Abstract
The impact of systemic therapy on survival of patients with metastatic melanoma is uncertain. This retrospective analysis aimed to compare the response rates and survival of patients without prior therapy with cisplatin, vinblastine, dacarbazine and interleukin (IL)-2 who were treated with biochemotherapy (containing these drugs plus interferon-α) with those of patients who received combination chemotherapy with and without interferon-α (chemotherapy ± IFN). Records for 616 chemo-naïve patients with unresectable metastatic melanoma who were treated on eight Phase II/III clinical trials between 1987 and 2001 were combined and reviewed The database included patients with melanoma of the skin (497 cases), unknown primary melanoma (83 cases), mucosal melanoma (21 cases), and uveal melanoma (15 cases). Two hundred sixty-four patients received biochemotherapy, and 352 received chemotherapy ± IFN. The overall response rate (complete and partial) of patients treated with biochemotherapy was 52% (138/264) compared with 35% (122/352) among patients treated with chemotherapy ± IFN regimens. The median survival times for patients in the biochemotherapy and chemotherapy ± IFN groups were 12.2 mo (95% CI: 10.9, 13.5) and 9.1 mo (95% CI: 8.1, 10.4), respectively (p < 0.0001). Five-year overall survival rates of patients treated with biochemotherapy and chemotherapy ± IFN were 17% and 7% (p = 0.0004), respectively. Ten-year overall survival rates were 15% and 5% (p = 0.0001), respectively. We conclude from these studies that patients benefited more from biochemotherapy than other regimens.
INTRODUCTION
The optimum therapy for metastatic melanoma remains uncertain. Dacarbazine (DTIC) alone or in multi-drug combinations has been extensively evaluated for efficacy as measured by the response rates and survival (Del Prete et al., Citation1984; Ahmann et al., Citation1989; Legha et al., Citation1989; Anderson et al., Citation1995; Middleton et al., Citation2000; Lotze et al., Citation2001; Millard et al., Citation2004). Prospectively-randomized Phase III clinical trials have failed to confirm the superiority of multi-drug chemotherapy regimens over dacarbazine alone (Buzaid et al., Citation1993; Chapman et al., Citation1999). Compelling evidence suggests that immune cells play an important role in the control of malignancy and that, in patients with metastatic cancer, the immune system is suppressed by growing tumor.
Active immunotherapy for cancer aims at eliciting an immune reaction that will eliminate or slow down the growth or spread of cancer. The advent of recombinant cytokines such as interferon-α (IFNα) and interleukin-2 (IL-2) has provided a more selective means for stimulating the immune system. Therapy with high-dose bolus IL-2 (600,000 IU/kg body weight, given as an intravenous [IV] bolus every 8 hr) as approved by the United States Food and Drug Administration (USFDA) has resulted in prolongation of disease-free survival and modest response rate of 15% in patients with metastatic melanoma.
During the past 15 years, we have evaluated the efficacy of cisplatin (C), vinblastine (V), dacarbazine (D), interferon, and IL-2 (biochemotherapy) combination against metastatic melanoma. The rationale for combining these drugs includes: (a) the capacity of interferon to activate host defense mechanisms through NK cells and macrophages, and up-regulation of HLA and tumor antigen expression; (b) the activation and proliferation of cytotoxic killer lymphocytes (LAK cells) by IL-2; and, (c) induction of widespread anti-tumor immune response by tumor cells killed by chemotherapeutic agents through release of DNA-binding nuclear protein that binds to toll-like receptor 4 on dendritic cells (Zitvogel et al., Citation2008).
We observed that biochemotherapy (combination of IL-2, interferon, and CVD chemotherapy regimen) is associated with better response rates and survival duration than other multi-drug regimens (Richards et al., Citation1992; Khayat et al., Citation1993; Atkins et al., Citation1994; Legha et al., Citation1996, Citation1998; Thompson et al., Citation1997; Johnston et al., Citation1998; O'Day et al., Citation1999; Flaherty et al., Citation2001; Hauschild et al., Citation2001; Atzpodien et al., Citation2002; Keilholz et al., 2005). However, the prospectively-randomized Phase III trials failed to firmly establish the superiority of IL-2-based regimens over chemotherapy (Rosenberg et al., Citation1999; Hauschild et al., Citation2001; Atzpodien et al., Citation2002; Eton et al., Citation2002; Atkins et al., Citation2003). In this retrospective study, we reviewed our 15-year experience with biochemotherapy and other regimens we used to treat patients with metastatic melanoma, and compared the outcomes.
