ABSTRACT
This work examines (a) the impact of electromagnetic fields (EMF) on heart rate variability (HRV), saliva cortisol, arterial blood oxygenation, and tympanic temperature, and (b) the potential effect of protective devices developed to counter EMF-induced stress. In a pilot study, recordings were taken during a 15-min mobile phone call emitting a high burden of EMF (electric, magnetic, high frequency) after a baseline measurement at rest with very low EMF. In a second visit, this was repeated with participants using three protective devices (insoles, pendant, mobile phone chip). In the main study, four experimental arms were employed, two of which replicated the experimental setup of the pilot study, and two of which examined the effect of only one mobile phone chip in an open-hidden-paradigm. In both experiments, exposure to EMF decreased HRV and increased salivary cortisol. In the protective experimental condition, HRV increased above and cortisol decreased below the level of the baseline measures. All differences were large and specific and not modulated by non-specific effects like placebo effects.
Introduction
An ever-growing exposure to electromagnetic fields (EMF) wireless telecommunication systems raises concern about their potentially harmful effects on health. The use of mobile communication devices has sparked particular interest due to their proximity to the head and ear. With EMF acting at the molecular, cellular, tissue, and organism level (Lai Citation2018), and living beings having an affinity of energy absorption, the central nervous system is regarded as being especially susceptible to detrimental effects (Zhang et al. Citation2017). A review of the literature, however, reveals contradictory empirical evidence, which arises for a variety of reasons, among which are, e.g., intensity and time of EMF exposure or parameters measured (Andrzejak et al. Citation2008; Braune et al. Citation2002; Parazzini et al. Citation2013; Tahvanainen et al. Citation2004). Overall, however, the body of evidence suggests that the autonomic nervous system, and particularly heart rate variability (HRV), may be very sensitive to EMF due to its role of responding to and dealing with stress.
The physiological origins of HRV are the fluctuations of the activity of cardiovascular vasoconstrictor and vasodilator centers in brain, which are directly exposed during mobile phone use. Some researchers link both habitual and short-term exposure to mobile phone radiation to reduced time domain HRV parameters, which is indicative of a shift to sympathetic activation under stress (Béres et al. Citation2018; Ekici et al. Citation2016). Others, in contrast, report an increase in parasympathetic nerve activity upon short-term EMF exposure (Misek et al. Citation2018). Besides methodological reasons, statistical problems may be the cause for such conflicting findings. For instance, the ‘highly significant’ differences reported in the Misek et al. study are in fact very small and reflect nil effects due to positive and negative confidence intervals. The problem of null hypothesis significance testing (NHST) overshadows much of EMF research and reflects a fundamental issue associated with NHST-based research findings, despite the shift that has ensued upon the so-called replication crisis in other research areas (Barry et al. Citation2019; Greenland et al. Citation2016; Kennedy-Shaffer Citation2019). Overall, however, both the pivotal role HRV plays for the development and progression of diseases in general as well as its significance as an indicator of stress makes it a relevant physiological parameter in the assessment of EMF-induced stress.
An additional detrimental EMF effect is the increase in cortisol secretion as part of biological stress adaptation. These effects on adrenal corticoid response were first studied in the early 1960s (Barnothy Citation1963) and research is prevalent in studies investigating the metabolism of various hormones primarily in rodents. The majority of studies suggest an elevation of cortisol under EMF exposure (de Kleijn et al. Citation2016; Kitaoka et al. Citation2016; Rauš Balind et al. Citation2016), but short-term exposure to extremely low-frequency EMF in humans is underresearched and has found less evidence for a direct relationship (Touitou and Selmaoui Citation2012). A recent study by Touitou et al. (Citation2022) involving long-term exposure of up to 20 years of workers in extra-high voltage (EHV) substations found a 10-time increase in blood cortisol secretion compared to an age-matched, unexposed control group. However, whether short-term exposure of HF EMF acts as an acute stressor in mobile phone users is less clear.
Another area of stress-induced effects of EMF can be found in hematological studies. For instance, erythrocytes have been found to form rouleaux under electromagnetic fields, resulting in a decrease in red blood cell counts (Kozma et al. Citation2011; Mousavy et al. Citation2009; Sebastián et al. Citation2005; Vagdatli et al. Citation2014). Animals exposed to 30-min mobile phone devices display color alterations in erythrocytes indicating lack of hemoglobin (Alghamdi and El-Ghazaly Citation2012). These authors suggested that a hypoxia-like status probably results from the oxygen-binding impairment of hemoglobin and the overproduction of reactive oxygen species caused by EMF. The effects of free radicals on the erythrocyte membrane may also contribute to the leak of hemoglobin out of the cells (Jbireal et al. Citation2018). Blood oxygenation is kept within relatively narrow margins to secure tissue oxygenation (McClatchey Citation2017), but impairment of hemoglobin binding may potentially result in a measurable decrease of arterial blood oxygenation. Whether short-term exposure to EMF emitted by mobile phones causes such effects, however, has not been systematically established.
