Abstract
Lung hyperinflation commonly accompanies expiratory flow-limitation in patients with Chronic Obstructive Pulmonary Disease (COPD) and contributes importantly to dyspnea and activity limitation. It is not surprising, therefore, that lung hyperinflation has become an important therapeutic target in symptomatic COPD patients. There is increasing evidence that acute dynamic increases in lung hyperinflation, under conditions of worsening expiratory flow-limitation and increased ventilatory demand (or both) can seriously stress cardiopulmonary reserves, particularly in patients with more advanced disease. Our understanding of the physiological mechanisms of dynamic lung hyperinflation during both physical activity and exacerbations in COPD continues to grow, together with an appreciation of its serious negative mechanical and sensory consequences. In this review, we will discuss the basic pathophysiology of COPD during rest, exercise and exacerbation so as to better understand how this can be pharmacologically manipulated for the patient's benefit. Finally, we will review current concepts of the mechanisms of symptom relief and improved exercise endurance following pharmacological lung volume reduction.
DETECTION, MEASUREMENT AND DEFINITIONS OF LUNG HYPERINFLATION
Lung hyperinflation is present when gas volume in the lungs, or in a region of the lung, is increased compared with the predicted value. Thoracic hyperinflation in patients with Chronic Obstructive Pulmonary Disease (COPD) can be detected by physical examination, particularly if it is severe. Recognized clinical features of severe hyperinflation include the inward motion of the lower lateral rib cage during inspiration, as originally described by Stokes (Citation[1]), and paradoxical inward motion of the anterior abdominal wall in synchrony with inspiratory flow (Citation[2], Citation[3]). However, hyperinflation in its earlier stages is often underestimated, even after assiduous clinical assessment. Lung hyperinflation can be detected by a variety of radiographic techniques (Citation[4], Citation[5], Citation[6], Citation[7], Citation[8]). The methodology for radiographic lung volume measurement (taken at total lung capacity) is not standardized and is rarely used in clinical practice for quantitative purposes. Future refinements in high-resolution computed tomography (HRCT) scanning promise to facilitate identification of regional distribution patterns of air space distention and lung volume quantification in COPD patients.
Body plethysmography remains the gold standard for the measurement of end-expiratory lung volume (EELV) and has been shown to be reliable. The term EELV is used interchangeably with the conventional term function residual capacity (FRC). Inert gas dilution techniques are also used extensively to measure hyperinflation, but may underestimate absolute lung volumes because of the effect of non-communicating airways. Conventionally, lung hyperinflation is said to exist when the total lung capacity (TLC) is greater than 120% of the predicted value and/or when the other volume compartments [i.e., EELV and residual volume (RV)] are above the upper limits of natural variability. In practice, values for these volume compartments exceeding 120% to 130% of the normal predicted value are deemed to be potentially clinically important, but these “cut-offs” remain arbitrary. No standardized stratification system currently exists for assessment of severity of hyperinflation. In the absence of any consensus on the definition or severity of lung hyperinflation, our practice when using the term is to specify the volume compartment referred to (i.e., TLC, EELV, RV), the method used in measurement, and to express the value as a percent of predicted normal (Citation[9], Citation[10]).
The natural history of the development of lung hyperinflation in COPD patients is unknown but clinical experience tells us that this is an insidious process that occurs over decades. It would appear that, in general, closing volume and RV are the first volume components to increase, reflecting increased airway closure. EELV increases thereafter, reflecting the effects of worsening expiratory flow-limitation and alteration in static lung mechanics. Eventually, TLC increases as lung compliance increases. However, longitudinal studies in large COPD populations are required to definitively chart the time course of change in the various volume compartments. There is preliminary evidence that significant increases in EELV may be present in patients with early stages of COPD, where severity is defined by stratification systems based on the measurement of forced expired volume in one second (FEV1) (Citation[11]). The existence of lung hyperinflation in patients with so-called early or mild disease, may have important clinical implications with respect to impairment and disability and this requires further study.
SPIROMETRIC ESTIMATIONS OF LUNG HYPERINFLATION
Indirect, spirometrically derived assessments of lung hyperinflation [i.e., vital capacity (VC) and inspiratory capacity (IC), which reflect RV and EELV, respectively] are more difficult to interpret, particularly in the absence of simultaneous plethysmographic EELV measurements. Slow or timed vital capacity (SVC) is less prone to measurement artifact in COPD than the forced vital capacity (FVC) and, therefore, provides a better estimation of the efficacy of lung emptying (Citation[12]). The IC is the maximal volume of air that can be inhaled after a spontaneous expiration to EELV. A reduced resting IC can indicate the presence of lung hyperinflation in the setting of expiratory flow-limitation (Citation[13]). The resting IC represents the operating limits for tidal volume expansion during the increased ventilation of exercise and can predict the peak symptom-limited oxygen uptake (V′O2) in patients with resting expiratory flow-limitation (Citation[14], Citation[15]). Moreover, a severely reduced IC/TLC ratio (i.e., < 25%) has recently been shown to be an independent predictor of poor survival in COPD (Citation[16]). The smaller the IC in flow-limited patients, the closer the operating tidal volume is to TLC and the upper reaches of the respiratory system's flattened pressure-volume relationship. In this respect, the IC is a crude surrogate for the extent of elastic loading of the respiratory muscles. However, the IC remains remarkably preserved in some patients with severe lung hyperinflation, since TLC and EELV can rise in parallel (Citation[17], Citation[18], Citation[19], Citation[20]). Clearly, in this circumstance, and in the setting of concomitant mechanical restriction or significant inspiratory muscle weakness, the IC alone is not a reliable marker of lung hyperinflation.
PATHOPHYSIOLOGY OF COPD: MECHANISMS OF LUNG HYPERINFLATION
Expiratory flow-limitation is the pathophysiological hallmark of COPD and arises because of the dual effects of permanent parenchymal destruction (emphysema) and airway dysfunction. The latter reflects the effects of small airway inflammation (mucosal edema, airway remodeling/fibrosis and mucous impaction) and possibly increased cholinergic airway smooth muscle tone. Emphysema results in reduced lung elastic recoil pressure which leads to a reduced driving pressure for expiratory flow through narrowed and poorly supported airways in which airflow resistance is significantly increased. Expiratory flow-limitation is said to be present when the expiratory flows generated during spontaneous tidal breathing represent the maximal possible flow rates that can be generated at that operating lung volume (Citation[21]). Under conditions of expiratory flow-limitation, expiratory flow rates are independent of expiratory muscle effort and are determined by the static lung recoil pressure and the resistance of the airways upstream from the flow-limiting segment (Citation[21], Citation[22]).
In health, the relaxation volume (Vr) of the respiratory system is dictated by the balance of forces between the inward elastic recoil pressure of the lung and the outward recoil pressure of chest wall. With advancing age, changes in the connective tissue matrix of the lung result in a reduction of the lung elastic recoil pressure, and the equilibrium point (where the net elastic recoil of the total respiratory system is zero) therefore occurs at a higher lung volume than in youth (Citation[23]). In COPD, the increased compliance of the lung, as a result of destructive emphysema, leads to a resetting of the respiratory system's relaxation volume to a higher level than in age-matched healthy individuals (Citation[24]) []. This has been termed “static” lung hyperinflation.
Figure 1 Pressure–volume (P–V) relationships of the total respiratory system in health and in COPD. Tidal pressure–volume curves during rest (filled area) and exercise (open area) are shown. In COPD, because of resting and dynamic hyperinflation (a further increased EELV), exercise tidal volume (VT) encroaches on the upper, alinear extreme of the respiratory system's P-V curve where there is increased elastic loading. In COPD, the ability to further expand VT is reduced, i.e., inspiratory reserve volume (IRV) is diminished. In contrast to health, the combined recoil pressure of the lungs and chest wall in hyperinflated patients with COPD is inwardly directed during both rest and exercise; this results in an inspiratory threshold load on the inspiratory muscles. Abbreviations:EELV = end-expiratory lung volume EILV = end-inspiratory lung volume; RV = residual volume; TLC = total lung capacity.