PATIENTS, MATERIALS, AND METHODS
Review Methods
We reviewed the database of consecutive clinical trials conducted at M. D. Anderson Cancer Center between 1987 and 2001 used for therapy of chemo-naïve patients with unresectable metastatic melanoma. The eligibility criteria of patients for the eight studies were similar in terms of eligibility requirements including a normal CBC and normal liver, renal, and cardiac functions and presence of measurable metastases and a Zubrod performance status (PS) 0–2. Only the Phase III clinical trial comparing CVD to biochemotherapy we completed 6 yr ago allowed patients with a PS of 3 that were excluded in the Phase II studies. Patients with symptomatic brain metastases were ineligible and those treated with IL-2 were required to be off steroid therapy. Baseline evaluation included history taking, physical examination, electrocardiography, and laboratory studies. Scans of the brain, chest, and abdomen were obtained in all patients. Selected patients receiving biochemotherapy had exercise thallium scan, cardiac scan or cardiac ultrasound to rule out marked myocardial dysfunction.
Trials and Regimens
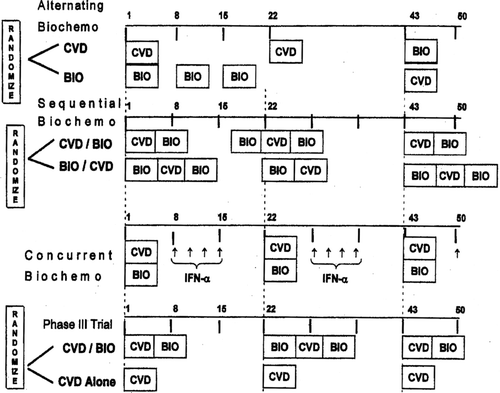
The CVD regimen trial (Study 1) was the basis for the subsequent clinical trials (Legha et al., Citation1989). In this trial, cisplatin was given intravenously (IV) at a dose of 20 mg/m2/d on Days 2–5; vinblastine was administered IV at a dose of 1.6 mg/m2/d on Days 1–5; and DTIC was given IV on Day 1 at a dose of 800 mg/m2. The courses were administered every 3 wk. In the next study (Study 2), IFN-α was added to the CVD chemotherapy (Legha et al., Citation1993). In addition, half the patients received Tamoxifen at 20 mg orally twice a day based on randomization. In the next study, vinblastine was replaced with paclitaxel (Taxol), yielding the CTD regimen (Papadopoulos et al., Citation2003). These three regimens did not include IL-2; thus, they are included in the “chemotherapy ± IFN” regimens ().
TABLE 1 Description of studies included in meta-analysis
Since 1989, we conducted five biochemotherapy trials with regimens that contained CVD, IFN-α, and IL-2 in different doses and schedules (). In these trials, the cisplatin and DTIC doses were kept the same as those in the CVD regimen, and the dose of vinblastine was reduced. The biotherapy (BIO) consisted of IL-2 at the equivalent dose of 9 MU/m2 by continuous IV infusion over 24 hr for 4 consecutive days and IFN-α (either Roferon [Roche, Basel, Switzerland] or Intron A [Schering-Plough Oncology, Kenilworth, NJ]) at a dose of 5 MU/m2 subcutaneously for 5 consecutive days starting on the first day of IL-2 (provided initially by Roche [Basel, Switzerland] or Chiron Biopharmaceuticals [Emeryville, CA], until it became commercially available).
In the first biochemotherapy study (Study 4), the patients were randomized to receive either two CVD courses over 6 wk and then three courses of BIO over 6 wk or the same treatments but in the reverse order; this regimen is referred to as alternating biochemotherapy (Legha et al., Citation1996) (). In the sequential biochemotherapy study (Study 5), single courses of BIO and CVD were administered sequentially (Legha et al., Citation1997). Patients were randomized to receive therapy either with schedule A (sequential CVD/BIO) or schedule B (sequential BIO/CVD). In the next concurrent biochemotherapy study (Study 6), both BIO and CVD were administered at the same time over 5 d (Legha et al., Citation1998). Study 7 was a prospectively-randomized Phase III clinical trial comparing CVD to sequential biochemotherapy (Eton et al., Citation2002). The high-dose biochemotherapy trial (Study 8) was designed to determine the maximum tolerable dose of each of the five drugs included in concurrent biochemotherapy (Kim et al., Citation2004).
All patients received appropriate supportive care. Biochemotherapy was given on an inpatient basis under close supervision as described in earlier publications (Legha et al., Citation1996, Citation1998; Eton et al., Citation2002; Kim et al., Citation2004). The response to treatment was assessed using the standard World Health Organization response criteria (WHO, 1979). These clinical trials were approved by the Institutional Review Board at the M. D. Anderson Cancer Center. The patients gave their informed written consent to participate in these studies in accordance with institutional and federal guidelines.
