Abstract
In a recent National Research Council document, new strategies for risk assessment were described to enable more accurate and quicker assessments.(Citation1) This report suggested that evaluating individual responses through increased use of bio-monitoring could improve dose-response estimations. Identi-fication of specific biomarkers may be useful for diagnostics or risk prediction as they have the potential to improve exposure assessments. This paper discusses systems biology, biomarkers of effect, and computational toxicology approaches and their relevance to the occupational exposure limit setting process.
The systems biology approach evaluates the integration of biological processes and how disruption of these processes by chemicals or other hazards affects disease outcomes. This type of approach could provide information used in delineating the mode of action of the response or toxicity, and may be useful to define the low adverse and no adverse effect levels. Biomarkers of effect are changes measured in biological systems and are considered to be preclinical in nature. Advances in computational methods and experimental -omics methods that allow the simultaneous measurement of families of macromolecules such as DNA, RNA, and proteins in a single analysis have made these systems approaches feasible for broad application.
The utility of the information for risk assessments from -omics approaches has shown promise and can provide information on mode of action and dose-response relationships. As these techniques evolve, estimation of internal dose and response biomarkers will be a critical test of these new technologies for application in risk assessment strategies. While proof of concept studies have been conducted that provide evidence of their value, challenges with standardization and harmonization still need to be overcome before these methods are used routinely.
INTRODUCTION
Currently, little toxicity data exist for most of the 82,000 chemicals used in the United States, which greatly hampers risk assessment and management activities.(Citation2,Citation3) In addition, it is rare for workers or the general public to be exposed to only to a single compound, but rather they are exposed to complex mixtures that may have additive, synergistic, or antagonistic actions. The complexity of exposure scenarios and lack of data make risk management decisions difficult and time consuming.
The systems biology approach is based on consideration of normal biological processes (including pathways leading to effects and homeostatic and adaptive responses) and how chemicals disrupt those processes.(Citation4,Citation5) This type of approach would provide integrated information that could be used in delineating the mode(s) of action (MOA) of the adverse response or toxicity.(Citation6,Citation7) Different doses can produce widely different responses in an organism. Some of the responses are of no consequence to the health or viability of the organism, others may be beneficial (e.g., antioxidant), and others are toxic.(Citation8)
Biomarkers have been defined by the National Academy of Sciences (NAS) as measurable changes in a biological system or organism or measured alterations in structure or function ().(Citation9) Biomarkers may be indicative of exposure, response or effect, and susceptibility and can be used to monitor exposures and a wide variety of responses ranging from abnormal development to early disease indicators.(Citation9,Citation10)
Occupational exposures are mainly by inhalation or through the dermal route, while the primary route for general environmental exposures is by ingestion. Route of exposure may affect the level of internal dose and therefore the toxicity. Biomarker measurements are an aggregate of all exposure pathways. One benefit of early response biomarkers is in their interpretation within the context of integrated systems models, which connect these biomarkers to adverse outcomes of regulatory concern. Advances in computational methods(Citation11) and experimental -omics methods that allow the simultaneous measurement of families of macromolecules such as DNA, RNA, and proteins in a single analysis(Citation12) have made these systems approaches feasible for broad application in both pharmaceutical discovery(Citation11) and environmental risk assessment.(Citation6) The promise is that information on hazard characterization, dose response, and risk characterization can be generated by -omics methods and used in risk assessments.(Citation7,Citation13)
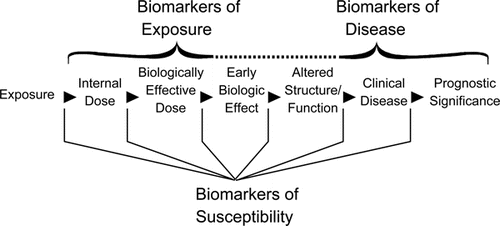
This article focuses on systems biology, biomarkers of effect/response, and computational toxicology approaches and their relevance to the occupational exposure limit (OEL) setting process. A glossary of key terms relating to this topic is provided in and acronyms are defined in .
Table 1 Glossary of Key Terms
Table NaN Glossary of Key Terms (Continued)
Key points of emphasis covered in this article include the following.
Many drivers exist for increased reliance on systems biology approaches that are pushing changes in health risk assessment methods, including for OEL setting.
Practical long-term implications of such approaches are many, including decreased uncertainty in OELs through improved understanding of biological responses at lower levels of chemical exposure.
Current methods and tools for consideration of effect biomarkers and the relationship with the toxic MOA within the framework of systems biology are being used and applied in OEL setting via proof of concept studies.
CURRENT EFFORTS ON EARLY RESPONSE BIOMARKERS AND RISK ASSESSMENT
Some efforts have been initiated in the global community to revise the way that risk assessments are conducted or to speed the data flow into risk assessments (Table III). The European Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH) program seeks to determine the risk of thousands of chemicals that are produced in quantities greater than 10 tons/year.(Citation14) Derived No Effect Levels are required for all chemicals that are classified as a health hazard. Traditional toxicity testing is unsustainable and unethical under this paradigm, because of the large number of animals that would be needed and the associated high cost.(Citation15) Efforts to establish a sustainable strategy for toxicity testing in the United States were accelerated when the National Research Council (NRC) defined a vision of toxicity testing in the 21st century that called for greater use of in vitro testing, computational system approaches, and a reduction of expensive animal testing.(Citation3,Citation16) In the NRC strategy, toxicity testing would evaluate specific perturbations in identified pathways rather than by direct evidence of adverse effects; therefore, risk assessments would be revised to incorporate this new information.(Citation17) These new technologies could be performed faster and cheaper and evaluate toxicity of a larger number of concentrations.
Shortly after the NRC report on toxicity testing,(Citation3) a separate NRC committee published recommendations on the use of toxicogenomic technologies and the need for more predictive toxicity testing for incorporation into risk assessments.(Citation1) Improvements in cross-species extrapolation, identification of vulnerable or sensitive populations, determination of life stage effects, investigation of mechanisms of action, and refinement of exposure assessments are all potential uses for toxicogenomic data.(Citation1)
Computational toxicology was the subject of a National Academy of Sciences Standing Committee on Use of Emerging Science for Environmental Health Decisions meeting in September 2009. The field of computational toxicology has emerged in an effort to build predictive models from biomarker of effect data generated by omics technologies.(Citation18) Computational toxicology identifies trends and patterns in biomarker and chemistry datasets.(Citation19) These models use chemical characterization to predict fate and transport as well as hazard identification. Computational toxicology also seeks to describe ways through which chemicals cause toxicity by developing computational tools that better utilize high throughput screening (HTS) and toxicogenomics data for hazard prediction. This includes models at varying levels of biological complexity, from relatively simple statistical models(Citation20–Citation22) to advanced dose-response and virtual tissue models.(Citation23,Citation24) The field of computational toxicology has rapidly expanded to include many more applications than HTS, which is still an evolving research area and in need of validation. Other applications are being utilized such as data mining the literature, in vitro- in vivo extrapolations, quantitative structure activity relationships, in silico models and use of National Health and Nutrition Examination Survey biomonitoring data for identification of populations at special risk of toxicity.(Citation25–Citation27)
Table 2 Definitions of Acronyms
In 2009, a third NRC publication was released that examined the EPA risk assessment process and how to practically improve it to assess human health risks.(Citation28) Two main areas of risk analysis were evaluated, technical analysis and utility of risk. Technical analysis is how scientific information is generated and used so that more accurate risk characterizations can be obtained. Utility of risk examines the relevance of the risk assessments for making risk management decisions. A key recommendation was to improve the upfront design of risk assessments to make them more useful to answer risk management needs. In particular, the report emphasized the importance of problem formulation in determining the scope of the assessment, issues needing consideration, and options so that the risk assessment can support risk management decision-making. The report also noted the importance of characterizing and communicating uncertainty and variability and of placing greater emphasis on the evaluation of risk from cumulative exposure scenarios.