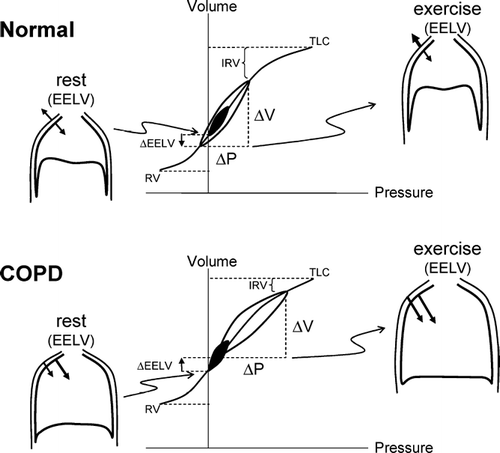
While in health the EELV during relaxed resting breathing corresponds with the actual equilibrium position of the respiratory system, this is often not the case in COPD (Citation[24]). During spontaneous resting breathing in patients with expiratory flow-limitation, EELV is also “dynamically” determined and is maintained at a level above the statically-determined relaxation volume of the respiratory system. In flow-limited patients, the mechanical time-constant for lung emptying (i.e., the product of compliance and resistance, “τ”) is increased in many alveolar units, but the expiratory time available (as dictated by the respiratory control centers) is often insufficient to allow EELV to decline to its normal relaxation volume, and gas accumulation and retention (often termed “air trapping”) results. In other words, lung emptying during expiration becomes incomplete because it is interrupted by the next inspiration and EELV therefore exceeds the natural relaxation volume of the respiratory system. EELV in COPD is a continuous dynamic variable that varies with the extent of expiratory flow-limitation and the degree of time-constant abnormalities, as well as the breathing pattern, as expressed by the following equation (25):where Vr = relaxation volume, TE = expiratory time, τrs = time constant for emptying of the respiratory system, VT = tidal volume, and base e = 2.718282.
In patients with expiratory flow-limitation and acute diffuse bronchoconstriction, severe lung hyperinflation is best explained by dynamic rather than static mechanisms (Citation[12], Citation[26], Citation[27], Citation[28], Citation[29]). The role of other potential contributors to lung hyperinflation such as re-setting of the passive elastic properties of the chest wall or active inspiratory muscle and laryngeal braking during expiration remains uncertain in COPD (Citation[30], Citation[31], Citation[32]).
DYNAMIC HYPERINFLATION DURING EXERCISE
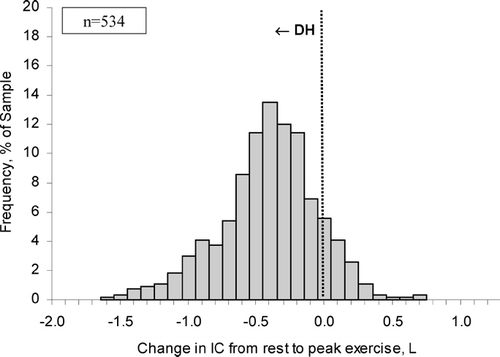
Acute “dynamic” lung hyperinflation (DH) is defined as a temporary and variable increase in EELV above its baseline value. Under any condition of increased ventilation in flow-limited patients with COPD (i.e., exercise, voluntary hyperventilation, anxiety, transient hypoxemia), inspiratory tidal volume increases and expiratory time diminishes further as breathing frequency increases above the baseline value, causing further acute-on-chronic DH (Citation[14], Citation[33], Citation[34], Citation[35], Citation[36]). It has been established for some time that DH occurs in flow-limited patients under conditions of increased ventilatory demand during exercise (Citation[37], Citation[38], Citation[39], Citation[40]) []. The rate and magnitude of DH during exercise is generally measured in the laboratory setting by serial IC measurements (Citation[17], Citation[18], Citation[19], Citation[20]). Changes in EELV during exercise can also be tracked by newer methods such as optoelectronic plethysmography or respiratory inductance plethysmography but their use is currently confined to the research setting (Citation[41], Citation[42]). Since TLC does not change during activity (Citation[39], Citation[43]), the change (reduction) in IC reflects the inverse change (increase) in dynamic EELV, or the extent of DH. This simple method has been shown to be reliable and recent multi-centre clinical trials have confirmed its reproducibility and responsiveness (Citation[17], Citation[18]). In several studies conducted in over 500 patients with moderate-to-severe COPD, the change in EELV during cycle ergometry averaged 0.4 L, representing a reduction in IC by ∼ 20 % of the resting value, but with wide variation in the range (Citation[14], Citation[19], Citation[20]) []. Over 80% of this population sample showed increases in EELV from rest to peak exercise, confirming the presence of significant DH (Citation[14], Citation[19], Citation[20]). The minority of patients who showed little reduction in IC with exercise demonstrated the most severe resting lung hyperinflation (Citation[14]). The rate of rise of DH was steeper in patients with the most severe expiratory flow-limitation (as estimated by the FEV1/FVC ratio), the lowest diffusing capacity for carbon monoxide and the highest ventilatory demand (reflecting greater ventilation-perfusion abnormalities), and generally reached a maximal value early in exercise (Citation[14]).
Figure 2 Changes in operating lung volumes are shown as ventilation increases with exercise in COPD (n = 105) and in age-matched normal subjects (n = 25). “Restrictive” constraints on tidal volume (VT, solid area) expansion during exercise are significantly greater in the COPD group from both below (reduced inspiratory capacity [IC]) and above (minimal inspiratory reserve volume [IRV], open area). Other abbreviations:EELV = end-expiratory lung volume; EILV = end-inspiratory lung volume; RV = residual volume; TLC = total lung capacity; VC = vital capacity. From reference 105, with permission.
![Figure 2 Changes in operating lung volumes are shown as ventilation increases with exercise in COPD (n = 105) and in age-matched normal subjects (n = 25). “Restrictive” constraints on tidal volume (VT, solid area) expansion during exercise are significantly greater in the COPD group from both below (reduced inspiratory capacity [IC]) and above (minimal inspiratory reserve volume [IRV], open area). Other abbreviations:EELV = end-expiratory lung volume; EILV = end-inspiratory lung volume; RV = residual volume; TLC = total lung capacity; VC = vital capacity. From reference 105, with permission.](/cms/asset/6a201c70-479e-490b-94cd-b9420d9efd07/icop_a_197648_uf0002_b.gif)
Figure 3 The distribution of the extent of change in inspiratory capacity (IC) during exercise is shown in moderate-to-severe COPD (n = 534). A reduction (negative change) in IC reflects dynamic hyperinflation (DH) during exercise. Each bar width corresponds to a change in IC range of 0.10 L. The majority of patients with COPD experienced significant DH during exercise. Graphs represent cumulative data from references 14, 19 and 20.
CONSEQUENCES OF ACUTE DYNAMIC HYPERINFLATION
The insidious development of flow-limitation and hyperinflation over many years allows for several adaptive mechanisms to come into play to preserve the functional strength of the overburdened inspiratory muscles, particularly the diaphragm (Citation[44]). A number of studies have shown several structural adaptations to chronic intrinsic mechanical loading, which include: (1) reduction in sarcomere length, which improves the ability of the muscle to generate force at higher lung volumes (Citation[45]); (2) an increase in the relative proportion of Type I fibres, which are slow-twitch and fatigue resistant (Citation[46], Citation[47]); and (3) an increase in mitochondrial concentration and efficiency of the electron transport chain, which improves oxidative capacity (Citation[45]). It is believed that the function of intercostal and sternomastoid muscles is less disadvantaged than that of the diaphragm in the presence of severe lung hyperinflation (Citation[26], Citation[48]). However, despite this temporal adaptation, the presence of severe hyperinflation means that the ability to increase ventilation, when this demand arises, is greatly limited in COPD.
The negative effects of acute DH during exercise are now well established (Citation[49]):
DH results in sudden increases in the elastic and threshold loads on the inspiratory muscles as tidal volume is forced to operate at the upper, alinear extreme of the respiratory system's pressure-volume relation, thus increasing the work and oxygen cost of breathing. In moderate-to-severe COPD, the inspiratory threshold load (ITL) at the peak of exercise, which reflects the force that the inspiratory muscles must generate to initiate inspiratory flow, can be considerable. Thus, the ITL reflects the force that the inspiratory muscles must generate to counterbalance the inward (expiratory) recoil of the lung and chest wall at end-expiration (Citation[50]).
DH results in functional inspiratory muscle weakness by maximally shortening the muscle fibers in the diaphragm (Citation[51]). The combination of excessive mechanical loading and increased velocity of shortening of the inspiratory muscles can predispose them to fatigue.
DH reduces the ability of tidal volume to expand appropriately during exercise and this leads to early mechanical limitation of ventilation (Citation[52]).
In some patients, this mechanical constraint on tidal volume expansion, in the setting of severe ventilation-perfusion abnormalities (i.e., high fixed physiological dead space), leads to carbon dioxide retention and arterial oxygen desaturation during exercise (Citation[52]).
Finally, DH adversely affects dynamic cardiac function by contributing to pulmonary hypertension, by reducing right ventricular pre-load (reduced venous return) and, in some cases, by increasing left ventricular afterload (Citation[53], Citation[54], Citation[55]). All of the above factors are clearly interdependent and contribute in a complex, integrated manner to dyspnea and exercise limitation in COPD.