Statistical Analyses
Data of the eight successive Phase II/III clinical studies were pooled by treatment regimen (biochemotherapy or chemotherapy ± IFN) as detailed in . Patient or tumor characteristics were summarized descriptively by treatment regimen using summary statistics (median, range) for continuous variables or frequency and percentage for categorical variables, as applicable. Distributions of demographic and clinical characteristics including response to treatment were compared between the two treatment groups using chi-square tests. For continuous variables such as age, Breslow thickness, and months from diagnosis to study therapy, Wilcoxon rank-sums tests were performed.
Overall survival was computed as the number of months from start of systemic therapy on the clinical trial to the date of death for the patients who died or the date of last contact for patients still alive. Patients still alive at the last contact date were censored. The Kaplan-Meier product limit method was used to construct survival curves and estimate median survival with the corresponding 95% confidence intervals (95% CI) (Kaplan and Meier, Citation1958). Survival was compared between the two therapy groups with the log-rank test (Mantel, Citation1966). In addition, for each treatment group, the probability of surviving 5 and 10 years as well as the corresponding standard errors were estimated using Kaplan-Meier methodology and compared between the two treatment groups using a Z test.
Cox proportional-hazards regression analysis was performed to calculate hazard ratios and 95% confidence intervals for demographic and clinical characteristics as well as treatment regimen (Cox, Citation1972). Initially, univariate models were fit to evaluate the predictive effect of each factor alone. Then, factors associated with survival at p ≤ 0.10 were included in a multivariate model. Using a step-wise backward selection procedure, individual variables were subsequently removed from the model to determine the most parsimonious model. All reported p-values are two-sided, and p-values less than 0.05 were considered statistically significant.
RESULTS
A total of 616 patients with a histologically-confirmed diagnosis of malignant melanoma with inoperable Stage III/IV disease were registered on eight successive Phase II/III clinical studies. Patients had cutaneous malignant melanoma (497 cases), mucosal melanoma (21 cases), uveal melanoma (15 cases) and unknown primary melanoma (83 cases). The survival of patients treated with regimens chemotherapy ± IFN group was not statistically significantly different than those treated with CVD (p = 0.56). Similarly, there was no statistically significant survival difference between various biochemotherapy regimens (p = 0.58). The characteristics of the patients in the biochemotherapy group were compared with those of the chemotherapy ± IFN group. The two groups were similar in terms of gender, site of primary tumor, Breslow thickness, baseline serum lactate dehydrogenase (LDH) level, number of metastatic sites, and disease stage (M1a, b, or c). Patients undergoing biochemotherapy were significantly younger (median, 46 years; range, 18–70 years) than patients treated with chemotherapy ± IFN (median, 50 years; range, 18–78 years) (p < 0.0001). Patients who received biochemotherapy had a baseline Zubrod performance status (PS) less than 2 significantly more often than patients treated with chemotherapy ± IFN (92% vs. 84%) (p < 0.003). In addition, more patients treated with chemotherapy ± IFN had abnormal baseline albumin levels compared to those who received biochemotherapy (14% vs. 7%; p < 0.003). However, more patients treated with chemotherapy ± IFN were free of brain metastasis compared to those who received biochemotherapy (93% vs. 88%; p = 0.016).
The overall response rate (complete + partial) of patients treated with biochemotherapy was about 52% compared with 35% among patients treated with chemotherapy ± IFN regimens ( and ). Specifically, the complete and partial response rates were higher in biochemotherapy groups compared with chemotherapy ± IFN groups (13% vs. 5%) and (40% vs. 30%), respectively. In both therapy groups, 26% of the patients had stable disease.
FIG. 2 Survival of patients (pts) treated with chemotherapy ± IFN by response. CR = complete response, PR = partial response, SD = stable disease, PD = progressive disease
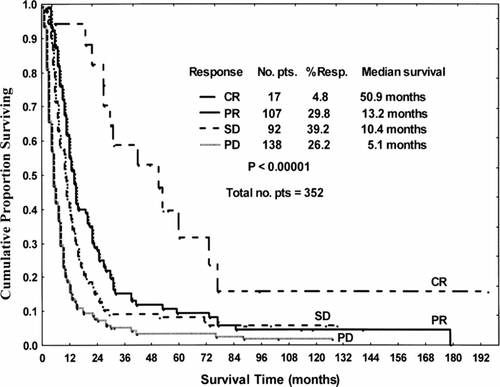
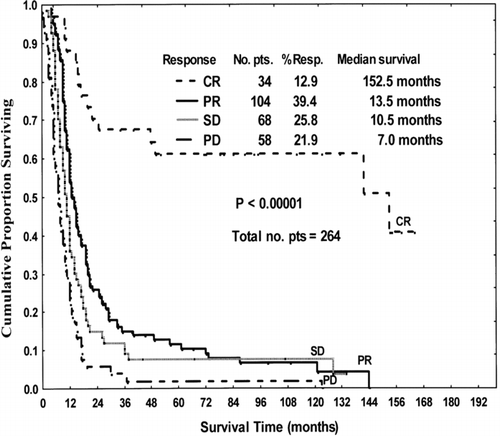
FIG. 3 Survival of patients (pts) treated with BioChemoRx by response. CR = complete response, PR = partial response, SD = stable disease, PD = progressive disease.
