abstract
Wound healing is a complex physiological process including overlapping phases (hemostatic/inflammatory, proliferating and remodeling phases). Every alteration in this mechanism might lead to pathological conditions of different medical relevance. Treatments for chronic non-healing wounds are expensive because reiterative treatments are needed. Regenerative medicine and in particular mesenchymal stem cells approach is emerging as new potential clinical application in wound healing.
In the past decades, advance in the understanding of molecular mechanisms underlying wound healing process has led to extensive topical administration of growth factors as part of wound care. Currently, no definitive treatment is available and the research on optimal wound care depends upon the efficacy and cost-benefit of emerging therapies.
Here we provide an overview on the novel approaches through stem cell therapy to improve cutaneous wound healing, with a focus on diabetic wounds and Systemic Sclerosis-associated ulcers, which are particularly challenging. Current and future treatment approaches are discussed with an emphasis on recent advances.
Abbreviations
| ADSCs | = | Adipose derived stem cells |
| APCs | = | antigen presenting cells |
| BM-MSCs | = | Bone Marrow Mesenchymal Stem Cells |
| EGF | = | Epidermal Growth Factor |
| E-MSCs | = | Endometrium Mesenchymal Stem Cells |
| FDA | = | Food and Drug Administration |
| FGF-2 | = | Fibroblast Growth Factor 2 |
| GM-CSF | = | Granulocyte Macrophage Colony-Stimulating Factor |
| GMP | = | Good Manufacturing Practices |
| IL | = | Interleukin |
| INF-ɣ | = | Interferon Gamma |
| MSCs | = | Mesenchymal stem cells |
| NK | = | Natural Killer |
| PDGF | = | Platelet derived growth factor |
| PGE2 | = | Prostaglandin E2 |
| SDF-1 | = | Stromal Cell-Derived Factor 1 |
| SSc | = | Systemic Sclerosis |
| SVF | = | stromal vascular fraction |
| TGF-β | = | Transforming Growth Factor Beta |
| TNF-α | = | Tumor Necrosis Factor Alpha |
| UCB-MSCs | = | Umbilical Cord Blood Mesenchymal Stem Cells |
| VEGF | = | Vascular Endothelial Growth Factor |
| vWF | = | Von Willebrand factor |
Introduction
A wound is defined as a disruption of the normal anatomic structure and functional integrity of the skin.Citation1 Chronic or non-healing wounds are wounds that do not progress through the normal wound healing process, resulting in an open laceration of varying degrees of severity.Citation2 These conditions may be associated to a number of different pathological conditions such as diabetes,Citation3 venous stasis,Citation4 autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, Crohn’s disease,Citation5 and and Systemic Sclerosis (SSc),Citation6 for which no definitive therapies are currently available. It has been suggested that all the aforementioned pathological conditions generally lead to a hyper-inflammatory environment that contributes to the impairment of the physiological healing processes.
It has been estimated that wound lesions affect more than 5.7 million people in the US, and that the relative annual health care costs account approximately for 20 billion US dollars.Citation7 Among the 150 millions of patients affected with diabetes worldwide, 15% suffer from foot ulcerations which often evolve into non-healing chronic wounds.Citation8 Furthermore, annually, 2.5 million pressure ulcers need treatment in the US,Citation9 600,000 patients suffer from venous ulcersCitation10 and 1.1 million burn injuries require medical attention.Citation11
Wound healing is characterized by a multi-step interactive process that leads to the restoration of a functional dermis/epidermis layer and revascularization of the skin.Citation12 The initial phase of healing is characterized by an inflammatory reaction aimed at controlling bleeding at the wound site through a complex interaction of activated cells with coagulation proteins and complement mediators. The latter help the recruitment and the infiltration of neutrophils and mast cells that clear the wound from dead cells, debris, foreign particles and bacteria. This process leads to the formation of granulation tissue that favors the transition from inflammation to repair.Citation12
Patients with autoimmune diseases, such as diabetes or SSc, have experienced vascular damage, that leads to severe complications such as ulcers often associated with severe pain strongly impairing daily life activities.Citation13,14 In these patients peripheral ulcerations are sustained by chronic local inflammation, that prevents healing processes.Citation14,15
Conventional therapies for non-healing wounds have been primarily targeted on the determinants of such chronic inflammation by enhancing tissue repair through the use of specific growth factors (GFs). Among these, becaplermin, a recombinant human platelets-derived growth factor (PDGF), has been approved for the treatment of diabetic wounds in association with good local care. Limitations to the use of this GFs are: malignancies, infections at the site of the ulcer and the contraindication in the case of ulcers with a diameter > than 5 cm2 or ulcers that require prolonged treatment ulcers to treat.Citation16 Furthermore, the clinical outcome of these approaches has been discouraging as the efficacy of these drugs is jeopardized by specific neutralization and/or biological degradation. Finally, a recent Cochrane Database Systematic Review found that many randomized clinical trials were conducted with a high risk of bias, and that further trials are needed before drawing a firm conclusion that GFs are able to increase the likelihood of a complete wound healing in diabetic foot ulcers.Citation17
Cell-based therapies are being gradually introduced into routine medical care to manage skin wounds because they can repair/replace damaged tissue with a normal one due to their natural ability to produce cytokines and molecules necessary for wound healing. Mesenchymal stem cell therapy, with particular focus on adipose derived stem cells (ADSCs), is a novel approach for the treatment of chronic non-healing wounds through: (1) structural repair via cellular differentiation; (2) immune-modulation; (3) secretion of growth factors that drive neovascularization and re-epithelialization; and (4) mobilization of resident stem cells.Citation11
Mesenchymal Stem Cells Description
Mesenchymal stem cells (MSCs) are multipotent and self-renewable progenitor cells, identified for the first time in the bone marrow in the ’50s as fibroblast precursors. After their discovery, MSCs have been isolated in several tissues, including adipose tissue, bone marrow, umbilical cord blood, peripheral blood, endometrium, dental pulp, dermis, amniotic fluid,Citation12-15,18,19 as well as in tumors.Citation20
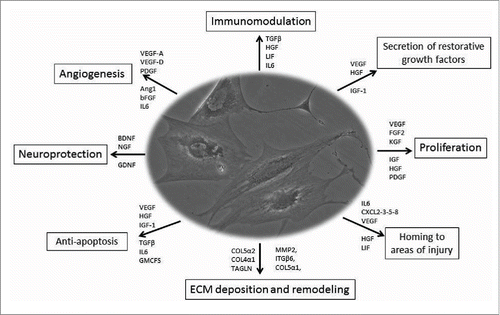
Regardless of their origin, MSCs exhibit a wide differentiating potential, since they are able to give rise to specialized cells of mesodermal origin (i.e., osteocytes, adipocytes, chondrocytes, myoblasts, and tenocytes), and to differentiate into cells of ectodermal origin.Citation19 Even though MSCs are usually defined by their ability to differentiate into tissues in vitro, their trophic, paracrine and immunomodulatory functions are those that may have the greatest therapeutic impact in vivo.Citation22 A large body of medical literature indicates that MSCs are able to repair damaged tissues, because they can migrate toward injured sites in response to inflammation, differentiate into cells and influence the microenvironment through the release of molecules involved in reparative processes and tissue regeneration such as cytokines (i.e., PGE2, GM-CSF, IL-1, RA, IL-7, IL-8, IL-10, and IL-11), chemokines (as SDF-1) and growth factorsCitation22–25 (). In addition, MSCs participate to tissue rescue through pro-angiogenic, anti-fibrotic, and anti-apoptotic pathwaysCitation26–28 and a strict correlation between MSCs and blood vessel density in stromal vascularized tissues exists.Citation29 Stem cells live and reproduce themselves in the morpho-functional unit called niche, in which a huge network of messages (i.e., the “secretome”), is fashioned through the embedded cells.Citation30 MSCs play a pivotal role in all the phases of the healing process that starts at the wound margin where epidermal cells proliferate and new blood capillaries grow to form granulation tissue. Furthermore, MSCs stimulate endothelial cell recruitment through the secretion of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), modulate tumor necrosis factor-α (TNF-α) production and reduce Natural Killer (NK) cell function in the inflammatory phase, lowering interferon-γ (IFN-γ) activity in the process. In the last phase of wound healing, MSCs modulate scar formation through PGE2 secretion, IL-10 up-regulation, IL-6 and IL-8 down-regulation and reduction of collagen production.Citation31–33 Finally, MSCs have immunomodulatory properties through the production of anti-inflammatory cytokines and the inhibition of CD4+ and CD+8 T cells, B-cells, and NK cells proliferation.Citation34 On the basis of safety and efficacy in preclinical and clinical preliminary reports,Citation21 MSC therapy represents a method to treat conditions that currently result in generally poor outcomes or invasive surgery. Indeed, MSC require further investigations to determine in vivo distribution of cells and their therapeutic mechanisms, to optimize its use in personalized regenerative medicine.
