the FRA and US versus UK room. These data were generally reflected in the results of the AhR activation assay. Five chemicals that activated the AhR (benzo[b]fluoranthene, benzo[a]anthracene, benzo[a]pyrene, chrysene, and indeno[1,2,3-cd]pyrene) were measured in smoke from the FRA and US furnished rooms, but none were found in the UK furnished room. Our data are in contrast to Blomqvist et al. (Citation2012) who noted that combustion of certain products containing brominated FR resulted in higher amounts of total PAH and a more toxic mixture of PAH. However, no assessment on biological activity was reported in their study. Blomqvist et al. (Citation2012) inferred toxicity from analytical chemistry data analysis and calculation of benzo[a]pyrene toxicity equivalents. Our findings demonstrate that measurement of biological activity, such as with AhR activation, is a more appropriate gauge of potential toxicity.
Two dark smoke samples from FRA furnishings and one of the dark smoke samples from US furnishings showed activation of the Bscl2-GFP genotoxicity reporter in ToxTracker® which is associated with DNA replication stress and induction of pro-mutagenic DNA adducts with subsequent inhibition of DNA replication suggesting chronic toxicity potential. Although the UK2 dark sample induced genes associated with oxidative stress, none of the UK samples activated the Bsc12-GFP reporter, suggesting a lower potential for chronic toxicity.
Conclusions
Under the conditions of this study, important differences in the chemical content and biological activity were noted between smoke from the combustion of the more flame retarded UK furnishings compared to either FRA or US furnishings (less flame retarded). Flame retarded furnishings such as present in the UK burns (1) slowed generation of smoke, (2) delayed the time of peak levels of acutely toxic gases, such as HCN, and (3) prolonged time to flashover. Although lethal levels of acutely toxic gases were achieved regardless of the source of furnishings and their FR content, delay in the production of toxic gases might provide occupants of the room time to escape prior to being subjected to the toxicity of the smoke. Firefighters make entry into a fully involved room fire only if there is a possibility of viable rescue of a resident. Thus, these individuals will not normally experience acute exposure to potentially lethal levels of gases during the burn. During post-fire salvage and overhaul operations firefighters might be exposed to particulates that have adsorbed high molecular weight chemicals, such as PAHs and halogenated dioxins and furans, which possess hazardous properties associated with chronic exposure.
Regarding potential chronic toxicity and cancer, the findings of this study suggest that chemicals in the smoke from the combustion of flame retarded furnishings may not enhance chronic toxicity hazards. This postulation may be in part due to the function of these FR chemicals inhibiting combustion of underlying materials thereby reducing the amounts and types of byproducts (Ravey et al. Citation1998b, Citation1998a).
Acknowledgments
We acknowledge the laboratory efforts of L. Higgins, L. Chatham, and F. Zhang with respect to the Ah Receptor assay conducted at Concept Life Sciences in Dundee, UK.
We acknowledge the North American Flame Retardant Alliance (a panel under the auspices of the American Chemistry Council), who funded the research and the production of this manuscript.
Disclosure statement
This work was funded by the American Chemistry Council’s North American Flame Retardant Alliance (NAFRA). T. Osimitz and W. Droege are employed by Science Strategies, LLC, a health and environmental sciences consulting firm that consults to the American Chemistry Council, which funded the production of this manuscript. M. Blais is employed by Southwest Research Institute, a nonprofit research institute that consults with the American Chemistry Council, which funded the production of this manuscript. G. Hendriks is the CEO of Toxys B.V., a Dutch biotech company that provides in vitro toxicity screening solutions to a variety of companies, including members of the American Chemistry Council. Toxys B.V. was paid for their work on this project by the American Chemistry Council.
The American Chemistry Council had no control of the experimental design or the results reported in this article or any influence on where this article was submitted for publication.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author (WD) upon reasonable request.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
Notes
1. It is of note that the United States uses several methods to assess the fire performance of furniture. California Technical Bulletin 117 and 133 are the two most widely recognized regulations regarding the flammability of upholstered furniture in the U.S. Furniture in the U.S. for other states is often tested to these regulations. There is currently no national standard for the assessment of flammability of furniture.