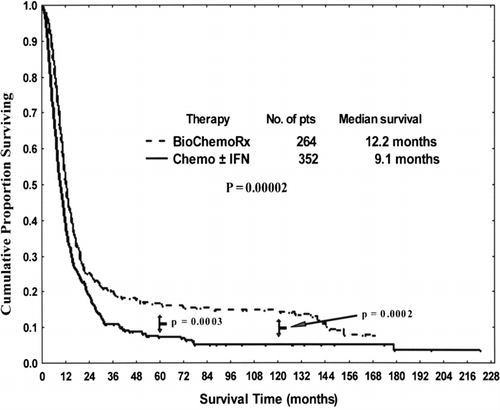
The median survival times for patients in the biochemotherapy and chemotherapy ± IFN groups were 12.2 mo (95% CI: 10.9, 13.5) and 9.1 mo (95% CI: 8.1, 10.4), respectively (p < 0.0001) (). The 5-yr survival rates for patients in biochemotherapy and chemotherapy ± IFN groups were 17% (95% CI: 12%, 22%) and 7% (95% CI: 5%, 10%), respectively (p = 0.0004). The 10-yr survival rates for biochemotherapy and chemotherapy ± IFN groups were 15% (95% CI: 11%, 20%) and 5% (95% CI: 3%, 8%), respectively (p = 0.0001). Furthermore, patients who responded to biochemotherapy survived longer (p = 0.0489) than did patients who responded to chemotherapy ± IFN (, ).
FIG. 4 Comparison of survival of patients (pts) by therapy and response. CR = complete response, PR = partial response, SD = stable disease, PD = progressive disease.
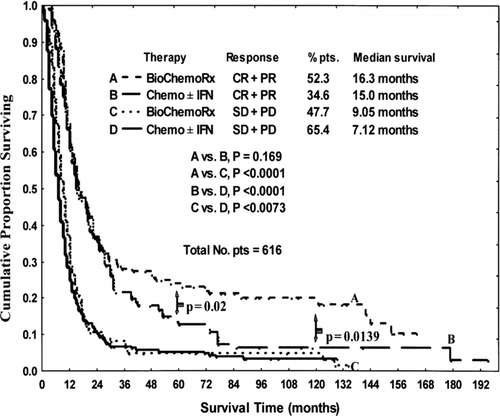
TABLE 2 Comparison of survival (months) by therapy group for responders, non-responders, and overall
Specifically, the 5-yr survival rate of responders to biochemotherapy was significantly better (p = 0.0026) than that of patients in the chemotherapy ± IFN group (26% [95% CI: 19%, 34%] vs. 12% [95% CI: 7%, 18%], respectively). The same trend continued for 10-yr survival (23% [95% CI: 16%, 30%] vs. 9% [95% CI: 4%, 15%] p = 0.0019, respectively). The 10-yr survival rate of the patients who had complete response with biochemotherapy was significantly higher (p < 0.05) than that of those who responded to chemotherapy ± IFN.
Multivariate Cox proportional hazards regression analysis identified treatment with chemotherapy ± IFN was significantly associated with shorter survival (Hazard Ratio [HR] 1.37; 95% CI: 1.14, 1.63; p = 0.0006), as was older age (HR 1.01; 95% CI 1.00, 1.01; p = 0.0424), a performance status of 2 or 3 (HR 2.08; 95% CI: 1.58, 2.75; p < 0.0001), abnormal baseline albumin level at start of systemic therapy (HR 1.44; 95% CI: 1.10, 1.89; p = 0.0085), abnormal baseline LDH level (HR 1.52; 95% CI: 1.26, 1.83; p < 0.0001), and one or more visceral metastasis sites. Compared to patients with no visceral metastatic sites (unresectable Stage III/M1a), patients with one visceral metastatic site had a HR of 1.51 (95%CI: 1.19, 1.91; p = 0.0006), patients with two metastatic visceral sites had a HR of 1.80 (95%CI: 1.38, 2.34; p < 0.0001), and patients with three or more visceral metastatic sites had a HR of 2.57 (95% CI: 1.88, 3.50; p < 0.0001).