DYNAMIC HYPERINFLATION AND EXERTIONAL DYSPNEA
A recent mechanistic study in our laboratory has determined that DH early in exercise allows flow-limited patients to increase ventilation while minimizing respiratory discomfort (Citation[56]). Thus, as a result of this early DH, the airways are maximally stretched at the higher lung volumes (close to TLC) and expiratory flow-limitation is attenuated allowing patients to maximize expiratory flow rates. Thus, patients with severe COPD could abruptly increase ventilation commensurate with increased metabolic demand, to approximately 40 L/min and generate tidal inspiratory pressures exceeding 40% of the maximal possible pressure generation while experiencing minimal increases in dyspnea (modified Borg dyspnea ratings 1–2). However, this advantage of DH was quickly negated when tidal volume expanded to reach a critically low inspiratory reserve volume (IRV) of approximately 0.5 L (or 10% predicted TLC) below TLC []. At this “threshold,” tidal volume becomes fixed on the upper less compliant extreme of the respiratory system's sigmoid-shaped pressure-volume relation, where there is increased elastic loading of the inspiratory muscles. At this operating volume, the diaphragm muscle fibers are maximally shortened and the increased breathing frequency leads to increased velocity of shortening and significant reductions in dynamic lung compliance. After reaching this minimal IRV, dyspnea (described as unsatisfied inspiration) soon rose to intolerable levels and reflected the widening disparity between inspiratory effort (reaching near maximal central neural drive) and the simultaneous tidal volume response, which becomes essentially fixed, i.e., increased effort/displacement ratio (Citation[14], Citation[56]). Dyspnea intensity correlated well with the increase in this effort/displacement ratio during exercise in COPD (Citation[14], Citation[56]).
Figure 4 The mechanical threshold of dyspnea is indicated by the abrupt rise in dyspnea after a critical “minimal” inspiratory reserve volume (IRV) is reached which prevents further expansion of tidal volume (VT) during exercise. Beyond this dyspnea/IRV inflection point during exercise, dyspnea intensity, breathing frequency (F), and the ratio of respiratory effort (Pes/PImax) to tidal volume displacement [VT standardized as a % of predicted vital capacity (VC)] ratio all continue to rise. Arrows indicate the dyspnea/IRV inflection point. Values are expressed as means ± SEM. IC = inspiratory capacity. Modified from reference 56, with permission.
![Figure 4 The mechanical threshold of dyspnea is indicated by the abrupt rise in dyspnea after a critical “minimal” inspiratory reserve volume (IRV) is reached which prevents further expansion of tidal volume (VT) during exercise. Beyond this dyspnea/IRV inflection point during exercise, dyspnea intensity, breathing frequency (F), and the ratio of respiratory effort (Pes/PImax) to tidal volume displacement [VT standardized as a % of predicted vital capacity (VC)] ratio all continue to rise. Arrows indicate the dyspnea/IRV inflection point. Values are expressed as means ± SEM. IC = inspiratory capacity. Modified from reference 56, with permission.](/cms/asset/8fa07ae8-5af3-4127-92c0-e454bd8de0d7/icop_a_197648_uf0004_b.gif)
Several studies have shown correlations between the intensity of dyspnea and the change in IC during exercise, suggesting that mechanical factors are contributory (Citation[14], Citation[17], Citation[50]). The contention that DH contributes importantly to exercise limitation in COPD has been bolstered by studies that have shown that pharmacological and surgical lung volume reduction are associated with consistent improvements in dyspnea and exercise endurance (Citation[17], Citation[19], Citation[56], Citation[57], Citation[58], Citation[59], Citation[60], Citation[61], Citation[62], Citation[63], Citation[64]).
DYNAMIC HYPERINFLATION DURING EXACERBATIONS OF COPD
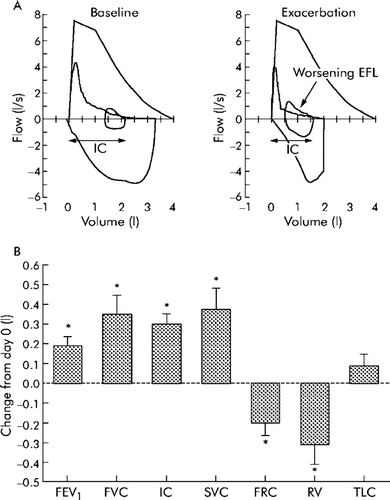
This topic has recently been reviewed more comprehensively elsewhere (Citation[34]). During acute exacerbations of COPD, airway resistance is increased (which effects the time-constant of the respiratory system) and ventilatory demand and breathing frequency may also be increased (reflecting increased ventilation-perfusion abnormalities) (Citation[65], Citation[66]). Together, these result in acute DH, which can stress the cardiopulmonary reserves of patients with more advanced disease and contribute to life-threatening respiratory failure (Citation[66]). Thus, acute DH results in excessive loading and functional weakness of the inspiratory muscles, which together with hypoxemia, acidosis and compromised cardiac function can culminate in mechanical failure of the respiratory pump (Citation[34], Citation[66], Citation[67], Citation[68]). We and others have recently argued that acute DH in the setting of an exacerbation contributes importantly to their most prominent symptom, i.e., worsening dyspnea (Citation[33], Citation[34], Citation[69]). The time course of recovery of dyspnea following exacerbations is closely associated with the reduction of DH, as measured by serial IC maneuvers (Citation[34]). This method of tracking hyperinflation appears to be reliable as TLC does not change significantly during acute exacerbations of COPD []. Further studies are required to determine if IC measurements during acute exacerbations of COPD can be used to evaluate clinical severity, to predict poor outcome and to monitor the course and recovery of the acute illness.
Figure 5 Magnitude of change in lung function parameters during recovery from exacerbation. (A) Representative flow-volume loops from a patient obtained at baseline (that is, before the exacerbation) and after onset of symptoms compatible with exacerbation. During exacerbation there is evidence of worsening expiratory flow limitation (EFL, arrow) resulting in hyperinflation with an increased end expiratory lung volume (EELV) and reduced inspiratory capacity (IC). (B) Change in lung function parameters during recovery from moderate exacerbations in 20 patients. Subjects were studied (day 0) within 72 hours of symptomatic deterioration. Data shown are change from initial (day 0) assessment. A bbreviations: FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; IC = inspiratory capacity; SVC = slow vital capacity; FRC = functional residual capacity; RV = residual volume; TLC = total lung capacity. From reference 34, with permission.
THE EFFECT OF BRONCHODILATOR THERAPY ON LUNG HYPERINFLATION AT REST
All classes of bronchodilators act by relaxing airway smooth muscle tone. Traditionally, improvement in airway function after bronchodilators is assessed by spirometric measurements of maximal expiratory flow rates (Citation[9]). Improvements in the FEV1 (beyond the natural variability of the measurement) after inhaled bronchodilators signify reduced resistance in the larger airways, as well as in alveolar units, with rapid time constants for lung emptying. In more advanced COPD (in contrast to asthma), post- bronchodilator increases in FEV1 mainly occur as a result of lung volume recruitment: the ratio of FEV1 to FVC is unaltered or actually decreases in response to bronchodilators (Citation[19], Citation[20], Citation[57], Citation[70], Citation[71]). Measurements of timed vital capacity or forced expiratory volume in 6 seconds (FEV6) appear to be more sensitive than FVC in detecting enhanced lung emptying after pharmacotherapy (Citation[72], Citation[73]). Improvement in small airway function is more difficult to measure, but reduced lung volumes (RV and EELV) as a consequence of reduced airway closure and enhanced gas emptying in alveolar units with slower time constants, provides indirect evidence of a positive effect. Recent studies have shown that substantial reductions (i.e., > 0.5 L) in EELV and improvements in IC (i.e., 10% predicted) can occur after acute short- and long-acting bronchodilator treatment in the presence of only modest improvements in FEV1 (Citation[13], Citation[19], Citation[20], Citation[57], Citation[70], Citation[71], Citation[74], Citation[75]) ().
Table 1 Bronchodilator-induced changes in spirometric variables and resting lung volumes
Long-acting bronchodilators have been shown to be associated with sustained lung volume reduction as measured by “trough” or morning, pre-bronchodilator EELV or IC (Citation[19], Citation[20], Citation[74]). Bronchodilator therapy is often associated with small but consistent increases in maximal volume-corrected expiratory flow rates in the effort-independent mid-volume range where tidal breathing occurs (Citation[19], Citation[57]). Bronchodilator therapy does not necessarily abolish resting expiratory flow-limitation (especially in more severe disease), but changes the conditions under which it occurs (Citation[13]). Thus, patients may remain flow-limited but can now accomplish the required alveolar ventilation at a lower operating lung volume and, therefore, at a reduced oxygen cost of breathing. Patients who show expiratory flow-limitation during spontaneous resting breathing (as determined by the negative expiratory pressure technique) and those with more severe resting lung hyperinflation have demonstrated the greatest lung volume reduction with bronchodilators (Citation[13], Citation[71], Citation[75]).