2. A wooden crib, made of dry wood stacked in a lattice formation weighing 17 g, was used as the ignition source.
3. measured as opacity in m2/second and integrated over time resulting in the unit m2
References
- Amakura, Y., T. Tsutsumi, M. Yoshimura, M. Nakamura, H. Handa, R. Matsuda, R. Teshima, and T. Watanabe. 2016. Detection of aryl hydrocarbon receptor activation by some chemicals in food using a reporter gene assay. Foods 5 (1):15. doi:https://doi.org/10.3390/foods5010015.
- Amon, F., M. McNamee, and P. Blomqvist. 2014. Fire effluent contaminants, predictive models, and gap analysis. Technical Report · January 2014. Brandforsk project 700-121.
- ASTM International. 2020. ASTM E800-20. Standard guide for measurement of gases present or generated during fires, Accessed May 20 2022. https://www.astm.org/e0800-20.html.
- Austin, C. C., D. Wang, D. J. Ecobichon, and G. Dussault. 2001. Characterization of volatile organic compounds in smoke at municipal structural fires. J Toxicol Environ Health Part A 63 (6):437–58. doi:https://doi.org/10.1080/152873901300343470.
- Behnisch, P. A., K. Hosoe, and S. Sakai. 2003. Brominated dioxin-like compounds: In vitro assessment in comparison to classical dioxin-like compounds and other polyaromatic compounds. Environ Int 29 (6):861–77. doi:https://doi.org/10.1016/S0160-4120(03)00105-3.
- Birnbaum, L. S. 1994. The mechanism of dioxin toxicity: Relationship to risk assessment. Environ Health Perspect 102 (9):157–67. doi:https://doi.org/10.1289/ehp.94102s9157.
- Blais, M., and K. Carpenter. 2015. Flexible polyurethane foams: A comparative measurement of toxic vapors and other toxic emissions in controlled combustion environments of foams with and without fire retardants. Fire Technol 51 (1):3–18. doi:https://doi.org/10.1007/s10694-013-0354-5.
- Blais, M. S. 2019. Letter to the editor for Chemosphere reference: Flame-retardants in UK furniture increase smoke toxicity more than they reduce fire growth rate. McKenna et al., 2017. Chemosphere 232:506–08.
- Blais, M. S., K. Carpenter, and K. Fernandez. 2020. ‘Comparative room burn study of furnished rooms from the United Kingdom, France and the United States. Fire Technol 56 (2):489–514. doi:https://doi.org/10.1007/s10694-019-00888-8.
- Blomqvist, P., M. S. McNamee, P. Andersson, and A. Lönnermark. 2012. Polycyclic aromatic hydrocarbons (PAHs) quantified in large-scale fire experiments. Fire Technol 48 (2):513–28. doi:https://doi.org/10.1007/s10694-011-0242-9.
- Bolstad-Johnson, D. M., J. L. Burgess, C. D. Crutchfield, S. Storment, R. Gerkin, and J. R. Wilson. 2000. Characterization of firefighter exposures during fire overhaul. AIHAJ 61 (5):636–41. doi:https://doi.org/10.1202/0002-8894(2000)061<0636:COFEDF>2.0.CO;2.
- Brandt-Rauf, P. W., L. F. Fallon Jr., T. Tarantini, C. Idema, and L. Andrews. 1988. Health hazards of fire fighters: Exposure assessment. Br J Ind Med 45 (9):606–12. doi:https://doi.org/10.1136/oem.45.9.606.
- Daniels, R. D., T. L. Kubale, J. H. Yiin, M. M. Dahm, T. R. Hales, D. Baris, S. H. Zahm, J. J. Beaumont, K. M. Waters, and L. E. Pinkerton. 2014. Mortality and cancer incidence in a pooled cohort of us firefighters from San Francisco, Chicago and Philadelphia (1950-2009). Occup Environ Med 71 (6):388–97. doi:https://doi.org/10.1136/oemed-2013-101662.
- Daniels, R. D., S. Bertke, M. M. Dahm, J. H. Yiin, T. L. Kubale, T. R. Hales, D. Baris, S. H. Zahm, J. J. Beaumont, K. M. Waters, et al. 2015. Exposure-response relationships for select cancer and non-cancer health outcomes in a cohort of U.S. firefighters from. Occup Environ Med 72:699–706.
- de Boer, J., and H. M. Stapleton. 2019. Toward fire safety without chemical risk. Science 364 (6437):231–32. doi:https://doi.org/10.1126/science.aax2054.