DISCUSSION
This retrospective study found that biochemotherapy was superior to chemotherapy ± IFN for metastatic melanoma. Biochemotherapy elicited responses in about 52% of patients, with a median survival of 12.2 mo, which are statistically superior to the 35% response rate and 9.1 mo median survival obtained in patients treated with chemotherapy ± IFN. More important, the 5-yr and 10-yr overall survival rates of patients who received biochemotherapy were statistically superior to those observed in patients treated with chemotherapy ± IFN.
The proportion of patients alive more than 5 yr from day of start of biochemotherapy is similar to that reported with high-dose bolus IL-2 (Ahmann et al., Citation1989; Rosenberg et al., Citation1994; Atkins et al., Citation1999) Patients who received biochemotherapy had complete remissions twice as often as that described for high-dose bolus IL-2 (12.5% vs. 6%) (Ahmann et al., Citation1989; Rosenberg et al., Citation1994). Since biochemotherapy includes significantly lower dose of IL-2, patients tolerate it better than high-dose bolus IL-2, which is usually given in specialized units. The contribution of biotherapy including IL-2 to the efficacy of biochemotherapy has been evaluated in several Phase III trials. These trials varied in sample size, choice of chemotherapy regimen, dose schedule of IL-2, and choice of control arm (Khayat et al., Citation1993; Johnston et al., Citation1998; Rosenberg et al., Citation1999; Hauschild et al., Citation2001; Atzpodien et al., Citation2002; Eton et al., Citation2002; Atkins et al., Citation2003; Keilholz et al., Citation2005).
In three prospectively randomized trials, patients received BCDT (dacarbazine, cisplatin, carmustine, tamoxifen [BCDT]) regimen with or without IL-2, the response rates and survival duration of the patients treated with either regimen were similar (Johnston et al., Citation1998; Hauschild et al., Citation2001; Keilholz et al., Citation2005). Keilholz et al. (Citation2005) reported on a Phase III trial involving 362 patients with metastatic melanoma who were randomized to receive the dacarbazine, cisplatin, and IFNα combination alone or with IL-2 (Atzpodien et al., Citation2002). The response and the median survivals of the patients in each group were similar. Rosenberg et al. (Citation1999) prospectively randomized 102 chemo-naïve patients with metastatic melanoma to receive chemotherapy, including cisplatin, dacarbazine, and tamoxifen, or this same chemotherapy regimen followed by IFNα 2b and IV bolus IL-2. Although the response rate to immunochemo-therapy was better than that to chemotherapy (44% vs. 27%, respectively; p = 0.071), there was a survival advantage in favor of chemotherapy alone (15.8 mo vs. 10.7 mo; p = 0.052). Information on the dose intensity and treatments the patients received after having tumor progression on this trial was not provided.
We reported the result of a prospectively randomized Phase III trial of 190 patients with metastatic melanoma that compared the efficacy of CVD or a sequential biochemotherapy (Rosenberg, et al., Citation1999). Biochemotherapy was superior to CVD therapy in response rate (48% vs. 25%; p = 0.001), time to treatment failure (median, 4.9 mo vs. 2.4 mo; p = 0.007), and overall survival (median, 12.2 mo vs. 9.1 mo, p < 0.0001) (Eton et al., Citation2002). Atkins et al. (Citation2003) reported on the results of a similar Phase III study that included 405 patients and compared CVD (201 patients) to concurrent biochemotherapy (204 patients). They showed the absence of significant difference in response rates (11% vs. 17%) and median survivals (8.7 mo vs. 8.4 mo) between CVD and biochemotherapy, respectively. Information about the dose intensity of the therapies was not provided.
The patients who receive biochemotherapy regimen experience a wide range of side-effects that reflected the combined toxicity of the chemotherapy combination and the biotherapy. The most frequent toxicities seen in most patients were those related to the interferon and IL-2, including asthenia that manifested as severe arthralgia/myalgia, anemia, fever, chills, nausea, and vomiting which are managed with symptomatic treatment. Hematological toxicities in the form of anemia, thrombocytopenia, and neutropenia are quite common. The dose-limiting toxicity is neutropenia that may be associated with fever and at times complicated with infection from central venous access line and sepsis. Symptomatic rash with macular eruption, covering a major part of the body surface area, facial and pedal edema and respiratory difficulty occur during IL-2 treatment. IL-2 related capillary leak syndrome causes hypotension, transient renal insufficiency that required management with plasma expanders and vasopressors (Legha et al., Citation1997).
