ABSTRACT
A large number of studies have reported that tumor cells are often out of sync with the surrounding healthy tissue. Exploiting this misalignment may be a way to obtain a substantial gain in the therapeutic window. Specifically, based on reports to date, we will assess whether radiotherapy outcomes differ depending on the administration time. Collectively, 24 studies met the inclusion criteria, out of which 12 at least reported that radiation therapy is less toxic when administered at a particular time, probably because there is less collateral damage to healthy cells. However, discrepancies exist across studies and urge further investigation. Mechanistic studies elucidating the relationship between radiotherapy, circadian rhythms, and cell cycle, combined with either our “digital” or “biological” chronodata, would help oncologists successfully chronotype individual patients and strategize treatment plans accordingly.
Introduction
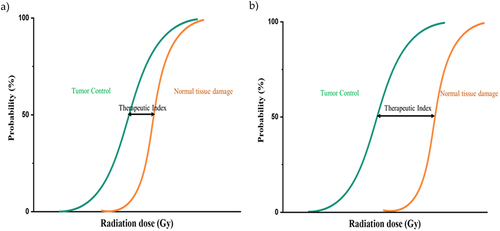
People might be exposed to ionizing radiation under different circumstances, naturally from radioactive elements in the earth’s crust (e.g., radon), accidentally through exposure to radioactive fallout, and deliberately in case of medical exposure for patient diagnosis or treatment. The American Cancer Society estimated that 1.8 million new invasive cancer cases were diagnosed in the United States in 2020 (excluding basal cell and squamous cell skin cancers and carcinomas in situ, except the urinary bladder).Citation1 Nearly two-thirds of these patients will receive radiation therapy (radiotherapy [RT]) as part of the treatment plan.Citation2 The main goal of RT is to deprive cancer cells of their multiplication (cell division) potential, possibly by damaging a cell’s DNA and inhibiting its ability to reproduce. A delay in cell division and suppression of mitosis have been confirmed after a single dose of γ-ray radiation in the order of 0.5–1.0 Gy. Moreover, the period of mitotic delay significantly varies depending on when the treatment was provided.Citation3 RT can be delivered in two ways, externally and internally, depending on the type of cancer and treatment goals. Despite the broad technical advances in imaging, planning, and delivery leading to the possibility of providing escalating radiation doses to the patient’s tumor, irradiating healthy tissue is largely unavoidable. Adverse effects from RT range in severity from transient acute effects, including xerostomia, dysgeusia, nausea, and painful mucositis to secondary cancers,Citation4 cardiac toxicity,Citation5 fertility problems, etc. These side effects that patients experience during and years after radiotherapy reduce the efficacy of treatment and severely affect an individual’s quality of life (QoL), leading to reduced life expectancy ().
Figure 1. Threshold-sigmoid dose response curve. A) High doses of ionizing radiation result in increased probability of tumor control that is often accompanied by an apparent increase in late complications as a result of inevitable irradiation of the surrounding healthy tissue. B) Low normal tissue complication risk in chronomodulated treatment. The therapeutic index is the difference between cure and toxicity. The larger the therapeutic ratio, the more likely the treatment is safe and effective.
Given that cell cycle progression, apoptosis, DNA repair pathway, antioxidant level, and immune system are under circadian control,Citation6–8 it is not surprising that several studies have found strong associations of an individual’s circadian clock with cancer incidence, chemotherapeutic treatment,Citation9,Citation10 and now RT. Notably, of the top 100 best-selling drugs in the United States, 56 target products of genes with rhythmic expression, demonstrating the ability of chronotherapy to positively impact drug tolerability and/or efficacy.Citation11 For example, circadian oscillations in the expression of the aspirin target gene, cyclooxygenase-1, known as Cox1 (Ptgs1), are responsible for rhythms in aspirin’s cardioprotective effects.Citation11 Furthermore, Clinical trials have shown that several adverse effects experienced by patients with cancer receiving cisplatin-based chemotherapy decrease when cisplatin is applied in a chronomodulated context.Citation12,Citation13 A short while ago, the results of the MEMOIR study showed that those receiving immunotherapy infusions more frequently in the morning or early afternoon had longer overall survival (OS) compared with those who received late afternoon or evening infusions.Citation14 These results were reported in 299 adults with stage IV melanoma who had received ≥4 infusions of ipilimumab, nivolumab, or pembrolizumab, either alone or in combination, between 2012 and 2020. Consistently, a retrospective study that examined disease control and treatment-related toxicity in patients undergoing gamma knife radiosurgery (GKRS) for metastatic non-small cell lung cancer (NSCLC) observed better local control (LC) and longer survival in patients who were given morning treatment appointments.Citation15 This literature review has been conducted to summarize the clinical outcomes of cancer chronoradiotherapy. Physiological mechanisms underlying the time-of-day effect and limitations are also highlighted to improve the future chronoradiotherapy study methodologies. Indeed, a sufficient number of new publications have appeared to warrant a new analysis of the evidence on time-of-day effects on radiotherapy outcomes.