Table 3 Efforts Affecting the Use of 21st Century Technologies and Risk Assessment
The EPA has initiated a program (http://www.epa.gov/risk/nexgen/) to evaluate the use of HTS, computational toxicology, and systems modeling for risk assessment and risk management for environmental exposures and the general population, though not necessarily occupational exposures.(Citation29) The vision is for a tiered system that provides risk estimates on the basis of available data as well as a formal means for recommending chemicals for higher tier investigation.
ROLE OF BIOMARKERS IN OCCUPATIONAL RISK ASSESSMENT
A major aim of biomarker research is to develop and validate biomarkers that reflect specific exposures or are quantitatively linked to adverse outcomes in humans to enable their use in risk prediction. Biomarkers have a number of advantages over “apical endpoints” typically observed in in vivo toxicology studies ().
Recent advances in biomedical technology have provided powerful tools to identify new biomarkers (Table IV). -omics technologies are increasingly being used and have brought capabilities to investigate adverse responses, underlying toxicity mechanisms, and key toxicity pathways that have the potential to be used in risk assessment (Table V).(Citation30–Citation32)
Environmental exposures can directly or indirectly cause alterations in gene expression at either the transcriptional (gene expression) or the translational level (proteomics). Development of gene expression profiles using oligonucleotide microarrays provides a view of perturbations at the transcript level and helps identify specific genes, pathways or networks that are specific to the toxic end point of interest.(Citation33) Identifying appropriate biomarkers can be difficult because interpretation of global gene expression changes is challenging as such changes may reflect nonspecific responses or overlapping/interacting molecular processes. The use of toxicogenomics data along with other types of supportive toxicological data has been considered for hazard characterization. Recent studies have shown that benchmark dose estimates, based on gene expression omics data, for non-cancer and cancer apical endpoints can be practically applied.(Citation22,Citation34)
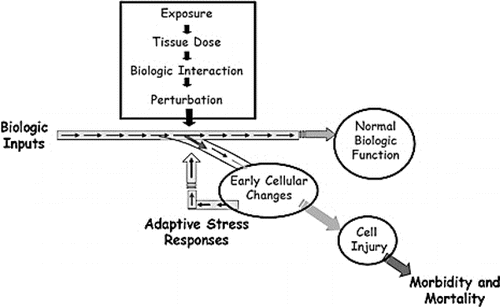
Determination of internal dose is important in risk assessment and provides highly relevant information that is more closely associated with disease response than external exposure estimates.(Citation35) The capability of -omics technologies to generate information that can be used for internal dose estimation and response markers will be important in their use in risk assessment.
Table 4 Different Types of Biomarkers
Epigenetics effects may also have an important role in the development of disease. For example, gene silencing, which is the interruption or suppression of the expression of a gene at transcriptional or translational levels, can occur with hypermethylation of DNA.(Citation36) Biomarkers of hypermethylation of DNA may be useful as early cancer detectors and therefore may have utility in risk assessments.
Although biomarkers have been identified using -omics technologies, there is no well-established standardized application of these technologies in using biomarkers in risk assessment (Table VI). Involvement of multiple molecular pathways in disease as in systems biology creates complex data analysis/interpretation challenges in validating associations between outcomes and sets of biomarkers.
The concept of the exposome, which encompasses all exposures over a lifetime, has the potential to improve risk assessment.(Citation37) The exposome will rely on -omics or other high throughput techniques for the identification of biomarkers of exposure and effect. Multiplex profiling (metabolomics, proteomics, and transcriptomics) is now being used along with complementary assays for the most comprehensive and informative views of biological systems.(Citation38) The exposome has the potential to offer more comprehensive exposure data that can be used to develop more accurate exposure profiles to improve risk assessments.
Table 5 Examples of -Omics Technologies
Most common chronic diseases involve the interaction of multiple exposures and biological pathways that ultimately lead to disease. System biology approaches have been used to study a variety of diseases such as epilepsy and metabolic syndrome and exposures such as particulate matter found in air pollution.(Citation39–Citation41) However, systems biology and how different biological processes may interact with one another to result in disease needs to be better understood to be useful in risk assessment.
The NRC(Citation3) identified several strategies to use biomarkers of effect to extrapolate dose and evaluate dose response. Physiological-based pharmacokinetic (PBPK) modeling can describe the relationship between external exposure and the internal dose (e.g., blood or tissue concentration of a toxicant) that simulate the toxicity pathways of a chemical. PBPK models can also be used to estimate an external dose (i.e., the relevant real world exposure) that would correspond to the doses used in in vitro and in vivo test systems, as well as in dose-response models to predict the environmental exposure needed to elicit a toxic response.(Citation3) Extrapolating in vitro dose-response data to predict responses in vivo has been a challenge because the doses applied in vitro have typically been much higher than cells in vivo (e.g., in the lungs) would experience even at occupational exposures.(42) An example of one approach is a range of in vitro doses (∼0.2–68 μg/ml) that was proposed based on estimates of the equivalent doses to human lung cells after either 24-hr or a 45-year working lifetime exposure to 1 mg/m3 of poorly soluble particles.(Citation42) Uncertainty about how well in vitro studies predict responses in in vivo systems includes the effect of dose rate and the role of other cells and processes in determining the in vivo response.(Citation43) An example of in vitro assays that show predictive trends of in vivo dose-response relationships is for biomarkers of inflammation in lung epithelial cell cultures and acute pulmonary inflammation in rats.(Citation44,Citation45)
Table 6 Uses of Biomarkers in Hazard Characterization and Dose-Response Analysis
Establishing a dose of concern is a primary goal of risk assessment. Several approaches that use an internal dose have also been described, including internal dose measures such as biological exposure indices(Citation46) and biomonitoring equivalents.(Citation47) Additionally, no observed effect levels and benchmark dose estimates can be applied for both internal and external dose measures.(Citation48–Citation50) These dose estimates may be used as points of departure to estimate exposures associated with lower (or presumed no) disease risk. and
Genotype-exposure interactions are particularly important for occupational and environmental diseases. Environmental and occupational triggers may interact with genetic factors to initiate the disease process or influence the clinical outcomes including time to onset, severity of the response, or dose. There has been little effort in incorporating genetic information into the risk assessment process, although the advantage of such data in improving accuracy has been discussed.(Citation51–Citation53)
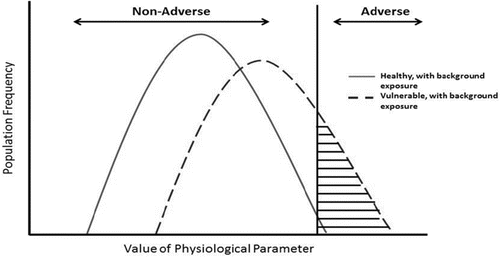
Currently, the default approach for addressing inter-individual variation in susceptibility for threshold effects is to apply a 10X uncertainty factor.(Citation54,Citation55) Note that this factor is not intended to cover the entire range of human variability. Instead, this factor addresses the difference between a “safe dose” estimate in the general population and the “safe dose” estimate in the population of interest.(Citation56) While the default size of the inter-individual factor is 10X, smaller or larger factors may be applied if supported by the available data, resulting in refined estimates of human variability. Criteria for the use of data to support other factors have been developed by the International Programme on Chemical Safety (IPCS).(Citation57) Biomarkers can play a critical role in describing the distribution of responses to a specified dose. This concept is illustrated in , in which the distribution (e.g., the biomarker for a physiologically important response) is shifted in the susceptible populations or life stages, resulting in a bi- or multi-modal overall distribution. The shape of the distribution for a given biomarker would depend on how it is distributed in the population (e.g., whether associated with specific gene alleles or results from multiple causes).