In recent years, technical solutions have emerged to counter adverse health effects of EMF. They are advertised as protective devices (e.g. mobile phone chips) which render EMF ‘biocompatible’, but there is very little research supporting such claims. In a recent study testing exposure to a 30-min EMF mobile phone call, increased activity in the theta, alpha, beta, and gamma EEG frequency bands was found when no chip or a placebo chip was used (Henz et al. Citation2018). The use of the mobile phone chip was additionally associated with an increase in cognitive performance in an attention test. However, the ‘significant’ differences reported by the authors do not reflect conclusive effect sizes due to positive and negative confidence intervals boundaries. It is thus unclear whether these results actually reflect a cognitive performance superiority effect caused by the chip.
The present work was designed to test three devices developed to protect against EMF-induced stress. First, a pilot study was conducted to determine the sensitivity of various physiological parameters with regard to EMF exposure and to estimate the protective effects of the devices. Based on these findings, the main study investigated their efficacy under different experimental boundary conditions addressing the following questions:
Do the EMF-protective devices have meaningful stress reducing effects?
Are these effects specific or dependent on placebo effects?
Methods
Study 1 (Pilot Study)
Participants
The sample consisted of N = 6 subjects (three females) who were on average 31 years old (22–46 years). The following inclusion criteria applied: (a) age of consent, (b) no disease of the cardiovascular system (e.g. cardiac arrhythmia, tachycardia/bradycardia, hyper-/hypotension), (c) no respiratory system disease (common cold, asthma, cystic fibrosis, COPD, COVID), (d) no current medication intake, (e) no food, caffeine, nicotine or alcohol consumption 2 h prior to the experiment, (f) no electrosmog sensitivity. All participants provided written informed consent and were remunerated with € 20. The study was run following the Ethical Principles for Medical Research Involving Human Subjects of the World Medical Association and the CONSORT guidelines.
Study design and procedure
All data recordings were conducted in a fully ventilated room (12 m2) with constant temperature (20°C), humidity (50%), and room illumination (200 lx). All sessions were run between 2:00 p.m. and 5:00 p.m. Participants were tested twice under two different conditions 1 week apart. In the first visit, they were exposed to EMF emitted by a Smartphone; in the second visit this was repeated but participants used three protective devices made of shungite. They wore insoles, a pendant that was placed around their neck, and the same type of Smartphone was equipped with a mobile phone chip (50 mm in diameter). Participants were first introduced to the purpose of the study and asked for their written consent. The recording devices were then attached and checked for accuracy of data sampling. Next, a baseline measurement ensued encompassing recording of the physiological measures for 15 minutes with the participants sitting upright in a comfortable armchair. They were encouraged to relax and breathe as normally as possible. After that, a tripod was placed in a position that allowed full contact of the attached Smartphone to the left ear of the participants. Then, EMF exposition with the Smartphone followed lasting again 15 minutes.
Assessment of EMF
Before each session, the experimental room was screened for EMF where the empty tripod and participants' head were to be placed (3-axis, 30 cm radius). The Trifield® EMF Meter Model TF2 was used (Alpha Lab Inc., Salt Lake City, USA) to assess three types of EMF: (1) electrical LF (true magnitude, frequency range: 40 Hz −100 kHz, accuracy: ± 5% @ 50/60 Hz, maximum range: 1000 V/m, resolution: 1 V/m), (2) magnetic LF (3-axis, true magnitude, frequency range: 40 Hz −100 kHz, accuracy: ± 4% @ 50/60 Hz, maximum range; 10000 nT, resolution: 10 nT), and (3) RF (radio)/microwave (1-axis, frequency range: 20 MHz – 6 GHz, accuracy: ± 20% @ 1 GHz, maximum range: 20000 µW/m2, resolution: 1 µW/m2). The maximum EMF readings were: AC electric: 1 V/m, AC magnetic: 40 nT, power density at radiofrequency: 0.05 µW/m2. In accordance with the critical limits recommended by the German Institute of Healthy and Ecological Building (SBM-2015/IBM) the EMF burden in the baseline recording condition was weak to very low.
Physiological measures
Arterial blood oxygen saturation
Peripheral arterial oxygen saturation (SpO2) was continually measured using the clinically validated pulse oximeter pulox PO-300 (Novidon, Cologne, Germany), which uses two light emitting diodes as energy source with the wavelengths 660 nm (red) and 920 nm (infrared). It displays a continuous real-time plethysmograph trace as well as arterial oxygen saturation (per cent) and heart rate (beats per minute) with a sample rate of one per second. The display automatically shut off after 30 seconds to prevent participants from tracking the recordings. The device was applied to the left index finger as probe location.