Figure 1. Mesenchymal stem cell therapy: role and function Depending on the microenvironment, MSCs are able to secrete several factors which may exert different functions via the release of different types of molecules involved in angiogenesis, immunomodelation, homing, ECM deposition and remodelling, proliferation, anti-apoptosis, and neuroprotection. Citation26-28
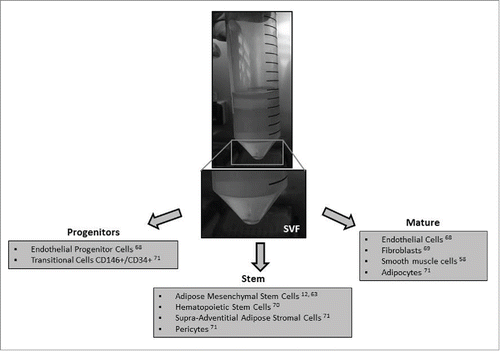
Types of mesenchymal stem cell
In 2006, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) defined the minimal criteria to define the phenotype of MCSs: i) ability to adhere in culture conditions, ii) surface expression of CD105, CD73 and CD90, but not of CD45, CD34 CD14, CD11b, CD79a, CD19 and HLA-DR, and iii) differentiation ability toward osteocytes, chondrocytes and adipocytes.Citation35 Although ISCT criteria require CD34 negativity, recent reports demonstrate that MSCs originated from adipose tissue express CD34 as a progenitor marker that distinguishes a distinct subset of cells with pronounced differentiation capacity.Citation36 MSCs can be derived from several tissues, but the best source to develop MSC-based regenerative therapies has not been identified yet.
Bone marrow mesenchymal stem cells (BM-MSCs)
Bone marrow is constituted by a heterogeneous cell population of stromal cells forming the niche responsible for the maintenance of haematopoietic stem cells. In vitro culture of BM-MSCs shows that this population is composed of a mix of tri-, bi-, and mono-potent cells. This heterogeneity could determine the BM-MSCs growth, senescence and differentiation potentials. Recent reports on direct injection of BM-MSCs into injured tissues demonstrated improved repair through mechanisms of differentiation and/or release of paracrine factors.Citation37–38
Although bone marrow represents the main source of MSCs, this has some limitations. Indeed, the aspiration of BM-MSCs is an invasive procedure, the amount of cells is modest and their differentiation potential decreases with age.Citation39,40
Umbilical cord blood mesenchymal stem cells (UCB-MSCs)
An alternative and attractive source of MSCs is represented by umbilical cord blood that is easier to be collected than bone marrowCitation41 and shows interesting immunoregulatory properties.Citation42 Several reports show the therapeutic potential of UCB-MSCs in humans. There is evidence that UCB-MSCs can improve wound healing and UCB-MSCs CD34+ cells were employed to treat skin wounds refractory to conventional treatment including surgery.Citation43 Moreover, several clinical trials are ongoing to evaluate the application of these cells in the treatment of burns (clinicaltrials.gov NCT01443689), and chronic diabetic wounds (clinicaltrials.gov NCT01413035).
Endometrium mesenchymal stem cells (E-MSCs)
Also human endometrium represents a promising alternative source of MSCs that can be retrieved after hysterectomy or diagnostic curettage and from menstrual blood.Citation44
Meng and co-workers demonstrated that endometrium-derived MSCs (E-MSCs) can be rapidly expanded in vitro and differentiated into several functional cells including cardiomyocytes, respiratory epithelium, neuronal cells, endothelial cells, pancreatic cells, myocytes, hepatocytes, adipose cells and osteocytes.Citation15 Murphy and colleagues demonstrated that E-MSCs show interesting regenerative capacities, especially at ischemic sites, where they are able to induce angiogenesis.Citation45 Recently, autologous tissue engineered scaffolds using artificial meshes and E-MSCs were prepared for regenerative therapy.Citation46 They were demonstrated to be suitable for fascial repair.Citation47 E-MSCs enhance neovascularization, reduce chronic inflammation, support tissue integration – likely because of their capability to modulate tissue response toward foreign materials – and promote distensibility of the artificial mesh.Citation48,49 Overall, these features make E-MSCs very suitable for wound repair.
Induced pluripotent stem (iPS) cells
Among the main sources of MSCs that might be used in the repair and regeneration of injured skin, induced Pluripotent Stem (iPS) cells have been used to study disease mechanisms, to test drugs and to develop personalized cell therapies. iPS cells are a type of pluripotent stem cell artificially derived from a non-pluripotent cell, typically an adult somatic cell, by inducing expression of a defined set of transcription factorsCitation49 or recombinant proteins channeled into the cells via poly-arginine anchors.Citation50 iPS cells were first produced in 2006 from mouse cells Citation51 and in 2007 from human cells.Citation52 MSCs derived from iPS cells (iPS-MSCs) offer the advantages of both MSCs and IPS cells: abundance, passaged >40 times in culture, sustain the self-renewal capacity, and they are also no longer tumorigenic.Citation53 In wounds, iPS-MSCs have been demonstrated to participate in tissue repair after autologous transplantation without immunological rejection.Citation53 The transplanted cells in hindlimb muscles and peripheral nerves of mouse model for diabetic polyneuropathy (DPN), ameliorated nerve conduction velocities, plantar skin blood flow, increased the number-to-muscle fiber ratios, suggesting that iPS-MSC transplantation might have therapeutic effects on DPN through secreting angiogenic/neurotrophic factors and differentiation to Schwann cell-like cells.Citation54 Moreover, recent studies have further support the use of iPS in skin regeneration. The authors successfully used human keratinocyte-derived iPSCs to reconstitute skin in vitro for the treatment of recessive dystrophic epidermolysis bullosa.Citation55 Indeed, active studies in both animal models and future clinical trials need be conducted to develop effective dosing, timing and delivery routes.
Adipose derived mesenchymal stem cells (ADSCs)
Basic research and preclinical studies of regenerative medicine have been mainly focused on adipose-derived mesenchymal stem cells (ADSCs). In the last years safety and efficacy of implanted ADSCs in different animal models have been investigated and promising preclinical studies with good perspectives for translational approaches are underway.
The adipose tissue is a highly specialized and complex connective tissue with important functions such as: i) protection or cushion from mechanical injury, ii) insulation against cold, iii) structural and metabolic support as an energy reservoir through fat accumulation, iv) dynamic participation in endocrine physiology. The adipose tissue is considered an important source of restorative growth factors,Citation56,57 it has multi-lineage differentiation capacity,Citation58 it can induce immunosuppression of activated immune cells,Citation59 it is able to homing to areas of injury,Citation60 and it has in vivo differentiation capacity to recreate a physiological condition when transplanted into a pathological microenvironment.Citation61
Unlike BM-MSCs, ADSCs can be obtained in large quantities at low risk. They are more abundant on a per gram basis (50,000 vs. 100–1,000) and more easily accessible than BM-MSCs.Citation62 However, studies have demonstrated that not all fat depots are equal in terms of quality of ADSCs, whereby the percentages of stem cells range from 1 to 10%, most likely depending on the donor and tissue harvesting site. ADSCs harvested from the superficial abdominal depot above Scarpa’s layer have been shown to be more resistant to apoptosis than other subcutaneous depots including the arm, hip, and thigh regions.Citation63 In addition, younger patients appear to have increased induction of their ADSCs than older patients.Citation64 In humans, subcutaneous adipose tissue can be obtained by liposuction aspirate (preferable option) or during reconstructive surgery. At variance with the latter during which adipose tissue is obtained in solid pieces, the isolation of adipose tissue after liposuction is rather simple, as the procedure yields already finely minced homogeneous tissue fragments on which the enzymatic digestion is more efficient. In 2001 Zuk et al. developed an ADSCs isolation protocol that has become the most widely used up to now.Citation58 More recently, Bianchi and colleaguesCitation65 described an innovative system, named Lipogems, providing a non-expanded, ready-to-use fat product. This system used mild mechanical forces in a completely closed system, avoiding enzymes, additives, and other manipulations. This innovative enzyme-free technology has been developed to process variable amounts of lipoaspirates, resulting in a non-expanded adipose tissue product that contains human ADSCs.Citation65,66
The functional cells of adipose tissue are adipocytes, which respond to insulin, secrete adipokines such as leptin and adiponectin, and store triglycerides in large, lipid-filled vacuoles.