- Döring, M., L. Greiner, and D. Goedderz. 2021. Flame retardants. In Plastics Flammability Handbook (fourth edition) (Hanser), ed. J. Troitzsch and E. Antonatus, 53–128. Munich, Germany: Hanser Publications.
- EPA. 1999. Compendium of methods for the determination of toxic organic compounds in ambient air. Second edition. Compendium method TO-15 determination of Volatile Organic Compounds (VOCs) in air collected in specially-prepared canisters and analyzed by gas chromatography/mass spectrometry (GC/MS),’ Accessed April 23 2021. https://www.epa.gov/amtic/compendium-methods-determination-toxic-organic-compounds-ambient-air.
- EPA. 2007. ‘SW-846 test method 8290A: Polychlorinated dibenzodioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs) by high-resolution gas chromatography/high resolution mass spectrometry (HRGC/HRMS),’ Accessed April 27 2021. https://www.epa.gov/hw-sw846/sw-846-test-method-8290a-polychlorinated-dibenzodioxins-pcdds-and-polychlorinated.
- EPA. 2017. ‘Method 23 determination of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans from stationary sources,’ Accessed April 26 2021. https://www.epa.gov/emc/method-23-dioxins-and-furans.
- EPA. 2021. ‘Vocabulary catalog. Integrated Risk Information System (IRIS) glossary,’ Accessed May 11 2022. https://sor.epa.gov/sor_internet/registry/termreg/searchandretrieve/glossariesandkeywordlists/search.do;jsessionid=nGEozfxIDFvnAeMpt1zv_Kcvr2EDICm7L3ZBcf9KQDZxN-WY7xwD!-441202046?details=&vocabName=IRIS%20Glossary.
- Evans, D. E., and K. W. Fent. 2015. Ultrafine and respirable particle exposure during vehicle fire suppression. Environ Sci Process Impacts 17 (10):1749–59. doi:https://doi.org/10.1039/C5EM00233H.
- Fabian, T. Z., J. L. Borgerson, P. D. Gandhi, C. S. Baxter, C. S. Ross, J. E. Lockey, and J. M. Dalton. 2014. Characterization of firefighter smoke exposure. Fire Technol 50 (4):993–1019. doi:https://doi.org/10.1007/s10694-011-0212-2.
- Fent, K. W., and D. E. Evans. 2011. Assessing the risk to firefighters from chemical vapors and gases during vehicle fire suppression. J Environ Monit Assess 13 (3):536–43. doi:https://doi.org/10.1039/c0em00591f.
- Fent, K. W., J. Eisenberg, J. Snawder, D. Sammons, J. D. Pleil, M. A. Stiegel, C. Mueller, G. P. Horn, and J. Dalton. 2014. Systemic exposure to PAHs and benzene in firefighters suppressing controlled structure fires. Ann Occup Hyg 58 (7):830–45. doi:https://doi.org/10.1093/annhyg/meu036.
- Fent, K. W., D. E. Evans, K. Babik, C. Striley, S. Bertke, S. Kerber, D. Smith, and G. P. Horn. 2018. Airborne contaminants during controlled residential fires. J Occup Environ Hyg 15 (5):399–412. doi:https://doi.org/10.1080/15459624.2018.1445260.
- Flatley, C. 2005. Flashover and Backdraft: A Primer, Accessed April 23 2021. https://www.fireengineering.com/firefighting/flashover-and-backdraft-a-primer/#gref.
- Guillaume, E., F. Didieux, A. Thiry, and A. Bellivier. 2014. Real-scale fire tests of one bedroom apartments with regard to tenability assessment. Fire Safety Journal 70:81–97. doi:https://doi.org/10.1016/j.firesaf.2014.08.014.
- Guillaume, E. 2021. Smoke and toxicity from fire effluents. In Plastics Flammability Handbook (fourth edition), ed. J. Troitzsch and E. Antonatus, 185–218. Munich, Germany: Hanser Publications.
- Harris, M. W., J. A. Moore, J. G. Vos, and B. N. Gupta. 1973. General biological effects of TCDD in laboratory animals. Environ Health Perspect 5:101–09. doi:https://doi.org/10.1289/ehp.7305101.