Heart rate variability and tympanic temperature
Heart rate variability and tympanic temperature were continually assessed with a portable in-ear sensor (cosinuss° One, Munich, Germany) attached to the right ear. Temperature was assessed to determine the well-documented thermal effects EMF may induce (Adair and Black Citation2003; Bortkiewicz et al. Citation2012). This device (45 x 38 × 18 mm, 6.5 grams) uses a green LED and photodiode (PPG) to determine heart rate and RR intervals, and a contact Pt 1000 resistance temperature sensor to measure body core temperature. Sampling rate is 256 Hz with a high accuracy of measurement (heart rate: ± 1 bpm, temperature: ± 0.2°C, RR intervals: ± 5 ms) according to several ECG validation studies. Recordings were transmitted via the ANT+ (Adaptive Network Topology) multicast wireless sensor technology to an Android device that tracked all parameters and stores it in CSV format. To obtain NN intervals, artifacts (e.g. ectopic beats) were identified using the algorithm proposed by Loimaala et al. (Citation1999) which considers as normal an adjacent RR interval that does not exceed a 30% difference between them. Artifacts were replaced by the mean of the three previous and valid RR intervals (Dos Santos Ribeiro et al. Citation2018). The following time-based target variables were assessed: mean NN, standard deviation of NN intervals (SDNN), root mean square of successive differences between normal heartbeats (RMSSD), NN50 (number of NN intervals exceeding 50 ms), and pNN50 (percentage of NN intervals exceeding 50 ms).
Neuroendocrine biomarker
Saliva samples were collected for assessing cortisol levels immediately after the baseline and treatment recordings and frozen at −18°C before being sent blinded to an independent laboratory (IBL, Hamburg, Germany). Cortisol levels were assessed using the enzyme immunoassay test (ELISA) following the typical competitive binding scenario between an unlabeled antigen and an enzyme-labeled antigen (conjugate) for a limited number of antibody-binding sites on the microplate. After washing and decanting procedures unbound materials were removed. After the washing step, the enzyme substrate was added. The enzymatic reaction was terminated by addition of the stopping solution. The absorbance was measured on a microtiter plate reader and expressed as µg/dl.
Treatment/intervention
Baseline
In the baseline phase, all recordings were performed in a non-stressful experimental setting with very low EMF.
Zero control
For the first visit, participants were exposed to a 15-min Smartphone call using the Samsung Galaxy J5, which was placed directly at the left ear using a mobile phone tripod. The device worked on a MHz LTE network using three standard band frequencies in Germany (800, 1800, and 2600) and has a SAR value of 0.61 W/kg at the head. Since the aim of the study was to mimic a typical mobile phone call the emitted field was not controlled during the experiments. The in-calling Smartphone was a Samsung Galaxy A22, which was located in an adjacent room operated by the experimenter.
Intervention
For the second visit, the Smartphone call was repeated, but three different protective devices (Beyond Matter, EssenceX, Berlin, Germany) were used: (a) insoles consisting of an upper layer made of natural leather, a middle layer made of shungite and a lower layer made of leather were placed in participants’ shoes prior to the EMF exposition, (b) a pendant consisting of a raw, shungite stone (ca. 30 mm x 10 mm x 15 mm) was placed around their neck, and c) a mobile phone chip consisting of shungite in round shape of 50 mm diameter was attached to the second Smartphone.
In each experimental session, the location where the mobile phone was placed was screened for EMF (5 cm proximity to the head/phone) during the first minute of each call. The readings yielded high emissions according to the SBM-2015/IBM guidelines: electrical field (3–18 V/m), magnetic field (200–500 nT), and radiofrequency (500–1500 µW/m2).
Data analysis
To assess treatment effects, the effect size d (Cohen Citation2008) and confidence intervals (95%) for within-group comparisons were calculated (Borenstein et al. Citation2009). To compare the experimental conditions, difference scores were computed (i.e. zero control minus baseline vs. intervention minus baseline). Calculation of effect sizes was in alignment with meta-analytical practice (Hunter and Schmidt Citation2004), and a consequence of the highly problematic use of NHST (Greenland et al. Citation2016).
Results
Arterial blood saturation
Compared to baseline, arterial blood oxygen increased in both experimental conditions (cf. ). The elevations of 0.45% in the control condition and 0.6% in the treatment condition, and did not differ statistically (d = 0.2, CI: −0.8 < d < 1.2).
Table 1. Mean and standard deviations of physiological parameters in study 1.
Tympanic temperature
There was a relative rise in the intervention condition, which was about five times larger than in the control condition, but the absolute temperature increases of 0.28°C versus 0.05°C did not differ statistically (d = 0.6; CI: −0 .4 < d < 1.6).
Heart rate variability
Mean NN
In both experimental conditions, there was an elevation of the mean NN intervals indicating a lowering of the heart rate. Although the increase was more than twice as high in the treatment condition (22 ms without versus 45 ms), this differential effect of d = 0.7 was statistically inconclusive (CI: −0.6 < d < 1.3).
SDNN
The standard deviation of the NN intervals yielded a large effect of with d = 2.4 (CI: 1 < d < 3.5). When exposed to the Smartphone-induced EMF without protection, SDNN decreased by about 6 ms but increased by about 3 ms when the participants were equipped with the protective devices.
RMSSD
A similar pattern of results was obtained for RMSSD, which dropped by about 7 ms without protection and rose by about 6 ms with the protective items (d = 3.4; CI: 1.8 < d < 4.8).