Despite the limitations of a retrospective review, we cannot ignore the presence of complete responses and long-term survival benefit from biochemotherapy in this study. Biochemotherapy, as delivered in our institution, has consistently given better results than regimens not containing IL-2. Only a large prospectively-randomized study comparing biochemotherapy, chemotherapy, and/or high-dose IL-2 could settle the superiority of one regimen over the others. However, such a study is prohibitive and impractical because it will require extensive resources in terms of patients, effort, time, and follow-up to answer the question related to impact of therapy on long-term survival. In view of the suboptimal results of these treatments for the majority of patients, a better understanding of the mechanisms of action for these IL-2-based therapies could identify approaches that can enhance the efficacy of these treatments. In conclusion, patients treated with biochemotherapy can have long-term survival similar to that previously reported for high-dose IL-2.
The authors would like to thank Susan McIntyre, RN, for updating survival information of the patients. Presented in part at the annual meeting of American Society of Clinical Oncology in Atlanta, 2005 and Immune-Mediated Diseases Congress, Moscow, 2007.
REFERENCES
- Ahmann D. L., Creagan E. T., Hahn R. G., Edmonson J. H., Bisel H. F., Schaid D. J. Complete responses and long-term survivals after systemic chemotherapy for patients with advanced malignant melanoma. Cancer 1989; 63: 224–227
- Allen I. E., Kupelnick B., Kumashiro M., Luo D., Ross S. D., Wolin M. J. Efficacy of interleukin-2 in the treatment of metastatic melanoma: Systematic review and meta-analysis. Cancer Ther. 1998; 1: 168–173
- Anderson C. M., Buzaid A. C., Legha S. S. Systemic treatments for advanced cutaneous melanoma. Oncology 1995; 9: 1149–1168
- Atkins M. B., Lee S., Flaherty L. E., Sosman J. A., Sondak V. K., Kirkwood J. M., for the U.S. Melanoma Intergroup. A prospective randomized Phase III trial of concurrent Biochemotherapy (BCT) with cisplatin, Vinblastine, DTIC (CVD), IL-2 and interferon α 2b (IFN) versus CVD alone in patients with metastatic melanoma (E3695): An ECOG-coordinated intergroup trial. Proc. ASCO 2003; 22, (Abstract #2847)
- Atkins M. B., Lotze M. T., Dutcher J. P., Fisher R. I., Weiss G., Margolin K., Abrams J., Sznol M., Parkinson D., Hawkins M., Paradise C., Kunkel L., Rosenberg S. A. High-dose recombinant interleukin-2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999; 17: 2105–2116
- Atkins M. B., O'Boyle K. R., Sosman J. A., Weiss G. R., Margolin K. A., Ernest M. L., Kappler K., Mier J. W., Sparano J. A., Fisher R. I., Eckardt J. R., Pereira C., Aronson F. R. Multi-institutional Phase II trial of intensive combination chemoimmunotherapy for metastatic melanoma. J. Clin. Oncol. 1994; 12: 1553–1560
- Atzpodien J., Neuber K., Kamanabrou D., Fluck M., Bröcker E. B., Neumann C., Rünger T. M., Schuler G., von den Driesch P., Müller I., Paul E., Patzelt T., Reitz M. Combination chemotherapy with or without subcutaneous IL-2 and 1FNα : Results of a prospectively randomized trial of the Cooperative Advanced Malignant Melanoma Chemoimmunotherapy Group (ACIMM). Br. J. Cancer 2002; 86: 179–184
- Buzaid A. C., Legha S., Winn R., Belt R., Pollock T., Wiseman C., Ensign L. G. Cisplatin (C), vinblastine (V), DTIC (D) (CVD) versus DTIC alone in metastatic melanoma: Preliminary results Phase III cancer community oncology program (CCOP). Proc. Am. Soc. Clin. Oncol. 1993; 12: 1328, (Abstract #1328)
- Chapman P. B., Einhorn L. H., Meyers M. L., Saxman S., Destro A. N., Panageas K. S., Begg C. B., Agarwala S. S., Schuchter L. M., Ernstoff M. S., Houghton A. N., Kirkwood J. M. Phase III multi-center randomized trial of the Dartmouth regimen versus DTIC in patients with metastatic melanoma. J. Clin. Oncol. 1999; 17: 2745–2751