A recent mechanical study on the mechanisms of dyspnea relief following tiotropium therapy showed that release of cholinergic tone was associated with improved airway conductance at all lung volumes from TLC to RV (Citation[56]). Static elastic recoil of the lung was unchanged after acutely administered tiotropium and expiratory timing during spontaneous resting breathing was unaffected. Lung deflation, therefore, primarily reflected improvements in the mechanical time constants for lung emptying (i.e., reduced airway resistance). This is in contrast to the situation following lung volume reduction surgery (Citation[76]), where increased elastic lung recoil pressure appears to be an important mechanism of lung deflation. The main impact of bronchodilator therapy is, therefore, on the dynamically determined resting EELV through pharmacologic manipulation of resistance in the airways upstream from the flow-limiting segments (Citation[21], Citation[22]).
EFFECT OF BRONCHODILATORS ON DYNAMIC VENTILATORY MECHANICS DURING EXERCISE
Improvements in resting IC have been shown to occur as a result of treatment with all classes of bronchodilators and this indirectly signifies reduced EELV (Citation[57], Citation[58], Citation[59], Citation[77]) (). A bronchodilator-induced increase in the resting IC (indicating reduced lung hyperinflation), in the order of 0.3 L or ∼ 10% predicted, appears to be clinically meaningful and corresponds with important improvements in exertional dyspnea and exercise endurance measured during constant work rate cycle exercise at 60–75% of the patients pre-determined maximal work rate (Citation[19], Citation[20], Citation[57], Citation[58], Citation[59], Citation[60], Citation[77]). Recent studies in patients with moderate to severe COPD have indicated that combined long-acting anticholinergic and β2-agonist bronchodilators have additive effects on airway function. A recent study by van Noord et al. (Citation[78]) showed that tiotropium (once daily) combined with formoterol (twice daily) was associated with sustained lung volume reduction, as assessed by serial IC measurements over a 24-hour period. The mean 0–24 hour increase in IC after this bronchodilator combination was 0.22 L, with an impressive daytime peak effect of 0.55 L. A recent study confirmed that the fluticasone propionate/salmeterol combination (FSC 250/50) was associated with reduced lung hyperinflation (increased IC) during rest and exercise, and increased cycle exercise endurance time when compared with placebo (Citation[60]).
A number of studies have shown that bronchodilator therapy, alone and in combination with inhaled corticosteroids, does not alter the rate of dynamic hyperinflation (or air trapping) during exercise (Citation[19], Citation[20], Citation[57], Citation[58], Citation[59], Citation[60], Citation[61], Citation[77]). In fact, the rest to peak exercise reduction in IC may actually increase as a result of the higher levels of ventilation permitted by bronchodilator therapy (Citation[19], Citation[20], Citation[57], Citation[58], Citation[59], Citation[60], Citation[61], Citation[77]). However, because of recruitment of the IC at rest, the dynamic EELV at peak exercise is lower, in absolute terms, than the value obtained at the breakpoint of exercise during the placebo arm of the treatment (Citation[19], Citation[20], Citation[57], Citation[58], Citation[59], Citation[60], Citation[61], Citation[77]). In other words, bronchodilator treatment (compared with placebo) is associated with is a parallel downward shift in the EELV over the course of the exercise test [].
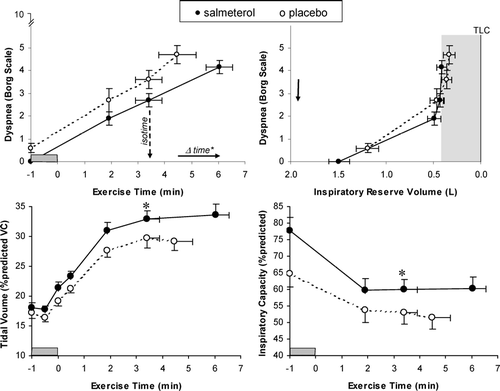
Figure 6 Relationships between Borg ratings of dyspnea intensity and each of exercise time and inspiratory reserve volume (IRV) are shown during constant-load cycle exercise at 75% of each patient's maximum work rate after salmeterol (closed circles, solid lines) and placebo (open circles, dotted lines). Dypnea-IRV relationships were unchanged after salmeterol, with dyspnea increasing rapidly once a critically reduced IRV (shaded area) was reached which prevented further expansion of tidal volume. At isotime during exercise, measurements of inspiratory capacity and tidal volume increased significantly after salmeterol compared with placebo (* p < 0.05). Values are means ± SEM (points measured at rest, at standardized times during exercise, and at end-exercise). Adapted from reference 59, with permission.
Improvements in the resting IC (standardized as a % of the predicted normal value) following bronchodilator therapy has been shown to correlate well with:
improved peak symptom-limited V′O2 (Citation[15], Citation[59]),
increased peak tidal volume (Citation[19], Citation[59]),
reduced dyspnea ratings (Citation[17], Citation[19], Citation[56], Citation[57], Citation[58], Citation[59], Citation[60], Citation[61]), and
increased endurance time during constant work-rate cycle exercise testing in moderate-to-severe COPD patients (Citation[15], Citation[17], Citation[19], Citation[56], Citation[57], Citation[58], Citation[59], Citation[60], Citation[61]).
In all of the studies, increased resting IC permitted greater tidal volume expansion with reduced breathing frequency throughout exercise (Citation[17], Citation[19], Citation[20], Citation[56], Citation[57], Citation[58], Citation[59], Citation[60], Citation[61]). Moreover, in a recent mechanical study, tiotropium was associated with reduced airways resistance and elastic loading of the inspiratory muscles which resulted in a reduced work and oxygen cost of breathing, compared with placebo (Citation[56]). A reduced inspiratory threshold load, reflecting reduced intrinsic positive end-expiratory pressure (PEEPi), would be expected to enhance neuromechanical coupling of the respiratory system during exercise. Lung volume deflation, by increasing sarcomere fiber length in the diaphragm, may also favorably affect this muscle's force generating capacity, which again would contribute to reduced effort requirements (and central neural drive) for a given tidal volume displacement. Thus, avoidance of “high end” mechanics (where the respiratory system's pressure-volume relation is relatively flat) as a result of bronchodilator-induced reductions in EELV and increases in IRV, should contribute importantly to alleviation of exertional dyspnea and improved exercise performance (Citation[19], Citation[57], Citation[70], Citation[71], Citation[74], Citation[75]).
IMPROVED VENTILATORY MECHANICS AND DYSPNEA RELIEF
The strongest correlate of improved exercise endurance in a number of bonchodilator trials has been reduced exertional dyspnea intensity (Citation[19], Citation[57]). Several bronchodilator studies have shown that reduced dyspnea ratings at a standardized exercise time correlated well with reduced operating lung volumes (i.e., reduced EELV and an increased IRV) and improved breathing pattern (i.e., increased tidal volume and reduced breathing frequency) (Citation[57], Citation[58], Citation[70], Citation[74]) []. The precise neurophysiological mechanisms of dyspnea relief remain speculative. In one study, treatment with the long-acting anticholinergic agent, tiotropium, was associated with consistent reductions in the ratio of respiratory effort to volume displacement throughout exercise, suggesting a more harmonious relation between neural drive and the mechanical response (Citation[56]) []. Bronchodilator-induced unloading of the ventilatory muscles must mean that less neural activation is required for a given force generation. Reduced motor command output (and central corollary discharge) may be sensed directly as a decrease in perceived inspiratory effort (Citation[79], Citation[80], Citation[81]). Improvement in the performance characteristics of inspiratory muscles secondary to lung deflation would be expected to enhance length-tension appropriateness—the muscle spindles may have an important role in sensing this (Citation[80], Citation[82]). To the extent that restriction of the normal volume response to the increased neural drive of exercise contributes to perceived respiratory discomfort (Citation[83]), then the newfound ability to increase tidal volume after bronchodilators may form the basis, at least in part, for reduced dyspnea intensity or for alteration in its quality. Interestingly, in two studies patients selected qualitative descriptors of dyspnea in the “unsatisfied inspiration” cluster less frequently after bronchodilators compared with placebo (Citation[56], Citation[59]). There are numerous mechanosensors throughout the respiratory system whose afferent inputs project to the cerebral cortex and can convey the sense of improved thoracic motion or volume displacement for a given electrical activation of the muscle (Citation[84], Citation[85]). Following bronchodilators, altered peripheral sensory inputs from these sources in the lung and chest wall, together with reduced central corollary discharge, culminate in reduced perceived respiratory discomfort during physical exertion in ways that are not yet fully understood. The emerging evidence supports the idea that the beneficial effects of bronchodilators on respiratory sensation in COPD patients are ultimately related to enhanced neuromechanical coupling of the respiratory system as a result of improved dynamic ventilatory mechanics.