NN50
On average, the number of NN intervals over 50 ms decreased by about 34 in the control condition and increased by about 54 in the intervention condition (d = 2.3; CI: 1.8 < d < 4.8).
pNN50
For the percentage of NN intervals over 50 ms there was a decrease of about 1.2% in the control condition and an elevation of about 7.3% when participants were protected (d = 1.7; CI: 0.6 < d < 2.9).
Cortisol
Salivary cortisol concentration rose by 0.015 µl/dl during the Smartphone call in the control condition and decreased by 0.0083 µl/dl in the treatment condition. This difference was statistically medium in size but fell within positive and negative confidence interval limits (d = 0.6; CI: −0.6 < d < 2.9).
Discussion
The pilot study suggested an impairing effect of EMF on the body’s autonomous nervous system during a 15-min Smartphone call close to the head. Three types of protective devices reversed this effect for HRV. The largest effects were found for RMSSD and SDNN which are considered to be reliable indicators of physiological resistance to short-duration stress (Shaffer and Ginsberg Citation2017). Both the sympathetic and parasympathetic nervous systems contribute to SDNN variability, but RMSSD represents the beat-to-beat variance in heart rate and is therefore regarded the primary time-domain measure used to estimate vagally mediated changes in HRV. This indicated that the parasympathetic nervous system counter-regulated the EMF-induced stress by increasing HRV when the devices were used. Rather than restoring HRV to ‘normal’ values (i.e. those observed during baseline measurement), it was ‘overcompensated’ during Smartphone exposure (i.e. increase of parasympathetic activity exceeding baseline).
The effects of the devices on arterial blood saturation, body temperature, and cortisol were less clear-cut. There was no indication that blood oxygenation was specifically elevated. Whether the relatively lower temperature rise indicated protection against harmful thermal effects was doubtful given that there was no clear differential effect. The use of the Smartphone did not trigger a strong adrenocortical release. However, despite the lack of a conclusive statistical effect, there was a descriptive reversal of hormone release, which could potentially have been indicative of a protection mechanism comparable to the one observed for HRV.
To account for alternative explanations of the study and to determine the effects based on a sample with sufficient power, a larger study was conducted which also explored additional boundary conditions. The aim of this study was to (a) conceptually replicate the findings of the pilot study, (b) vary the type of protection used, and (c) explore the role of potential placebo effects.
Materials and method
Study 2
Based on the effect size of HRV (RMSSD) found in the pilot study, the sample size of each experimental group was set at n = 15 to achieve a power of 1- β ≥ .95 (Faul et al. Citation2009). In total, 60 participants (28 females) were enrolled in study 2. Their mean age was 37.6 years (SD = 13.9). Inclusion criteria were set to identically reflect those of the pilot study. All participants provided written informed consent and were remunerated with € 20.
Study design and procedure
The experimental conditions were adopted from study 1. In contrast, participants were randomly assigned to four arms using a randomized block design containing five-digit random number sequences that were ranked in ascending succession and assigned to the experimental arms. Before each experiment, the experimenter opened an envelope containing the assignment. To replicate the findings of study 1, a zero control condition was employed which mimicked the Smartphone set-up without protection. In the second condition (Treatment 1), participants used the same protective items as in study 1 (insoles, pendant, chip) when using the Smartphone (Samsung Galaxy J5). The third and fourth conditions employed the open-hidden paradigm using only the mobile phone chip in the open condition (Treatment 2). In the hidden condition (Treatment 3), participants were not aware of the mobile phone chip, which was placed under a hard cover mobile phone case. In the open condition, the chip was visible on the Smartphone and introduced by the experimenter. Data recording sequences were adopted from study 1, starting with an introduction and gathering of the written consent, experimental setup check-up, baseline measurement for 15 minutes, installation of the Smartphone tripod and EMF exposition for 15 minutes. Participants in the hidden condition were unblinded upon completion of the study.
Assessment of EMF
EMF screenings of the experimental room were weak and did not exceed the following values: AC electric: 1 V/m, AC magnetic: 30 nT; radiofrequency: 5 µW/m2. In contrast, exposure to EMF in the experimental conditions changed as follows: electrical field: 0–2 V/m; magnetic field: 700–1800 nT; power density at radiofrequency: 700–13000 µW/m2.
Physiological measures
HRV and salivary cortisol were chosen as target parameters. Sampling methods corresponded to those of study 1. Saliva samples were blindly analyzed by Synlab (Echterdingen, Germany) and expressed as ng/ml.
Data analysis
Eta square (η2) was calculated as the effect measure for the ANOVAs and Cohen’s d and confidence intervals (95%) were calculated for mean comparisons. To compare the experimental conditions, difference scores were computed (i.e. treatment minus baseline).