Circadian rhythm
The endogenous circadian system operates with a periodicity of 24 h to maintain proper rhythms in sleep–wake cycles, behavior, metabolism, hormone secretion, and cell cycle. Light that is initially collected by the rod, cone, and ganglion cells in the retina passes into the central pacemaker, the suprachiasmatic nucleus in the hypothalamus, for proper coupling of the physiological and behavioral processes in the body with the external environment. Most peripheral tissues and cells also contain self-sustained circadian oscillators that are regularly synchronized with the central pacemaker to provide them with information about external time and coordinates their rhythmic output accordingly.Citation16,Citation17
The Nobel Assembly at Karolinska Institutet has awarded the 2017 Nobel Prize in Physiology or Medicine jointly to Jeffrey C. Hall, Michael Rosbash, and Michael W. Young for their discoveries of molecular mechanisms controlling the circadian rhythm. The cloning and characterization of the period gene in the early 1980s, independently from the Young and Rosbash laboratories, paved the way for a series of further discoveries of additional genes and proteins, culminating in the establishment of the so-called transcriptional translational feedback loop (TTFL) model. In the primary TTFL, CLOCK-BMAL1 heterodimers bind to E-boxes in the promoters of Period (Per1, Per2, Per3) and Cryptochrome (Cry1, Cry2), activating their transcription. After translation, PERs and CRYs dimerize and undergo nuclear translocation to inhibit their own transcription, forming a negative feedback loop. A second loop involves additional pairs of transcription factors that regulate Bmal1 gene through ROR elements: REV-ERBα and RORα.Citation18 REV-ERBα is a transcriptional repressor, whereas RORα is an activator.Citation19 Genetic disruption and physiologic perturbation, jet lag, of circadian homeostasis in experimental animals accelerates tumorigenesis and progression of specific cancers.Citation20,Citation21 Moreover, prolonged nocturnal activity, long-term and frequent shift work, and sleep deprivation (activities known as 24/7 days) can disrupt endogenous circadian timing and create potentially harmful health effects attributable to the suppression of melatonin release.Citation22–26 It has been suggested that the decrease in melatonin production induces an increase in the levels of reproductive hormones, such as estrogens, which would then stimulate the growth and proliferation of hormone-sensitive cells in the breast, prostate, colon, and rectum.Citation27 As a result of both population and laboratory-based findings, circadian disruption has been designated a likely carcinogen, which was stated in a note from the World Health Organization.Citation28
Chronotype is an attribute of humans that describes the time-of-day preferences for performing daily activities. Individuals who wake up early, are more alert in the earlier part of the day, and choose earlier bedtimes are classified as morning chronotype. Conversely, evening types prefer later rising times, perform better in the evening or night, and have later bedtimes.Citation29 The length of the Per3 repeat region is used to differentiate between extreme morning preference and extreme evening preference. The longer allele is associated with morningness, and those with shorter allele tend to be “evening people.”Citation30
Chronoradiotherapy
Clinical attempts of chronotherapy began approximately 40 years ago with chronomodulated infusion of cisplatin to reduce nephrotoxicity in patients with cancer without compromising its anticancer activity.Citation31 This optimally timed therapy has also been shown effective in the adjuvant setting, delays and even prevents local and distant recurrence of locally advanced bladder cancer. Adjuvant chemotherapy regimen was provided to 13 patients with bladder cancer in a circadian-timed plan: doxorubicin (morning)–cisplatin (evening) in full doses for nine courses. Of the 13 patients, 10 did not show recurrence of disease after a median follow-up period of 3.5 years.Citation32 Improved tolerability and/or better antitumor activity have been confirmed for large patient cohorts and different malignancies.Citation10,Citation12,Citation33–36
Success in chronochemotherapy studies stimulates more researchers to focus on the chronomodulated RT area. To date, 20 studies examined the correlation between radiation treatment time and outcomes, including LC, OS, and side effects. Oral mucositis is a common side effect observed in patients with cancer who were treated with radiation fields involving the oral cavity. Radiation-induced oral mucositis starts as an inflammation of the oral mucosa, tongue, and pharynx, possibly due to the recruitment of various inflammatory cells and release of inflammatory cytokines, chemotactic mediators, and growth factors. As the RT continues, mucositis can progress to a life-threatening stage as a result of severe physical obstruction of nutritional intake with subsequent weight loss.Citation37 Severe oral mucositis developed in 29%–66% of patients receiving RT for head and neck cancer.Citation38,Citation39 Among the 24 studies used for the main analysis, five investigated the potential impact of the morning (AM) vs. afternoon (PM) RT on the severity and prevalence of radiation-induced oral mucositis in patients treated for head and neck squamous cell carcinoma (HNSCC).Citation40–44 A single-center retrospective study (n = 240) by Jiang et al.Citation40, did not demonstrate a significant difference in toxicity of AM vs. PM RT. AM and PM were dichotomized by noon. Additionally, a prospectively randomized trial carried out by Bjarnason et al.Citation41 could not show statistically significant results either. Of 205 evaluable patients, 53% and 62% developed grade 3–4 mucositis (RTOG score) after AM and PM RT, respectively (p = .17). However, their subgroup analyses of patients (n = 111) receiving 66–70 Gy showed that morning RT resulted in a significant reduction in grade III/IV mucositis (45% vs. 67%, p = .022) and longer interval to its development (median, >7.9 vs. 5.6 weeks, p = .033). In the same year, another randomized prospective study published by Goyal et al.Citation42 defined the primary endpoint as the occurrence of grade ≥ III (RTOG classification) mucositis during and after conventional RT with parallel opposed fields for non-metastatic stage II–IV HNSCC. Moreover, 212 patients were randomized to the AM (08:00–11:00) and PM (15:00–18:00) groups. Conventional RT was given with curatively intended in fractions of 2.0 Gy once daily (five times per week) and without concomitant or induction chemotherapy. The authors reported a marginally higher incidence of grade III/IV mucositis in the evening-irradiated group (38% vs. 26%, p = .08). Furthermore, the evening-irradiated group showed a rapid progression in the grade of mucositis from the fourth week after treatment (p < .05). Specifically, a meta-analysis on the last two articles revealed that morning treatment significantly reduces the risk of developing grade III and IV oral mucositis by 19% (risk ratio, 0.81; 95% confidence interval, 0.66–0.99;p= .04).Citation45 Fortunately, a recent prospective trial found that, among patients treated with RT before 09:30, 50% developed severe mucositis compared to 72% and 57.1% among those treated before 15:00 and late afternoon, respectively, suggesting that chronotherapy is a simple, cost-free way to limit the severity of oral mucositis.Citation42 Interestingly, new data have emerged suggesting that RT delivered in the DARKER half of the year for HNSCC, in particular, resulted in higher acute toxicity compared with RT in LIGHT (1.98 vs. 1.61; p = .0127).Citation44 Indeed, each year was divided into DARK and LIGHT by the March and September equinoxes.
