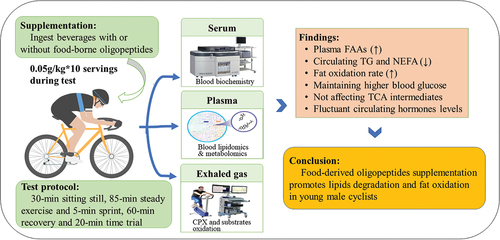
ABSTRACT
Background
Accumulation of body fat and dyslipidemia are associated with the development of obesity and cardiometabolic diseases. Moreover, the degree to which lipids can be metabolized has been cited as a determinant of cardiometabolic health and prolonged endurance capacity. In the backdrop of increasing obesity and cardiometabolic diseases, lipid metabolism and its modulation by physical activity, dietary adjustments, and supplementation play a significant role in maintaining health and endurance. Food-derived oligopeptides, such as rice and soybean peptides, have been shown to directly regulate abnormal lipid metabolism or promote hypolipidemia and fat oxidation in cell culture models, animal models, and human studies. However, whether supplementation with oligopeptides derived from multiple food sources can promote lipid degradation and fat oxidation in athletes remains unclear. Therefore, in a randomized controlled crossover trial, we investigated the impact of food-derived oligopeptide supplementation before and during exercise on lipid metabolism in young male cyclists.
Methods
Sixteen young male cyclists (age: 17.0 ± 1.0 years; height: 178.4 ± 6.9 cm; body mass: 68.7 ± 12.7 kg, body mass index: 21.5 ± 3.4 kg/m2; maximum oxygen uptake: 56.3 ± 5.8 mL/min/kg) participated in this randomized controlled crossover trial. Each participant drank two beverages, one containing a blend of three food-derived oligopeptides (treatment, 0.5 g/kg body weight in total) and the other without (control), with a 2-week washout period between two experiments. The cyclists completed a one-day pattern protocol that consisted of intraday fasting, 30 min of sitting still, 85 min of prolonged exercise plus a 5-min sprint (PE), a short recovery period of 60 min, a 20-min time trial (TT), and recovery till next morning. Blood samples were collected for biochemical analyses of serum lipids and other biomarkers. We analyzed plasma triglyceride species (TGs), free amino acids (FAAs), and tricarboxylic acid (TCA) cycle intermediates using omics methods. In addition, exhaled gas was collected to assess the fat oxidation rate.
Results
Five of 20 plasma FAAs were elevated pre-exercise (pre-Ex) only 20 min after oligopeptide ingestion, and most FAAs were markedly increased post PE and TT. Serum levels of TG and non-esterified fatty acids were lower in the experimental condition than in the control condition at the post PE and TT assessments, respectively. Further, the omics analysis of plasma TGs for the experimental condition demonstrated that most TGs were lower post PE and at the next fasting when compared with control levels. Simultaneously, the fat oxidation rate began to increase only 20 min after ingestion and during the preceding 85 min of PE. Levels of TCA cycle intermediates did not differ between the conditions.
Conclusions
The study noted that continuous ingestion of food-derived oligopeptides accelerated total body triglyceride breakdown, non-esterified fatty acid uptake, and fat oxidation during both sedentary and exercise states. Elevated circulating and intracellular FAA flux may modulate the selection of substrates for metabolic pathways in conjunction with the release of neuroendocrinological factors that slow down carbohydrate metabolism via acetyl coenzyme A feedback inhibition. This may increase the availability of fatty acids for energy production, with FAAs supplying more substrates for the TCA cycle. The findings of this study provide novel insight into strategies for promoting lipid metabolism in populations with dyslipidemia-related metabolic disorders such as obesity and for improving physiological functioning during endurance training. However, the absence of a non-exercising control group and verification of long-term supplementation effects was a limitation. Future studies will emphasize the impacts of whole protein supplementation as a control and of combined food-derived peptides or oligopeptides with probiotics and healthy food components on lipid metabolism in individuals who exercise.