METHODS AND APPROACHES
Risk Assessment Methods and Issues
The goal of human health risk assessment is to predict the likelihood of adverse health effects before they manifest in a population. Different types of studies can provide information that has utility in risk assessments. Epidemiologic studies are important for the assessment of toxic effects directly in humans because no interspecies extrapolation is needed. Such studies are also important in estimating population-based, exposure-attributable risks. The most important challenges related to epidemiological studies are the difficulties in precise estimation of exposure, existence of confounding variables such as other exposures and considerable inter-individual variation including genetic make-up, physiological, nutritional, and lifestyle differences. Such studies are also costly and time consuming and have limitations in characterizing dose-response relationships, causal mechanisms, and extrapolating to low doses in risk assessment.(Citation58,Citation59)
Extrapolations from animal studies to humans are confounded by a number of issues, including species-specific differences in uptake and response, homogeneity of test animals as compared to heterogeneity of human populations and short-term testing as compared to complex lifetime exposure, as well as uncertainties due to gaps in the available data. Extrapolation is further complicated by levels and routes of exposure, as these factors can differ greatly between animal models and real-life exposure scenarios. With regard to interspecies extrapolation, several factors must be considered, such as dose normalization for the differences in body size, metabolic rate, variability in toxicokinetics of the chemical, and sensitivity of the target for toxicity. Occupationally exposed populations have considerable physiological and genetic variability in such factors as metabolic capacity and in toxicity response.
Efforts have been undertaken to harmonize dose-response relationships for cancer and non-cancer endpoints(Citation60) focusing on MOA as the basis for selecting dose-response models and determining extrapolation approaches.(Citation61,Citation62) In general, the default science policy choice based on MOA assumes that a threshold would not exist for substances that interact directly with DNA. This is based on the idea that damage to one DNA molecule could be fixed as a mutation and clonally expand to cancer or result in other effects, such as developmental toxicity. MOAs that do not involve direct DNA reactivity (e.g., cytotoxicity leading to either necrosis or to regenerative cell proliferation and cancer) are generally considered to have biological response thresholds, due to the existence of repair and redundant cellular processes.(Citation63) However, when data are available that provide strong evidence for alternative modes of action, these data may replace default assumptions in risk assessment and OEL derivation. For example, NIOSH(Citation64) used evidence concerning a secondary genotoxic MOA (via persistent inflammation) to inform selection of the nonlinear (but also non-threshold) dose-response models used to estimate the working lifetime risk of lung cancer from inhalation exposure to the poorly-soluble particulate titanium dioxide (TiO2).
There has been considerable discussion in the risk assessment community recently concerning the observation of non-threshold behavior for chemicals that do not interact with DNA.(Citation28,Citation65,Citation66) A threshold response for a given agent may be difficult to detect in a population (e.g., a statistical dose-response model may not be able to exclude zero as a possible threshold dose), even if the MOA evidence indicates a threshold is plausible. Reasons for observing non-threshold behavior in a population for non-carcinogens include variability in individual threshold responses or exposures that contribute to an existing disease process.(Citation67,Citation68) Rhomberg et al.(Citation66) suggested some alternative explanations, such as measurement error at low exposures in epidemiology studies, for not detecting a threshold in human studies when a threshold is observed in animal studies. Additionally, the observation of a threshold may be influenced by factors including variability and sample size in both animal studies and epidemiology studies.
Risk estimates based on extrapolating high-dose animal studies to humans may be particularly sensitive to assumptions about the MOA and shape of dose-response relationships including threshold/non-threshold assumptions. Thus, there may be considerable uncertainty in extrapolation from animal studies when the doses are considerably higher than those relevant to OEL development (e.g., if the MOA that occurs at a high dose is not relevant to that occurring at a much lower dose). Additional uncertainty may occur from temporal extrapolations, which could result in over- or under-estimation of the risk of long-term exposure.(Citation69,Citation70) Even when the MOA is known, statistical arguments cannot resolve the uncertainty in low-dose extrapolation, and so science policy choices (e.g., default approach of linear low dose extrapolation for carcinogens in the absence of strong evidence indicating otherwise) are needed in risk assessment.
One of the advantages of using biomarkers of effect is that they can help to reduce the need for extrapolation, allowing instead evaluation of effects in the dose range of interest and in the species of interest (e.g., when human cells are tested in vitro). Under ideal situations, the MOA is used to identify appropriate biomarkers, which are then evaluated sufficiently close to the dose range of interest, so that mathematical curve fitting can be used to more directly estimate risk, rather than relying on the cruder approaches of linear extrapolation (assuming no threshold) or uncertainty factors (assuming a threshold response).
The use of precursor effect data or biomarkers of early effect is gaining increased scrutiny for use in risk assessments.(Citation22, Citation71) A challenge is that many of these biomarkers lack validation.(Citation72) The basis for extrapolation between the biomarker and the toxicological outcome needs to be established so that a dose associated with a low risk of an adverse health effect can be estimated.(Citation71)
Direct Dose-Response Using Early Effects Data
The analysis of -omic dose-response studies has traditionally utilized analysis of variance (ANOVA) approaches together with pair-wise comparisons between dose groups and the corresponding control.(Citation73,Citation74) The ANOVA identifies genes that are significantly altered as a function of dose while the pair-wise comparisons identify genes that are significantly altered between specific dose pairs. The ANOVA approach for analyzing -omic dose-response studies is analogous to the methods used to define lowest observed adverse effect levels (LOAEL) or no observed adverse effect levels (NOAEL) for other toxicological endpoints (Table VII). For applying -omic dose-response data to chemical risk assessment, the traditional ANOVA approach faces several challenges in that dose spacing and the experimental sample size can have a dramatic impact on the final NOAEL and LOAEL, and the approach does not account for variability in the estimate of the dose-response or the slope of the dose-response curve.