- Cox D. R. Regression models and lifetables. J. Royal Stat. Soc. B 1972; 34: 187–202
- Del Prete S. A., Maurer L. H., O'Donnell J., Forcier R. J., LeMarbre P. Combination chemotherapy with cisplatin, carmustine, DTIC, and tamoxifen in metastatic melanoma. Cancer Treat. Rep. 1984; 68: 1403–1405
- Dudley M. E., Wunderlich J. R., Robbins P. F., Yang J. C., Hwu P., Schwartzentruber D. J., Topalian S. L., Sherry R., Restifo N. P., Hubicki A. M., Robinson M. R., Raffeld M., Duray P., Seipp C. A., Rogers-Freezer L., Morton K. E., Mavroukakis S. A., White D. E., Rosenberg S. A. Cancer regression and autoimmunity in patients after clonal repopulation with anti-tumor lymphocytes. Science 2002; 298: 850–854
- Eton O., Legha S. S., Bedikian A. Y., Lee J. J., Buzaid A. C., Hodges C., Ring S. E., Papadopoulos N. E., Plager C., East M. J., Zhan F., Benjamin R. S. Sequential biochemotherapy versus chemotherapy for metastatic melanoma: Results from a Phase III randomized trial. J. Clin. Oncol. 2002; 20: 2045–2052
- Flaherty L. E., Atkins M., Sosman J., Weiss G., Clark J. I., Margolin K., Dutcher J., Gordon M. S., Lotze M., Mier J., Sorokin P., Fisher R. I., Appel C., Du W. Outpatient Biochemotherapy with interleukin-2 and interferon-α 2b in patients with metastatic malignant melanoma: Results of two Phase II cytokine working group trials. J. Clin. Oncol. 2001; 19: 3194–3202
- Hauschild A., Garbe C., Stolz W., Ellwanger U., Seiter S., Dummer R., Ugurel S., Sebastian G., Nashan D., Linse R., Achtelik W, Mohr P., Kaufmann R., Fey M., Ulrich J., Tilgen W. Dacarbazine and interferon-α with or without interleukin 2 in metastatic melanoma: A randomized Phase III multicenter trial of the Dermatologic Oncology Group (DeCOG). Br. J. Cancer 2001; 84: 1036–1042
- Johnston S. R., Constenia D. O., Moore J., Atkinson H., A'Hern R. P., Dadian G., Riches P. G., Gore M. E. Randomized Phase II trial of BCDT [carmustine (BCNU), cisplatin, DTIC (DTIC), and Tamoxifen] with or without interferon-alpha (IFN〈) and interleukin-2 (IL-2) in patients with metastatic melanoma. Br. J. Cancer 1998; 77: 1280–1286
- Kaplan E. L., Meier P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958; 53: 457–481
- Keilholz U., Punt C. J., Gore M., Kruit W., Patel P., Lienard D., Thomas J., Proebstle T. M., Schmittel A., Schadendorf D., Velu T., Negrier S., Kleeberg U., Lehman F., Suciu S., Eggermont A. M. DTIC, cisplatin and interferon-〈 2b with or without interleukin-2 in metastatic melanoma: A randomized Phase III trial (18951) of the European Organization for Research and Treatment of Cancer Melanoma. J. Clin. Oncol. 2005; 23: 6747–6755
- Khayat D., Borel C., Tourani J. M., Benhammouda A., Antoine E., Rixe O., Vuillemin E., Bazex P. A., Thill L., Franks R., Auclerc G., Soubrane C., Banzet P., Well M. Sequential chemoimmunotherapy with cisplatin, interleukin-2 and interferon-〈 2a for metastatic melanoma. J. Clin. Oncol. 1993; 11: 2173–2180
- Kim B., Eton O., East M. J., Hodges C., Plager C., Papadopoulos N. E., Rohl C., Grimm E. A., Bedikian A. Y. Pilot study of high dose concurrent biochemotherapy for advanced melanoma. Cancer 2004; 101: 596–603
- Legha S., Ring S., Bedikian A. Y., Eton O., Plager C., Papadopoulos N. E., Ensign L. G., Benjamin R. S. Lack of benefit from tamoxifen (T) added to a regimen of cisplatin (C), vinblastine (V), DTIC (D) and α -interferon (IFN) in patients (pts) with metastatic melanoma (MM). Proc. Am. Soc. Clin. Oncol. 1993; 12: 388
- Legha S. S., Ring S., Bedikian A. Y., Plager C., Eton O., Buzaid A. C., Papadopoulos N. E. Treatment of metastatic melanoma with combined chemotherapy containing cisplatin, vinblastine and DTIC (CVD) and biotherapy using interleukin-2 and interferon-α. Ann. Oncol. 1996; 7: 827–835