GRAPHICAL ABSTRACT
1. Introduction
Excess accumulation of body fat is associated with obesity, dyslipidemia, and cardiometabolic diseases including, hypertension, stroke, type 2 diabetes, and several types of cancer [Citation1]. In general, strategies for improving lipolysis/fat oxidation include increased participation in physical activities, adjustments to dietary patterns, and supplementation [Citation2]. Physical activity improves lipid metabolism by enhancing lipolysis and fat oxidation. Researchers have observed a higher fat oxidation rate in athletes than in untrained or non-elite participants during high-intensity exercise [Citation3,Citation4]. Endogenous triacylglycerol is an important source of fuel for endurance exercise, and its oxidation progressively increases during exercise [Citation5,Citation6]. In competitive long-distance runners, an increased ratio of fat utilization can partly spare glycogen for the later sprint and improve exercise performance. Healthy dietary patterns and nutritional supplements are also beneficial for improving fat intake and lipid metabolism.
Protein or amino acid supplementation is prevalent in sports nutrition. Evidence shows that high-protein diets or dietary supplements, particularly amino acid mixtures containing branched chain amino acids (BCAAs), can reduce body fat and improve dyslipidemia when combined with physical activity by stimulating lipolysis and maximizing fat oxidation [Citation7–11]. Furthermore, elevated circulating BCAA levels in individuals with low physical activity are linked to a high body fat percentage, low fat oxidation, and impaired metabolism [Citation12]. At the same time, BCAA deprivation impairs lipid oxidation in C2C12 myotubes [Citation13]. Moreover, leucine/valine metabolites such as β-hydroxy-β-methylbutyrate and β-aminoisobutyric acid improve fat oxidation and increase energy expenditure [Citation14,Citation15] in adipose tissue and skeletal muscles. Individual amino acids such as arginine (Arg), tyrosine (Tyr), and tryptophan (Trp) modulate circulating levels of neurotransmitters or hormones such as growth hormones and catecholamines, which may regulate glucose and lipid metabolism during exercise [Citation16].
Food-derived peptides are increasingly used in ordinary food products, sports nutrition products, nutraceuticals, specific medical supplements, and therapeutic food products [Citation17–21]. Food-derived oligopeptides formed by condensing 2–10 amino acids can be absorbed as free amino acids (FAAs) or partial oligopeptides [Citation22]. Food-derived oligopeptides such as rice and soybean peptides directly regulate abnormal lipid metabolism or promote hypolipidemia and fat oxidation in cell culture models, animal models, and human studies [Citation23–26].
The effect of supplementation with multi-food-derived oligopeptides on lipid degradation and fat oxidation in athletes/fitness enthusiasts has not been explored. We hypothesized that this would improve lipid metabolism under various physiological states in relatively static conditions during moderate–intensity exercise. Therefore, in this study, we examined the effects of ingesting beverages with and without a blend of three food-derived oligopeptides, which have been shown to have the beneficial effects of alleviating exercise-induced fatigue and skeletal muscle damage [Citation27,Citation28] and improving immunity and metabolism [Citation29,Citation30], on lipid degradation and fat oxidation under various physiological conditions. These included sitting still (SS), prolonged exercise (PE), a time trial (TT) after a short rest period, and recovery till the next morning in young cyclists.
Our findings will help endurance athletes, fitness enthusiasts, and populations with metabolic disorders in choosing dietary supplements. Our findings will also be essential and referentially significant to future studies, along with the increasing development and broad utilization of food-derived oligopeptides as popular dietary supplements, especially combined with probiotics and other nutritional components in various physical activities.
2. Materials and methods
2.1. Study design
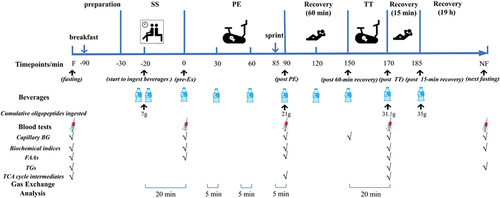
In this single-blind randomized placebo-controlled crossover trial, each participant drank two beverages, one containing a blend of three food-derived oligopeptides (treatment group, T) and one with no such peptides (control group, C), with a 2-week washout period between the experiments. The study design is presented in .
