Abstract
Interpreting changes in outcomes of clinical trials in chronic obstructive pulmonary disease should be viewed from a broader perspective than only the statistical significance of the findings. The minimal clinical difference in outcome measures provides a conceptual framework to assist in clinical trial interpretation and a methodology to assess the clinical relevance of study results. Use of distribution-based techniques, comparison with other external measures, and opinions from experts, clinicians and patients can assist in minimal clinically important difference development. Although the minimal clinically important difference has been suggested for a wide range of outcomes of importance in chronic obstructive pulmonary disease, many have not been subjected to rigorous analysis. For newer tools such as activity monitors and questionnaires and measures not widely employed such as laboratory-based exercise tests, minimal clinically important differences remain to be determined.
INTRODUCTION
The focus of this article is on the assessment of three important outcomes of new therapies for patients with chronic obstructive pulmonary disease (COPD): exercise, health-related quality of life, and activity. It is tempting and seemingly logical to suggest that all 3 constructs are closely related. Other articles in this supplement indicate reasonable correlations between quality of life and activity, and exercise and quality of life (Citation[1], Citation[2]). However, the evidence indicates relations are less clear between exercise and activity, and exercise and quality of life (Citation[3]). If all 3 outcomes tracked in a similar manner in response to therapeutic interventions, then it might only be necessary to assess 1 of the 3 in clinical trials. In the absence of close correlations between these distinct outcomes, clinical trials may be difficult to plan and their results difficult to interpret. Several factors combine to further complicate the use of these outcomes.
The concepts of exercise, quality of life and activity are not well understood, the measures used to assess these parameters are not routinely employed in clinical practice, there is only a fair relationship of these measures to patient symptoms, and the outcome tools and their scoring are not readily apparent to patients and physicians. In addition, the clinical significance of changes in these outcomes may not be readily apparent. This article reviews the use of MCIDs as a potential tool to interpret the results of clinical trials using exercise, activity and quality-of-life outcomes. Certainly the MCID is not the only possible way to assess results of clinical trials; other approaches such as number needed to treat and effect size may also provide valuable information in the interpretation of clinical trials.
Minimal clinically important difference
The usual standard for interpretation of clinical trials is a statistical analysis indicating that there is less than a 5% chance that the results may have occurred by chance alone. This analysis provides a clear-cut answer as to whether the results should be used to influence patient care. However, small changes in outcomes may be statistically significant but may nonetheless lack clinical relevance. In this context, the FEV1 has traditionally been a favored outcome of clinical trials of bronchodilator pharmacotherapy in COPD. Changes in FEV1 in subjects receiving the medication compared to placebo have been used as the primary outcome measure of efficacy. However, this well validated measure of lung function has not rigorously been compared to other outcomes that are of more importance to patients. It could be argued that unless a change in FEV1 was associated with corresponding improvements in shortness of breath, the major symptom leading patients to seek medical care, limitations in physical function such as walking and patient-centered outcomes such as health-related quality of life, the therapy might not be used by patients. Thus, other methods to interpret clinical trial results may be useful (Citation[4]).
The concept of MCID incorporates information about outcome interpretation that can be useful in assessing the findings of clinical trials. MCID has been defined as
the smallest difference in score of a domain of interest that patients perceive as beneficial and which would mandate, in the absence of troublesome side effects and excessive cost, a change in the patient's management (Citation[5]).
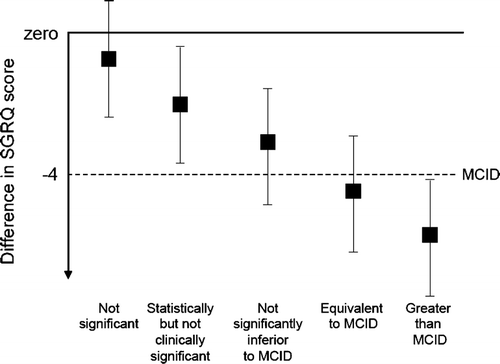
Three approaches can be initially employed to estimate an MCID: distribution (statistical), anchor (external measure), and opinion approaches () (Citation[6], Citation[7], Citation[8]). Although all approaches can provide valid estimates of MCID, the most useful final MCID might be one that has considered a multitude of approaches, tested in multiple settings, and most importantly tempered by clinical relevance (Citation[7]). Leidy and Wyrwich advocate triangulation methodology to integrate the estimates from various approaches to arrive at a final MCID (Citation[9]). It should also be recognized that a single value for MCID might not be applicable for all populations and might be specific for the disease and population under investigation. The MCID might best be considered as a range rather than a single numerical value (Citation[10]). For example, Jones recently reviewed the literature to calculate an MCID of the St. George's Respiratory Questionnaire, a disease-specific quality of life measure commonly used in COPD clinical trials (Citation[11]). This information was used to provide a range of values for the MCID and a graphic representation of how to use this MCID to interpret the results of COPD trials ().
Table 1 Methods for estimating MCID
Figure 1 A suggested taxonomy for changes in SGRQ score relative to the minimum clinically important difference (MCID). Lower score indicates better health. Error bars indicate 95% Confidence Intervals. Reproduced with permission from Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005; 2:75–79.