- Hendriks, G., R. S. Derr, B. Misovic, B. Morolli, F. M. Calléja, and H. Vrieling. 2016. The extended ToxTracker assay discriminates between induction of DNA damage, oxidative stress, and protein misfolding. Toxicol Sci 150 (1):190–203. doi:https://doi.org/10.1093/toxsci/kfv323.
- Hirschler, M. M. 2019. Rebuttal to” Flame retardants in UK furniture increase smoke toxicity more than they reduce fire growth rate” by S. McKenna, R. Birtles, K. Dickens, R. Walker, M. Spearpoint, A. Stec and R. Hull (2018 Apr; 196: 429-439). Chemosphere 232:509–511. doi: https://doi.org/10.1016/j.chemosphere.2017.12.017.
- IARC. 2010. Painting, firefighting, and shiftwork. IARC Monogr Eval Carcinogen Risks Human 98: 9–764.
- IARC. 2022. ‘IARC monographs on the identification of carcinogenic hazards to humans. List of classifications. Agents Classified by the IARC Monographs, Volumes 1131,’ Accessed May 23 2021. https://monographs.iarc.who.int/list-of-classifications/.
- ISO. 2012. ‘ISO 13571:2012. Life-threatening components of fire — Guidelines for the estimation of time to compromised tenability in fires’, Accessed May 11 2022. https://www.iso.org/standard/56172.html.
- ISO. 2015a. ‘ISO 5660-1:2015. Reaction-to-fire tests — Heat release, smoke production and mass loss rate — Part 1: Heat release rate (cone calorimeter method) and smoke production rate (dynamic measurement),’ Accessed May 10 2022. https://www.iso.org/standard/57957.html.
- ISO. 2015b. ‘ISO 13344:2015. Estimation of the lethal toxic potency of fire effluents,’ Accessed May 11 2022. https://www.iso.org/standard/68029.html#:~:text=ISO%2013344%3A2015%20provides%20a,of%20a%20physical%20fire%20model.
- ISO. 2016a. ‘ISO 9705-1:2016. Reaction to fire tests — Room corner test for wall and ceiling lining products — Part 1: Test method for a small room configuration,’ Accessed November 26 2021. https://www.iso.org/standard/59895.html.
- ISO. 2016b. ‘ISO/TS 19700:2016. Controlled equivalence ratio method for the determination of hazardous components of fire effluents - Steady-state tube furnace,’ Accessed May 10 2022. https://www.iso.org/standard/70630.html.
- ISO. 2017. ‘ISO 5659-2:2017. Plastics — Smoke generation — Part 2: Determination of optical density by a single-chamber test’, Accessed May 10 2022. https://www.iso.org/standard/65243.html.
- Keir, J. L. A., U. S. Akhtar, D. M. J. Matschke, P. A. White, T. L. Kirkham, H. M. Chan, and J. M. Blais. 2020. Polycyclic aromatic hydrocarbon (PAH) and metal contamination of air and surfaces exposed to combustion emissions during emergency fire suppression: Implications for firefighters’ exposures. Sci Total Environ 698:134211. doi:https://doi.org/10.1016/j.scitotenv.2019.134211.
- Kirk, K. M., and M. B. Logan. 2015. Firefighting instructors’ exposures to polycyclic aromatic hydrocarbons during live fire training scenarios. J Occup Environ Hyg 12 (4):227–34. doi:https://doi.org/10.1080/15459624.2014.955184.
- Larsson, M., D. Fraccalvieri, C. D. Andersson, L. Bonati, A. Linusson, and P. L. Andersson. 2018. Identification of potential aryl hydrocarbon receptor ligands by virtual screening of industrial chemicals. Environ Sci Pollut Res 25 (3):2436–49. doi:https://doi.org/10.1007/s11356-017-0437-9.
- McKenna, S. T., R. Birtles, K. Dickens, R. G. Walker, M. J. Spearpoint, A. A. Stec, and T. R. Hull. 2018. Flame retardants in UK furniture increase smoke toxicity more than they reduce fire growth rate. Chemosphere 196:429–39. doi:https://doi.org/10.1016/j.chemosphere.2017.12.017.
- McNamee, M., B. Truchot, G. Marlair, and B. J. Meacham. 2020. Research roadmap: Environmental impact of fires in the built environment. Final Report, Accessed May 13 2021. https://www.nfpa.org/-/media/Files/News-and-Research/Fire-statistics-and-reports/US-Fire-Problem/RFRoadmapEnvironmentalImpactFires.pdf.