Gastrointestinal (GI) mucositis is caused by crypt cell death, which is observed in RT for the abdominopelvic region. Clinical signs most often consist of nausea, vomiting, and diarrhea. A total of 229 patients with cervical cancer were allocated into two groups to compare the severity of acute GI mucositis after RT delivered in the morning and afternoon.Citation46 Patients received 50 Gy in 25 fractions of pelvic external beam RT using teletherapy cobalt-60 machine and had not received chemotherapeutic drugs. Toxicity was scored weekly and graded in terms of diarrhea. The overall prevalence of mucositis of any grade was higher in the morning group. Additionally, grade III and IV mucositis was statistically significantly more frequent in morning patients (overall, 87.39% vs. 68.18%, p < .01; higher grade: 14.29% vs. 5.45%, p < .05). Similar results were found by Chang et al.Citation47 who evaluated 67 patients with cervical cancer who received brachytherapy combined with external beam RT. These authors observed that patients undergoing radiation in the morning showed a higher incidence of overall and high-grade (III–IV) diarrhea (75.0% vs. 57.6% and 12.5% vs. 6.1%, respectively). Conversely, RT in the evening was associated with severe hematological toxicity together with higher apoptosis. Acute and late GI toxicity was also assessed in 419 patients who underwent high-dose RT (HDRT) (median, 78 Gy) for non-metastatic prostate adenocarcinoma.Citation48 Patients were dichotomized by 17:00, before 17:00 for daytime arm, and after 17:00 for the evening group. Evening HDRT was significantly associated with a higher incidence of both acute GI toxicities (56% vs. 42% for daytime group p = .01) and acute genitourinary toxicities (52% vs. 32%; p < .001) of any grade. Moreover, evening treatment was significantly correlated with worse freedom from grade ≥ II late GI complications (hazard ratio, 2.96; p < .001), especially in patients aged ≥70 years (6-year rate, evening RT 74% vs. daytime RT 93%, p < .0001). This association was not statistically significant in younger patients (p = .63). Worse toxicity in evening therapy was consistent with the finding of Negoro et al., who did a retrospective study on the effects of time-of-day treatment on the severity of lower urinary tract symptoms (LUTS) in a cohort of patients undergoing proton beam therapy (PBT) for localized prostate cancer. Morning PBT has been reported to significantly ameliorate worsening LUTS and improve patient’s QoL compared with treatment delivered in noon or late afternoon.Citation49
Patients with breast cancer were also evaluated for acute toxicity after whole breast or chest wall RT, delivered morning (before 10:00 am) and late afternoon (after 3:00 pm).Citation50 Grade II or higher acute skin reaction was significantly more frequent in patients treated in the afternoon than in the morning arm (13.7% vs. 5.8%, p = .0088). However, a second study involving 140 breast cancer patients who RT-treated in the morning, afternoon, and evening times showed no statistically significant differences in the incidence of radiation-associated dermatitis or fatigue,Citation51 consistent with others’ findings.Citation52 Indeed, fatigue and treatment time have no relationship, even among patients receiving RT to other anatomic locations.Citation53 Interestingly, a radiogenomic study identified a genetic association between the severity of RT toxicity and treatment time. RT-associated toxicity was assessed in two breast cancer patient cohorts, LeND and REQUITE cohorts, who were treated with adjuvant irradiation. Additionally, patients were genotyped for three clock gene polymorphisms: VNTR polymorphism in PER3, SNP in CLOCK (rs1801260), and SNP in NOC (rs13116075). It has been reported that patients harboring 4/4 PER3 VNTR and/or AA NOCT (Nocturnin) rs13116075 genotypes were more likely to experience worse side effects if RT was administered in the morning.Citation54 It is more likely to reduce the severity of adverse events associated with breast cancer RT by identifying genetic variants of circadian genes and adjusting treatment time accordingly.
While RT timing modified the tolerability of healthy tissues in those trials mentioned above (), no difference was found for efficacy. More recent study showed that the daytime (i.e., AM/ PM) of RT delivery did not yield any prognostic impact on progression-free survival (PFS) or OS in high-grade glioma patients.Citation55 In contrast, patients with HNSCC experienced significant improvements in cause-specific survival when RT was administered in the AM vs. PM (hazard ratio: 1.837, 95% confidence interval: 1.075–3.141; p = .0262).Citation43 Interestingly, a retrospective study (n = 617) evaluating the impact of season of RT application for HNSCC found a higher 5-year locoregional control (DARK vs. LIGHT, 73% vs. 61%; p = .0108) and PFS (DARK vs. LIGHT, 51% vs. 43%; p = .0374) when RT was administered in DARK.Citation56 Earlier, seasonal variations have been explained by circannual variations of certain hormones. An analysis of the Norwegian cancer registry concluded that survival is superior in patients diagnosed with breast, colon, and prostate cancers in summer and autumn compared to winter and spring, which was attributed to the fair amount of vitamin D received from sunlight exposure.Citation57 Another study out of the UK also found generally higher mortality in cancers diagnosed in winter.Citation58 It is also worth mentioning that a retrospective analysis of the relevance of timing for GKRS for brain metastasis of NSCLC revealed that patients irradiated in the morning had better LC at 3 months (97% vs. 67%), lower rate of central nervous system (CNS)-related cause of death, and nearly double median survival time (9.5 months vs. 5 months) compared to those who underwent afternoon GKRS.Citation15 However, three subsequent retrospective studies attempt to validate these results in larger patient populations, treated with the same technique for the same medical indication, failed to observe any significant correlations between treatment time and LC or OS.Citation59–61 Badiyan et al.Citation61 had mixed results in patients with metastatic NSCLC; their univariate analysis, looking only at the impact of time of day, demonstrated a positive effect of morning treatment. One-year LC and median OS were significantly higher for patients treated before 11:41 (74% vs. 54%, p = .016; 10 vs. 8 months, p = .012, respectively). When matched pair analyses were performed accounting for additional factors, Karnofsky performance status and graded prognostic assessment score for analysis of OS, and number of shots of radiation and total tumor volume for analysis of LC, treatment time failed to remain significantly correlated to OS (p = .29) or LC (p = .19). Statistical power in Badiyan’s study is inadequate for identifying significant changes in the multivariate analysis after accounting for imbalances between treatment groups, which could be overcome by large data resources from multi-institutional setting or prospective trials. The inconsistencies in these studies indicate that further clinical and mechanistic investigation is necessary.