Figure 1. Test protocol. Riding start time (pre-exercise, pre-Ex) was set as 0 min. Breakfast: immediately after fasting blood collection; preparation: 90 min post breakfast; SS: sitting still (20 min); PE: prolonged exercise (90 min), which consisted of a steady-state cycling period (85 min) and a sprint (5 min); TT: time trial (20 min); recovery period including three durations of 60 min between PE and TT, 15 min post TT, and 19 h till next fasting. Using a crossover design, each participant performed the same test two times (ingestion of a food-derived oligopeptide supplement or control beverage) with a 2-week washout period, respectively. F: fasting; NF: next fasting. BG: blood glucose; FAAs: free amino acids; TGs: triglyceride species (omics analysis); TCA: tricarboxylic acid.
2.2. Participants
Sixteen healthy, young male cyclists (age: 17.0 ± 1.0 years; height: 178.4 ± 6.9 cm; body weight: 68.7 ± 12.7 kg, body mass index: 21.5 ± 3.4 kg/m2; maximum oxygen uptake [VO2max]: 56.3 ± 5.8 mL/min/kg) with >1 year of experience with cycling training and >10 h cycling training/week participated in the study lasting 24 h. Participants with acute sports-related injuries and those using any prescription medication, or ooligopeptide or protein supplements were excluded. Participants were randomly and equally allocated to the experimental or control supplement.
The study was approved by the ethics committee of National Institute of Sports Medicine in China (No. 202006). All procedures were conducted in accordance with the ethical standards of the 1964 Helsinki Declaration. In addition, the trial was pre-registered at ClinicalTrials.gov (website: http://www.chictr.org.cn/; Clinical Trial Identification Number: ChiCTR2100052885) on 6 November 2021. Furthermore, written informed consent was obtained from all participants before commencing the study. No additional consent for publication was required.
2.3. Exercise protocol, training, and diets
Individual VO2max was determined using an incremental workload (Watts) on Gravat 2 trainer (accuracy: 2500 Watts ± 3%) (Magene, Qingdao, China) 2 weeks before the trial. A 100-watt initial workload and 20-watt increment per minute were applied. Participants’ fasting (F) blood was collected in the early morning. To avoid measuring error, two participants (one from each group) were paired to perform the test in the morning or in the afternoon, approximately 90 min after a standardized breakfast or lunch on the test day. After emptying their bladder and bowels, the participant completed the subsequent test, consisting of a 30-min SS component, an 85-min PE component at an intensity of 60–70% of the individual VO2max, and a 5-min sprint on an ergometer bicycle (Wattbike Ltd, Nottingham, United Kingdom), followed by a rest period in the supine position for 60 min and a 20-min TT at approximately 85% VO2max on the same bicycle. The exercise test was conducted at 22 ± 2°C and 60 ± 5% humidity. Moreover, each participant was arranged to ride on the same ergometer bicycle and collected respiratory gas using the same Cortex MetaMax® 3B remote-controlled system (Cortex Biophysik, Leipzig, Germany) for gas exchange analysis in the two tests
All participants lived the same training camp with only one canteen and trained together using the same training plan, which comprised 80–120 km of road or bike trainer (if raining) riding for 3–4 days per week at an intensity with a heart rate of 130–180 beats/min and resistance training for 1–2 days per week during the study, including the 2-week washout period, except on the exercise test day. One of the researchers were arranged to record participants’ training and advise participants to follow a balanced diet during the study.
2.4. Food-derived oligopeptide supplementation
Each participant consumed two beverages containing 6% carbohydrate (CHO) and electrolytes with or without food-derived oligopeptides (2.7%) in a counterbalanced single-blind manner in two separate tests with a 2-week washout period. The added raw oligopeptide powder was a blend (weight ratio: 1:1:0.5) of enzymatic hydrolyzates of soybean, wheat, and fish skin collagen proteins containing 75–85% oligopeptides (dry weight) composed of 2–10 amino acids with a molecular weight of 200–1,000 Da. The contents of essential amino acids (EAAs) and BCAAs were up to 21.42 g and 9.53 g per 100 g blend (). The ratio of the three hydrolyzates was applied on the base of consideration with more balanced array of EAAs (21.42 g of EAAs and 9.53 g of BCAAs and 4.29 g of Leu per 100 g) () and purine [Citation29] in the blend than in a single hydrolyzate. The total volume of the beverages was calculated according to the dosage (0.5 g/kg body weight) of food-derived oligopeptide supplementation (FOPS), divided into 10 servings (0.05 g/kg body weight, each) ingested at various timepoints. Approximately 7 g of oligopeptides was ingested 20 min before exercise, and a total of approximately 35 g of oligopeptides containing 7.5 g of EAAs and 1.5 g of Leu was consumed per athlete during the trial (), which was consistent with a recommendation of protein ingestion (an absolute dose of 20–40 g per serving or 0.25 g/kg body weight per hour of endurance exercise) by the International society of sports nutrition [Citation31] that provided an ideal EAAs and Leu consumption. All beverages, which were independently packaged into aluminum cans, were manufactured and donated by Jianlibao Group Co., Ltd, China. Participants were told to refrain from taking any other nutritional supplements during the study.