- National Institute for Occupational Safety and Health. 2019. Immediately Dangerous to Life or Health (IDLH) values, Accessed April 26 2021. https://www.cdc.gov/niosh/idlh/default.html.
- Nebert, D. W., and T. P. Dalton. 2006. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 6 (12):947–60. doi:https://doi.org/10.1038/nrc2015.
- NFPA. 2003. Fire protection handbook. 19th. vol. II, In: National Fire Protection Association, Inc, 8–24.
- NTP. 2021. ‘Report on carcinogens, fifteenth edition’, Accessed April 23 2021. https://ntp.niehs.nih.gov/go/roc15.
- Peeters, K., M. Ursič, Č. Tavzes, and F. Knez. 2021. Review: The use of bench-scale tests to determine toxic organic compounds in fire effluents and to subsequently estimate their impact on the environment. Fire Technol 57 (2):625–56. doi:https://doi.org/10.1007/s10694-020-01065-y.
- Petkov, P. I., J. C. Rowlands, R. Budinsky, B. Zhao, M. S. Denison, and O. Mekenyan. 2010. Mechanism-based common reactivity pattern (COREPA) modelling of aryl hydrocarbon receptor binding affinity. SAR QSAR Environ Res 21 (1–2):187–214. doi:https://doi.org/10.1080/10629360903570933.
- Purser, D., and J. L. McAllister. 2016. Assessment of hazards to occupants from smoke, toxic gases, and heat in. In SFPE Handbook of Fire Protection Engineering, 2308–428. 5th ed. New York: Springer.
- Purser, D. 2016. Combustion toxicity in. In SFPE Handbook of Fire Protection Engineering, 2207–307, 5th ed. New York: Springer.
- Ravey, M., I. Keidar, E. D. Weil, and M. P. Eli. 1998a. Flexible polyurethane foam. II. Fire retardation by tris(1,3-dichloro-2-propyl) phosphate. Part A. Examination of the vapor phase (the flame). J Appl Polym Sci 68 (2):217–29. doi:https://doi.org/10.1002/(SICI)1097-4628(19980411)68:2<217::AID-APP5>3.0.CO;2-T.
- Ravey, M., D. W. Edward, I. Keidar, and M. P. Eli. 1998b. Flexible polyurethane foam. II. Fire retardation by tris(1,3-dichloro-2-propyl) phosphate. Part b. Examination of the condensed phase (the pyrolysis zone). J Appl Polym Sci 68 (2):231–54. doi:https://doi.org/10.1002/(SICI)1097-4628(19980411)68:2<231::AID-APP6>3.0.CO;2-R.
- Safe, S. H. 1986. Comparative toxicology and mechanism of action of polychlorinated dibenzo-p-dioxins and dibenzofurans. Annu Rev Pharmacol Toxicol 26 (1):371–99. doi:https://doi.org/10.1146/annurev.pa.26.040186.002103.
- Safe, S. 1990. Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: Environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit Rev Toxicol 21 (1):51–88. doi:https://doi.org/10.3109/10408449009089873.
- Stec, A. A., and T. R. Hull. 2011. Assessment of the fire toxicity of building insulation materials. Energy Build 43 (2–3):498–506. doi:https://doi.org/10.1016/j.enbuild.2010.10.015.
- Wakefield, J. C. 2010. A toxicological review of the products of combustion. Health Protection Agency Centre for Radiation, Chemical and Environmental Hazards, Chemical Hazards and Poisons Division, Chilton, Didcot, Oxfordshire, UK, Accessed April 25 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/458052/HPA-CHaPD-004_for_website.pdf.
- Zhao, B., D. E. Degroot, A. Hayashi, G. He, and M. S. Denison. 2010. CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor. Toxicol Sci 117 (2):393–403. doi:https://doi.org/10.1093/toxsci/kfq217.
- Zhao, H., L. Chen, T. Yang, N. D. V. Ya-Long Feng, B. Liu, Q. Liu, Y. Zhao, Y. Zhao, and -Y.-Y. Zhao. 2019. Aryl hydrocarbon receptor activation mediates kidney disease and renal cell carcinoma. J Transl Med 17 (1):302. doi:https://doi.org/10.1186/s12967-019-2054-5.