Table 1. Timing – it’s the secret ingredient of RT treatment
Further studies of chronotherapy efficacy found significant variance when patients were dichotomized into subgroups by age, sex, and tumor site. In older women with brain metastasis, whole-brain RT in the noon was significantly associated with longer OS (2.12 vs. 1.23 and 1.18 months for morning and late afternoon treatment, respectively; p = .019), while no significant differences between cohorts have been detected in either young women or men.Citation62 A subsequent study by the same group found that female patients had a better response to therapy by retrospectively reviewed data of patients who were treated with RT for bone metastases.Citation63 In contrast, male patients had improved pathological tumor response following long-course neoadjuvant chemoradiotherapy for rectal adenocarcinoma,Citation64 indicating that age and sex differences should be further investigated for a role in circadian regulation of the response to RT. Indeed, this sex specificity has been observed even in the case of chemotherapy. A meta-analysis of three international randomized trials that involved 842 patients with metastatic colorectal cancer revealed that a chronomodulated chemotherapy protocol led to better treatment response and prolonged OS in men but not in women.Citation65 Guo et al.Citation66 detected a greater tumor control probability with evening RT for cervical and esophageal cancer. However, the benefit disappeared at the treatment of either lung cancer or nasopharyngeal carcinoma, indicating that the optimal timing for RT varies between organs. Supporting these findings, approximately 1,400 genes that have been characterized in a previous transcriptome study were phase-shifted with respect to themselves by at least 6 h between two organs, with 131 genes completely antiphase. For example, the transcript levels of vascular endothelial growth factor, Vegfa, peaked in brown fat but reached trough level in the heart.Citation11
Chronobiological mechanisms
The circadian clock system has been shown to regulate several physiological processes, such as sleep–wake cycle, hormone secretion, cell cycle, and inflammatory mediators. Previous studies have indicated that the circadian system regulates cell proliferation through control transcription of the genes regulating cell cycle transition points, such as MYC (G0/G1 transition), cyclin-D1 (G1/S transition), and WEE1 (G2/M transition).Citation6,Citation67 Biopsy specimens were obtained from normal-looking oral mucosa to assess cell cycle regulatory proteins expression and, consequently, determine the timing of cell-cycle phases over 24 h. Quantitative immunohistochemistry revealed that cyclin A and cyclin B1 expressions, whose markers for G2/M phase, peak at 16:00 and 21:00, respectively.Citation68 Accumulated studies on different body tissues (e.g., intestinal epithelium, skin, and bone marrow) have shown that the two main cellular oscillators–circadian clock and cell cycle–are closely connected.Citation69–71 These studies demonstrate that the circadian clock controls the speed of the cell cycle, regulating cell division and growth in synchronization with the day and night cycles. RT effectiveness at the cell level depends to a large degree on the stage of the cell cycle during which irradiation occurs. Bjarnason’s findingsCitation68 indicated that most radiosensitive phase of the cell cycle (G2–M) occurs in late afternoon/evening in human oral mucosa. Thus, the higher incidence of mucositis (grade III/IV) that has been recorded after evening RT for head and neck carcinomaCitation40,Citation41 could be explained by variation in the cell cycle.
Nuclear factor erythroid 2‐related factor 2 (Nrf2) functions as a master regulator of intracellular antioxidant response through orchestrating the transcription of a number of antioxidant response elements‐containing genes encoding antioxidants and phase II detoxification enzymes/proteins.Citation72 Strikingly, induction of Nrf2 decreased total body irradiation-induced myelosuppression and mortality in mice,Citation73 probably related to its established function in mediating cytoprotection in response to reactive oxygen species. Various studies have reported that the level of Nrf2 protein fluctuates in a daily rhythm, which underlies daily transcriptional rhythms in oxidative-responsive genes, including those that are responsible for glutathione biosynthesis that is a predominant guardian against oxidative stress,Citation74–76 suggesting that susceptibility to oxidative challenge is gated by the circadian clock. For example, bleomycin treatment leads to severe fibrotic phenotype when administered at time nadir in Nrf2 levels.Citation76 Similarly, hepatotoxicity of carbon tetrachloride was observed to be greater when administered in the afternoon,Citation77 indicating that the vulnerability to toxicity due to radical inducers shows defined periodicity.Citation78 These data suggest that it may be possible to mitigate RT‐induced injury of normal tissues by scheduling for treatment time corresponding to temporal Nrf2 activity.
Robust DNA repair mechanism faithfully preserves genome stability by either removing or tolerating the damage to ensure better survival rates. Experimental findings indicate that the circadian clock controls the expression of DNA repair proteins and, as a consequence, guarantees a variant response to exogenous and endogenously generated genotoxins. Base excision repair activity changed by twofold over the course of the day that ascribed to the oscillatory pattern of 8-oxoguanine DNA glycosylase (OGG1) protein level.Citation79 In support of this interpretation, xeroderma pigmentosium A exhibited a robust circadian pattern of expression in various mouse tissues, and this oscillation is in phase with the nucleotide excision repair activity,Citation80 suggesting to confine cancer treatment to the moment of the day when the treatment-mediated risks on normal tissue are at its lowest level.
Key parameters of the immune system, including the number of immune cells, cytokines, and hormones, exhibit circadian rhythm in the blood and tissues.Citation8 These immunity-mediators oscillate to synchronize with the rest–activity phase of species.Citation81 Consequently, numerous studies show that mice are highly sensitive and subject to greatly reduced survival when exposed to various pathogens at the beginning of the physical activity phase, that is, early evening.Citation82–84 Therefore, circadian variation of postradiotherapy toxicities, including mucositis, diarrhea, lymphedema, erythema, and fibrosis, could be related to the cyclic change in immune mediators.
Limitation
Studies on timed administration of RT have striking discrepancies that may be due to the following limitations in study design. Of all 24 studies in the review, 20 (~80%) compared only two timepoints, morning versus either afternoon or evening, which might miss circadian effects at other times of the day. Additionally, there is no consensus when defining morning, afternoon, or evening cohorts; some studies described morning cohort by those treated before 10:00, but others extend the morning span to 12:30. Such inconsistency does not indeed allow direct comparisons to be made between studies. Furthermore, most studies did not account for possible inter-individual variability in internal circadian phase, chronotype, that arises from genetic differences and defines preferred sleep, wake, and activity timing. “Eight a.m. on the wall clock is not ‘morning’ for everyone”.Citation85
The timing of cell-cycle events is mostly specific to each body region as inferred from differences in peak height and timing of DNA synthesis among five different regions of the digestive tract in male mice,Citation86 suggesting that the optimal timing for RT may not be identical for different areas of the GI tract. This finding is consistent with the result of a previous study that has not reported a significant difference in response among the timed treatment groups as several sites were analyzed.Citation63 Therefore, chronomodulation delivery of therapeutic radiation beams should be based on experiments that study the circadian rhythms of the regions being treated.
Finally, the studies conducted to date had used variable endpoints; some used OS, while others referred to adverse events as a clinical surrogate endpoint. Yet, non-tumor-related factors, such as sex and age, influence the effectiveness of chronotherapy in the radiation setting. Therefore, prospective trials of matched arms are necessary to address the effects of these covariates. Additionally, further investigation into the underlying mechanisms may be a way of developing optimal treatment plans for all patients.