Table 7 Effect Levels, by Severity, That are Considered in the Derivation of Exposure Limits
To utilize -omic dose-response data within the existing risk assessment paradigm, benchmark dose (BMD) methods have been used to fit a statistical model to the dose-response data and to identify a dose that causes a defined change in the endpoint of interest.(Citation22,Citation34,Citation75) The application of the BMD method provides several advantages including better use of dose-response information, more appropriate reflection of experimental sample sizes, and the lack of constraint to experimental doses.(Citation76) In this analysis, the dose and individual gene response data are fit with the standard set of statistical models used in BMD analysis. A single model is selected for each gene based on fit, modeling complexity, and the BMD and associated lower confidence limit (BMDL).
To allow investigators to interpret the -omic data and provide context for the observed BMD values, public and commercial databases are used to group genes into functional processes and signalling pathways.(Citation77,Citation78) The choice of database depends on the context required for interpreting the -omic dose response study. For certain studies, a pathway-based analysis may provide a better understanding of the underlying perturbations in the signaling networks while in other studies, an analysis focused on cellular-processes may provide better linkage with the phenotypic effects of the chemical. The BMD and BMDL values for the individual genes are summarized to represent the general behavior of the process or signaling pathway as a function of dose. In most cases, the mean or median BMD and BMDL are sufficient to capture the general dose-related perturbation of the category or pathway. In certain studies, the transcriptional BMD values for specific cellular biological processes and pathways showed a high degree of correlation with traditional non-cancer and cancer-related apical BMD values.(Citation22,Citation79) Many of the correlated processes and pathways had been implicated in non-cancer and cancer disease pathogenesis. Subsequent studies have demonstrated a high degree of correlation between transcriptional BMD values for the most sensitive pathway response and traditional non-cancer and cancer-related apical BMD values.(Citation13)
Early effects data can provide evidence about the MOA and the shape of the dose-response relationship for disease development. Epigenetic effects may alter down-stream responses and outcomes. However, to most effectively use early response and systems biology data in risk assessment and OEL derivation, predictive models are needed to link the early response with the probability of developing the frank effect (conditional on the early effect).
An example MOA involving early responses and frank effect is persistent lung inflammation associated with development of cancer.(Citation80) This effect has also been observed in animals related to inhaled, poorly-soluble particles(Citation81) including TiO2. The MOA for rat lung cancer from inhaled poorly soluble particles is generally considered to involve persistent pulmonary inflammation, which causes oxidative DNA damage.(Citation81) Driscoll et al.(Citation82) observed an increased mutation frequency in the hypoxanthine-guanine phosphoribosyl transferase gene (hprt mutations are detrimental lesions caused by oxidative damage to DNA) in alveolar type II cells from rats treated with a high mass dose (100 mg/kg) of fine-sized TiO2 or other types of poorly-soluble particles. In vitro, hprt mutation frequency was also increased in an alveolar epithelial cell line (RLE-6TN) following co-incubation with inflammatory cells (alveolar macrophages and neutrophils) derived from bronchoalveolar lavage fluid from particle-treated rats.(Citation82) Addition of catalase (an enzyme which protects cells against oxidative damage) to these co-incubations inhibited the increase in hprt mutations. These studies support a role of inflammatory cell-derived oxidants in particle-associated mutagenesis.
In risk assessment and development of recommended exposure limits for fine and ultrafine TiO2, NIOSH(Citation64) used statistical models of animal dose-response data for lung cancer and pulmonary inflammation to estimate the working lifetime risks. On the basis of a secondary genotoxic mechanism, prevention of persistent lung inflammation would be expected to prevent lung cancer by that mechanism. However, evaluation of the rat subchronic inflammation data did not show evidence of a threshold (although the dose-response relationship was nonlinear). Rat- and human-based excess risk estimates for lung cancer from working lifetime exposures to inhaled poorly-soluble particles were compared. The particles evaluated include those for which long-term dose-response data are available in both species, i.e., coal dust, carbon black, titanium dioxide, silica, and diesel exhaust particulate. The excess risk estimates derived from the rat data were generally lower than those derived from the human studies, and none of the rat- and human-based risk estimates were significantly different (all p-values>0.05).(Citation83) Given the limited data available to quantitatively evaluate the relationship between inflammation and lung cancer in rats or humans, NIOSH derived the Recommended Exposure Limits on the basis of rat dose-response data for lung tumors. NIOSH estimated the human-equivalent, 8-hr time-weighted average concentrations associated with <1/1000 excess risk of lung cancer over a working lifetime, derived from the nonlinear dose-response models fit to the rat data.(Citation64)
Biologically Based Dose Response (BBDR) Models
Risk estimates that rely on default assumptions may be uncertain to the extent that the true relationships differ from those assumptions. This uncertainty arises from the limited data that are available to inform the selection of the dose-response models and the assumptions used in interspecies and low-dose extrapolations. Risk estimates on the basis of default assumptions may overestimate the risk for a population because the default approaches are intended to be conservative in the absence of chemical-specific data.(Citation84) They may also underestimate risk in other cases (e.g., if greater individual variability exists than accounted for in the default assumptions).(Citation85,Citation86)
By utilizing measurements of biological pathway perturbations, uncertainties in the target tissue dose across species and the influence of exposure routes may be decreased, resulting in more reliable risk assessments.(Citation84) An advantage of a BBDR model is that, by describing key steps in the development of toxic effects, alternative mechanisms of action can be evaluated and compared to the data, to test hypotheses and evaluate the importance of specific assumptions. BBDR models also have the advantage of directly predicting the response at doses of interest, avoiding the threshold/non-threshold dichotomy, but they may require assumptions about the connections between dose and key events. These models can also incorporate inter-individual susceptibility and confounders such as existing diseases and background exposures.(Citation65) Although BBDR models have a number of advantages, a key issue in their use is the uncertainties associated with the parameters used in the model, as well as the substantial sensitivity of the model results to the assumptions regarding the underlying mathematical form for intermediate steps in the mechanism of action.(Citation87) However, identification of biomarkers corresponding to these intermediate steps would provide an opportunity to directly address these assumptions and reduce the uncertainty of key parameters. Verification of BBDR model predictions, as well as incorporation of population-based distributions of parameter values, may be needed for wider acceptance of these models in risk assessment and development of OELs. The International Programme on Chemical Safety(Citation88) guidance on use of physiologically based pharmacokinetic (PBPK) models in risk assessment provides a template to facilitate understanding of models by risk assessors; a key consideration is comparing the uncertainties of the PBPK/BBDR model with those of the default approach.