Table 1. The content of amino acids in raw food-derived oligopeptide powder (g/100 g).
2.5. Blood tests
2.5.1. Blood sample collection
Whole blood was drawn from the forearm vein into vacutainer tubes at F (7:00 a.m.), pre-Ex (i.e. the end of 30-min SS period or the start of exercise), post PE and TT, and at the next fasting (NF) (). Blood samples were allowed to clot for 1 h at 24 ± 2°C before centrifugation for 20 min at 3,500 rpm. Serum or plasma for timely biochemical analysis was loaded into analytical tube, and the rest was stored at −80°C until serum biochemical and plasma omics assays. Capillary blood was collected for monitoring blood glucose (BG) at various timepoints ().
2.6. Biochemical analyses
BG was tested using a handheld BG analyzer (Accu-Chek, Roche, Germany). Serum levels of beta-hydroxybutyric acid (β-HBA), non-esterified fatty acid (NEFA), triglycerides (TG), total cholesterol (TCH), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and blood urea (BU) were determined using an automatic biochemical analyzer (Beckman coulter AU5800, USA). Serum glucagon, insulin, cortisol, and testosterone levels were measured using an immunofluorescence assay on the Unicel® DxI 800 Access® Immunoassay System using commercial kits (for insulin, cortisol, and testosterone: Leadman Biochemical Technology Co., Ltd., Beijing, China; for glucagon: J&L Biological, Shanghai, China). The intraplate coefficient of variation was less than 9%, and the interplate coefficient of variation was less than 11.0%. All tests were performed in accordance with the manufacturer’s instructions in the laboratories certified by ISO/IEC 17,025 and by ISO 15,189.
2.7. Quantitative metabolomics analyses
Quantitative omics analyses for plasma triglyceride species (TGs), FAAs and their metabolites, and tricarboxylic acid (TCA) cycle intermediates were performed using ultra-performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS). We identified 101 TGs, 32 FAAs and their metabolites, and 8 TCA cycle metabolites. Sample preparation and the UPLC-MS/MS procedure have been previously described [Citation32,Citation33].
2.8. Sample preparation for metabolomics
The samples were not thawed prior to analysis. In brief, for the extraction of plasma FAAs, 10 μL of the plasma sample was mixed with 40 μL isopropyl alcohol and 5 μL internal standard. After vortexing, the mixture was centrifuged at 12,000 rpm for 10 min at 4°C. Then, 10 μL of the supernatant was mixed with 10 μL double-distilled water (ddH2O). Subsequently, 10 μL of the mixture was mixed with 70 μL boric acid buffer (AccQ•Tag kit, Waters, Milford, MA, USA) and 20 μL of AccQ•Tag derivative reagent (Kairos amino acid kit), after which the new mixture was shocked for 10 s. The centrifuge tube was heated at 55℃ for 10 min; then, 500 μL water was added and transferred to autosampler vials (DIKMA, Beijing, China) for testing. TGs in plasma samples (50 μL) were extracted by adding 20 μL of the inter-standard solution (Avanti Polar Lipids, Alabaster, AL, USA), 280 μL methanol, and 1 mL methyl tertiary butyl ether (Fisher Scientific, Waltham, MA, USA). Subsequently, the mixture was ultrasonicated at 22 ± 2°C for 1 h, and 100 µL of ddH2O was added and mixed well. To extract plasma TCA cycle intermediates, 150 μL of methanol was added to 50 μL of plasma samples. The mixture was vortexed and centrifuged. Subsequently, 50 μL of supernatant was taken and mixed with 50 μL of internal standard (Sigma-Aldrich, St. Louis, MO).