Results
Heart rate variability
Mean NN
The ANOVA of the mean NN intervals yielded a small effect of η2 = 0.03 (F(3,59) = 0.8). While mean NN of the intervals in the three treatment conditions decreased (Treatment 1: −9.7 ms; Treatment 2: −1.1 ms; Treatment 3: −3.5 ms), it increased by 7.4 ms in the control condition (cf and ). However, there was no statistically meaningful effect for any of the individual comparisons. The largest effect of d = 0.5 was found for the comparison of the control condition and Treatment 1, but it fell within positive and negative confidence interval boundaries (CI: −0.2 < d < 1.2).
Figure 1. Mean difference scores and standard errors for neurocardiac (HRV) and neuroendocrine (saliva cortisol) parameters in study 2; Zero Control: High EMF exposure; Treatment 1: Three protective devices (insoles, penchant, cell phone chip); Treatment 2: open mobile phone chip; Treatment 3: hidden mobile phone chip.
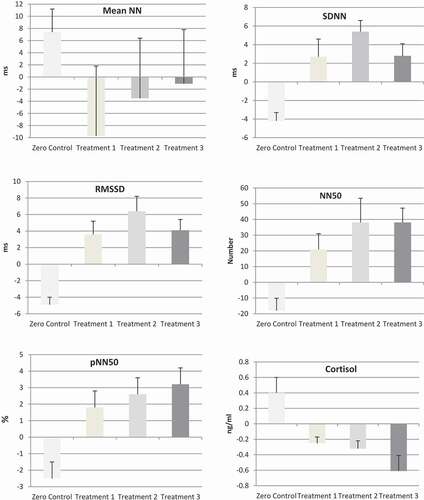
Table 2. Mean and standard deviations of physiological parameters in study 2.
SDNN
There was a large effect for the standard deviation of the NN intervals of η2 = 0.32 (F(3,59) = 8.9). Mean comparisons revealed that SDNN dropped by 4.2 ms in the control condition and rose in the three intervention arms (Treatment 1: 2.7 ms; Treatment 2: 2.8 ms; Treatment 3: 5.4 ms). Comparable to the findings in the pilot study, there was a reversal of effects when the protective items were used with SDNN increasing above the levels of the baseline recordings. Compared to the control condition, the effects were: Treatment 1: d = 1.2 (CI: 0.4 < d < 2), Treatment 2: d = 1.7 (CI: 0.9 < d < 2.5), and Treatment 3: d = 2.2 (CI: 1.3 < d < 3.1). Although the effect for the hidden treatment was largest, there was no statistical difference between the three interventions. The largest difference observed was between Treatment 3 (hidden chip) and Treatment 2 (open chip), but it was statistically inconclusive (d = 0.5; CI: −0.2 < d < 1.3).
RMSSD
The ANOVA for RMSSD also showed a large effect (η2 = 0.38 (F(3,59) = 11.7). Analogous to SDNN, RMSSD was reduced by 4.9 ms in the control condition and heightened in the three intervention arms (Treatment 1: 3.6 ms; Treatment 2: 4.1 ms; Treatment 3: 6.4 ms). The differential effects for the comparisons with the control condition were: Treatment 1: d = 1.2 (CI: 0.4 < d < 2); Treatment 2: d = 1.7 (CI: 0.9 < d < 2.5), Treatment 3: d = 2.9 (CI: 1.8 < d < 3.9). The three treatments did not differ statistically (largest difference: Treatment 3 vs. Treatment 2; d = 0.5; CI: −0.2 < d < 1.2).
NN50
Analyses for the number of NN intervals over 50 ms yielded a large effect (η2 = 0.24 (F(3,59) = 5.8) revealing that it decreased in the control group by 18, while it rose in the three intervention groups (Treatment 1: 20.9; Treatment 2: 38.1; Treatment 3: 38.1). The effects for the mean comparisons were d = 1.1 (CI: 0.3 < d < 1.9) for Treatment 1, d = 1.2 (CI: 0.4 < d < 2) for Treatment 2, and d = 1.7 (CI: 0.8 < d < 2.5) for Treatment 3. The largest difference between the intervention arms were found for Treatment 3 and Treatment 1, but they were statistically inconclusive (d = 0.5: CI: −0.3 < d < 1.2).
pNN50
The ANOVA of the mean percentage of NN50 showed a large effect (η2 = 0.15 (F(3,59) = 3.3). While it decreased in the control condition by 2.5%, it rose in the intervention arms (Treatment 1: 1.8%; Treatment 2: 3.2%; Treatment 3: 2.6%). The differential effects were: Treatment 1: d = 1.1 (CI: 0.3 < d < 1.8); Treatment 2: d = 1 (CI: 0.2 < d < 1.8); Treatment 3: d = 1.1 (CI: 0.3 < d < 1.8). There were no differences between these groups.
Cortisol
Three samples of saliva had to be excluded from the analyses due to high viscosity. For the remaining sample, a large effect was found (η2 = 0.26 (F(3,56) = 6.3). Analogous to the HRV results, the cortisol level in the control group increased by 0.39 ng/ml, while it decreased during intervention (Treatment 1: −0.25 ng/ml; Treatment 2: −0.39 ng/ml; Treatment 3: −0.61 ng/ml). The differential effect for the treatments were identical (d = 1.2; CI: 0.4 < d < 2). There were no statistical differences between the intervention groups (largest difference: Treatment 3 and Treatment 1: d = 0.5, CI: −0.2 < d < 1.2).