Figure 7 (A) Campbell diagrams are shown at a standardized time during constant-load exercise for a patient with severe COPD (FEV1 = 29 % predicted). After tiotropium compared with placebo, there were decreases in the inspiratory threshold load (ITL), the elastic work of breathing (shaded areas), and the resistive work of breathing (area within volume-Pes loops). Abbreviations: CLdyn = dynamic compliance, Cw = chest wall compliance, IC = inspiratory capacity, Pes = esophageal pressure. (B) The relationship between respiratory effort (Pes/PImax) and tidal volume displacement [VT standardized as a fraction of predicted vital capacity (VC)], an index of neuromechanical coupling, is shown during constant work rate exercise after tiotropium and placebo in patients with moderate to severe COPD (data from reference 56). Data from a group of age-matched healthy subjects during exercise is also shown (data from reference 50). Compared with placebo, tiotropium enhanced neuromechanical coupling throughout exercise in COPD. Values are means ± SEM.
![Figure 7 (A) Campbell diagrams are shown at a standardized time during constant-load exercise for a patient with severe COPD (FEV1 = 29 % predicted). After tiotropium compared with placebo, there were decreases in the inspiratory threshold load (ITL), the elastic work of breathing (shaded areas), and the resistive work of breathing (area within volume-Pes loops). Abbreviations: CLdyn = dynamic compliance, Cw = chest wall compliance, IC = inspiratory capacity, Pes = esophageal pressure. (B) The relationship between respiratory effort (Pes/PImax) and tidal volume displacement [VT standardized as a fraction of predicted vital capacity (VC)], an index of neuromechanical coupling, is shown during constant work rate exercise after tiotropium and placebo in patients with moderate to severe COPD (data from reference 56). Data from a group of age-matched healthy subjects during exercise is also shown (data from reference 50). Compared with placebo, tiotropium enhanced neuromechanical coupling throughout exercise in COPD. Values are means ± SEM.](/cms/asset/523ce2ba-3991-4037-a26d-ef88eca5792e/icop_a_197648_uf0007_b.gif)
OXYGEN BREATHING AND REDUCED DYNAMIC HYPERINFLATION
Since the extent of DH during exercise in flow-limited patients depends on minute ventilation (V′E), it follows that therapeutic interventions, such as oxygen, that reduce submaximal ventilation should reduce and/or delay the rate of onset of DH and therefore the onset of critical ventilatory constraints that limit exercise. However, the effects of ambulatory oxygen (O2) on DH, dyspnea and exercise performance in any given individual with symptomatic COPD is difficult to predict (Citation[86], Citation[87], Citation[88], Citation[89], Citation[90]). A reduced rate of DH has been shown to be associated with a hyperoxia-induced reduction of submaximal ventilation (i.e., breathing frequency) during exercise in hypoxemic COPD patients (36). The effects of hyperoxia on the rate of exercise-induced DH in normoxic patients is less pronounced (Citation[61], Citation[91]). A placebo-controlled, crossover study, where patients with advanced COPD received either 60% oxygen or room air during constant work rate exercise showed that hyperoxia more than doubled the time taken to reach ventilatory limitation compared with when breathing room air (Citation[36]) []. At a standardized submaximal work rate, V'E decreased by approximately 3 L/min and the IRV increased by 0.3 L during 60% oxygen compared with room air (Citation[36]). Somfay et al. (Citation[92]) examined the dose-response relationship between increasing concentrations of supplemental oxygen and dynamic operating lung volumes in 10 patients with advanced normoxic COPD who had clinical characteristics of emphysema (i.e., diffusion lung for carbon monoxide of 40% predicted). In that study, improvements in IRV and endurance time increased as inspired O2 concentrations increased from 21 to 50%, with no further improvements thereafter. Reductions in dyspnea with supplemental O2 administration were correlated with changes in EELV (r = 0.48, p = 0.002) and end-inspiratory lung volume (EILV, r = 0.43 and p = 0.005) during exercise (Citation[92]).
Figure 8 Dyspnea, ventilation, breathing frequency (F), and operating lung volumes plotted against exercise time in patients randomized to breathe either room air (RA) or 60% oxygen. While breathing oxygen, exercise endurance increased significantly in conjunction with significant decreases in dyspnea, ventilation, F, end-expiratory lung volume (EELV, i.e., increased IC) and end inspiratory lung volume (EILV, i.e. increased IRV) at isotime during exercise (*p < 0.05, **p < 0.01). From reference 36, with permission.
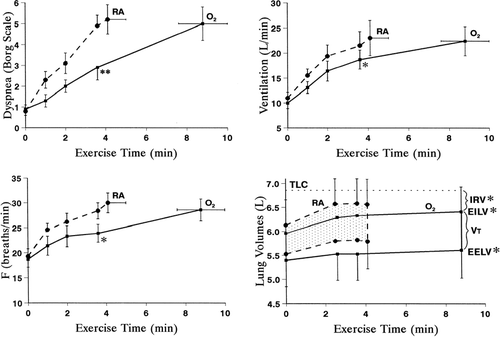
By contrast, we examined the effects of O2 (60%) in 11 patients with COPD of comparable severity but with mild arterial oxygen desaturation, and found that there were no consistent effects on operating lung volumes throughout exercise (Citation[91]). In two studies designed to examine the effects of hyperoxia in normoxic patients with COPD (Citation[61], Citation[91]), TE prolongation at a standardized time during exercise with hyperoxia averaged only 6% (∼ 0.16 s) and 9% (0.11 s), respectively, compared with the room air value. This is in contrast with increases of TE found in two studies associated with consistent reductions in operating lung volumes: in Somfay's paper (Citation[92]) and our previous paper in hypoxic patients (Citation[36]), TE increased by ∼ 27% (0.39 s) and 23% (0.35 s), respectively. These data suggest that the overall effect of hyperoxia on operating lung volumes in a given individual with COPD likely depends on the net time constant for emptying of the lungs and the extent of TE prolongation that accompanies altered neural inputs in response to added oxygen. Patients who reduce DH in response to hyperoxia appear to have significantly greater baseline airway obstruction, greater ventilatory constraints during exercise, and poorer exercise performance with steeper dyspnea/ventilation slopes (Citation[61]). It follows that such patients should experience greater dyspnea relief in response to even modest reductions in V'E following supplemental O2.
It must be remembered that the effects of O2 administration in COPD are multifactorial and include improvements in peripheral muscle and cardiac function as well as reduced ventilatory drive. A recent study has shown that significant dyspnea relief and improvement in exercise endurance can occur even in the absence of an effect on DH (Citation[61]).
THE IMPACT OF HELIUM/OXYGEN MIXTURES ON DYNAMIC HYPERINFLATION
The use of helium-oxygen mixtures (usually 79% helium and 21% oxygen, also called heliox) for the treatment of asthma and COPD was introduced in 1935 by Barach (Citation[93]). Maximum flow rate is determined by airway size and compliance (at the choke point) as well as by physical gas properties (Citation[22]). During heliox breathing, the density of which is about one third of air, expired flow rate is expected to increase as a result of decreased turbulence within the large airways (Citation[94]). While Grapé et al. found a significant decrease in pulmonary resistance with heliox, Wouters et al. found no change in total respiratory resistance (Citation[95], Citation[96]). Also controversial is the effect of heliox on lung hyperinflation. Grapé et al. (Citation[95]) found no significant reduction in EELV with heliox, while Swidwa et al. (Citation[97]) found a substantial fall in EELV. This discrepancy may reflect variation in the response to heliox within the COPD populations studied. Indeed, Meadows et al. (Citation[98]) found that only half of their COPD patients exhibited an increase of maximal expiratory flows with heliox therapy. Similarly, inconsistent results have been found in mechanically ventilated COPD patients (Citation[99], Citation[100]).
By contrast, studies conducted in healthy older volunteers during physical exercise documented that the effects of low gas density are mostly evident at high work loads where expiratory flow limitation can occur during tidal breathing and EELV tends to increase. Under these conditions, breathing heliox allows higher ventilation since it allows tidal volume to increase at the expense of a reduced EELV (Citation[101]). Recently, Palange et al. (Citation[102]) reasoned that resistive unloading of the airways during heliox breathing could also be beneficial in COPD patients. They tested this hypothesis in a group of moderate-to-severe COPD patients exercising at 80% of their maximal power output, while breathing either heliox or room air, in random order. Heliox was associated with a two-fold increase in exercise endurance time, with significant increases in maximal expiratory flow rates, tidal volume and ventilation at peak exercise. Operating lung volumes were reduced together with dyspnea ratings at as standardized time []. Interestingly, the increase in endurance time achieved with heliox appears to be superior to that reported in studies using oxygen supplementation alone (Citation[36]) or single bronchodilatation treatments (Citation[19]). The beneficial effects of heliox on exercise tolerance in COPD has been confirmed in subsequent studies (Citation[103], Citation[104]), where the authors showed that the effects of heliox alone or in combination with supplementary oxygen, are additive in nature but vary with disease severity (Citation[104]).