Biologically informed empirical dose-response modeling provides a bridge between strictly empirical models and full BBDRs. Such approaches are analogous perhaps to compartmental pharmacokinetic models, but can incorporate pharmacodynamic data using biomarkers. Like the compartmental pharmacokinetic models, the biologically-informed empirical dose-response models incorporate some chemical-specific data, but include empirical curve-fitting. The goal of such analytical methods is to improve the qualitative and quantitative description of the biological processes determining the shape of the dose-response curve, without investing the resources needed to develop and verify a BBDR model. An advantage of these approaches is the use of quantitative data on early events (biomarkers) to extend the overall dose-response curve to lower doses using biology, rather than being limited to the default choices of linear extrapolation or uncertainty factors. Using biomarkers to extend the dose-response curve towards the dose region of interest also offers the potential for better description of the dose-response relationship of chemicals with a MOA that includes contributions from both DNA-reactive and non-DNA reactive components.
Allen et al.(Citation71) developed such a model as a proof of concept for predicting risk of lung cancer given persistent lung inflammation from chronic inhalation of TiO2 in rats. A series of cause and effect functions, fit using a likelihood estimation approach, were utilized to describe the relationships between successive key events leading to the ultimate tumor response. This approach was used to evaluate a hypothesized pathway for progression from a biomarker of exposure (lung burden), through several intermediate potential biomarkers of effect, to the clinical effect of interest (lung tumor production).
Another approach to biologically informed empirical dose-response modeling was demonstrated by Hack et al.,(Citation89) who used a Bayesian network model to integrate exposure biomarkers to conduct an exposure-dose-response assessment for acute myeloid leukemia resulting from exposure to benzene. The network approach was used to evaluate and compare individual biomarkers and quantitatively link the biomarkers along the exposure-disease continuum. This work provides a quantitative approach for linking changes in biomarkers of effect both to exposure information and to changes in disease response. Such linkage can provide a scientifically valid point of departure that incorporates precursor dose-response information without being dependent on the difficult issue of a definition of adversity for precursors.
More classical mathematical approaches also have the potential for linking biomarkers to adverse effects. For example, the Hill model describes the biology of a chemical binding to a receptor, a key event in many receptor-mediated MOAs. Budinsky et al.(Citation90) used the Hill model to compare the dose-response for aryl hydrocarbon receptor-mediated CYP1A1 and CYP1A2 messenger RNA induction and enzyme activity in rat and human hepatocytes exposed to 2,3,7,8-tetrac-hlorodibenzo-p-dioxin, 2,3,4,7,8-pentachlorodibenzofuran, or 2,3,7,8-tetrachlorodibenzofuran. In an extended analysis of genome-wide transcriptomic data from the same experiment, BMD analysis of the gene expression changes showed an average 18-fold cross-species difference in potency among differentially expressed orthologs and similar differences were observed for signaling pathways.(Citation91) The data were used to support the conclusion that humans are less sensitive than rats to these aryl hydrocarbon receptor-dependent end points and to support the use of a modified uncertainty factor for extrapolating between rats and humans.
More general approaches to empirical dose-response modeling are incorporated in standard modeling methods where the mathematical form used for empirical curve-fitting is based on the presumed shape of the biological response. Thus, for example, probit modeling is typically used for modeling lethality data. Similarly, a multistage model has been used for tumor modeling, based on the multi-stage model for cancer. In an example of modifying the standard choice based on biology, Dourson et al.(Citation92) used the probit model to describe the dose-response for thyroid tumors in rats orally exposed to acrylamide. This choice was based on both improved empirical model fit compared to the multistage model, and the observation that the shape of the probit model better reflected (compared with the default linear extrapolation approach) the mixed MOA of DNA reactivity at low doses and growth stimulation at the higher doses tested in the animal bioassay.
CONCLUSIONS
Advantages and Limitations
Several key advantages to the use of biomarkers in risk assessments exist. Biomarkers are used to identify the MOA and can support the MOA in risk assessments rather than relying on general default approaches. Additionally, biomarkers can be used to characterize inter-individual variability by helping to ensure that sensitive populations are identified and adequately addressed in the assessments and to reduce uncertainty in the extrapolation of animal data to humans.(Citation53,Citation93) Another advantage of biomarkers is hypotheses are tested at doses relevant to human exposures. One ultimate goal of the use of biomarkers is to extend the dose-response curve to the range (or near the range) of the exposures of interest. This would allow one to use the biomarker data more directly to evaluate dose-response, without having to go to default approaches of linear or nonlinear extrapolation. Such data could be used to establish more appropriate OELs to protect individuals who are at high risk. Systems biology and MOA approaches will also lead to new hypotheses and ways of thinking about chemical risk assessments and hence move the entire field of risk assessment forward.
While early biomarkers of effect have great promise, many limitations and challenges need to be overcome before early effect biomarkers can be reliably used. The whole field of computational toxicology and systems biology is still evolving and results have not been validated in human populations. Appropriate interpretation and validation of biomarker results is lacking.
Special Issues in Applying These Approaches for OEL Setting
Developing OELs on the basis of early effects dose-response data means that more sensitive, relevant endpoints could be targeted for prevention. If these biomarkers can be validated to ensure they represent an adverse effect, it may be possible to reverse a deleterious exposure before the disease has progressed. These precursor events (i.e., detected using a biomarker) might be preclinical but could be associated with an increased susceptibility to develop the disease effect. Setting OELs to prevent early adverse effects may help to prevent material impairment of health and functional capacity as a result of workplace exposure. However, a challenge is to determine the linkage between early effects, which may not yet constitute material impairment of health and functional capacity, and the later adverse outcomes.
Since the risk of preclinical responses have not been well defined with respect to what those biomarkers mean to health, this presents a challenge in how to utilize early effects data in a standardized, harmonized risk assessment strategy across agents and cancer and non-cancer endpoints, as recommended by the NRC.(Citation28) The use of BBDR models to quantitatively link early preclinical changes to apical endpoints of regulatory concern may mitigate this problem in the future.
Standardization is an important issue in the use of biomarkers, although the issue is not unique to the biomarker-based risk assessments. In an approach based on the NOAEL/LOAEL with uncertainty factors, the NOAEL may be based on a range of different responses or severity of response at the corresponding LOAEL. This severity of the endpoint may be addressed in the magnitude of the uncertainty factor applied to the LOAEL, but this is a relatively crude approach. One of the advantages of the BMDL is that it is based on a response level, but differences in severity of the endpoint can still lead to inconsistencies.
Early biological effects using a systems biology approach and computational toxicology efforts offer great promise for the future of risk assessment. Information on these effects can be generated using HTS providing needed information quicker and cheaper than conventional animal testing. Proof of concept studies in computational toxicology provide early evidence of their promise in utilizing early biomarkers in establishment of dose.(Citation22,Citation34) However, challenges such as standardization and validation still need to be overcome before these methods are used in routine risk assessments.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Andrew Maier for his contribution and thoughtful insight in the areas of systems biology and risk assessment.
FUNDING
Funding for Russell Thomas's contribution to this document was provided by the American Chemistry Council's Long Range Research Initiative.