Conclusion
Timing of RT matters. Twelve trials have shown that patients had fewer complications when exposed to radiation at a particular time, which is thought to be when non-cancerous cells are less vulnerable to injury. To come up with a new approach to an old problem, 2 h duration for the morning, afternoon, and evening cohorts, separated by at least 4 h (i.e., morning 8:00–10:00; afternoon, 14:00–16:00; evening, 20:00–22:00) would be helpful to evaluate whether it is possible to capture significant outcomes derived from the circadian rhythm. It can also be useful to in-depth study the circadian rhythms of the region being treated, such as transcripts of genes involved in cell cycle progression, to guide clinical trials. Our objective is to encourage consistency in the “time range” definitions, and to enable data across studies to be compared. Ample evidence exists that large variation among patients’ clocks affects the statistical power of trials comparing chronotherapy to conventional treatment in patients with varying chronotypes. For this reason, clinicians need to assess and consider the chronotype in the design of chrono-modulated radiation therapy. Chronotype is mainly measured using self-report questionnaires, such as the Morningness–Eveningness Questionnaire and the more recent Munich ChronoType Questionnaire. In fact, smartwatches have gained significant attention as a next-generation tool for identifying chronotype.
Subjects in inpatient care have a disrupted circadian rhythm as they are often deprived of natural environmental fluctuations for extended periods. Besides light, noise levels and frequent awakenings are also sources of circadian disruption. Simple therapeutic approaches, including reestablishing the natural day–night cycle and minimizing sleep interruptions overnight, could be implemented to restore circadian function prior to radiation administration. This critical consideration leads many to rethink hospital layouts from the vantage of circadian biology.
Author contributions
YA and N-A formed the work idea. YA and ZH literature collect and draft the article. N-A and GZ did the critical revision of the article. Eventually, ZH, N-A and GZ gave their final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. 2020. Cancer statistics, 2020. CA Cancer J Clin. 70(1):7–30. doi:10.3322/caac.21590.
- Berkey FJ. Managing the adverse effects of radiation therapy. Am Fam Physician. 2010;82(4):381–394.
- Rubin NH. Influence of the circadian rhythm in cell division on radiation-induced mitotic delay in vivo. Radiat Res. 1982;89(1):65–76. doi:10.2307/3575685.
- Ali YF, Cucinotta FA, Ning-Ang L, Zhou G. 2020. Cancer risk of low dose ionizing radiation. Front Phys. 8:234. doi:10.3389/fphy.2020.00234.
- Gupta D, Shukla P, Munshi A, Aggarwal JP. 2012. Cardioprotective radiotherapy: the circadian way. Med Hypotheses. 78(3):353–355. doi:10.1016/j.mehy.2011.12.009.
- Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. 2006. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 22(3):375–382. doi:10.1016/j.molcel.2006.03.038.
- Fu L, Pelicano H, Liu J, Huang P, Lee C. 2002. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo [published correction appears in Cell 2002 Dec 27;111(7):1055]. Cell. 111(1):41–50. doi:10.1016/s0092-8674(02)00961-3.
- Scheiermann C, Kunisaki Y, Frenette PS. 2013. Circadian control of the immune system. Nat Rev Immunol. 13(3):190–198. doi:10.1038/nri3386.
- Xiong H, Yang Y, Yang K, Zhao D, Tang H, Ran X. 2018. Loss of the clock gene PER2 is associated with cancer development and altered expression of important tumor-related genes in oral cancer. Int J Oncol. 52(1):279–287. doi:10.3892/ijo.2017.4180.
- Ezzat A, Raja MA, Berry J, Zwaan F, Rahal M, El-Warith A. 1997. A phase II trial of circadian-timed paclitaxel and cisplatin therapy in metastatic breast cancer. Ann Oncol. 8(7):663–667. doi:10.1023/a:1008228121390.
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. 2014. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 111(45):16219–16224. doi:10.1073/pnas.1408886111.
- Li J, Chen R, Ji M, Zou SL, Zhu LN. 2015. Cisplatin-based chronotherapy for advanced non-small cell lung cancer patients: a randomized controlled study and its pharmacokinetics analysis. Cancer Chemother Pharmacol. 76(3):651–655. doi:10.1007/s00280-015-2804-x.
- Zhang PX, Jin F, Li ZL, Wu WL, Li YY, Long JH, Chen GY, Chen XX, Gan JY, Gong XY, et al. 2018. A randomized phase II trial of induction chemotherapy followed by cisplatin chronotherapy versus constant rate delivery combined with radiotherapy. Chronobiol Int. 35(2):240–248. doi:10.1080/07420528.2017.1397684.
- Qian DC, Kleber T, Brammer B, Xu KM, Switchenko JM, Janopaul-Naylor JR, Zhong J, Yushak ML, Donald Harvey R, Paulos CM, et al. 2021. Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): a propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol. 22(12):1777–1786. doi:10.1016/S1470-2045(21)00546-5.
- Rahn DA 3rd, Ray DK, Schlesinger DJ, Steiner L, Sheehan JP, O’Quigley JM, Rich T. 2011. Gamma knife radiosurgery for brain metastasis of nonsmall cell lung cancer: is there a difference in outcome between morning and afternoon treatment? Cancer. 117(2):414–420. doi:10.1002/cncr.25423.
- Soták M, Sumová A, Pácha J. 2014. Cross-talk between the circadian clock and the cell cycle in cancer. Ann Med. 46(4):221–232. doi:10.3109/07853890.2014.892296.
- Ikegami K, Refetoff S, Van Cauter E, Yoshimura T. 2019. Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol. 15(10):590–600. doi:10.1038/s41574-019-0237-z.
- Westgate EJ, Cheng Y, Reilly DF, Price TS, Walisser JA, Bradfield CA, FitzGerald GA. 2008. Genetic components of the circadian clock regulate thrombogenesis in vivo. Circulation. 117(16):2087–2095. doi:10.1161/CIRCULATIONAHA.107.739227.
- Kumar S, Chen D, Jang C, Nall A, Zheng X, Sehgal A. 2014. An ecdysone-responsive nuclear receptor regulates circadian rhythms in Drosophila. Nat Commun. 5(1):5697. doi:10.1038/ncomms6697.
- Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. 2010. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One. 5(6):e10995. doi:10.1371/journal.pone.0010995.
- Papagiannakopoulos T, Bauer MR, Davidson SM, Heimann M, Subbara L, Bhutkar A, Bartlebaugh J, Vander Heiden MG, Jacks T. 2016. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 24(2):324–331. doi:10.1016/j.cmet.2016.07.001.