After centrifugation, 100 μL isopropanol/acetonitrile (1:1, v/v) (Fisher Scientific, Beijing China) was added, and the supernatant was dried using a centrifugal vacuum evaporator (Thermo Fisher Scientific, Waltham, MA, USA) for 4 h at 4°C and dissolved via ultrasonication (Kunshan Hechuang, Shanghai, China). We repeated the centrifugation procedure, and 100 μL of supernatant was collected.
2.9. UPLC-MS/MS procedure
Treated samples were analyzed using reverse phase chromatography, performed on UHPLC (UPLCI-CLASS, waters, UK). Mass spectrometer analysis was performed in a positive polarity mode with a tandem quadrupole mass spectrometer equipped with a thermoelectric spray ion source (XEVO TQ-XS, waters, USA). The chromatographic conditions and mass spectrometric parameters are shown in Table S1.
2.10. Data analysis and quality control
Masslynx software 4.1 (Waters, USA) and analysis software (SCIEX, Boston, MA, USA) were used for different systemic control and data acquisition, respectively. The content of the corresponding substances in the peak area of each chromatographic peak was calculated using Skyline analysis software (MacCoss, University of Washington). Regarding FAAs and TCA cycle intermediates, quantitative results were obtained using the standard curve method. Concentrations of individual TGs were obtained using the isotopic internal standard. Quality control analyses were performed every 6–8 samples to monitor the stability and repeatability of the entire liquid-mass system. This method gave a satisfactory precision and linearity with relative standard deviation less than 30% and pearson correlation coefficients greater than 0.99.
2.11. Gas exchange analysis
Expired gas was collected for 30 min during SS, 5 min every 30 min during PE, and for 20 min during TT using Cortex MetaMax® 3B remote-controlled system for analyses of oxygen uptake (VO2), respiratory exchange rate (RER), CHO, and fat oxidation rates using the breath-by-breath method. The system was calibrated with a standard gas (5% CO2, 15% O2, BAL.n2; Air Liquide Healthcare America Corporation, Plumsteadville, PA, USA) before use.
2.12. Data processing and statistical analysis
Data are expressed as the mean ± standard error (S.E.) unless otherwise specified. Repeated-measures analysis of variance (ANOVA) followed by post hoc least significant difference testing was performed to determine statistical differences of biochemical analyses and metabolomic results with characteristics over time between different timepoints intragroup and between groups. A paired Student’s t-test was used to compare the difference in gas exchange analysis between groups. GraphPad Prism 8.0 software (GraphPad Software, Inc., La Jolla, CA, USA) was used to generate graphs. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS software (IBM Corp., Armonk, NY).
Statistical analysis for TGs omics data was performed using MetaboAnalyst 5.0. Data were log-transformed and standardized to a normal distribution before multivariate statistical analysis. The variable importance in projection (VIP) generated in the orthogonal partial least squares discriminant analysis (OPLS-DA) processing represents the contribution to the discrimination of each metabolite ion between groups. The differential TG species were determined based on VIP > 1.0 and p < 0.05.
Pathway enrichment analysis of plasma FAAs was performed according to the p values from hypergeometric tests and pathway impact values from pathway topology analysis (relative-betweenness centrality). The standard for potentially targeted pathway was set as false-discovery rate <0.05 and pathway impact value >0.4. FAAs were mapped to pathways in the Kyoto Encyclopedia of Genes and Genomes.