DISCLAIMER
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health. This manuscript has been subjected to review by the National Health and Environmental Effects Research Laboratory and the National Center for Environmental Assessment of the Environmental Protection Agency and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does the mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- National Research Council (NRC): Applications of Toxicogenomic Technologies to Predictive Toxicology and Risk Assessment. Washington, D.C.: The National Academies Press, 2007.
- U.S. Government Accounting Office: Chemical Regulation: Options Exist to Improve EPA's Ability to Assess Health Risks and Manage its Chemical Review Program, 2005. Available at http://www.gao.gov/new.items/d05458.pdf) (accessed January 7, 2013).
- National Research Council (NRC): Toxicity Testing in The 21st Century: A Vision and a Strategy. Washington, D.C.: The National Academies Press, 2007.
- Kitano, H.: Computational systems biology. Nature 420(6912):206–210 (2002).
- Kohl, P., E.J. Crampin, T.A. Quinn, and D. Noble: Systems biology: an approach. Clin. Pharmacol. Ther. 88(1):25–33 (2010).
- Edwards, S.W., and R.J. Preston: Systems biology and mode of action based risk assessment. Toxicol. Sci. 106(2):312–318 (2008).
- McHale, C.M., L. Zhang, A.E. Hubbard, and M.T. Smyth: Toxicogenomic profiling of chemically exposed humans in risk assessment. Mutat. Res. 705(3):172–183 (2010).
- Holsapple, M.P., and K.B. Wallace: Dose response considerations in risk assessment–an overview of recent ILSI activities. Toxicol. Lett. 180(2):85–92 (2008).
- National Research Council (NRC): Biological markers in environmental health research. Environ. Health Perspect. 74:3–9 (1987).
- Schulte, P.A., and F.P. Perera: Validation. In Molecular Epidemiology: Principles and Practices, P.A. Schulte and F.P. Perera (eds.). San Diego, CA: Academic Press, 1993.
- Schadt, E.E.: Molecular networks as sensors and drivers of common human diseases. Nature 461(7261):218–223 (2009).
- Aardema, M.J., and J.T. MacGregor: Toxicology and genetic toxicology in the new era of “toxicogenomics”: Impact of “-omics” technologies. Mutat. Res. 499(1):13–25 (2002).
- Thomas, R.S., S.C. Wesselkamper, N.C. Wang et al.: Temporal concordance between apical and transcriptional points of departure for chemical risk assessment. Toxicol. Sci. 134(1):180–194 (2013).
- Boogaard, P.J., S.M. Hays, and L.L. Aylward: Human biomonitoring as a pragmatic tool to support health risk management of chemicals: examples under the EU REACH programme. Regul. Toxicol. Pharmacol. 59(1):125–132 (2011).
- Stephens, M.L.: An animal protection perspective on 21st century toxicology. J. Toxicol. Environ. Health B Crit. Rev. 13(2–4):291–298 (2010).
- Andersen, M.E., and D. Krewski: Toxicity testing in the 21st century: bringing the vision to life. Toxicol. Sci. 107(2):324–330 (2009).
- Krewski, D., M. Westphal, M. Al-Zoughool, M.C. Croteau, and M.E. Andersen: New directions in toxicity testing. Ann. Rev. Publ. Health 32:161–178 (2011).
- Kavlock, R.J., G. Ankley, J. Blancato et al.: Computational toxicology: a state of the science mini review. Toxicol. Sci. 103(1):14–27 (2008).
- Rusyn, I., and G.P. Daston: Computational toxicology: realizing the promise of the toxicity testing in the 21st century. Environ. Health Perspect. 118(8):1047–1050 (2010).
- Reif, D.M., M.T. Martin, S.W. Tan et al.: Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ. Health Perspect. 118(12):1714–1720 (2010).
- Judson, R.S., R.J. Kavlock, R.W. Setzer et al.: Estimating toxicity-related biological pathway altering doses for high-throughput chemical risk assessment. Chem. Res. Toxicol. 24(4):451–62 (2011).
- Thomas, R.S., H.J. Clewell, III, B.C. Allen et al.: Application of transcriptional benchmark dose values in quantitative cancer and noncancer risk assessment. Toxicol. Sci. 120(1):194–205 (2011).
- Shah, I., and J. Wambaugh: Virtual tissues in toxicology. J. Toxicol. Environ. Health B Crit. Rev. 13(2–4):314–328 (2010).
- Zhang, Q., S. Bhattacharya, M.E. Andersen, and R.B. Conolly: Computational systems biology and dose-response modeling in relation to new directions in toxicity testing. J. Toxicol. Environ. Health B Crit. Rev. 13(2–4):253–276 (2010).
- Ruiz, P., M. Mumtaz, J. Osterloh, J. Fisher, and B.A. Fowler: Interpreting NHANES biomonitoring data, cadmium. Toxicol. Lett. 198(1):44–48 (2010).
- Fowler, B.A.: Biomarkers in Toxicology and Risk Assessment. In Molecular, Clinical and Environmental Toxicology, A. Luch (ed.). Heidelberg, Germany: Springer Basel AG, 2012. pp. 459–470.
- Fowler, B.A.: Computational Toxicology: Applications for Risk Assessment. Amsterdam, The Netherlands: Elsevier Publishers, 2013.
- National Research Council (NRC): Science and Decisions: Advancing Risk Assessment. Washington, D.C.: The National Academies Press, 2009.
- Cote, I., P.T. Anastas, L.S. Birnbaum et al.: Advancing the next generation of health risk assessment. Environ. Health Perspect. 120(11):1499–1502 (2012).
- Dix, D.J., K.A. Houck, M.T. Martin, A.M. Richard, R.W. Setzer, and R.J. Kavlock: The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol. Sci. 95(1):5–12 (2007).
- Casciano, D.A., and J. Woodcock: Empowering microarrays in the regulatory setting. Nat. Biotechnol. 24(9):1103 (2006).
- Fostel, J.M.: Future of toxicogenomics and safety signatures: balancing public access to data with proprietary drug discovery. Pharmacogenomics 8(5):425–430 (2007).
- Brown, P.O., and D. Botstein: Exploring the new world of the genome with DNA microarrays. Nat. Genet. 21(1 Suppl):33–37 (1999).
- Thomas, R.S., B.C. Allen, A. Nong et al.: A method to integrate benchmark dose estimates with genomic data to assess the functional effects of chemical exposure. Toxicol. Sci. 98(1):240–248 (2007).
- Aylward, L.L., and S.M. Hays: Biomonitoring-based risk assessment for hexabromocyclododecane (HBCD). Int. J. Hyg. Environ. Health 214(3):179–187 (2011).
- Fukushige, S., and A. Horii: DNA methylation in cancer: a gene silencing mechanism and the clinical potential of its biomarkers. Tohoku J. Exp. Med. 229(3):173–185 (2013).
- Wild, C.P.: Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol. Biomark. Prev. 14(8):1847–1850 (2005).
- Lesko, L.J., and A.J. Atkinson, Jr.: Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Ann. Rev. Pharmacol. Toxicol. 41:347–366 (2001).