Although adipocytes constitute almost 90% of adipose tissue volume,Citation67 adipose tissue yields a heterogeneous population of many other cell types including ADSCs, preadipocytes, endothelial cells, pericytes, haematopoietic -lineage cells, and fibroblasts. Approximately 0.5×104 –2×105 ADSCs can be isolated per gram of adipose tissue.Citation63
Most sources indicate that in the SVF, ADSC frequency is of 5.1–20%. Endothelial cells (mature and progenitors) are identified through the expression of CD146+/CD31+/CD144+/VEGFR2+ and could represents from 7% up to ~30% of SVF.Citation68 Depending on processing, fibroblasts could represent up to 50% of SVF.Citation69 CD34+ cells are present at large number and could compose up to 63% of SVF. It has also been described that the SVF is composed of nearly 11% CD14+ cells, ~2% CD31+ cells, ~7% CD34+, ~9% CD45+ cells, ~29% CD90+, and ~47% 146+ cells.Citation70 Other studies indicated that SVF of human adipose tissue contained: endothelial progenitors, pericytes, CD146+/CD34+ transitional cells, and supra-adventitial adipose stromal cellsCitation71 ().
In summary, the stromal vascular fraction (SVF) of adipose tissue is composed of many mature, progenitor and stem cell types. Therefore, depending on adipose tissue processing method, the composition of SVF and relative values of each cell population can differ significantly.
In the following sections we will examine the recent results by ADSC therapy in chronic wound-pathologies as diabetes, and autoimmune diseases, with particular attention for systemic sclerosis (SSc).
Wound Healing
Wound healing is a complex and dynamic process of replacing devitalized and missing cellular structures and tissue layers. The human adult wound healing process can be divided into 3 distinct programmed phases: i) hemostasis/inflammation, ii) proliferation, and iii) remodelling. These phases and their biophysiological functions must occur in the proper sequence, at a specific time, and continue for a specific duration at an optimal intensity by the actions of the main actors represented by all skin cells, keratinocytes, fibroblasts, endothelial cells, nerve cells, immune cells as well as blood cells (i.e., white blood cells, red blood cells , platelets).
hemostasis/Inflammation begins at the time of injury and lasts for 24 to 48 hours. This phase begins with hemostasis and leads to inflammation. Platelets form the initial thrombus release growth factors that induce the chemotaxis and proliferation of neutrophils and macrophages, which then become the prominent cell of this phase and release various growth factors and cytokines that change the moderately acellular wound into a highly cellular environment.
Fibroblasts proliferate to become the dominant cell of the proliferative phase. They produce collagen, which provides structure to the wound and replaces the fibronectin–fibrin matrix with angiogenesis of new capillaries and with the epithelialization support of Keratinocytes.
In the remodelling phase collagen synthesis and degradation reach equilibrium. Fibroblasts organize and cross-link the collagen, wound strength gradually increases, wound contraction occurs, and fibroblast density decrease.
Impaired wound healing occurs for defects in the normal tissue response to injury due to local or systemic factors and to poor treatment of the wound, resulting in chronic skin lesions, or ulcers.
Chronic or non-healing wounds are wounds that do not follow the normal wound healing process, resulting in an open laceration of varying degrees of severity.Citation72 This disorder may be associated to a number of different pathological conditions such as diabetes,Citation73 venous stasis,Citation74 and chronic autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, Crohn’s disease, Citation75 and SSc,Citation76 for which effective therapies are no currently available.
It has been suggested that all these diseases generally contribute to the generation of a hyper-inflammatory environment that further impairs the physiological healing processes. Chronic ulcers affect over 6 million people in the United States, with an incidence that is expected to grow mirroring the increasing mean age of general population and the number of subjects affected by diabetes. Chronic ulcers strongly affect the quality of life and productivity of the patients, representing a financial burden to the health care system. The average cost of treatment per patient is about $ 1,000 and in 46% of patients the healing process lasts 26 weeks, in 15% of subjects may last up to 2 yCitation77
Cell-based therapies are slowly gaining ground in routine medical care and, especially, in wound management of skin. They offer promise for the repair and/or replacement of damaged tissue and the restoration of lost functionality because they possess many of the criteria necessary for wound healing.Citation78
ADSCs Application for Wound Healing Therapy
The ability of ADSCs to play a role in wound healing seems strictly related to their anti-inflammatory properties, which also aids the tolerance of transplanted cells even in the case of allogenic ADSC both in acute and in chronic conditions, as they exhibit pleiotropic immune regulatory activities (e.g., inhibit the function of different immune cell subpopulations of the innate and adaptive immunity). These properties are mediated by the release of soluble paracrine factors and by direct cell-to-cell interactions with professional antigen presenting cells (APCs) such as dendritic cells,Citation79 T cells,Citation80,81 B cellsCitation82 and macrophages.Citation83 ADSCs are able to block APCs maturation in a contact-dependent manner, to induce the expression of anti-inflammatory cytokines such as IL-10 and to enhance TGF-β activity.Citation57
The application of ADSCs in wound repair and tissue regeneration has been demonstrated in a number of experimental models both in vitro and in vivo. ADSCs in cutaneous wounds significantly accelerated the re-epithelization by promoting human dermal fibroblast proliferation through direct cell–cell contact or via paracrine secretion of a variety of growth factors. In a full thickness excisional injury model in rats, ADSCs were shown to enhance neovasculogenesis and to accelerate wound closure via secretion of VEGF-A, hepatic growth factor, and FGF-2,Citation84 and thus promoting subsequent angiogenesis and proliferation of keratinocytes or dermal fibroblast.Citation57 This study also validated the differentiation potential of ADSCs into endothelial and epithelial cell types, supporting the applicability of ADSCs to tissues regeneration.
Focus on ADSCs Application for Wound Healing in Diabetes
Impaired wound healing is a major clinical problem in diabetic patients leading to limb amputation in several cases.Citation85 Cell-based therapies are promising in this field and ADSCs are good candidates.Citation84 Recent preclinical studies, including animal models of diabetes, showed the beneficial effect of ADSC administration in promoting wound healing.Citation86
Kuo and colleagues investigated the effect of ADSCs transplantation into streptozotocin-induced diabetes rodent model wounds. Results revealed that the complete wound healing time was significantly decreased in the ADSC-treated group compared to controls. Moreover, histological examination revealed that the ADSC-treated group showed a significant reduction in the pro-inflammatory reaction, with significantly increased levels of EGF, VEGF, rPH, and Ki67 expression with increased angiogenesis via vWF and VEGF expression. The authors hypothesized that ADSC treatment significantly stimulates neo-angiogenesis and increases tissue regeneration through paracrine and autocrine mechanisms.Citation87
Kim et al demonstrated that the administration of ADSCs enhanced wound healing in a mouse model, and that ADSCs promote human dermal fibroblast proliferation, not only by cell-to-cell direct contact, which was confirmed by co-culture experiment, but also by paracrine activation through secretory factors, resolved by transwell co-culture and culturing with conditioned medium of ADSCs.Citation88 Therefore, it was postulated that ADSCs did not directly influence wound healing as previously thought, but worked indirectly via local mediators.
Indeed, the release of growth factors at the wound site favors the activation of resident cell function, the recruitment of cells, and the differentiation into resident cell types. In fact, the ability of topically applied ADSCs to differentiate into endothelial and epithelial cells as well as their capacity to release large amounts of proangiogenic growth factors have been described.Citation89
Evidence of an effective treatment with ADSCs in diabetic ulcers derives from several clinical trials.Citation90 Nevertheless, there are some limitations to the use of autologous ADSCs, due to an altered phenotype of MSCs in diabetic patients: when characterized phenotypically and functionally, diabetic MSCs were less potent than normal ones, with a decreased expression of VEGF-A and chemokine receptor CXCR4 in fibroblast positive ADSCs. High expression of fibroblast markers associated with reduced expression of VEGF-A may affect the effectiveness of autologous cell therapies in diabetic patients. Therefore, in diabetic patients an allogenic donor could be the optimal source.Citation91
Focus on ADSCs Application for Wound Healing in Systemic Sclerosis
Scleroderma, or systemic sclerosis (SSc), is a chronic multisystem autoimmune disease characterized by vasculopathy, diffuse fibrosis of skin and various organs and immune abnormalities. Patient suffering for SSc have often hand disability for the presence of digital ulcers that severely interfere with daily life.Citation8
Although the pathophysiology of SSc is undoubtedly complex and remains incompletely understood, progresses have been made in elucidating at least some of the multiple mechanisms which are likely to contribute to the vascular and fibrotic alterations.Citation92 Most research on the changes in vascular and fibrotic features in SSc has focused on the MSCs with conflicting results.Citation93 BM-MSCs from patients with SSc are similar to those from healthy donors in terms of their phenotype and capacity to differentiate into adipogenic and osteogenic lineages,Citation94 showed an upregulation of pericyte-specific markers and a decreased proliferation capacity.Citation95 It has also been shown that SSc MSCs constitutively over-express and release pro-angiogenic mediators compared with healthy control MSCs.Citation96
In 2013 an open-label and single arm study was performed in 12 SSc patients in order to evaluate the number of adverse events related to SVF injection.Citation97 This procedure improved manifestations of peripheral vasculitis as Raynaud’s phenomenon, ameliorate digital ulcers and consequently had an impact on hand pain. These results suggest that SVF may improve vasomotor tone and microvascular perfusion. This hypothesis is further substantiated by the significant reduction of avascular areas and dystrophic capillaries evaluated using nailfold capillaroscopy.