3. Results
3.1. Plasma FAAs
Exercise with FOPS resulted in a greater change in plasma FAAs for the T group condition than for the C group. For five of the 20-species FAAs categorized as glucogenic amino acids (GAAs), levels were increased in the T group (Table S2) during the pre-Ex period (i.e. only 20 min after ingesting 2 servings, totaling approximately 7 g of oligopeptides per participant). However, total FAA levels were mildly increased. Further, 17 of the 20-species FAAs categorized as EAAs, BCAAs, ketogenic amino acids (KAAs)/glucogenic and KAAs (GKAAs), aromatic amino acids (AAAs) ( and Table S2), and some of their metabolites ( and Table S2) were markedly elevated post PE (i.e. after ingesting 21 g oligopeptides within 110 min, totaling approximately 14 g [0.05 g/kg × 4 servings] during the 90-min PE plus the preceding 7 g per participant). Additionally, although levels of FAAs and their metabolites were significantly reduced post TT vs. post PE in the T group, reaching levels close to the pre-Ex levels, most were still higher than those in the C group ( and Table S2). In contrast, levels of EAAs, BCAAs, and KAAs/GKAAs, but not AAAs, gradually decreased in the control group over time. Moreover, these levels were significantly decreased post TT vs. pre-Ex (), while post PE levels of BU were higher than pre-Ex levels. Meanwhile, individual FAAs exhibited a clear, gradual separation according to group at various timepoints (). Quantitative omics analysis of FAAs demonstrated differential FAAs at three timepoints between the C and T groups, including 17 differential FAAs at post PE and 10 differential FAAs at post TT. Seven FAAs (Arg, Gln, Glu, Lys, Asp, Ala, Met) did not significantly differ between the groups post TT but were consumed more during the 60-min recovery and 20-min TT periods ().
Figure 2. FOPS led to alterations in levels of plasma FAAs and their metabolic intermediates. a, total FAAs; b-g, plasma FAA levels for diverse categories; h, BU level; i, a heatmap of plasma FAAs and their metabolite levels from low (light blue) to high (deep red), with values and significant changes between groups at each timepoint listed in Table S2; j, a Venn Diagram of the OPLS-DA score plot; k, plasma differential FAAs at diverse timepoints; b overview of pathway enrichment analysis for plasma FAAs. Significant pathway names are listed. Data are presented as mean±S.E. (n = 16 per group). C, control; T, FOPS; Ex, exercise; F: fasting, NF: next fasting. Compared between groups: *p < .05, **p < .01, ***p < .001. FOPS: food-derived oligopeptide supplementation; FAAs: free amino acids.
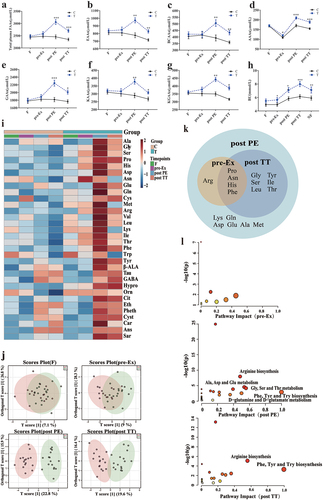
The enrichment analysis revealed the following for markedly differential FAAs: 1) pre-Ex: no significant pathway enrichment; slight predominance for biosynthesis of AAAs and Arg; 2) post PE: biosynthesis of Arg and AAAs; metabolism of alanine (Ala), aspartate (Asp), glutamate (Glu), glutamine (Gln), glycine (Gly), serine (Ser), and threonine (Thr); 3) post TT: biosynthesis of AAAs and Arg ().
3.2. Lipid degradation and fat oxidation rate
3.2.1. Lipid degradation
The ANOVA for serum lipid profiles revealed marked changes in TG, LDL-C, TCH, HDL-C, β-HBA (p < 0.0001 for all), and NEFA (p = 0.019) levels with breakfast, exercise, and FOPS in both groups (). Serum TG levels were markedly elevated pre-Ex in both groups. Serum TG levels were lower (p = 0.067) in the T group than in the C group both post PE and post TT and were significantly lower at NF than at F (). Mildly lower serum TG levels were observed only 20 min after ingesting oligopeptides, coinciding with the change in plasma FAAs (). Post PE, serum NEFA levels were lower in the T group than in the C group (), while LDL-C levels at NF were higher in the T group than in the C group (p = 0.051) (). In addition, a greater decrease in LDL-C and TCH levels was observed at NF in the C group but not in the T group (). Furthermore, greater increases in NEFA (p = 0.046) () and β-HBA (p = 0.007) () were observed at NF than at F in the T group. No significant differences were observed in HDL-C levels between the groups ().