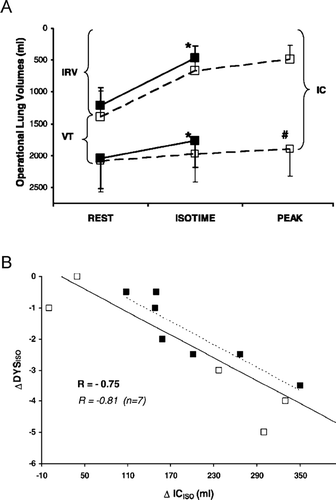
Figure 9 (A) Operating lung volumes during high intensity, constant work rate exercise are shown while breathing heliox (□) and air (▪) in 12 patients with COPD. At isotime during heliox breathing, inspiratory capacity (IC) and inspiratory reserve volume (IRV) increased significantly compared with air (* p < 0.01); at peak exercise on heliox, IC was significantly higher compared with air (#p < 0.01). VT = tidal volume. (B) The increase in IC at isotime during exercise correlated with the concurrent decrease in ratings of dyspnea intensity (r = − 0.75, p < 0.01). From reference 102, with permission.
SUMMARY
In COPD, lung hyperinflation is associated with expiratory flow-limitation and becomes further amplified under conditions of acutely increased ventilatory demand. Acute-on-chronic dynamic hyperinflation during exercise and exacerbation leads to excessive loading and functional weakness of the inspiratory muscles and can impose a critical mechanical limitation to ventilation. Ultimately, acute dynamic hyperinflation contributes to neuromechanical uncoupling of the respiratory system with attendant respiratory discomfort and activity limitation. Relief of dyspnea and improvement of exercise endurance following bronchodilator therapy is explained, at least in part, by reduced lung hyperinflation, with release of tidal volume restriction and improved neuromechanical coupling. Measurement of lung volumes can therefore provide useful complementary information in clinical trials designed to evaluate bronchodilator efficacy. Other interventions that reduce ventilatory drive or improve airway function, such as oxygen and helium, alone or in combination, have been shown to slow the rate of dynamic hyperinflation during exercise, with positive sensory and mechanical consequences. Collectively, all of these interventions have the potential to augment exercise re-conditioning which, arguably, has the highest impact on the pivotal patient-centered outcomes in the COPD population. It now appears that we have the capability of achieving sustained lung volume reduction by pharmacologic means that is comparable in magnitude (in terms of inspiratory capacity recruitment) to that obtained by lung volume reduction surgery. What remains to be determined is whether long term pharmacological lung volume reduction will favorably alter the natural history of this devastating disease.
REFERENCES
- Stokes W. A treatise on the diagnosis and treatment of diseases of the chest. Part 1. Diseases of the lung and windpipe. The New Sydenham Society, London 1837; 168–169
- Sharp J T. The respiratory muscles in emphysema. Clin Chest Med 1983; 4: 421–432, [INFOTRIEVE], [CSA]
- Sharp J T, Goldberg N B, Druz W S, Fishman H C, Danon J. Thoraco-abdominal motion in chronic obstructive lung disease. Am Rev Resp Dis 1977; 115: 47–56, [INFOTRIEVE], [CSA]
- Simon G. Principles of Chest X-ray Diagnosis, 3rd ed. Butterworths, London 1971
- Simon G, Pride N B, Jones N L, Raimondi A C. Relation between abnormalities in the chest radiograph and changes in pulmonary function in chronic bronchitis and emphysema. Thorax 1973; 28: 15–23, [INFOTRIEVE], [CSA]
- Burki N K, Krumpelman J L. Correlation of pulmonary function with the chest roentgenogram in chronic airway obstruction. Am Rev Respir Dis 1980; 121: 217–223, [INFOTRIEVE], [CSA]
- Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Paré P D, Hogg J C, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000; 162: 1102–1108, [INFOTRIEVE], [CSA]
- de Jong P A, Müller N L, Paré P D, Coxson H O. Computed tomographic imaging of the airways:relationship to structure and function. Eur Respir J 2005; 26: 140–152, [INFOTRIEVE], [CROSSREF], [CSA]
- Pellegrino R, Viegi G, Brusasco V, Crapo R O, Burgos F, Casaburi R, Coates A, van der Grinten C PM, Gustafsson P, Hankinson J, Jensen R, Johnson D C, MacIntyre N, McKay R, Miller M R, Navajas D, Pedersen O F, Wanger J. ATS/ERS Task Force: Standardisation of lung function testing: Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968, [INFOTRIEVE], [CROSSREF], [CSA]
- Quanjer P H, Tammeling G J, Cotes J E, Pedersen O F, Peslin R, Yernault J C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J 1993; 6: 5–40, Suppl. 16[CSA]
- He Z, Lam M, Webb K, O'Donnell D E. Ventilatory Constraints during Exercise in Patients with Mild COPD. Am J Respir Crit Care Med 2005; 171: A304, [CSA]
- Leith D E, Brown R. Human lung volumes and the mechanisms that set them. Eur Respir J 1999; 13: 468–472, [INFOTRIEVE], [CROSSREF], [CSA]
- Tantucci C, Duguet A, Similowski T, Zelter M, Derenne J P, Milic-Emili J. Effect of salbutamol on dynamic hyperinflation in chronic obstructive pulmonary disease patients. Eur Respir J 1998; 12: 799–804, [INFOTRIEVE], [CROSSREF], [CSA]
- O'Donnell D E, Revill S, Webb K A. Dynamic hyperinflation and exercise intolerance in COPD. Am J Respir Crit Care Med 2001; 164: 770–777, [INFOTRIEVE], [CSA]
- Diaz O, Villafranca C, Ghezzo H, Borzone G, Leiva A, Milic-Emil J, Lisboa C. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitations at rest. Eur Respir J 2000; 16: 269–275, [INFOTRIEVE], [CROSSREF], [CSA]
- Casanova C, Cote C, de Torres J P, Aguirre-Jaime A, Marin J M, Pinto-Plata V, Celli B R. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005 15; 171: 591–597, [CROSSREF], [CSA]
- O'Donnell D E, Lam M, Webb K A. Measurement of symptoms, lung hyperinflation and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158: 1557–1565, [INFOTRIEVE], [CSA]
- O'Donnell D E, He Z, Lam M, Webb K, Fluge T, Hamilton A. Reproducibility of measurements in inspiratory capacity, dyspnea intensity and exercise endurance in multicentre trials in COPD (abs). Eur Respir J 2004; 24(48)P2151, [CSA]
- O'Donnell D, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23: 832–840, [INFOTRIEVE], [CSA]
- Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba F C, Richter K, Kesten S, O'Donnell D. Improvements in symptom-limited exercise performance over eight hours with once-daily tiotropium in patients with COPD. Chest 2005; 128: 1168–1178, [INFOTRIEVE], [CROSSREF], [CSA]
- Hyatt R E. Expiratory flow limitation. J Appl Physiol 1983; 55: 1–8, [INFOTRIEVE], [CSA]
- Dawson S V, Elliott E A. Wave-speed limitation on expiratory flow—a unifying concept. J Appl Physiol 1977; 43: 498–515, [INFOTRIEVE], [CSA]
- Pride N B. Ageing and changes in lung mechanics. Eur Respir J 2005; 26: 563–565, [INFOTRIEVE], [CROSSREF], [CSA]
- Pride N B, Macklem P T. Lung mechanics in disease. Handbook of Physiology, Section 3, Vol III, Part 2: The Respiratory System, A P Fishman. American Physiological Society, Bethesda, MD 1986; 659–692
- Vinegar A, Sinnett E E, Leith D E. Dynamic mechanisms determine functional residual capacity in mice. J Appl Physiol 1979; 46: 867–891, [INFOTRIEVE], [CSA]
- Sharp J T. The respiratory muscles in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 134: 1089–1091, [INFOTRIEVE], [CSA]
- Demedts M. Mechanisms and consequences of hyperinflation. Eur Respir J 1990; 3: 617–618, [INFOTRIEVE], [CSA]
- Macklem P T. Hyperinflation (editorial). Am Rev Respir Dis 1984; 129: 1–2, [INFOTRIEVE], [CSA]