Correlational analyses
Given the reversal of effects found for HRV and salivary cortisol in the control and treatment conditions, their association was explored in more detail. There was a positive correlation between HRV and cortisol in the control condition such that a decrease of RMSSD was associated with an increase of cortisol secretion when exposed to EMF (r = 0.4; d = 0.9). In contrast, there was a negative correlation in the three intervention groups indicating a decrease of cortisol release when HRV was elevated (r = −0.3; d = 0.6). The difference between the two correlations corresponded to a large effect (Cohen’s q = 0.7). The correlational analyses for Intervention 3, where the largest effects were observed, yielded an even greater association of inhibition of cortisol release and increase of RMSSD of r = −0.6 (d = 1.5). The corresponding difference between this correlation and the correlation in the control group was Cohen’s q = 1.1. Thus, the neurocardiac and neuroendocrine responses were inversely proportional upon use of the protective devices suggesting a counter-regulation of EMF stress.
Discussion
The main study aimed at replicating the pilot study using a sample with sufficient power. Two study arms mimicked the experimental setup of the pilot study (i.e. duration and intensity of EMF exposure, duration, and type of intervention), and two study arms tested only a mobile phone chip, which was either known to the participants or concealed (open-hidden paradigm). EMF produced marked changes in the autonomic nervous system suggesting a shift from sympathovagal balance to sympathetic activation. This indicated that a mobile phone placed near the head for 15 minutes influenced the medulla oblongata where the vagal nerve originates. The decrease in parasympathetic nerve and heightening of sympathetic activity is a typical physiological response to stressors, which in this study, showed in the vast majority of participants. RMSSD decreased in 14 out of 15 individuals from 2.9% to 22.8% (average: 9.5%) relative to a low EMF environment (baseline). The stress response also showed in an average elevation of cortisol secretion of 22.7%. The hypothalamic–pituitary–adrenal axis controls cortisol to alter the availability of glucose as part of a fight or flight response. The study’s results are hence in alignment with the stress-response hypothesis put forward by Touitou et al. (Citation2022) which posits elevated cortisol secretion in relation to the intensity of EMF exposure. However, it extends it with regard to exposure time needed to produce elevated secretion, which in this study, was relatively short.
In contrast, there were no indications of an elevated stress response in the conditions with EMF protection, but instead a reversal of effects that was noticeably consistent across the HRV parameters. As in the pilot study, the effect was largest for RMSSD which represents vagally mediated changes in HRV. Surprisingly, HRV increased above the activity observed during baseline recording. In 37 out of 45 participants (82% responder), RMSSD was elevated by 1.5% to 36.6% (average: 10.3%) which suggested that there was some type of EMF-related ‘overcompensation’ of autonomic nervous system regulation during the use of the devices.
In alignment with the findings for HRV, the pattern of cortisol release yielded comparable effects. In 11 out of 15 individuals cortisol increased from 9.6% to 119.6% (mean: 40.9%) when participants were exposed to EMF without protection. Conversely, salivary cortisol decreased in 36 out of 42 participants from −2.1% to −76% (mean: −26.9%) when the devices were present.
These effects were specific and indicated no modulation by placebo effects. To the contrary, the absolute effect sizes for HRV were largest in the experimental condition where individuals were nescient of the presence of a mobile phone chip (hidden condition). Although the three treatment arms did not differ statistically the absolute effect size differences were noticeable. Future research should investigate this issue in more detail and examine whether there is an overregulation upon use of all three protective items, or whether there is a ‘paradoxical nocebo effect’ which potentially mitigates the full effect, e.g. when users are suspicious of the devices.
The devices acted on the neurocardiac and neuroendocrine level, but it is unclear how these changes were modulated. The aim of this study was to establish whether the devices are associated with meaningful stress-reducing effects, but more studies are needed to elucidate the modulating functional mechanisms. Given the continued technological advancement of mobile phone technology and new developments in telecommunication (e.g. roll-out of 5 G), these findings await confirmation and generalization by investigations addressing these issues. Furthermore, both short-term and long-term exposure to EMF may result in an overstimulation of various brain areas, which not only may cause stress responses as the ones examined in this work, but which may impair cognitive and mental faculties, result in psychophysiological overstimulation, or impede recuperative functions. This work did not relate EMF effects to any of these functions and more empirical evidence is needed to understand the implications of the herein reported effects.
In sum, the results of this work contributed to the body of evidence showing biological stress responses after short-term mobile phone use. It adds the finding that new technologies are able to counter or even reverse such adverse effects. Apart from recommendations of a reduction of exposure time to EMF and a sensible use of mobile phones, this technology could offer a meaningful solution to counter harmful EMF effects.