In 2014, an Italian group performed a study on finger injection of ADSCs in 15 SSc patients, having a long-lasting digital ulcer in only one fingertip, unresponsive to intensive systemic and local treatment. An improvement in healing time, a significant reduction of pain intensity and an increase in the number of capillaries has been showed after a 6-month follow up period.Citation98
Other authors reported data on the therapeutic effects of MSC local or regional transfer in patients with SSc suffering from ischemic lesions in their fingers or limbs. Although only few cases were described, it is noticeable that the adopted procedures have constantly induced an improvement on ischemic lesions.Citation99
Focus on ADSCs Application for Wound Healing in Autoimmune Diseases
ADSCs have been proposed as candidates for the treatment of wound healing in of different immune-mediated diseases,Citation100,101 however the greater experience is available with non-ADSCs (generally, BM and UCB-MSCs).Citation102
Studies on preclinical efficacy of ADSCs in autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis (mainly collagen-induced arthritis), Crohn’s disease (experimental colitis), experimental autoimmune hearing loss, experimental autoimmune thyroiditis, experimental autoimmune encephalomyelitis (model of multiple sclerosis), and immune thrombocytopenia have been carried out in different animal modelsCitation103–124 (). The current evidences of cellular therapy for autoimmune diseases in humans are mainly based on non-controlled trials. Few clinical studies on the immunomodulatory properties of ADSCs have been performed; they are mainly case reports based on compassionate-use treatments for rheumatoid arthritis, multiple sclerosis, polymyositis, autoimmune inner ear disease, atopic dermatitis, and autoimmune thrombocytopenic purpura. Moreover, the adipose tissue-derived SVFCitation58 has been successfully administered instead of ex vivo culture-expanded ADSCs in rheumatoid arthritis and multiple sclerosis, showing encouraging results.Citation124 Also in the case of autoimmune diseases other than diabetes, MSCs have an altered phenotype and function. An allogenic rather than autologous MSC-based therapy might be preferable for treatment. Indeed, autologous MSCs are characterized by an early senescence, but they preserve immunomodulatory properties that support their use anyway.Citation125
Table 1. Studies on preclinical efficacy of MSCs in animal models for autoimmune diseases
Tissue Bioengineering
Regarding skin wound healing, tissue engineering is making strides in creating new biomedical skin substitutes.Citation126 Indeed, the combination of cell therapy with biomaterials is one of the main challenges to treat wound healing. Numerous studies of biomaterials for wound dressings have been performed for the improvement of the functions that support wound healing. Materials for wound dressings are required to have good biocompatibility, wound-sealing capability, and to maintain a humid environment to inhibit drying of the wound. Additionally, fabrication is desired for sponge, film, and gel forms to adjust to the wound shape or size. Wound dressings are also required to have an absorption capability of wound exudates fluid, which includes important growth factors to stimulate cells of the immune system. As reported by our group, silk fibroin can possess all these properties and combination of human ADSCs with silk fibroin resulted in accelerated wound healing of diabetic ulcers in impaired db/db diabetic mice.Citation89
Moreover, topical application of human ADSCs seeded on a silk fibrin-chitosan scaffold was shown to improve wound repair and these cells were shown to differentiate and contribute to fibrovascular, endothelial, and epithelial components of the reconstituted tissue .Citation127 Very recent evidence coming from animal models suggest that also allogenic ADSC sheets,Citation128 or ADSC spheroids assembled on polymer membranes could be a future therapeutic approach.Citation129 The advantage of such bioengineering techniques is to provide ADSCs with a more favorable milieu for cytokine and chemokine production, as demonstrated by animal models.Citation129 Furthermore, the use of antibacterial materials such as chitosan may reduce the risk of bacterial growth, thus reducing the incidence of side effect.Citation130
Clinical Studies with ADSCs as Cell-based Therapy
Stem cell research is in its early stages of development and the market is therefore still behind. Approximately 4 million people affected by wound healing impairments for diabetes, autoimmune disease or burns in the US would benefit from cell therapy products.
The US. Food and Drug Administration (FDA) defines somatic cell therapy as the administration of autologous, allogenic or xenogenic non-germ cells excluding blood products for transfusion, which have been manipulated, processed, propagated, expanded, selected ex vivo, or drug-treated.Citation131 Cell therapy products are considered as drugs, so they follow the same regulations, adhering to the Current Good Manufacturing Practices (GMP), which establish minimum quality requirements for their manipulation. The key points of the current FDA regulation for cell therapy products include: demonstrations of preclinical safety and efficacy; no risk of transmission of infectious or genetic diseases from donors; no risk of contamination or other adverse effects of cells or sample processing; specific and detailed determination of cell type, purity and potency of the final product; in vivo safety and efficacy of the final product.Citation132 Clinical applications using ADSCs for wound healing, burns, diabetic foot and chronic limb ischemia are underway throughout Asia, Europe and North and South America. Some of these can be found on the clinicaltrials.gov website [clinicaltrials.gov (Accessed on 20 November 2015)], where 10 studies are listed under the search term “adipose stem cell” AND “wound” (as of 20 November 2015). Of these, 7 studies actually are recruiting patients, 1 is complted, 1 is active and 1 is unknown because it has not been updated (clinicaltrials.gov) ().
Table 2. clinical studies from clinicaltrials.gov as of 20 November 2015
Limitations
For translational medicine, safety remains a major issue. First of all, although stem cells have been largely studied in vitro and in animal models, in vivo mechanisms are largely obscure and the biological implications in humans still remain to be proven, Another limitation in the use of MSCs is that they are found in a very small amount in the tissue of origin, thus requiring expansion protocols in vitro. During the protocol the risk of contamination of the cells is low, but still possible. Furthermore, also the risk of exposure to prions and of immunological should be considered. These risks are due to the fact that supplements often have an animal origin and they could be overcome with the use of synthetic media. Another risk is the exposure to toxic agents such as endotoxin. Then, unpredictable fluctuations of the milieu of MSCs during expansion or after transplantation, could affect their biological functions .Citation133 Although acute toxicity seems not to be a major concern, based on findings in >2,000 patients so far exposed to MSCs,Citation134 and fewer to ADSCs,Citation135 evidence from animal models suggests that the approach might be associated with loss of endogenous tumor surveillance. In fact, descriptions of the possible involvement of ADSCs in tumorigenesis have been reported in vitro e in vivo,Citation135–137 and biological mechanisms are poorly understood. ADSCs are appealing thanks to their ability to proliferate and differentiate also secreting cytokines and growth factors and their immunoregulatory function. Overall, these characteristics may expose the patients both to oncogenic/tumor-supporting risk and to ectopic differentiation risk.Citation138 There is growing evidence that MSC treatment is safe in humans, although cancer recurrence after fat grafts have been reported in patients whose history was notable for sarcoma,Citation139 and breast cancer.Citation140 Finally, the possibility that cell expansion could give rise to genomic abnormalities is still debated, although major concerns derive more from the possibility of an accelerated cell senescence, that could expose the patient to more side effects or to less efficacy. Indeed, Importantly, long-term safety data are lacking and implementation of devoted registries is critical, especially regarding tumor surveillance.
Therefore, despite the encouraging data regarding therapeutic applications, the opportunity of stem cell therapy should be carefully evaluated in each patient, balancing the risks and benefits of a relatively novel technique.
Conclusions
In recent years, basic and translation research held great hope for this new field with significant progress in the modulation of stem cell commitment in vitro and providing protocols for targeted clinical applications. Recent advances in bioengineering and nanotechnology have allowed researchers to manipulate microenvironments in increasingly precise spatial and temporal scales, recapitulating key homeostatic cues that may drive regeneration. MSCs are able to secrete a large number of trophic factors capable of repairing the recipient tissue through angiogenic, anti-apoptotic and anti-fibrotic mechanisms. In this context, adipose tissue is emerging as a clinically relevant and easy to harvest source of multipotent progenitors to develop regenerative therapies. The application of ADSCs in wound repair and tissue regeneration has been shown in a number of experimental models both in vitro and in vivo. The positive outcome obtained with this therapeutic approach, although promising, is limited to small cohorts of patients and needs to be confirmed in larger and controlled studies.
Based on the interesting available evidence in the literature, we are confident that MSCs cell therapy will be a promising and important strategy for chronic wound repair in a next future. Understanding the dynamics that regulate MSCs homeostasis, especially their anti-inflammatory effect and immunomodulatory capacity, has led to challenge a number of consolidated beliefs on their therapeutic mechanisms. Since the precise mechanism which allows this effect is not completely understood, more studies, focused on the role of adult stem cells in wound healing, are needed in order to address this question and improve the efficacy of this therapy.