- Knutsson A, Alfredsson L, Karlsson B, Åkerstedt T, Fransson EI, Westerholm P, Westerlund H. 2013. Breast cancer among shift workers: results of the WOLF longitudinal cohort study. Scand J Work Environ Health. 39(2):170–177. doi:10.5271/sjweh.3323.
- Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. 2003. Night-shift work and risk of colorectal cancer in the nurses’ health study. J Natl Cancer Inst. 95(11):825–828. doi:10.1093/jnci/95.11.825.
- Viswanathan AN, Hankinson SE, Schernhammer ES. 2007. Night shift work and the risk of endometrial cancer. Cancer Res. 67(21):10618–10622. doi:10.1158/0008-5472.CAN-07-2485.
- Parent MÉ, El-Zein M, Rousseau MC, Pintos J, Siemiatycki J. 2012. Night work and the risk of cancer among men. Am J Epidemiol. 176(9):751–759. doi:10.1093/aje/kws318.
- Blakeman V, Williams JL, Meng QJ, Streuli CH. 2016. Circadian clocks and breast cancer. Breast Cancer Research. 18(1):1–9. doi:10.1186/s13058-016-0743-z.
- Anjum B, Singh B, Verma R, Singh N, A Mahdi R, K Singh A, De Meester R, Wilczynska F, Takahashi A, Dharwadkar T, et al. 2012. Associations of circadian disruption of sleep and nutritional factors with risk of cancer. Open Nutraceuticals J. 5(1):124–135. doi:10.2174/1876396001205010124.
- Shafi AA, Knudsen KE. 2019. Cancer and the circadian clock. Cancer Res. 79(15):3806–3814. doi:10.1158/0008-5472.CAN-19-0566.
- Roden LC, Rudner T, Rae D. 2017. Impact of chronotype on athletic performance: current perspectives. Chronophysiol Ther. 7:1–6. doi:10.2147/CPT.S99804.
- Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt J, von Schantz M. 2003. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 26(4):413–415. doi:10.1093/sleep/26.4.413.
- Roemeling RV, Olshefski R, Langevin T, Berestka J, Reusch JJ, Reusch JE, Lakatua D, Wick MR, Hrushesky WJ. 1986. Cisplatin chronotherapy and disulfiram rescue reduce toxicity without interfering with anticancer activity: animal findings and preliminary clinical experiences. Chronobiol Int. 3(1):55–64. doi:10.3109/07420528609083160.
- Hrushesky WJ, Roemeling RV, Wood PA, Langevin TR, Lange P, Fraley E. High-dose intensity, circadian-timed doxorubicin and cisplatin adjuvant chemotherapy for bladder cancer. Cancer Treat Rep. 1987;71(10):915–919. PMID: 3652055.
- Lévi FA, Zidani R, Vannetzel JM, Perpoint B, Focan C, Faggiuolo R, Chollet P, Garufi C, Itzhaki M, Dogliotti L, et al. 1994. Chronomodulated versus fixed-infusion-rate delivery of ambulatory chemotherapy with oxaliplatin, fluorouracil, and folinic acid (leucovorin) in patients with colorectal cancer metastases: a randomized multi-institutional trial. J Natl Cancer Inst. 86(21):1608–1617. doi:10.1093/jnci/86.21.1608.
- Focan C, Denis B, Kreutz F, Focan-Henrard D, Levi F. Ambulatory chronotherapy with 5-fluorouracil, folinic acid, and carboplatin for advanced non-small cell lung cancer. A phase II feasibility trial. J Infus Chemother. 1995;5(3 Suppl 1):148–152. PMID: 8528976.
- Miglietta L, Bruzzone M, Ghione G, Pepe A, Marenghi C, Ragni N, Boccardo F. Chemotherapy with cisplatin and 5-fluorouracil chronomodulated infusion in locally advanced or metastatic/recurrent carcinoma of the cervix. Tumori. 2002;88(3):204–208. PMID: 12195758. doi:10.1177/030089160208800305.
- Innominato PF, Ballesta A, Huang Q, Focan C, Chollet P, Karaboué A, Giacchetti S, Bouchahda M, Adam R, Garufi C, et al. 2020. Sex-dependent least toxic timing of irinotecan combined with chronomodulated chemotherapy for metastatic colorectal cancer: randomized multicenter EORTC 05011 trial. Cancer Med. 9(12):4148–4159. doi:10.1002/cam4.3056.
- Maria OM, Eliopoulos N, Muanza T. 2017. Radiation-induced oral mucositis. Front Oncol. 7:89. doi:10.3389/fonc.2017.00089.
- Vera-Llonch M, Oster G, Hagiwara M, Sonis S. 2006. Oral mucositis in patients undergoing radiation treatment for head and neck carcinoma. Cancer. 106(2):329–336. doi:10.1002/cncr.21622.
- Lalla RV, Sonis ST, Peterson DE. 2008. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 52(1):61–viii. doi:10.1016/j.cden.2007.10.002.
- Jiang D, Mierzwa ML, Huth B, Xie C, Barrett WL, Redmond KP, Forster N. 2015. Does time of daily treatment matter in radiation therapy for head and neck cancer? Int J Radiat Oncol Biol Phys. 93(3):E348. doi:10.1016/j.ijrobp.2015.07.1434.
- Bjarnason GA, Mackenzie RG, Nabid A, Hodson ID, El-Sayed S, Grimard L, Brundage M, Wright J, Hay J, Ganguly P, et al. 2009. National cancer institute of Canada clinical trials group (HN3). Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: a prospective randomized trial of the national cancer institute of Canada clinical trials group (HN3). Int J Radiat Oncol Biol Phys. 73(1):166–172. doi:10.1016/j.ijrobp.2008.07.009.
- Goyal M, Shukla P, Gupta D, Bisht SS, Dhawan A, Gupta S, Pant MC, Verma NS. 2009. Oral mucositis in morning vs. evening irradiated patients: a randomised prospective study. Int J Radiat Biol. 85(6):504–509. doi:10.1080/09553000902883802.
- Gu F, Farrugia MK, Duncan WD, Feng Y, Hutson AD, Schlecht NF, Repasky EA, Antoch MP, Miller A, Platek A, et al. 2020. Daily time of radiation treatment is associated with subsequent oral mucositis severity during radiotherapy in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 29(5):949–955. doi:10.1158/1055-9965.EPI-19-0961.