Conclusion
The use of mobile phones induces physiological stress on a neurocardiac and neuroendocrine level after a short exposure time of 15 minutes. These physiological stress responses can be offset with specially designed protected devices. The technology tested (EssenceX shungite) produces a ‘super-optimization’ of HRV and cortisol inhibition exceeding normoregulation in non-stressful conditions. These results encourage further investigation of long-term effects of this technology in different environments.
Acknowledgments
I thank Nick Singer and George Taylor for helpful remarks regarding the paper’s intelligibility.
Disclosure statement
The study was commissioned by EssenceX, Berlin, Germany. The company had no role in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication. The principal investigator did not interfere with the treatments and had no personal contact with the participants.
Data Availability Statement
The datasets generated and analyzed are not publicly available due to funding source’s policies but are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Adair, E. R., and D. R. Black. 2003. Thermoregulatory responses to RF energy absorption. Bioelectromagnetics 24:17–38. doi:10.1002/bem.101334.
- Alghamdi, M. S., and N. A. El-Ghazaly. 2012. Effects of exposure to electromagnetic field on some hematological parameters in mice. Open J Med Chem 2:30–42.
- Andrzejak, R., R. Poreba, M. Poreba, A. Derkacz, R. Skalik, P. Gac, B. Beck, A. Steinmetz-Beck, and W. Pilecki. 2008. The influence of the call with a mobile phone on heart rate variability parameters in healthy volunteers. Ind Health 46:409–17. doi:10.2486/indhealth.46.409.
- Barnothy, M. F. 1963. Biological effects of magnetic fields on small mammals. Biomed. Sci. Instrum. 1:127–35.
- Barry, A. E., D. Valdez, P. Goodson, L. Szucs, and J. V. Reyes. 2019. Moving college health research forward: Reconsidering our reliance on statistical significance testing. J Am Coll Health 67:181–88. doi:10.1080/07448481.2018.1470091.
- Béres, S., Á. Németh, Z. Ajtay, I. Kiss, B. Németh, and L. Hejjel. 2018. Cellular phone irradiation of the head affects heart rate variability depending on inspiration/expiration ratio. In Vivo 32(5), 1145–53. doi:10.21873/invivo.11357.
- Borenstein, M., L. V. Hedges, J. P. T. Higgins, and H. R. Rothstein. 2009. Introduction to meta-analysis. Chichester: John Wiley & Sons.
- Bortkiewicz, A., E. Gadzicka, W. Szymczak, and M. Zmyslony. 2012. Changes in tympanic temperature during the exposure to electromagnetic fields emitted by mobile phone. Int J Occup Med Environ Health 25:145–50. doi:10.2478/s13382-012-0013-y.
- Braune, S., A. Riedel, J. Schulte-Monting, and J. Raczek. 2002. Influence of a radio frequency electromagnetic field on cardiovascular and hormonal parameters of the autonomic nervous system in healthy individuals. Radiat. Res. 158:352–56. doi:10.1667/0033-7587(2002)158[0352:IOAREF]2.0.CO;2.
- Cohen, J. 2008. Statistical power analysis for the behavioral sciences. Hillsdale: Laurence Erlbaum Associates.
- de Kleijn, S., G. Ferwerda, M. Wiese, J. Trentelman, J. Cuppen, T. Kozicz, L. de Jager, P. W. M. Hermans, and B. M. L. Verburg-van Kemenade. 2016. A short-term extremely low frequency electromagnetic field exposure increases circulating leukocyte numbers and affects HPA-axis signaling in mice. Bioelectromagnetics 37:433–43. doi:10.1002/bem.21998.
- Ekici, B., A. Tanindi, G. Ekici, and E. Diker. 2016. The effects of the duration of mobile phone use on heart rate variability parameters in healthy subjects. Anatol J Cardiol 16:833–38. doi:10.14744/AnatolJCardiol.2016.6717.
- Faul, F., E. Erdfelder, A. Buchner, and A.-G. Lang. 2009. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods 41:1149–60. doi:10.3758/BRM.41.4.1149.
- Greenland, S., S. J. Senn, K. J. Rothmann, J. B. Carlin, C. Poole, S. N. Goodman, and D. G. Altman. 2016. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 31:337–50.
- Henz, D., W. I. Schöllhorn, and B. Poeggeler. 2018. Mobile phone chips reduce increases in EEG brain activity induced by mobile phone-emitted electromagnetic fields. Front. Neurosci. 12:190. doi:10.3389/fnins.2018.00190.
- Hunter, J. E., and F. Schmidt. 2004. Methods of meta-analysis: Correcting error and bias in research findings. London: Sage Publishers.
- Jbireal, J. M., A. E. Azab, and A. S. I. Elsayed. 2018. Disturbance in haematological parameters induced by exposure to electromagnetic fields. Hematol Transfus Int J 6:242–51. doi:10.15406/htij.2018.06.00193.
- Kennedy-Shaffer, L. 2019. Before p < 0.05 to beyond < 0.05: Using history to contextualize < 0.05 to Beyond p < .05: Using History to Contextualize p-Values and Significance Testing. Am Stat 73:82–90. doi:10.1080/00031305.2018.1537891.