Furthermore the possibility to obtain a final cell product containing viable adipocyte, pre-adipocyte, and stem cells, eliminating problems related to enzymatic digestion and other manipulations, exhibits a great appeal not only for its application in plastic and reconstructive medicine, but also in research, and regenerative medicine.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
Bibliography
- Atiyeh BS, Ioannovich J, Al-Amm CA, El-Musa KA. Management of acute and chronic open wounds: the importance of moist environment in optimal wound healing. Curr Pharm Biotechnol 2002; 3:179-95; PMID:12164477; http://dx.doi.org/10.2174/1389201023378283
- Branski LK, Gauglitz GG, Herndon DN, Jeschke MG. A review of gene and stem cell therapy in cutaneous wound healing. Burns 2009; 35:171-80; PMID:18603379; http://dx.doi.org/10.1016/j.burns.2008.03.009
- Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005; 366:1719-24; PMID:16291066; http://dx.doi.org/10.1016/S0140-6736(05)67698-2
- Reddy M, Gill SS, Rochon PA. Preventing pressure ulcers: a systematic review. Jama 2006; 296:974-84; PMID:16926357; http://dx.doi.org/10.1001/jama.296.8.974
- Abbade LP, Lastoria S. Venous ulcer: epidemiology, physiopathology, diagnosis and treatment. Int J Dermatol 2005; 44:449-56; PMID:15941430; http://dx.doi.org/10.1111/j.1365-4632.2004.02456.x
- Gibran NS, Heimbach DM. Current status of burn wound pathophysiology. Clin Plast Surg 2000; 27:11-22; PMID:10665353
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999; 341:738-46; PMID:10471461; http://dx.doi.org/10.1056/NEJM199909023411006
- Gualtierotti R, Adorni G, Lubatti C, Zeni S, Meroni PL, Ingegnoli F. Digital ulcer management in patients with systemic sclerosis. OA Arthritis 2014; 2:2
- Silva I, Almeida J, Vasconcelos C. A PRISMA-driven systematic review for predictive risk factors of digital ulcers in systemic sclerosis patients. Autoimmun Rev 2015; 14:140-52; PMID:25449678; http://dx.doi.org/10.1016/j.autrev.2014.10.009
- Tingey T, Shu J, Smuczek J, Pope J. Meta-analysis of healing and prevention of digital ulcers in systemic sclerosis. Arthritis Care Res (Hoboken) 2013; 65:1460-71; PMID:23554239; http://dx.doi.org/10.1002/acr.22018
- Pikuła M, Marek-Trzonkowska N, Wardowska A, Renkielska A, Trzonkowski P. Adipose tissue-derived stem cells in clinical applications. Expert Opin Biol Ther 2013; 13:1357-70; PMID:23919743; http://dx.doi.org/10.1517/14712598.2013.823153
- Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001; 7:211-28; PMID:11304456; http://dx.doi.org/10.1089/107632701300062859
- Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol 2000; 109:235-42; PMID:10848804; http://dx.doi.org/10.1046/j.1365-2141.2000.01986.x
- Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol 2004; 36:585-97; PMID:15010325
- Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med 2007; 5:57; PMID:18005405; http://dx.doi.org/10.1186/1479-5876-5-57
- Fang RC, Galiano RD. A review of becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Biologics 2008; 2:1-12; PMID:19707423
- Martí-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, Cedeño-Taborda J. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev 2015; 10:CD008548
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 2000; 97:13625-30; PMID:11087820; http://dx.doi.org/10.1073/pnas.240309797
- Sessarego N, Parodi A, Podesta M, Benvenuto F, Mogni M, Raviolo V, Lituania M, Kunkl A, Ferlazzo G, Bricarelli FD, et al. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica 2008; 93:339-46; PMID:18268281; http://dx.doi.org/10.3324/haematol.11869
- Yan XL, Fu CJ, Chen L, Qin JH, Zeng Q, Yuan HF, Nan X, Chen HX, Zhou JN, Lin YL, et al. Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat 2012; 132:153-64; PMID:21584665; http://dx.doi.org/10.1007/s10549-011-1577-0
- Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 2013; 45:e54; PMID:24232253; http://dx.doi.org/10.1038/emm.2013.94
- García-Gómez I, Elvira G, Zapata AG, Lamana ML, Ramírez M, Castro JG, Arranz MG, Vicente A, Bueren J, García-Olmo D. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther 2010; 10:1453-68; http://dx.doi.org/10.1517/14712598.2010.519333
- Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair-current views. Stem Cells 2007; 25:2896-902; PMID:17901396; http://dx.doi.org/10.1634/stemcells.2007-0637
- Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev 2009; 20:419-27; PMID:19926330; http://dx.doi.org/10.1016/j.cytogfr.2009.10.002
- Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther 2015; 23:812-23; PMID:25868399; http://dx.doi.org/10.1038/mt.2015.44
- Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng 2005; 11:1198-211; PMID:16144456; http://dx.doi.org/10.1089/ten.2005.11.1198
- Petrie Aronin CE, Tuan RS. Therapeutic potential of the immunomodulatory activities of adult mesenchymal stem cells. Birth Defects Res C Embryo Today 2010; 90:67-74; PMID:20301222; http://dx.doi.org/10.1002/bdrc.20174
- Haynesworth SE, Baber MA and Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol 1996; 166:585-92; PMID:8600162; http://dx.doi.org/10.1002/(SICI)1097-4652(199603)166:3%3c585::AID-JCP13%3e3.0.CO;2-6
- Blocki A, Wang Y, Koch M, Peh P, Beyer S, Law P, Hui J, Raghunath M. Not all MSCs can act as pericytes: functional in vitro assays to distinguish pericytes from other mesenchymal stem cells in angiogenesis. Stem Cells Dev 2013; 22:2347-55; PMID:23600480; http://dx.doi.org/10.1089/scd.2012.0415
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature 2001; 414:98-104; PMID:11689954; http://dx.doi.org/10.1038/35102160
- Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 2014; 10:29-37; PMID:24322872; http://dx.doi.org/10.4161/org.27405
- Wang Y, Crisostomo PR, Wang M, Markel TA, Novotny NM, Meldrum DR. TGF-alpha increases human mesenchymal stem cell-secreted VEGF by MEK- and PI3-K- but not JNK- or ERK-dependent mechanisms. Am J Physiol Regul Integr Comp Physiol 2008; 295:R1115-23; PMID:18685072; http://dx.doi.org/10.1152/ajpregu.90383.2008
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005; 105:1815-22; PMID:15494428; http://dx.doi.org/10.1182/blood-2004-04-1559
- Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, Mao N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005; 105:4120-6; PMID:15692068; http://dx.doi.org/10.1182/blood-2004-02-0586
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315-7; PMID:16923606; http://dx.doi.org/10.1080/14653240600855905
- Scherberich A, Di Maggio ND, McNagny KM. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J Stem Cells 2013; 5:1-8; PMID:23362435; http://dx.doi.org/10.4252/wjsc.v5.i1.1
- Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 2006; 208:64-76; PMID:16557516; http://dx.doi.org/10.1002/jcp.20636
- Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007; 15:S18-26; PMID:17727462; http://dx.doi.org/10.1111/j.1524-475X.2007.00221.x
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science 1999; 284:143-7; PMID:10102814; http://dx.doi.org/10.1126/science.284.5411.143
- Rao MS, Mattson MP. Stem cells and aging: expanding the possibilities. Mech Ageing Dev 2001; 122:713-34
- Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A 1989; 86:3828-32; PMID:2566997; http://dx.doi.org/10.1073/pnas.86.10.3828
- Kim JY, Jeon HB, Yang YS, Oh W, Chang JW. Application of human umbilical cord blood-derived mesenchymal stem cells in disease models. World J Stem Cells 2010; 2:34-8; PMID:21607114; http://dx.doi.org/10.4252/wjsc.v2.i2.34
- Valbonesi M, Giannini G, Migliori F, Dalla Costa R, Dejana AM. Cord blood (CB) stem cells for wound repair. Preliminary report of 2 cases. Transfusion Apheresis Sei 2004; 30; 153-6; http://dx.doi.org/10.1016/j.transci.2003.11.006