- Lougheed D M, Webb K A, O'Donnell D E. Breathlessness during induced lung hyperinflation in asthma:the role of the inspiratory threshold load. Am J Respir Crit Care Med 1995; 152: 911–920, [INFOTRIEVE], [CSA]
- Cormier Y. Mechanisms of hyperinflation in asthma. Eur Respir J 1990; 3: 619–624, [INFOTRIEVE], [CSA]
- Martin J G. Inspiratory muscle activity during induced hyperinflation. Respir Physiol 1980; 39: 303–313, [INFOTRIEVE], [CROSSREF], [CSA]
- Cole P, Savard P, Miljeteig H, Haight J S. Resistance to respiratory airflow of the extrapulmonary airways. Laryngoscope 1993; 103(4 Pt. 1)447–450, [INFOTRIEVE], [CSA]
- Stevenson N J, Walker P P, Costello R W, Calverley P M. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 1510–1516, [INFOTRIEVE], [CROSSREF], [CSA]
- O'Donnell D E, Parker C M. COPD exacerbations · 3: Pathophysiology. Thorax 2006; 61: 354–361, [INFOTRIEVE], [CROSSREF], [CSA]
- Gelb A F, Guitierrez C A, Weisman I M, Newsom R, Taylor C F, Zamel N. Simplified detection of dynamic hyperinflation. Chest 2004; 126: 1855–1860, [INFOTRIEVE], [CROSSREF], [CSA]
- O'Donnell D E, D'Arsigny C, Webb K A. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 892–898, [INFOTRIEVE], [CSA]
- Grimby G, Bunn J, Mead J. Relative contribution of rib cage and abdomen to ventilation during exercise. J Appl Physiol 1968; 24: 159–166, [INFOTRIEVE], [CSA]
- Potter W A, Olafsson S, Hyatt R E. Ventilatory mechanics and expiratory flow limitation during exercise in patients with obstructive lung disease. J Clin Invest 1971; 50: 910–919, [INFOTRIEVE], [CSA]
- Stubbing D G, Pengelly L D, Morse J LC, Jones N L. Pulmonary mechanics during exercise in subjects with chronic airflow obstruction. J Appl Physiol 1980; 49: 511–515, [INFOTRIEVE], [CSA]
- Dodd D S, Brancatisano T, Engel L A. Chest wall mechanics during exercise in patients with severe chronic airway obstruction. Am Rev Respir Dis 1984; 129: 33–38, [INFOTRIEVE], [CSA]
- Aliverti A, Stevenson N, Dellaca R L, Lo Mauro A, Pedotti A, Calverley P M. Regional chest wall volumes during exercise in chronic obstructive pulmonary disease. Thorax 2004; 59: 210–216, [INFOTRIEVE], [CROSSREF], [CSA]
- Clarenbach C F, Senn O, Brack T, Kohler M, Bloch K E. Monitoring of ventilation during exercise by a portable respiratory inductive plethysmograph. Chest 2005; 128: 1282–1290, [INFOTRIEVE], [CROSSREF], [CSA]
- Vogiatzis I, Georgiadou O, Golemati S, Aliverti A, Kosmas E, Kastanakis E, Geladas N, Koutsoukou A, Nanas S, Zakynthinos S, Roussos C. Patterns of dynamic hyperinflation during exercise and recovery in patients with severe chronic obstructive pulmonary disease. Thorax 2005; 60: 723–729, [INFOTRIEVE], [CROSSREF], [CSA]
- Similowski T, Yan S, Gauthier A P, Macklem P T, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med 1991; 325: 917–923, [INFOTRIEVE], [CSA]
- Orozco-Levi M, Gea J, Lloreta J L, Félez M, Minguella J, Serano S, Broquetas J M. Subcellular adaptation of the human diaphragm in chronic obstructive pulmonary disease. Eur Respir J 1999; 13: 371–378, [INFOTRIEVE], [CROSSREF], [CSA]
- Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphgram in chronic obstructive pulmonary disease. N Engl J Med 1997; 337: 1799–1806, [INFOTRIEVE], [CROSSREF], [CSA]
- Mercadier J J, Schwartz K, Schiaffino S, Wisnewsky C, Ausoni S, Heimburger M, Marrash R, Pariente R, Aubier M. Myosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflation. Am J Physiol 1998; 274: L527–534, [INFOTRIEVE], [CSA]
- Decramer M. Hyperinflation and respiratory muscle interaction. Eur Respir J 1997; 10: 934–941, [INFOTRIEVE], [CSA]
- O'Donnell D E, Webb K A. Exercise. Chronic Obstructive Pulmonary Disease, 2nd edition, P MA Calverley, W MacNee, N B Pride, S I Rennard. Arnold, London 2003; 243–269, Chapter 18
- O'Donnell D E, Chau L KL, Bertley J C, Webb K A. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med 1997; 155: 109–115, [INFOTRIEVE], [CSA]
- Sinderby C, Spahija J, Beck J, Kaminski D, Yan S, Comtois N, Sliwinski P. Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 1637–1641, [INFOTRIEVE], [CSA]
- O'Donnell D E, D'Arsigny C, Fitzpatrick M, Webb K A. Exercise Hypercapnia in advanced chronic obstructive pulmonary disease: the role of lung hyperinflation. Am J Respir Crit Care Med 2002; 166: 663–668, [INFOTRIEVE], [CROSSREF], [CSA]
- Montes de Oca M, Rassulo J, Celli B R. Respiratory muscle and cardiopulmonary function during exercise in very severe COPD. Am J Respir Crit Care Med 1996; 154: 1284–1289, [INFOTRIEVE], [CSA]
- Light R W, Mintz W M, Linden G S, Brown S E. Hemodynamics of patients with severe chronic obstructive pulmonary disease during progressive upright exercise. Am Rev Respir Dis 1984; 130: 391–395, [INFOTRIEVE], [CSA]
- Vizza C D, Lynch J P, Ochoa L L, Richardson G, Trulock E P. Right and left ventricular dysfunction in patients with severe pulmonary disease. Chest 1998; 113: 576–583, [INFOTRIEVE], [CSA]
- O'Donnell D E, Hamilton A L, Webb K A. Sensory-mechanical relationships during high-intensity, constant-work-rate exercise in COPD. J Appl Physiol 2006; 101: 1025–1035, [INFOTRIEVE], [CSA]
- O'Donnell D E, Lam M, Webb K A. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in COPD. Am J Respir Crit Care Med 1999; 160: 524–549, [CSA]
- Belman M J, Botnick W C, Shin J W. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153: 967–975, [INFOTRIEVE], [CSA]
- O'Donnell D E, Voduc N, Fitzpatrick M, Webb K A. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J 2004; 24: 86–94, [INFOTRIEVE], [CROSSREF], [CSA]
- O'Donnell D E, Sciurba F, Celli B, Mahler D A, Webb K A, Kalberg C J, Knobil K. Effect of fluticasone propionate/salmeterol on lung hyperinflation and exercise endurance in COPD. Chest 2006; 130: 647–656, [INFOTRIEVE], [CROSSREF], [CSA]
- Peters M M, Webb K A, O'Donnell D E. Combined physiological effects of bronchodilators and hyperoxia on exertional dyspnea in normoxic COPD. Thorax 2006; 61: 559–567, [INFOTRIEVE], [CROSSREF], [CSA]
- O'Donnell D E, Bertley J, Webb K A, Conlan A A. Mechanisms of relief of exertional breathlessness following unilateral bullectomy and lung volume reduction surgery in advanced chronic airflow limitation. Chest 1996; 110: 18–27, [INFOTRIEVE], [CSA]
- Martinez F J, Montes de Oca M, Whyte R I, Stetz J, Gay S E, Celli B R. Lung-volume reduction improves dyspnea, dynamic hyperinflation and respiratory muscle function. Am J Respir Crit Care Med 1997; 155: 1984–1990, [INFOTRIEVE], [CSA]
- Laghi F, Jurban A, Topeli A, Fahey P J, Garrity E, Jr, Archids J M, DePinto D J, Edwards L C, Tobin M J. Effect of lung volume reduction surgery on neuromechanical coupling of the diaphragm. Am J Respir Crit Care Med 1998; 157: 475–483, [INFOTRIEVE], [CSA]
- Barbera J A, Roca J, Ferrer A, Felez M A, Diaz O, Roger N, Rodriguez-Roisin R. Mechanisms of worsening gas exchange during acute exacerbations of chronic obstructive pulmonary disease. Eur Respir J 1997; 10: 1285–1291, [INFOTRIEVE], [CROSSREF], [CSA]
- Calverley P M. Respiratory failure in chronic obstructive pulmonary disease. Eur Respir J Suppl 2003; 47: 26s–30s, [INFOTRIEVE], [CROSSREF], [CSA]