- Mittal, S., A.K. Shah, D.T. Barkmeier, and J.A. Loeb: Systems biology of human epilepsy applied to patients with brain tumors. Epilepsia 54(Suppl 9):35–39 (2013).
- Lusis, A.J., A.D. Attie, and K. Reue: Metabolic syndrome: from epidemiology to systems biology. Nat. Rev. Genet. 9(11):819–830 (2008).
- Wang, T., J.G. Garcia, and W. Zhang: Epigenetic regulation in particulate matter-mediated cardiopulmonary toxicities: a systems biology perspective. Curr. Pharmacogen. Person Med. 10(4):314–321 (2012).
- Gangwal, S., J.S. Brown, A. Wang et al.: Informing selection of nanomaterial concentrations for ToxCast in vitro testing based on occupational exposure potential. Environ. Health Perspect. 119(11):1539–1546 (2011).
- Oberdörster, G.: Nanotoxicology: in vitro-in vivo dosimetry. Environ. Health Perspect. 120(1):A13 (2012); author reply A.
- Donaldson, K., P.J. Borm, G. Oberdorster, K.E. Pinkerton, V. Stone, and C.L. Tran: Concordance between in vitro and in vivo dosimetry in the proinflammatory effects of low-toxicity, low-solubility particles: the key role of the proximal alveolar region. Inhal. Toxicol. 20(1):53–62 (2008).
- Rushton, E.K., J. Jiang, S.S. Leonard et al.: Concept of assessing nanoparticle hazards considering nanoparticle dosemetric and chemical/biological response metrics. J. Toxicol. Environ. Health A 73(5-6):445–461 (2010).
- American Conference of Governmental Industrial Hygienists (ACGIH): “TLV/BEI Resources.” Available at http://www.acgih.org/TLV/ (accessed January 6, 2014).
- Hays, S.M., L.L. Aylward, J.S. LaKind et al.: Guidelines for the derivation of biomonitoring equivalents: report from the Biomonitoring Equivalents Expert Workshop. Regul. Toxicol. Pharmacol. 51(3 Suppl):S4–S15 (2008).
- Crump, K.S.: A new method for determining allowable daily intakes. Fundam. Appl. Toxicol. 4(5):854–871 (1984).
- Crump, K.S.: Calculation of benchmark doses from continuous data. Risk Anal. 15(1):79–89 (1995).
- U.S. Environmental Protection Agency: Benchmark Dose Technical Guidance. 2012. (http://www.epa.gov/raf/publications/pdfs/benchmark_dose_guidance.pdf). (Accessed January 6, 2014).
- Lohmueller, K.E., C.L. Pearce, M. Pike, E.S. Lander, and J.N. Hirschhorn: Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat. Genet. 33(2):177–182 (2003).
- Demchuk, E., B. Yucesoy, V.J. Johnson et al.: A statistical model for assessing genetic susceptibility as a risk factor in multifactorial diseases: lessons from occupational asthma. Environ. Health Perspect. 115(2):231–234 (2007).
- Scinicariello, F., A. Yesupriya, M.H. Chang, and B.A. Fowler: Modification by ALAD of the association between blood lead and blood pressure in the U.S. population: results from the Third National Health and Nutrition Examination Survey. Environ. Health Perspect. 118(2):259–264 (2010).
- Renwick, A.G., and N.R. Lazarus: Human variability and noncancer risk assessment: an analysis of the default uncertainty factor. Regul. Toxicol. Pharmacol. 27(1):3–20 (1998).
- U.S. Environmental Protection Agency: “A Review of The Reference Dose and Reference Concentration Processes.” 2002. Available at http://www.epa.gov/raf/publications/pdfs/rfd-final.pdf (accessed January 6, 2014).
- Dourson, M., G. Charnley, and R. Scheuplein: Differential sensitivity of children and adults to chemical toxicity. II. Risk and regulation. Regul. Toxicol. Pharmacol. 35(3):448–467 (2002).
- International Programme on Chemical Safety (IPCS): “Harmonization Project Document No. 2: Chemical-Specific Adjustment Factors for Interspecies Differences and Human Variability: Guidance Document for Use of Data in Dose/Concentration-Response Assessment.” 2005. Available at http://whqlibdoc.who.int/publications/2005/9241546786_eng.pdf (accessed January 7, 2014).
- Samet, J.M., R. Schnatter, and H. Gibb: Epidemiology and risk assessment. Am. J. Epidemiol. 148(10):929–936 (1998).
- Stayner, L.T., and R.J. Smith: Methodologic issues in using epidemiologic studies of occupational cohorts for cancer risk assessment. Epidemiol. Prev. 14(53):32–39 (1992).
- Bogdanffy, M.S., G. Daston, E.M. Faustman et al.: Harmonization of cancer and noncancer risk assessment: proceedings of a consensus-building workshop. Toxicol. Sci. 61(1):18–31 (2001).
- U.S. Environmental Protection Agency: Guidelines for Carcinogen Risk Assessment. Washington, D.C.: U.S. Environmental Protection Agency, 2005, publication no. Report #EPA/630/P-03/001b)
- Boobis, A.R., J.E. Doe, B. Heinrich-Hirsch et al.: IPCS framework for analyzing the relevance of a noncancer mode of action for humans. Crit. Rev. Toxicol. 38(2):87–96 (2008).
- Haber, L.T., J.E. Strawson, A. Maier, I.M. Baskerville-Abraham, A. Parker, and M.L. Dourson: Noncancer Risk Assessment: Principles and Practice in Environmental and Occupational Settings. In Patty's Toxicology Sixth edition, E. Bingham, and B. Cohrssen (eds.). New York: John Wiley & Sons, Inc., 2012. pp. 89–132.
- U.S. Department of Health and Human Services: Current Intelligence Bulletin 63: Occupational Exposure to Titanium Dioxide. Cincinnati, OH: Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, 2011, (Rice F publication no. 2011-160)
- White, R.H., I. Cote, L. Zeise et al.: State-of-the-science workshop report: issues and approaches in low-dose-response extrapolation for environmental health risk assessment. Environ. Health Perspect. 117(2):283–287 (2009).
- Rhomberg, L.R., J.E. Goodman, L.T. Haber et al.: Linear low-dose extrapolation for noncancer heath effects is the exception, not the rule. Crit. Rev. Toxicol. 41(1):1–19 (2011).
- Crump, K.S., D.G. Hoel, C.H. Langley, and R. Peto: Fundamental carcinogenic processes and their implications for low dose risk assessment. Cancer Res. 36(9 pt.1):2973–9 (1976).
- Lutz, W.K.: Susceptibility differences in chemical carcinogenesis linearize the dose-response relationship: threshold doses can be defined only for individuals. Mutat. Res. 482(1–2):71–76 (2001).
- Jarabek, A.M.: Considerations of temporal toxicity challenges current default assumptions. Inhal. Toxicol. 7:927–946 (1995).
- Kalberlah, F., U. Föst, and K. Schneider: Time extrapolation and interspecies extrapolation for locally acting substances in case of limited toxicological data. Ann. Occup. Hyg. 46(2):175–185 (2002).