- Lin J, Xiang D, Zhang JL, Allickson J, Xiang C. Plasticity of human menstrual blood stem cells derived from the endometrium. J Zhejiang Univ Sci B 2011; 12:372-80; PMID:21528491; http://dx.doi.org/10.1631/jzus.B1100015
- Murphy MP, Wang H, Patel AN, Kambhampati S, Angle N, Chan K, Marleau AM, Pyszniak A, Carrier E, Ichim TE, Riordan NH. Allogeneic endometrial regenerative cells: an "Off the shelf solution" for critical limb ischemia? J Transl Med 2008; 19: 6:45
- Verdi J, Tan A, Shoae-Hassani A, Seifalian AM. Endometrial stem cells in regenerative medicine. J Biol Eng 2014; 8:20; PMID:25097665; http://dx.doi.org/10.1186/1754-1611-8-20
- Su K, Edwards SL, Tan KS, White JF, Kandel S, Ramshaw JA, Gargett CE, Werkmeister JA. Induction of endometrial mesenchymal stem cells into tissue-forming cells suitable for fascial repair. Acta Biomater 2014; 10:5012-5020; PMID:25194931; http://dx.doi.org/10.1016/j.actbio.2014.08.031
- Ulrich D, Edwards SL, White JF, Supit T, Ramshaw JA, Lo C, Rosamilia A, Werkmeister JA, Gargett CE: A preclinical evaluation of alternative synthetic biomaterials for fascial defect repair using a rat abdominal hernia model. PLoS One 2012; 7:e50044; PMID:23185528; http://dx.doi.org/10.1371/journal.pone.0050044
- Edwards SL, Werkmeister JA, Rosamilia A, Ramshaw JA, White JF, Gargett CE: Characterisation of clinical and newly fabricated meshes for pelvic organ prolapse repair. J Mech Behav Biomed Mater 2013; 23:53-61; PMID:23651550; http://dx.doi.org/10.1016/j.jmbbm.2013.04.002
- Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 2007; 1:39-49; PMID:18371333; http://dx.doi.org/10.1016/j.stem.2007.05.012
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009; 4(5):381-4; PMID:19398399; http://dx.doi.org/10.1016/j.stem.2009.04.005
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007; 131(5):861-72; PMID:18035408; http://dx.doi.org/10.1016/j.cell.2007.11.019
- Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, et al. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010; 121(9):1113-23; PMID:20176987; http://dx.doi.org/10.1161/CIRCULATIONAHA.109.898312
- Himeno T, Kamiya H, Naruse K, Cheng Z, Ito S, Kondo M, Okawa T, Fujiya A, Kato J, Suzuki H, et al. Mesenchymal stem cell-like cells derived from mouse induced pluripotent stem cells ameliorate diabetic polyneuropathy in mice. Biomed Res Int. 2013; 2013:259187; PMID:24319678; http://dx.doi.org/10.1155/2013/259187
- Sebastiano V, Zhen HH, Haddad B, Bashkirova E, Melo SP, Wang P, Leung TL, Siprashvili Z, Tichy A, Li J, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014; 6(264):264ra163; PMID:25429056
- Kapur SK, Katz AJ. Review of the adipose derived stem cell secretome. Biochimie 2013; 95:2222-8; PMID:23770442; http://dx.doi.org/10.1016/j.biochi.2013.06.001
- Salgado AJ, Reis RL, Sousa NJ, Gimble JM. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther 2010; 5:103-10; PMID:19941460; http://dx.doi.org/10.2174/157488810791268564
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002; 13:4279-95; PMID:12475952; http://dx.doi.org/10.1091/mbc.E02-02-0105
- McIntosh KR, Frazier T, Rowan BG, Gimble JM. Evolution and future prospects of adipose-derived immunomodulatory cell therapeutics. Expert Rev Clin Immunol 2013; 9:175-84; PMID:23390948; http://dx.doi.org/10.1586/eci.12.96
- Qin JB, Li KA, Li XX, Xie QS, Lin JY, Ye KC, Jiang ME, Zhang GX, Lu XW. Long-term MRI tracking of dual-labeled adipose-derived stem cells homing into mouse carotid artery injury. Int J Nanomedicine 2012; 7:5191-203; PMID:23125528
- Marfia G, Campanella R, Navone SE, Zucca I, Scotti A, Figini M, Di Vito C, Alessandri G, Riboni L, Parati E. Potential use of human adipose mesenchymal stromal cells for intervertebral disc regeneration: a preliminary study on biglycan-deficient murine model of chronic disc degeneration. Arthritis Res Ther 2014; 16:457; PMID:25293819; http://dx.doi.org/10.1186/s13075-014-0457-5
- Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 2006; 24:150-4; PMID:16488036; http://dx.doi.org/10.1016/j.tibtech.2006.01.010
- Jurgens, WJ, Oedayrajsingh-Varma MJ, Helder MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, Ritt MJ, van Milligen FJ. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res 2008; 332:415-26; PMID:18379826; http://dx.doi.org/10.1007/s00441-007-0555-7
- Schipper, BM, Marra KG, Zhang W, Donnenberg AD, Rubin JP. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann Plast Surg 2008; 60:538-44; PMID:18434829; http://dx.doi.org/10.1097/SAP.0b013e3181723bbe
- Bianchi, F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C, Ventura C. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 2013; 22:2063-77; PMID:23051701; http://dx.doi.org/10.3727/096368912X657855
- Giori A, Tremolada C, Vailati R, Navone S E, Marfia G, Caplan AI. Recovery of Function in Anal Incontinence After Micro-Fragmented Fat Graft (Lipogems→) Injection: Two Years Follow Up of the First 5 Cases. CellR4 2015; 3:e1544
- Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast Reconstr Surg 2009; 124:1087-97; PMID:19935292; http://dx.doi.org/10.1097/PRS.0b013e3181b5a3f1
- Hager G, Holnthoner W, Wolbank S, Husa AM, Godthardt K, Redl H, Gabriel C. Three specific antigens to isolate endothelial progenitor cells from human liposuction material. Cytotherapy 2013; 15(11):1426-35; PMID:24094492; http://dx.doi.org/10.1016/j.jcyt.2013.06.018
- Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Cervelli V. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med 2012; 1(3):230-6; PMID:23197782; http://dx.doi.org/10.5966/sctm.2011-0054
- Astori G, Vignati F, Bardelli S, Tubio M, Gola M, Albertini V, Bambi F, Scali G, Castelli D, Rasini V, et al. "In vitro" and multicolour phenotypic characterization of cell subpopulations identified in fresh human adipose tissue stromal vascular fraction and in the derived mesenchymal stem cells. J Transl Med 2007; 5:55; PMID:17974012; http://dx.doi.org/10.1186/1479-5876-5-55
- Zimmerlin L, Donnenberg VS, Rubin JP, Donnenberg AD. Mesenchymal markers on human adipose stem/progenitor cells. Cytometry A. 2013;83:134-40; PMID:23184564; http://dx.doi.org/10.1002/cyto.a.22227
- Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol 2007; 25:19-25; PMID:17276197; http://dx.doi.org/10.1016/j.clindermatol.2006.12.005
- Fahey TJ, 3rd, Sadaty A, Jones WG, 2nd, Barber A, Smoller B, Shires GT. Diabetes impairs the late inflammatory response to wound healing. J Surg Res 1991; 50:308-13; PMID:2020184; http://dx.doi.org/10.1016/0022-4804(91)90196-S
- Stanley AC, Park HY, Phillips TJ, Russakovsky V, Menzoian JO. Reduced growth of dermal fibroblasts from chronic venous ulcers can be stimulated with growth factors. J Vasc Surg 1997; 26:994-9; PMID:9423715; http://dx.doi.org/10.1016/S0741-5214(97)70012-0
- Khatami M. Unresolved inflammation: 'immune tsunami' or erosion of integrity in immune- privileged and immune-responsive tissues and acute and chronic inflammatory diseases or cancer. Expert Opin Biol Ther 2011; 11(11):1419-32; PMID:21663532; http://dx.doi.org/10.1517/14712598.2011.592826
- Moran ME. Scleroderma and evidence based non-pharmaceutical treatment modalities for digital ulcers: a systematic review. J Wound Care. 2014; 23(10):510-6; PMID:25296352; http://dx.doi.org/10.12968/jowc.2014.23.10.510
- Greer N, Foman NA, MacDonald R, Dorrian J, Fitzgerald P, Rutks I, Wilt TJ. Advanced wound care therapies for nonhealing diabetic, venous, and arterial ulcers: a systematic review. Ann Intern Med 2013; 15; 159(8):532-42; http://dx.doi.org/10.7326/0003-4819-159-8-201310150-00006
- Jackson WM, Nesti LJ, Tuan RS. A review: mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res Ther 2012; 3:20; PMID:22668751; http://dx.doi.org/10.1186/scrt111
- Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood 2009; 113(26):6576-83; PMID:19398717; http://dx.doi.org/10.1182/blood-2009-02-203943
- Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther 2011; 2:34; PMID:21861858
- Gonzalez-Rey E, Gonzalez MA, Varela N, O'Valle F, Hernandez-Cortes P, Rico L, Büscher D, Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis 2010; 69:241-8; PMID:19124525; http://dx.doi.org/10.1136/ard.2008.101881
- Franquesa M, Mensah FK, Huizinga R, Strini T, Boon L, Lombardo E, DelaRosa O, Laman JD, Grinyó JM, Weimar W, et al. Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells 2015; 33:880-91; PMID:25376628; http://dx.doi.org/10.1002/stem.1881
- González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology 2009; 136:978-89; http://dx.doi.org/10.1053/j.gastro.2008.11.041
- Nie C, Yang D, Xu J, Si Z, Jin X, Zhang J. Locally administered adipose-derived stem cells accelerate wound healing through differentiation and vasculogenesis. Cell Transplant 2011; 20:205-16; PMID:20719083; http://dx.doi.org/10.3727/096368910X520065
- Siitonen OI, Niskanen LK, Laasko M, Siitonen JT, Pyörälä K. Lower-extremity amputations in diabetic and nondiabetic patients. Diabetes care 1993; 16:16-20; PMID:8422771; http://dx.doi.org/10.2337/diacare.16.1.16
- Amos PJ, Kapur SK, Stapor PC, Shang H, Bekiranov S, Khurgel M, Rodeheaver GT, Peirce SM, Katz AJ. Human adipose-derived stromal cells accelerate diabetic wound healing: impact of cell formulation and delivery. Tissue Eng Part A 2010; 16:1595-606; PMID:20038211; http://dx.doi.org/10.1089/ten.tea.2009.0616
- Kuo YR, Wang CT, Cheng JT, Kao GS, Chiang YC, Wang CJ. Adipose-derived stem cells accelerate diabetic wound healing through the induction of autocrine and paracrine effects. Cell Transplant 2015 Apr 7. [Epub ahead of print]
- Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, Park JS. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007; 48:15-24; PMID:17643966; http://dx.doi.org/10.1016/j.jdermsci.2007.05.018
- Navone SE, Pascucci L, Dossena M, Ferri A, Invernici G, Acerbi F, Cristini S, Bedini G, Tosetti V, Ceserani V, et al. Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice. Stem Cell Res Ther 2014; 5:7; PMID:24423450; http://dx.doi.org/10.1186/scrt396
- Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract 2011; 92(1):26-36; PMID:21216483; http://dx.doi.org/10.1016/j.diabres.2010.12.010
- Kočí Z, Turnovcová K, Dubský M, Baranovičová L, Holáň V, Chudíčková M, Syková E, Kubinová S. Characterization of human adipose tissue-derived stromal cells isolated from diabetic patient's distal limbs with critical ischemia. Cell Biochem Funct 2014; 32(7):597-604; http://dx.doi.org/10.1002/cbf.3056
- Dumoitier N, Lofek S, Mouthon L. Pathophysiology of systemic sclerosis: state of the art in 2014. Presse Med 2014; 43:e267-78; PMID:25179277; http://dx.doi.org/10.1016/j.lpm.2014.08.001
- Cipriani P, Ruscitti P, Di Benedetto P, Carubbi F, Liakouli V, Berardicurti O, Ciccia F, Triolo G, Giacomelli R. Mesenchymal stromal cells and rheumatic diseases: new tools from pathogenesis to regenerative therapies. Cytotherapy 2015; 17:832-49; PMID:25680301; http://dx.doi.org/10.1016/j.jcyt.2014.12.006
- Larghero J, Farge D, Braccini A, Lecourt S, Scherberich A, Foïs E, Verrecchia F, Daikeler T, Gluckman E, Tyndall A, et al. Phenotypical and functional characteristics of in vitro expanded bone marrow mesenchymal stem cells from patients with systemic sclerosis. Ann Rheum Dis 2008; 67:443-9; PMID:17526552; http://dx.doi.org/10.1136/ard.2007.071233
- Cipriani P, Marrelli A, Benedetto PD, Liakouli V, Carubbi F, Ruscitti P, Alvaro S, Pantano I, Campese AF, Grazioli P, et al. Scleroderma Mesenchymal Stem Cells display a different phenotype from healthy controls; implications for regenerative medicine. Angiogenesis 2013; 16:595-607; PMID:23413114; http://dx.doi.org/10.1007/s10456-013-9338-9
- Guiducci S, Manetti M, Romano E, Mazzanti B, Ceccarelli C, Dal Pozzo S, Milia AF, Bellando-Randone S, Fiori G, Conforti ML, et al. Bone marrow-derived mesenchymal stem cells from early diffuse systemic sclerosis exhibit a paracrine machinery and stimulate angiogenesis in vitro. Ann Rheum Dis 2011; 70:2011-21; PMID:21821866; http://dx.doi.org/10.1136/ard.2011.150607
- Granel B, Daumas A, Jouve E, Harlé JR, Nguyen PS, Chabannon C, Colavolpe N, Reynier JC, Truillet R, Mallet S, et al. Safety, tolerability and potential efficacy of injection of autologous adipose-derived stromal vascular fraction in the fingers of patients with systemic sclerosis: an open-label phase I trial. Ann Rheum Dis 2015; 74:2175-82; PMID:25114060; http://dx.doi.org/10.1136/annrheumdis-2014-205681
- Del Papa N, Di Luca G, Sambataro D, Zaccara E, Maglione W, Gabrielli A, Fraticelli P, Moroncini G, Beretta L, Santaniello A, et al. Regional implantation of autologous adipose tissue-derived cells induces a prompt healing of long-lasting indolent digital ulcers in patients with Systemic Sclerosis. Cell Transplant 2015; 24:2297-305; PMID:25506730; http://dx.doi.org/10.3727/096368914X685636
- Guiducci S, Porta F, Saccardi R, Guidi S, Ibba-Manneschi L, Manetti M, Mazzanti B, Dal Pozzo S, Milia AF, Bellando-Randone S, et al. Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: a case report. Ann Intern Med 2010; 153:650-4; PMID:21079220; http://dx.doi.org/10.7326/0003-4819-153-10-201011160-00007
- Guo K Ikehara S, Meng X. Mesenchymal stem cells for inducing tolerance in organ transplantation. Front Cell Dev Biol 2014; 2:8; PMID:25364716; http://dx.doi.org/10.3389/fcell.2014.00008
- Kebriaei P, Robinson S. Treatment of graft-versus-host-disease with mesenchymal stromal cells. Cytotherapy 2011; 13:262-8; PMID:21231805; http://dx.doi.org/10.3109/14653249.2010.549688
- Tyndall A. Mesenchymal stem cell treatments in rheumatology: a glass half full? Nat Rev Rheumatol 2014; 10:117-24; PMID:24217581; http://dx.doi.org/10.1038/nrrheum.2013.166
- Choi EW, Shin IS, Park SY, Park JH, Kim JS, Yoon EJ, Kang SK, Ra JC, Hong SH. Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum 2012; 64:243-53; PMID:21904997; http://dx.doi.org/10.1002/art.33313
- Choi EW, Yun TW, Song JW, Lee M, Yang J, Choi KS. Preventive effects of CTLA4Ig-overexpressing adipose tissue-derived mesenchymal stromal cells in rheumatoid arthritis. Cytotherapy 2015; 17:271-82; PMID:25541299; http://dx.doi.org/10.1016/j.jcyt.2014.10.010
- Park MJ, Kwok SK, Lee SH, Kim EK, Park SH, Cho ML. Adipose tissue-derived mesenchymal stem cells induce expansion of Interleukin-10 producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant 2015; 24:2367-77; PMID:25506685; http://dx.doi.org/10.3727/096368914X685645
- González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 2009; 60:1006-19; http://dx.doi.org/10.1002/art.24405
- Zhou B, Yuan J, Zhou Y, Ghawji M Jr, Deng YP, Lee AJ, Lee AJ, Nair U, Kang AH, Brand DD, et al. Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis. Clin Immunol 2011; 141:328-37; PMID:21944669; http://dx.doi.org/10.1016/j.clim.2011.08.014
- Serratrice N, Bruzzese L, Magalon J, Véran J, Giraudo L, Aboudou H, Ould-Ali D, Nguyen PS, Bausset O, Daumas A, et al. New fat-derived products for treating skin-induced lesions of scleroderma in nude mice. Stem Cell Res Ther 2014; 5:138; PMID:25519759; http://dx.doi.org/10.1186/scrt528
- González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum 2009; 60:1006-19; http://dx.doi.org/10.1002/art.24405
- Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 2009; 58:929-39; PMID:19136511; http://dx.doi.org/10.1136/gut.2008.168534
- Gonçalves Fda C, Schneider N, Pinto FO, Meyer FS, Visioli F, Pfaffenseller B, Lopez PL, Passos EP, Cirne-Lima EO, Meurer L, et al. Intravenous vs intraperitoneal mesenchymal stem cells administration: What is the best route for treating experimental colitis? World J Gastroenterol 2014; 20:18228-39; http://dx.doi.org/10.3748/wjg.v20.i48.18228