Figure 3. FOPS resulted in changes in serum lipids and plasma TG species. a-f, serum lipids (TG, LDL-C, TCH and HDL-C) and TG degradation products (NEFA and β-HBA); g, a heatmap of net changes in plasma TGs from low (light blue) to high (deep red) at 170 min and at NF vs. F; h, average alteration in plasma TG species relative to that at F. Data are presented as mean±S.E. (n = 16 per group). C, control; T, FOPS; Ex, exercise; F: fasting, NF: next fasting. *p < .05, **p < .01, ***p < .001, ****p < .0001. FOPS: feed-derived oligopeptide supplementation; TG: triglycerides; LDL-C: low-density lipoprotein cholesterol; TCH: total cholesterol; HDL-C: high-density lipoprotein cholesterol; NEFA: non-esterified fatty acid; β-HBA: β-hydroxybutyric acid.
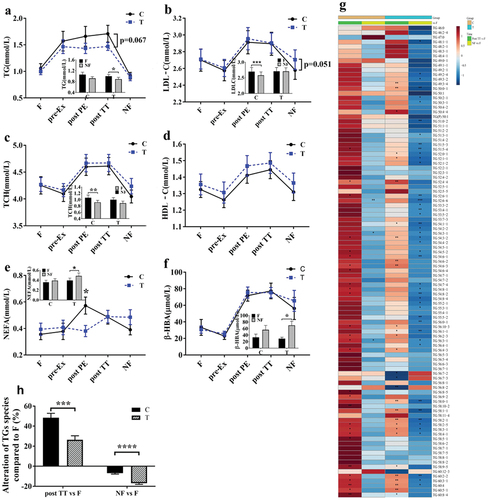
The heatmap of the omics analysis for 101 TGs indicated that levels of most TGs were lower in the T group than in the C group at post TT and at NF ( and ). The significant differences relative to the values at F clearly demonstrated the changes post TT and at NF, in which 45 of 101 TGs (45%) in the T group and only three of the 101 TGs (3%) in the C group were significantly decreased at NF ( and ). Consequently, the average percentage changes post TT and at NF when compared with those at F were −22% and −10%, respectively, in the T and C groups (Table S3–2 and ).
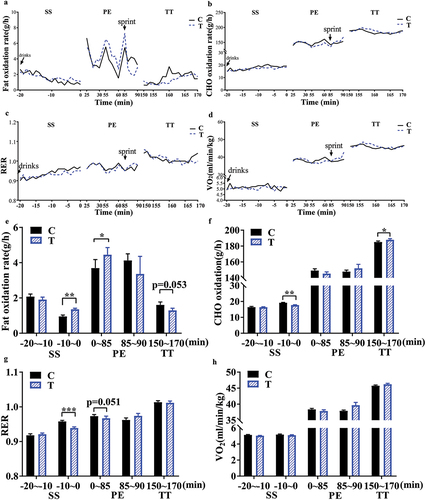
3.3. Fat oxidation rate
The average RER was lower in the T group than in the C group during SS and former 85 min during PE, although there was no significant difference between the groups at durations (). Accordingly, the calculated fat oxidation rate during the PE period (0–85 min) was higher in the T group than in the C group, although it was slightly lower during the latter 5 min of PE (sprint time) and 20 min all-out TT (). Corresponding changes in the CHO oxidation rate were also observed (p = .0017, ). Fat oxidation began to change to a statistically significant extent within only 10–20 min following the first two servings of FOPS (0.1 g/kg, approximately 7 g per participant) during SS (p = .0016, ). However, there was no statistically significant change in VO2 () owing to the same energy demand during an equal exercise intensity in each group.
Figure 4. Results of gas exchange analysis. a-d: dynamic curves for average fat and CHO oxidation rates, RER, and VO2 during SS, PE, and TT, respectively. e-h: average fat and CHO oxidation rates, RER, and VO2 during separate durations of SS, PE, and TT. Data are presented as mean±S.E. (n = 16 per group). C, control; T, FOPS. F: fasting, NF: next fasting. SS: sitting still (20 min), PE: prolonged exercise (90 min) including a steady-state cycling period (85 min) and a sprint (5 min), TT: time trial (20 min). A paired samples t-test was performed to compare the difference during the same duration between two groups. *p < 0.05, **p < 0.01, ***p < 0.001. CHO: carbohydrate; RER: respiratory exchange rate.