- Legha S. S., Ring S., Eton O., Bedikian A., Buzaid A. C., Plager C., Papadopoulos N. Development of a biochemotherapy regimen with concurrent administration of cisplatin, vinblastine, DTIC, interferon-α, and interleukin-2 for patients with metastatic melanoma. J. Clin. Oncol. 1998; 16: 1752–1759
- Legha S. S., Ring S., Eton O., Bedikian A., Plager C., Papadopoulos N. Development and results of biochemotherapy in metastatic melanoma: The University of Texas M.D. Anderson Cancer Center Experience. Cancer J. Sci. Am. 1997; 3: S9–S15
- Legha S. S., Ring S., Papadopoulos N., Plager C., Chawla S., Benjamin R. A prospective evaluation of a triple-drug regimen containing cisplatin, vinblastine, and DTIC (CVD) for metastatic melanoma. Cancer 1989; 64: 2024–2029
- Lotze M. T., Dallal R. M., Kirkwood J. M., Flickinger J. C. Cutaneous melanoma. Cancer: Principles & Practice of Oncology, 6th Edition, V. T. DeVita, Jr, S. Hellman, S. A. Rosenberg. Lippincott, Williams & Wilkins, Philadelphia 2001; 2:2012–2069
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966; 50: 163–170
- Middleton M. R., Grob J. J., Aaronson N., Fierlbeck G., Tilgen W., Seiter S., Gore M., Aamdal S., Cebon J., Coates A., Dreno B., Henz M., Schadendorf D., Kapp A., Weiss J., Fraass U., Statkevich P., Muller M., Thatcher N. Randomized Phase III study of temozolomide vs. DTIC in the treatment of patients with advanced metastatic malignant melanoma. J. Clin. Oncol. 2000; 18: 158–166
- Millard M. J., Bedikian A. Y, Conry R. M., Gore M. E., Pehamberger H. E., Sterry W., Pavlick A. C., De Conti R. C., Gordon D., Itri L. M. Randomized multinational Phase 3 trial of DTIC (DTIC) with or without Bcl-2Antisense (oblimersen sodium) in patients (pts) with advanced malignant melanoma (MM): Analysis of long-term survival. Proc. Am. Soc. Clin. Oncol. 2004; 23: 708, (#7505)
- O'Day S. J., Gammon G., Boasberg P. D., Martin M. A., Kristedja T. S., Guo M., Stern S., Edwards S., Fournier P., Weisberg M., Cannon M., Fawzy N. W., Johnson T. D., Essner R., Foshag L. J., Morton D. L. Advantages of concurrent Biochemotherapy modified by decrescendo interleukin-2, granulocyte colony-stimulating factor, and tamoxifen for patients with metastatic melanoma. J. Clin. Oncol. 1999; 17: 2752–2761
- Papadopoulos N., Bedikian A. Y., Ring S., Kim K. B., Camacho L. H., Eton O. Phase II study of CTD (cisplatin, paclitaxel, DTIC) in metastatic melanoma (MM). Proc. Am. Soc. Clin. Oncol. 2003; 22, (Abstract #2889)
- Richards J. M., Mehta N., Ramming K., Skosey P. Sequential chemoimmunotherapy in treatment of metastatic melanoma. J. Clin. Oncol. 1992; 10: 1338–1343
- Rosenberg S. A., Yang J. C., Schwartzentruber D. J., Hwu P., Marincola F. M., Topalian S. L., Seipp C. A., Einhorn J. H., White D. E., Steinberg S. M. Prospective randomized trial of the treatment of patients with metastatic melanoma using chemotherapy with cisplatin, DTIC, and tamoxifen alone or in combination with interleukin-2 and interferon α 2b. J. Clin. Oncol. 1999; 17: 968–975
- Rosenberg S. A., Yang J. C., Topalian S. L., Schwartzentruber D. J., Weber J. S., Parkinson D. R., Seipp C. A., Einhorn J. H., White D. E. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin-2. JAMA 1994; 271: 907–913
- Thompson J. A., Gold P. J., Fefer A. Outpatient chemoimmunotherapy for the treatment of metastatic melanoma. Sem. Oncol. 1997; 24(S14)S44–S48
- WHO. World Health Organization. Handbook for Reporting Results of Cancer Treatment. World Health Organization, Geneva 1979
- Yee C., Thompson J. A., Byrd D., Riddell S. R., Roche P., Celis E., Greenberg P. D. Adoptive T-cell therapy using antigen-specific CD8+ T-cell clones for the treatment of patients with metastatic melanoma: In vivo persistence, migration, and anti-tumor effect of transferred T-cells. Proc. Natl. Acad. Sci. USA 2002; 99: 16168–16173
- Zitvogel L., Apetoh L., Ghiringhelli F., Kroemer G. Immunological aspects of cancer chemotherapy. Nature Med. 2008; 8: 59–73