- Zhou Y, Yuan J, Zhou B, Lee AJ, Lee AJ, Ghawji M Jr, Yoo TJ. The therapeutic efficacy of human adipose tissue-derived mesenchymal stem cells on experimental autoimmune hearing loss in mice. Immunology 2011; 133:133-40; PMID:21366561; http://dx.doi.org/10.1111/j.1365-2567.2011.03421.x
- Choi EW, Shin IS, Lee HW, Park SY, Park JH, Nam MH, Kim JS, Woo SK, Yoon EJ, Kang SK, et al. Transplantation of CTLA4Ig gene-transduced adipose tissue-derived mesenchymal stem cells reduces inflammatory immune response and improves Th1/Th2 balance in experimental autoimmune thyroiditis. J Gene Med 2011; 13:3-16; PMID:21259404; http://dx.doi.org/10.1002/jgm.1531
- Choi EW, Lee JM, Lee HW, Yang J, Youn HY. Therapeutic effects of CTLA4Ig gene-transduced adipose tissue-derived mesenchymal stem cell transplantation on established autoimmune thyroiditis. Cell Transplant 2015; 24:2221-36; PMID:25299180; http://dx.doi.org/10.3727/096368914X685122
- Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 2009; 27:2624-35; PMID:19676124; http://dx.doi.org/10.1002/stem.194
- Payne NL, Sun G, McDonald C, Moussa L, Emerson-Webber A, Loisel-Meyer S, Medin JA, Siatskas C, Bernard CC. Human adipose-derived mesenchymal stem cells engineered to secrete IL-10 inhibit APC function and limit CNS autoimmunity. Brain Behav Immun 2013; 30:103-14; PMID:23369732; http://dx.doi.org/10.1016/j.bbi.2013.01.079
- Scruggs BA, Semon JA, Zhang X, Zhang S, Bowles AC, Pandey AC, Imhof KM, Kalueff AV, Gimble JM, Bunnell BA. Age of the donor reduces the ability of human adipose-derived stem cells to alleviate symptoms in the experimental autoimmune encephalomyelitis mouse model. Stem Cells Transl Med 2013; 2:797-807; PMID:24018793; http://dx.doi.org/10.5966/sctm.2013-0026
- Yousefi F, Ebtekar M, Soleimani M, Soudi S, Hashemi SM. Comparison of in vivo immunomodulatory effects of intravenous and intraperitoneal administration of adipose-tissue mesenchymal stem cells in experimental autoimmune encephalomyelitis (EAE). Int Immunopharmacol 2013; 17:608-16; PMID:23973288; http://dx.doi.org/10.1016/j.intimp.2013.07.016
- Semon JA, Zhang X, Pandey AC, Alandete SM, Maness C, Zhang S, Scruggs BA, Strong AL, Sharkey SA, Beuttler MM, et al. Administration of murine stromal vascular fraction ameliorates chronic experimental autoimmune encephalomyelitis. Stem Cells Transl Med 2013; 2:789-96; PMID:23981726; http://dx.doi.org/10.5966/sctm.2013-0032
- Zhang X, Bowles AC, Semon JA, Scruggs BA, Zhang S, Strong AL, Gimble JM, Bunnell BA. Transplantation of autologous adipose stem cells lacks therapeutic efficacy in the experimental autoimmune encephalomyelitis model. PLoS One 2014; 9:e85007; PMID:24465465; http://dx.doi.org/10.1371/journal.pone.0085007
- Tafreshi AP, Payne N, Sun G, Sylvain A, Schulze K, Bernard C. Inactive GSK3beta is disturbed in the spinal cord during experimental autoimmune encephalomyelitis, but rescued by stem cell therapy. Neuroscience 2014; 277:498-505; PMID:25064057; http://dx.doi.org/10.1016/j.neuroscience.2014.07.013
- Semon JA, Maness C, Zhang X, Sharkey SA, Beuttler MM, Shah FS, Pandey AC, Gimble JM, Zhang S, Scruggs BA, et al. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res Ther 2014; 5:2; PMID:24405805; http://dx.doi.org/10.1186/scrt391
- Bassi EJ, Moraes-Vieira PM, Moreira-Sa CS, Almeida DC, Vieira LM, Cunha CS, Hiyane MI, Basso AS, Pacheco-Silva A, Câmara NO. Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes 2012; 61:2534-45; PMID:22688334; http://dx.doi.org/10.2337/db11-0844
- Xiao J, Zhang C, Zhang Y, Zhao J, Liang J, Zhong X, Chen Y. Transplantation of adipose-derived mesenchymal stem cells into a murine model of passive chronic immune thrombocytopenia. Transfusion 2012; 52:2551-8; PMID:22486546; http://dx.doi.org/10.1111/j.1537-2995.2012.03642.x
- Cras A, Farge D, Carmoi T, Lataillade JJ, Wang DD, Sun L. Update on mesenchymal stem cell-based therapy in lupus and scleroderma. Arthritis Res Ther. 2015; 17(1):301; PMID:26525582; http://dx.doi.org/10.1186/s13075-015-0819-7
- Trottier V, Marceau-Fortier G, Germain L, Vincent C, Fradette J.. IFATS collection: Using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells 2008; 26:2713-23; PMID:18617689; http://dx.doi.org/10.1634/stemcells.2008-0031
- Altman AM, Yan Y, Matthias N, Bai X, Rios C, Mathur AB, Song YH, Alt EU. IFATS collection: Human adipose-derived stem cells seeded on a silk fibroin-chitosan scaffold enhance wound repair in a murine soft tissue injury model. Stem Cells 2009; 27:250-8; PMID:18818439; http://dx.doi.org/10.1634/stemcells.2008-0178
- Kato Y, Iwata T, Morikawa S, Yamato M, Okano T, Uchigata Y. Allogeneic Transplantation of an Adipose-Derived Stem Cell Sheet Combined With Artificial Skin Accelerates Wound Healing in a Rat Wound Model of Type 2 Diabetes and Obesity. Diabetes. 2015; 64(8):2723-34; PMID:25795216; http://dx.doi.org/10.2337/db14-1133
- Hsu SH, Hsieh PS. Self-assembled adult adipose-derived stem cell spheroids combined with biomaterials promote wound healing in a rat skin repair model. Wound Repair Regen. 2015; 23(1):57-64; PMID:25421559; http://dx.doi.org/10.1111/wrr.12239
- Tan HB, Wang FY, Ding W, Zhang Y, Ding J, Cai DX, Yu KF, Yang J, Yang L, Xu YQ. Fabrication and Evaluation of Porous Keratin/chitosan (KCS) Scaffolds for Effectively Accelerating Wound Healing. Biomed Environ Sci. 2015; 28(3):178-89; PMID:25800442
- FDA Proposed Approach to Regulation of Cellular and Tissue-Based Products. Available online:http://www.fda.gov/downloads/BiologicasBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/UCM062601.pdf (Accessed on 14 May 2015).
- Halme DG, Kessler DA. FDA regulation of stem-cell-based therapies. N Engl J Med 2006; 355:1730-5; PMID:17050899; http://dx.doi.org/10.1056/NEJMhpr063086
- Pacini S. Deterministic and stochastic approaches in the clinical application of mesenchymal stromal cells (MSCs). Front Cell Dev Biol. 2014; 2:50; PMID:25364757
- Martin, I, Ireland H, Baldomero H, Passweg J. The survey on cellular and engineered tissue therapies in europe in 2012. Tissue Eng Part A 2015; 21:1-13; PMID:25425342; http://dx.doi.org/10.1089/ten.tea.2014.0515
- Ra JC, Shin IS, Kim SH, Kang SK, Kang BC, Lee HY, Kim YJ, Jo JY, Yoon EJ, Choi HJ, et al. Safety of intravenous infusion of human adipose tissue-derived mesenchymal stem cells in animals and humans. Stem Cells Dev 2011; 20:1297-308; PMID:21303266; http://dx.doi.org/10.1089/scd.2010.0466
- Tolar J, Nauta AJ, Osborn MJ, Panoskaltsis Mortari A, McElmurry RT, Bell S, Xia L, Zhou N, Riddle M, Schroeder TM, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells 2007; 25:371-9; PMID:17038675; http://dx.doi.org/10.1634/stemcells.2005-0620
- Zimmerlin L, Donnenberg AD, Rubin JP, Basse P, Landreneau RJ, Donnenberg VS. Regenerative therapy and cancer: in vitro and in vivo studies of the interaction between adipose-derived stem cells and breast cancer cells from clinical isolates. Tissue Eng Part A 2011; 17:93-106; PMID:20673000; http://dx.doi.org/10.1089/ten.tea.2010.0248
- Herberts CA, Kwa MS, Hermsen HP. Risk factors in the development of stem cell therapy. J Transl Med. 2011 Mar 22; 9:29; http://dx.doi.org/10.1186/1479-5876-9-29
- Perrot P, Rousseau J, Bouffaut AL, Rédini F, Cassagnau E, Deschaseaux F, Heymann MF, Heymann D, Duteille F, Trichet V, et al. Safety concern between autologous fat graft, mesenchymal stem cell and osteosarcoma recurrence. PLoS One 2010; 5:e10999; PMID:20544017; http://dx.doi.org/10.1371/journal.pone.0010999
- Chaput B, Foucras L, Le Guellec S, Grolleau JL, Garrido I. Recurrence of an invasive ductal breast carcinoma 4 months after autologous fat grafting. Plast Reconstr Surg 2013; 131:123e-4e; PMID:23271541; http://dx.doi.org/10.1097/PRS.0b013e318272a1f6