- Brolese EK, Cihoric N, Bojaxhiu B, Sermaxhaj B, Schanne DH, Mathier E, Lippmann J, Shelan M, Eller Y, Aebersold DM, et al. 2021. The impact of delivery daytime and seasonality of radiotherapy for head and neck cancer on toxicity burden. Radiother Oncol. 158:162–166. doi:10.1016/j.radonc.2021.02.039.
- Chan S, Rowbottom L, McDonald R, Bjarnason GA, Tsao M, Danjoux C, Barnes E, Popovic M, Lam H, DeAngelis C, et al. 2017. Does the time of radiotherapy affect treatment Outcomes? A review of the literature. Clin Oncol (R Coll Radiol). 29(4):231–238. doi:10.1016/j.clon.2016.12.005.
- Shukla P, Gupta D, Bisht SS, Pant MC, Bhatt ML, Gupta R, Srivastava K, Gupta S, Dhawan A, Mishra D, et al. 2010. Circadian variation in radiation-induced intestinal mucositis in patients with cervical carcinoma. Cancer. 116(8):2031–2035. doi:10.1002/cncr.24867.
- Chang L, Li L, Li W, Jiang M, Jv Y, Wang L, Hou Y, Long Q, Yu S. 2016. Research on radiotherapy at different times of the day for inoperable cervical cancer. Int J Clin Pharmacol Ther. 54(11):856–864. doi:10.5414/CP202654.
- Hsu FM, Hou WH, Huang CY, Wang CC, Tsai CL, Tsai YC, Yu HJ, Pu YS, Cheng JC. 2016. Differences in toxicity and outcome associated with circadian variations between patients undergoing daytime and evening radiotherapy for prostate adenocarcinoma. Chronobiol Int. 33(2):210–219. doi:10.3109/07420528.2015.1130049.
- Negoro H, Iizumi T, Mori Y, Matsumoto Y, Chihara I, Hoshi A, Sakurai H, Nishiyama H, Ishikawa H. Chronoradiation therapy for prostate cancer: morning proton beam therapy ameliorates worsening lower urinary tract symptoms. J Clin Med. 2020;9(7):2263. Published 2020 Jul 16. doi:10.3390/jcm9072263.
- Noh JM, Choi DH, Park H, Huh SJ, Park W, Seol SW, Jeong BK, Nam SJ, Lee JE, Kil WH. 2014. Comparison of acute skin reaction following morning versus late afternoon radiotherapy in patients with breast cancer who have undergone curative surgical resection. J Radiat Res. 55(3):553–558. doi:10.1093/jrr/rrt141.
- Ishaq O, Valdimarsdottir H, Cooper BT, Modrek A, Redd W, Formenti SC. 2015. Effect of treatment time of day on radiation fatigue and toxicity in early-stage breast cancer patients after breast conserving surgery. Int J Radiat Oncol Biol Phys. 93(3):E54. doi:10.1016/j.ijrobp.2015.07.679.
- LaRiviere MJ, Chao HH, Doucette A, Kegelman TP, Taunk NK, Freedman GM, Vapiwala N. 2020. Factors associated with fatigue in patients with breast cancer undergoing external beam radiation therapy. Pract Radiat Oncol. 10(6):409–422. doi:10.1016/j.prro.2020.05.011.
- Hasan S, Renz P, Packard M, Horrigan S, Gresswell S, Kirichenko AV. 2019. Effect of daily and every other day stereotactic body radiation therapy schedules on treatment-related fatigue in patients with hepatocellular Carcinoma. Pract Radiat Oncol. 9(1):e38–e45. doi:10.1016/j.prro.2018.06.005.
- Johnson K, Chang-Claude J, Critchley AM, Kyriacou C, Lavers S, Rattay T, Seibold P, Webb A, West C, Symonds RP, et al. 2019. REQUITE consortium. genetic variants predict optimal timing of radiotherapy to reduce side-effects in breast cancer patients. Clin Oncol (R Coll Radiol). 31(1):9–16. doi:10.1016/j.clon.2018.10.001.
- Sapienza LG, Nasra K, Berry R, Danesh L, Little T, Abu-Isa E. 2021. Clinical effects of morning and afternoon radiotherapy on high-grade gliomas. Chronobiol Int. 38(5):732–741. doi:10.1080/07420528.2021.1880426.
- Elicin O, Koller Brolese E, Bojaxhiu B, Sermaxhaj B, Schanne DH, Mathier E, Lippmann J, Shelan M, Eller Y, Aebersold DM, et al. 2021. The prognostic impact of daytime and seasonality of radiotherapy on head and neck cancer. Radiother Oncol. 158:293–299. doi:10.1016/j.radonc.2021.04.004.
- Robsahm TE, Tretli S, Dahlback A, Moan J. 2004. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway). Cancer Causes Control. 15(2):149–158. doi:10.1023/B:CACO.0000019494.34403.09.
- Roychoudhuri R, Robinson D, Coupland V, Holmberg L, Møller H. 2009. Season of cancer diagnosis exerts distinct effects upon short- and long-term survival. Int J Cancer. 124(10):2436–2441. doi:10.1002/ijc.24213.
- Kabolizadeh P, Wegner R, Bernard M, Heron D, Mintz A, Burton S. 2011. The effect of treatment time on outcome in non-small cell lung cancer brain metastases treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 81(2):S301. doi:10.1016/j.ijrobp.2011.06.1784.
- Badiyan SN, Ferraro DJ, Yaddanapudi S, Drzymala RE, Lee AY, Simpson JR, DeWees T, Rich KM, Robinson CG. 2012. Ep-1293 impact of time of day of gamma knife radiosurgery on survival for non-small cell lung cancer brain metastases. Radiotherapy and Oncology. 103(103):S491–2. doi:10.1016/S0167-8140(12)71626-2.
- Badiyan SN, Ferraro DJ, Yaddanapudi S, Drzymala RE, Lee AY, Silver SA, Dyk P, DeWees T, Simpson JR, Rich KM, et al. 2013. Impact of time of day on outcomes after stereotactic radiosurgery for non-small cell lung cancer brain metastases. Cancer. 119(19):3563–3569. doi:10.1002/cncr.28237.
- Chan S, Rowbottom L, McDonald R, Zhang L, Bjarnason GA, Tsao M, Danjoux C, Barnes E, Lam H, Popovic M, et al. 2016. Could time of whole brain radiotherapy delivery impact overall survival in patients with multiple brain metastases? Ann Palliat Med. 5(4):267–279. doi:10.21037/apm.2016.09.05.