The distribution based or statistical approach to estimating MCID is based on the distribution of the outcome measure in a population of untreated subjects. In short, the wider the distribution of initial values or the more heterogeneous the population, the larger the change in the outcome measure needs to be in order to be considered clinically relevant. Statistical measures describing the baseline population such as the standard error of the outcome measure under investigation can be used to derive a MCID distribution estimate. One of the most widely used MCID statistical estimates uses a half standard deviation, but other methods have also been described (Citation[12], Citation[13], Citation[14]).
The anchor-based MCID estimate correlates the changes in the outcome measure of interest with other external measures. This approach is best employed if the other external measures can be considered as “gold standard” tools upon which there is universal agreement about their importance. External measures are most useful if they have established clinical relevance and if respective MCIDs have been validated. In COPD, there are few such measures that have been rigorously investigated and are universally considered of primary importance by patients and health-care providers. Nevertheless, quality of life is not infrequently used as an external anchor.
Opinions of patients and health care providers can be used to estimate MCID. Consensus approaches using group approaches in an iterative manner has been suggested as the most rigorous methodology (Citation[15], Citation[16], Citation[17], Citation[18]). One of the more intriguing uses of this approach was developed by Redelmeier et al for estimation of the MCID for the 6-minute walk test in COPD (Citation[16]). They studied 112 patients with stable COPD. Subjects were asked to rate their perceived functional capacity by rating themselves a little better or a little worse or about the same compared to other patients. They found that patients needed to differ by 54 meters (95% confidence interval of 37 to 71 meters) to start rating themselves as different than their peers. The strength of this approach has led to this range being accepted as the MCID for the 6-minute walk test.
SUMMARY
This article reviews 3 important outcomes of therapies for COPD: exercise, activity, and quality of life. Although there is a large body of literature on the use of health-related quality of life in COPD, exercise and activity as outcomes of therapies have not been as widely used in clinical trials. The meaning of changes in exercise and activity outcomes using newly developed tools to measure them is not well understood. Since these outcomes are increasingly recognized as important, efforts to define the MCID would provide patients and clinicians useful information to incorporate the results of clinical trials into practice. However, the MCID has not been rigorously investigated for all of the outcomes of importance in COPD.
The information that is currently available is summarized in . If activity and exercise are to be meaningful to patients and physicians, additional efforts should be made to define their relevance. One approach to achieve this goal is to more accurately assess the MCID of activity and exercise measures using the 3 approaches (distribution, anchor and opinion) described here.
Table 2 Suggested MCID of commonly used outcome measures in COPD2
REFERENCES
- Kaplan R M, Ries A L. Quality of life: Concept and definition. J COPD 2007, (In press)
- Jones P W. Activity limitation and quality of life in COPD. J COPD 2007, (In press)
- Berry M J. The relationship between exercise tolerance and other outcomes in COPD. J COPD 2007, (In press)
- Cohen J. Statistical power analysis for the behavioural sciences. Academic Press, LondonEngland 1969
- Jaeschke R, Singer J, Guyatt G H. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989; 10: 407–415
- Man-Son-Hing M, Laupacis A, O'Rourke K, Molnar F J, Mahon J, Chan K B, Wells G. Determination of the clinical importance of study results. J Gen Intern Med 2002; 17: 469–476
- Sloan J A. Assessing the minimally clinically significant difference: Scientific considerations, challenges, and solutions. J COPD 2005; 2: 57–62
- Guyatt G H, Osoba D, Wu A W, Wyrwich K W, Norman G R. Methods to explain the clinical significance of health status measures. Mayo Clin Proc 2002; 77: 371–383
- Leidy N K, Wyrwich K W. Bridging the gap: Using triangulation methodology to estimate minimal clinically important differences (MCIDs). J COPD 2005; 2: 157–165
- Make B, Casaburi R, Leidy N K. Interpreting results from clinical trials: Understanding minimal clinically important differences in COPD outcomes. J COPD 2005; 2: 1–5
- Jones P W. St. George's respiratory questionnaire: MCID. J COPD 2005; 2: 75–79
- Norman G R, Sloan J A, Wyrwich K W. Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care 2003; 41: 582–592
- Wells G, Beaton D, Shea B, Boers M, Simon L, Strand V, Brooks P, Tugwell P. Minimal clinically important differences: Review of methods. J Rheumatol 2001; 28: 406–412
- Wyrwich K W, Tierney W M, Wolinsky F D. Further evidence supporting a sem-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol 1999; 52: 861–873
- Hays R D, Farivar S S, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. J COPD 2005; 2: 63–67
- Redelmeier D A, Bayoumi A M, Goldstein R S, Guyatt G H. Interpreting small differences in functional status: The six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med 1997; 155: 1278–1282
- Redelmeier D A, Guyatt G H, Goldstein R S. Assessing the minimal important difference in symptoms: A comparison of two techniques. J Clin Epidemiol 1996; 49: 1215–1219
- Wyrwich K W, Fihn S D, Tierney W M, Kroenke K, Babu A N, Wolinsky F D. Clinically important changes in health-related quality of life for patients with chronic obstructive pulmonary disease: An expert consensus panel report. J Gen Intern Med 2003; 18: 196–202