- Tantucci C, Corbeil C, Chasse M, Braidy J, Matar N, Milic-Emili J. Flow resistance in patients with chronic obstructive pulmonary disease in acute respiratory failure. Am Rev Respir Dis 1991; 144: 384–389, [INFOTRIEVE], [CSA]
- Guerin C, Coussa M L, Eissa N T, Corbeil C, Chasse M, Braidy J, Matar N, Milic-Emili J. Lung and chest wall mechanics in mechanically ventilated COPD patients. J Appl Physiol 1993; 74: 1570–1580, [INFOTRIEVE], [CSA]
- Parker C M, Voduc N, Aaron S D, Webb K A, O'Donnell D E. Physiological changes during symptom recovery from moderate exacerbations of COPD. Eur Resp J 2005; 26: 420–428, [CROSSREF], [CSA]
- O'Donnell D E. Assessment of bronchodilator efficacy in symptomatic COPD. Is spirometry useful?. Chest 2000; 117: 42S–47S, [INFOTRIEVE], [CROSSREF], [CSA]
- Newton M, O'Donnell D E, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest 2002; 121: 1042–1050, [INFOTRIEVE], [CROSSREF], [CSA]
- Rabe K F, Bateman E D, O'Donnell D, Witte S, Bredenbröker D, Bethke T D. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005; 366: 563–571, [INFOTRIEVE], [CROSSREF], [CSA]
- Swanney M P, Jensen R L, Crichton D A, Beckert L E, Cardno L A, Crapo R O. FEV6 is an acceptable surrogate for FVC in the spirometric diagnosis of airway obstruction and restriction. Am J Respir Crit Care Med 2000; 162: 917–919, [INFOTRIEVE], [CSA]
- Celli B, ZuWallack R, Wang S, Kesten S. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest 2003; 124: 1743–1748, [INFOTRIEVE], [CROSSREF], [CSA]
- O'Donnell D E, Forkert L, Webb K A. Evaluation of bronchodilator responses in patients with “irreversible emphysema”. Eur Respir J 2001; 18: 914–920, [INFOTRIEVE], [CROSSREF], [CSA]
- Sciurba F C, Rogers R M, Keenan R J, Slivka W A, Gorscan J, III, Ferson P F, Holbert J M, Brown M L, Landreneau R J. Improvement in pulmonary function and elastic recoil after lung-reduction surgery for diffuse emphysema. N Engl J Med 1996; 334: 1095–1099, [INFOTRIEVE], [CROSSREF], [CSA]
- Chrystyn H, Mulley B A, Peake M D. Dose response relation to oral theophylline in severe chronic obstructive airways disease. Brit Med J 1988; 297: 1506–1510, [INFOTRIEVE], [CSA]
- van Noord J A, Aumann J L, Janssens E, Verhaert J, Smeets J J, Mueller A, Cornellisen P JG. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest 2006; 129: 509–517, [INFOTRIEVE], [CROSSREF], [CSA]
- Gandevia S C. The perception of motor commands on effort during muscular paralysis. Brain 1982; 105: 151–195, [INFOTRIEVE], [CSA]
- Chen Z, Eldridge F L, Wagner P G. Respiratory associated rhythmic firing of midbrain neurons in cats:relation to level of respiratory drive. J Physiol 1991; 437: 305–325, [INFOTRIEVE], [CSA]
- Campbell E JM, Howell J BL. The sensation of breathlessness. Br Med Bull 1963; 18: 36–40, [CSA]
- Chen Z, Eldridge F L, Wagner P G. Respiratory-associated thalamic activity is related to level of respiratory drive. Respir Physiol 1992; 90: 99–113, [INFOTRIEVE], [CROSSREF], [CSA]
- O'Donnell D E, Hong H H, Webb K A. Respiratory sensation during chest wall restriction and dead space loading in exercising men. J Appl Physiol 2000; 88: 1859–1869, [INFOTRIEVE], [CSA]
- Davenport P W, Friedman W A, Thompson F J, Franzen O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. J Appl Physiol 1986; 60: 1843–1848, [INFOTRIEVE], [CSA]
- Zechman F R, Jr., Wiley R L. Afferent inputs to breathing:respiratory sensation. Handbook of Physiology, Section 3, Volume II, Part 2: The Respiratory System, A P Fishman. American Physiological Society, Bethesda, MD 1986; 449–474
- Chronos N, Adams L, Guz A. Effect of hyperoxia and hypoxia on exercise-induced breathlessness in normal subjects. Clin Sci 1988; 74: 531–537, [INFOTRIEVE], [CSA]
- Lane R, Cockcroft A, Adams L, Guz A. Arterial oxygen saturation and breathlessness in patients with chronic obstructive airways disease. Clin Sci 1987; 72: 693–698, [INFOTRIEVE], [CSA]
- Woodcock A A, Gross E R, Geddes D M. Oxygen relieves breathlessness in “pink puffers”. Lancet 1981; 1: 907–909, [INFOTRIEVE], [CROSSREF], [CSA]
- Stein D A, Bradley B L, Miller W. Mechanisms of oxygen effects on exercise in patients with chronic obstructive pulmonary disease. Chest 1982; 81: 6–10, [INFOTRIEVE], [CSA]
- Libby D M, Briscoe W A, King T KC. Relief of hypoxia-related bronchoconstriction by breathing 30 percent oxygen. Am Rev Respir Dis 1981; 123: 171–175, [INFOTRIEVE], [CSA]
- O'Donnell D E, Bain D J, Webb K A. Factors contributing to relief of exertional breathlessness during hyperoxia in chronic airflow limitation. Am J Respir Crit Care Med 1997; 155: 530–535, [INFOTRIEVE], [CSA]
- Somfay A, Porszasz J, Lee S M, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxemic COPD patients. Eur Respir J 2001; 18: 77–84, [INFOTRIEVE], [CROSSREF], [CSA]
- Barach A L. The use of helium in the treatment of asthma and obstructive lesions in the larynx and trachea. Ann Intern Med 1935; 9: 739–765, [CSA]
- Pedley T J, Schroter R C, Sudlow M F. Gas flow and mixing in the airways. Bioengineering aspects of the lung, J B West. Marcel Dekker, New York 1977; 163–265
- Grapé B, Channin E, Tyler J M. The effect of helium and oxygen mixtures on pulmonary resistances in emphysema. Am Rev Respir Dis 1960; 81: 823–829, [CSA]
- Wouters E F, Landser F J, Polko A H, Visser B F. Impedance measurement during air and helium-oxygen breathing before and after salbutamol. Clin Exp Pharmacol Physiol 1992; 19: 95–101, [INFOTRIEVE], [CSA]
- Swidwa D M, Montenegro H D, Goldman M D, Lutchen K R, Saidel G M. Helium-oxygen breathing in severe chronic obstructive pulmonary disease. Chest 1985; 87: 790–795, [INFOTRIEVE], [CSA]
- Meadows J A, 3rd, Rodarte J R, Hyatt R E. Density dependence of maximal expiratory flow in chronic obstructive pulmonary disease. Am Rev Respir Dis 1980; 121: 47–53, [INFOTRIEVE], [CSA]
- Marini J J. Heliox in chronic obstructive pulmonary disease: time to lighten up?. Crit Care Med 2000; 28: 3086–3088, [INFOTRIEVE], [CROSSREF], [CSA]
- Chevrolet J C. Helium oxygen mixtures in the intensive care unit. Crit Care 2001; 5: 179–181, [INFOTRIEVE], [CROSSREF], [CSA]
- McClaran S R, Wetter T J, Pegelow D F, Dempsey J A. Role of expiratory flow limitation in determining lung volumes and ventilation during exercise. J Appl Physiol 1999; 86: 1357–1366, [INFOTRIEVE], [CSA]
- Palange P, Valli G, Onorati P, Antonucci R, Paoletti P, Rosato A, Manfredi F, Serra P. Effect of heliox on lung dynamic hyperinflation, dyspnea, and exercise endurance capacity in COPD patients. J Appl Physiol 2004; 97: 1637–1642, [INFOTRIEVE], [CROSSREF], [CSA]
- Goto S, Porszasz J, Sakurai S, et al. Effect of helium breathing on dynamic hyperinflation, minute ventilation and exercise tolerance in severe COPD patients. Am J Respir Crit Care Med 2004; 169: 467A, [CSA]
- Laude E A, Duffy N C, Baveystock C, Dougill B, Campbell M J, Lawson R, Jones P W, Calverley P M. The effect of helium and oxygen on exercise performance in COPD: a randomised crossover trial. Am J Respir Crit Care Med 2006; 173: 865–870, [INFOTRIEVE], [CROSSREF], [CSA]
- O'Donnell D E, Webb K A. Mechanisms of dyspnea in COPD. Dyspnea: Mechanisms, Measurement, and Management, 2nd edition, D Mahler, D E O'Donnell. Lung Biology in Health and Disease Series, Taylor & Francis Group, Boca Raton, FL 2005; Volume 208: 29–58, Chapter 3