- Chan S, Zhang L, Rowbottom L, McDonald R, Bjarnason GA, Tsao M, Barnes E, Danjoux C, Popovic M, Lam H, et al. 2017. Effects of circadian rhythms and treatment times on the response of radiotherapy for painful bone metastases. Ann Palliat Med. 6(1):14–25. doi:10.21037/apm.2016.09.07.
- Squire T, Buchanan G, Rangiah D, Davis I, Yip D, Chua YJ, Rich T, Elsaleh H. 2017. Does chronomodulated radiotherapy improve pathological response in locally advanced rectal cancer? Chronobiol Int. 34(4):492–503. doi:10.1080/07420528.2017.1301462.
- Giacchetti S, Dugué PA, Innominato PF, Bjarnason GA, Focan C, Garufi C, Tumolo S, Coudert B, Iacobelli S, Smaaland R, et al. 2012. Sex moderates circadian chemotherapy effects on survival of patients with metastatic colorectal cancer: a meta-analysis. Ann Oncol. 23(12):3110–3116. doi:10.1093/annonc/mds148.
- Guo P, Wang H, Jiang R, Wang Z. The clinical effect study on malignant tumors with chronoradiotherapy. Biol Rhythm Res. 2015;46(2):249–255. doi:10.1080/09291016.2014.985001.
- Griniatsos J, Michail OP, Theocharis S, Arvelakis A, Papaconstantinou I, Felekouras E, Pikoulis E, Karavokyros I, Bakoyiannis C, Marinos G, et al. 2006. Circadian variation in expression of G1 phase cyclins D1 and E and cyclin-dependent kinase inhibitors p16 and p21 in human bowel mucosa. World J Gastroenterol. 12(13):2109–2114. doi:10.3748/wjg.v12.i13.2109.
- Bjarnason GA, Jordan RC, Sothern RB. 1999. Circadian variation in the expression of cell-cycle proteins in human oral epithelium. Am J Pathol. 154(2):613–622. doi:10.1016/S0002-9440(10)65306-0.
- Buchi KN, Moore JG, Hrushesky WJ, Sothern RB, Rubin NH. 1991. Circadian rhythm of cellular proliferation in the human rectal mucosa. Gastroenterology. 101(2):410–415. doi:10.1016/0016-5085(91)90019-h.
- Brown WR. 1991. A review and mathematical analysis of circadian rhythms in cell proliferation in mouse, rat, and human epidermis. J Invest Dermatol. 97(2):273–280. doi:10.1111/1523-1747.ep12480379.
- Sothern RB, Smaaland R, Moore JG. 1995. Circannual rhythm in DNA synthesis (S-phase) in healthy human bone marrow and rectal mucosa. FASEB J. 9(5):397–403. doi:10.1096/fasebj.9.5.7896010.
- Wang X, Tomso DJ, Chorley BN, Cho HY, Cheung VG, Kleeberger SR, Bell DA. 2007. Identification of polymorphic antioxidant response elements in the human genome [published correction appears in Hum Mol Genet. 2007 Nov 15;16(22):2780]. Hum Mol Genet. 16(10):1188–1200. doi:10.1093/hmg/ddm066.
- Kim JH, Thimmulappa RK, Kumar V, Cui W, Kumar S, Kombairaju P, Zhang H, Margolick J, Matsui W, Macvittie T, et al. 2014. NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J Clin Invest. 124(2):730–741. doi:10.1172/JCI70812.
- Wible RS, Ramanathan C, Sutter CH, Olesen KM, Kensler TW, Liu AC, Sutter TR. 2018. NRF2 regulates core and stabilizing circadian clock loops, coupling redox and timekeeping in Mus musculus. Elife. 7:e31656. doi:10.7554/eLife.31656.
- Xu YQ, Zhang D, Jin T, Cai DJ, Wu Q, Lu Y, Liu J, Klaassen CD. 2012. Diurnal variation of hepatic antioxidant gene expression in mice. PLoS One. 7(8):e44237. doi:10.1371/journal.pone.0044237.
- Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, et al. 2014. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 28(6):548–560. doi:10.1101/gad.237081.113.
- Skrzypińska-Gawrysiak M, Piotrowski JK, Sporny S. Circadian variations in hepatotoxicity of carbon tetrachloride in mice. Int J Occup Med Environ Health. 2000;13(2):165–173.
- Bruckner JV, Ramanathan R, Lee KM, Muralidhara S. 2002. Mechanisms of circadian rhythmicity of carbon tetrachloride hepatotoxicity. J Pharmacol Exp Ther. 300(1):273–281. doi:10.1124/jpet.300.1.273.
- Manzella N, Bracci M, Strafella E, Staffolani S, Ciarapica V, Copertaro A, Rapisarda V, Ledda C, Amati M, Valentino M, et al. Circadian modulation of 8-Oxoguanine DNA Damage Repair. Sci Rep. 2015;5(1):13752. Published 2015 Sep 4. doi:10.1038/srep13752.
- Kang TH, Lindsey-Boltz LA, Reardon JT, Sancar A. 2010. Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc Natl Acad Sci U S A. 107(11):4890–4895. doi:10.1073/pnas.0915085107.
- Zhao Y, and Chen Q. Diurnal variations in immunity. In eLS, John Wiley & Sons, Ltd (Ed.). https://doi.org/10.1002/9780470015902.a0028361.
- Feigin RD, Middelkamp JN, Reed C. 1972. Circadian rhythmicity in susceptibility of mice to sublethal Coxsackie B3 infection. Nat New Biol. 240(97):57–58. doi:10.1038/newbio240057a0.
- Feigin RD, San Joaquin VH, Haymond MW, Wyatt RG. 1969. Daily periodicity of susceptibility of mice to pneumococcal infection. Nature. 224(5217):379–380. doi:10.1038/224379a0.
- Shackelford PG, Feigin RD. 1973. Periodicity of susceptibility to pneumococcal infection: influence of light and adrenocortical secretions. Science. 182(4109):285–287. doi:10.1126/science.182.4109.285.
- Ruben MD, Smith DF, FitzGerald GA, Hogenesch JB. 2019. Dosing time matters. Science. 365(6453):547–549. doi:10.1126/science.aax7621.
- Scheving LE, Burns ER, Pauly JE, Tsai TH. 1978. Circadian variation in cell division of the mouse alimentary tract, bone marrow and corneal epithelium. Anat Rec. 191(4):479–486. doi:10.1002/ar.1091910407.