- Allen, B., A.M. Maier, A. Willis, and L.T. Haber: “Use of Early Effect Biomarker Data to Enhance Dose-Response Models of Lung Tumors in Rats Exposed to Titanium Dioxide.” Cincinnati, OH: Toxicology Excellence for Risk Assessment (TERA); 2013. Available at http://www.tera.org/Publications/Publications.html (accessed January 6, 2014).
- Maier, A., R.E. Savage, Jr., and L.T. Haber: Assessing biomarker use in risk assessment–a survey of practitioners. J. Toxicol. Environ. Health A 67(8–10):687–695 (2004).
- Kerr, M.K., and G.A. Churchill: Statistical design and the analysis of gene expression microarray data. Genet. Res. 77(2):123–128 (2001).
- Stekel, D.: Microarray Bioinformatics. Cambridge, U.K.: Cambridge University Press, 2003.
- Yang, L., B.C. Allen, and R.S. Thomas: BMDExpress: a software tool for the benchmark dose analyses of genomic data. BMC Genom. 8:387 (2007).
- Filipsson, A.F., S. Sand, J. Nilsson, and K. Victorin: The benchmark dose method–review of available models, and recommendations for application in health risk assessment. Crit. Rev. Toxicol. 33(5):505–542 (2003).
- Kanehisa, M., S. Goto, M. Furumichi, M. Tanabe, and M. Hirakawa: KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 38(Database issue):D355–360 (2010).
- Bureeva, S., and Y. Nikolsky: Quantitative knowledge-based analysis in compound safety assessment. Expert. Opin. Drug Metab. Toxicol. 7(3):287–298 (2011).
- Thomas, R.S., M.B. Black, L.Li et al.: A comprehensive statistical analysis of predicting in vivo hazard using high-throughput in vitro screening. Toxicol. Sci. 128(2):398–417 (2012).
- Katabami, M., H. Dosaka-Akita, K. Honma et al.: Pneumoconiosis-related lung cancers: preferential occurrence from diffuse interstitial fibrosis-type pneumoconiosis. Am. J. Respir. Crit. Care Med. 162(1):295–300 (2000).
- Schins, R.P., and A.M. Knaapen: Genotoxicity of poorly soluble particles. Inhal. Toxicol. 19 Suppl 1:189–198 (2007).
- Driscoll, K.E., L.C. Deyo, J.M. Carter, B.W. Howard, D.G. Hassenbein, and T.A. Bertram: Effects of particle exposure and particle-elicited inflammatory cells on mutation in rat alveolar epithelial cells. Carcinogenesis 18(2):423–430 (1997).
- Kuempel, E.D., R.J. Smith, D.A. Dankovic, and L.T. Stayner: Rat- and human-based risk estimates of lung cancer from occupational exposure to poorly-soluble particles: a quantitative evaluation. J. Phys. Conf. Ser. 151:012011 (2009).
- Conolly, R.B.: The use of biologically based modeling in risk assessment. Toxicology 181–182:275–279 (2002).
- Hattis, D., P. Banati, and R. Goble: Distributions of individual susceptibility among humans for toxic effects. How much protection does the traditional tenfold factor provide for what fraction of which kinds of chemicals and effects?. Ann. NY Acad. Sci. 895:286–316 (1999).
- Hattis, D., and K. Silver: Human interindividual variability–a major source of uncertainty in assessing risks for noncancer health effects. Risk Anal. 14(4):421–431 (1994).
- Crump, K.S., C. Chen, W.A. Chiu et al.: What role for biologically based dose: response models in estimating low-dose risk?. Environ. Health Perspect. 118(5):585–588 (2010).
- International Programme on Chemical Safety (IPCS): “Characterization and Application of Physiologically Based Pharmacokinetic Models in Risk Assessment.” 2010. Available at http://www.who.int/ipcs/methods/harmonization/areas/pbpk_models.pdf (accessed January 7, 2014).
- Hack, C.E., L.T. Haber, A. Maier et al.: A Bayesian network model for biomarker-based dose response. Risk Anal. 30(7):1037–1051 (2010).
- Budinsky, R.A., E.L. LeCluyse, S.S. Ferguson, J.C. Rowlands, and T. Simon: Human and rat primary hepatocyte CYP1A1 and 1A2 induction with 2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3,7,8-tetrachlorodibenzofuran, and 2,3,4,7,8-pentachlorodibenzofuran. Toxicol. Sci. 118(1):224–235 (2010).
- Black, M.B., R.A. Budinsky, A. Dombkowski et al.: Cross-species comparisons of transcriptomic alterations in human and rat primary hepatocytes exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 127(1):199–215 (2012).
- Dourson, M., R. Hertzberg, B. Allen et al.: Evidence-based dose-response assessment for thyroid tumorigenesis from acrylamide. Regul. Toxicol. Pharmacol. 52(3):264–289 (2008).
- Demchuk, E., P. Ruiz, S. Chou, and B.A. Fowler: SAR/QSAR methods in public health practice. Toxicol. Appl. Pharmacol. 254(2):192–197 (2011).
- Andersen, M.E., J.E. Dennison, R.S. Thomas, and R.B. Conolly: New directions in incidence-dose modeling. Trends Biotechnol. 23(3):122–127 (2005).
- Woodruff, T., E. Wells, E. Holt, D.E. Burgin, and D.A. Axelrad: Estimating risk from ambient concentrations of acrolein across the United States. Environ. Health Perspect. 115(3):410–415 (2007).
- U.S. Environmental Protection Agency: “Terminology Services.” 2012. Available at http://ofmpub.epa.gov/sor_internet/registry/termreg/home/overview/home.do (accessed January 7, 2014).
- National Research Council (NRC): Human Biomonitoring for Environmental Chemicals. Washington, D.C.: The National Academies Press, 2006.
- Collins, F.B., and V.S. Vaidya: Novel technologies for the discovery and quantitation of biomarkers of toxicity. Toxicology 245:167–174 (2008).
- Kaddurah-Daouk, R., B.S. Kristal, and R.M. Weinshilboum: Metabolomics: a global biochemical approach to drug response and disease. Ann. Rev. Pharmacol. Toxicol. 48:653–683 (2008).
- Wishart, D.S.: Applications of metabolomics in drug discovery and development. Drugs R&D 9(5):307–322 (2008).
- U.S. Environmental Protection Agency: “Advancing the Next Generation (NexGen) of Risk Assessment.” 2013. Available at http://www.epa.gov/risk/nexgen/) (accessed January 7, 2014).
- World Health Organization (WHO): “Environmental Health Criteria 222: Biomarkers in Risk Assessment: Validity and Validation.” 2001. Available at http://www.inchem.org/documents/ehc/ehc/ehc222.htm (accessed January 17, 2014).
- U.S. Environmental Protection Agency: Methods for Derivation of Inhalation Reference Concentrations and Application of Inhalation Dosimetry. Research Triangle Park, NC: U.S. Environmental Protection Agency, 1994, publication no. Report #EPA/600/8-90/066F.