- Kitaoka, K., S. Kawata, T. Yoshida, F. Kadoriku, M. Kitamura, and Y. Tsuji. 2016. Exposure to an extremely low-frequency magnetic field stimulates adrenal steroidogenesis via inhibition of phosphodiesterase activity in a mouse adrenal cell line. PLoS ONE 11:e0154167. doi:10.1371/journal.pone.0154167.
- Kozma, N., H. Speletz, U. Reiter, G. Lanzer, and T. Wagner. 2011. Impact of 13.56-MHz radiofrequency identification systems on the quality of stored red blood cells. Transfusion 51:2384–90. doi:10.1111/j.1537-2995.2011.03169.x.
- Lai, H. 2018. A summary of recent literature (2007-2017) on neurobiological effects of radio frequency radiation. In Mobile communications and public health, ed. M. Markov, 185–220. New York: CRC Press.
- Loimaala, A., H. Sievanen, R. Laukkanen, J. Parkka, I. Vuori, and H. Huikuri. 1999. Accuracy of a novel real-time microprocessor QRS detector for heart rate variability assessment. Clin Physiol 19:84–88. doi:10.1046/j.1365-2281.1999.00152.x.
- McClatchey, K. D. 2017. Clinical laboratory medicine. Philadelphia: Lippincott Williams & Wilkins.
- Misek, J., I. Belyaev, V. Jakusova, I. Tonhajzerova, J. Barabas, and J. Jakus. 2018. Heart rate variability affected by radiofrequency electromagnetic field in adolescent students. Bioelectromagnetics 39:277–88. doi:10.1002/bem.22115.
- Mousavy, S. J., G. H. Riazi, and M. Kamarei. 2009. Before p < .05 to Beyond p < .05: Using History to Contextualize p-Values and Significance Testing. Int. J. Biol. Macromol. 44:278–85. doi:10.1016/j.ijbiomac.2009.01.001.
- Parazzini, M., P. Ravazzani, G. Thuroczy, F. B. Molnar, G. Ardesi, A. Sacchettini, and L. T. Mainardi. 2013. Nonlinear heart rate variability measures under electromagnetic fields produced by GSM cellularphones. Electromagn Biol Med 32:173–81. doi:10.3109/15368378.2013.776424.
- Rauš Balind, S., M. Manojlović-Stojanoski, V. Milošević, D. Todorović, L. Nikolić, and B. Petković. 2016. Short- and long-term exposure to alternating magnetic field (50 Hz, 0.5 mT) affects rat pituitary ACTH cells: Stereological study. Environ. Toxicol. 31:461–68. doi:10.1002/tox.22059.
- Ribeiro, D. S., G. V. Ribeiro Neves, L. Deresz, R. Domingues Melo, P. Dal Lago, and M. Karsten. 2018. Can RR intervals editing and selection techniques interfere with the analysis of heart rate variability? Braz J Phys Ther 22:383–90. doi:10.1016/j.bjpt.2018.03.008.
- Sebastián, J. L., S. Muñoz San, M. Martín, J. Sancho, M. Miranda, and G. Alvarez. 2005. Erythrocyte rouleaux formation under polarized electromagnetic fields. Phys Rev E Stat Nonlin Soft Matter Phys 72:031913. doi:10.1103/PhysRevE.72.031913.
- Shaffer, F., and J. P. Ginsberg. 2017. An overview of heart rate variability metrics and norms. Front Public Health 5:258. doi:10.3389/fpubh.2017.00258.
- Tahvanainen, K., J. Nino, P. Halonen, T. Kuusela, T. Laitinen, E. Lansimies, J. Hartikainen, M. Hietanen, and H. Lindholm. 2004. Cellular phone use does not acutely affect blood pressure or heart rate of humans. Bioelectromagnetics 25:73–83. doi:10.1002/bem.10165.
- Touitou, Y., and B. Selmaoui. 2012. The effects of extremely low-frequency magnetic fields on melatonin and cortisol, two marker rhythms of the circadian system. Dialogues Clin Neurosci 14:381–99. doi:10.31887/DCNS.2012.14.4/ytouitou.
- Touitou, Y., B. Selmaoui, and J. Lambrozo. 2022. Assessment of cortisol secretory pattern in workers chronically exposed to ELF-EMF generated by high voltage transmission lines and substations. Environ Int 161:107103. doi:10.1016/j.envint.2022.107103.
- Vagdatli, E., V. Konstandinidou, N. Adrianakis, I. Tsikopoulos, A. Tsikopoulos, and K. Mitsopoulou. 2014. Effects of electromagnetic fields on automated blood cell measurements. J Lab Autom 19:362–65. doi:10.1177/2211068213520492.
- Zhang, J., A. Sumich, and G. Y. Wang. 2017. Acute effects of radiofrequency electromagnetic field emitted by mobile phone on brain function. Bioelectromagnetics 38:329–38. doi:10.1002/bem.22052.