3.4. TCA cycle intermediates
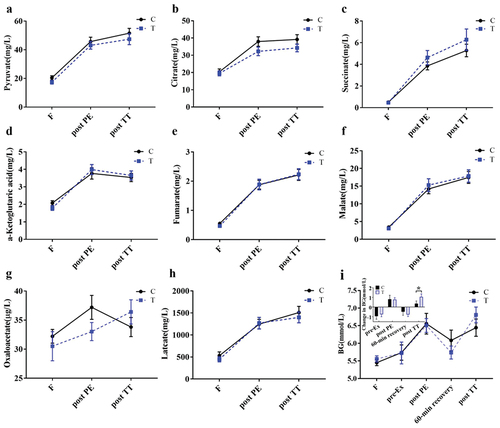
The levels of detected TCA intermediates, including pyruvate, fumarate, succinate, malate, α-ketoglutaric acid, citrate, and lactate (but not oxaloacetate), increased post PE and were maintained post TT, without any difference between the groups ().
Figure 5. Levels of plasma TCA cycle intermediates and blood glucose. a-h, intermediates; i, blood glucose and net change relative to previous timepoint (inserted graph). Data are presented as mean±S.E. (n = 16 per group). C, control; T, FOPS. F: fasting, NF: next fasting. *p < 0.05. TCA: tricarboxylic acid cycle.
3.5. BG and serum hormones
BG levels had a main effect with exercise and FOPS, and the average BG level post TT was higher in the T group than in the C group (p = 0.041) ( and inserted graph). In both conditions, several hormone levels fluctuated with exercise, without any statistically significant difference between groups at any time point (Fig. S1).
4. Discussion
Accumulated fat is a health risk. Some amino acids [Citation11,Citation34] and protein peptides [Citation24–26,Citation35] improve lipid metabolism. Bioactive peptides are more effective in decreasing serum cholesterol and LDL-C content than dietary proteins [Citation17]. Food products containing food-derived peptides or oligopeptides may be preferred by consumers who believe that peptides can take effect more rapidly on supplying fuel, repairing impaired skeletal muscles, and promoting protein synthesis.
The blend of multi-source oligopeptides used in the present study, which comprised 2–10 amino acids with high biological value (), may exert rapid and sustaining effects on lipid metabolism. In the present study, we recognized that FOPS promoted TG breakdown and NEFA uptake under various physiological states in young cyclists. Our findings revealed that improved fat metabolism was closely linked with FOPS-induced alterations in plasma FAAs. The underlying mechanisms might be involved in three pathways as described in : 1) globally neuroendocrinological modulation; 2) increased substrate flux of elevated FAAs and FAAs-driven FFA for energy production; 3) regulation of intracellular FAAs and FFA metabolic intermediates on CHO metabolism.
Figure 6. A draft of the proposed mechanism for FOPS-promoted lipolysis and fat oxidation during prolonged exercise. ↑: increased; thin blue solid line: enhanced or accelerated; thick blue/red solid line: strongly enhanced; thin blue dotted line: inhibition 
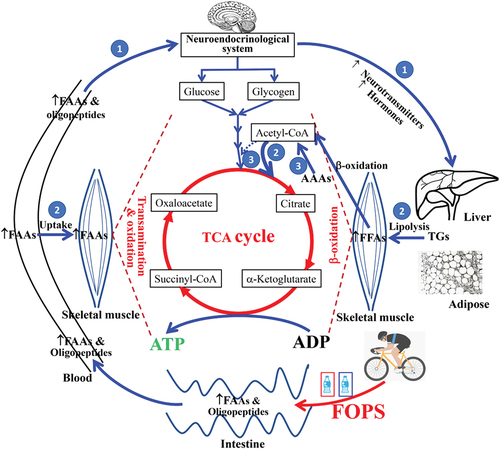
First, our findings suggest that the amino acids, dipeptides, and/or tripeptides [Citation22] absorbed following ingestion of the FOPS can promote TG breakdown to free fatty acids (i.e. NEFA) and fat oxidation in a short time, as changes were observed only 20 min after ingesting 7 g of oligopeptides even without exercise, and only five species of FAA were markedly elevated ( and Table S2). The effect on lipid metabolism might involve globally rapid and sustained neuroendocrinological modulation by amino acids and/or bioactive peptides from FOPS (①).
Proteins are first degraded into peptides or amino acids prior to absorption in the small intestine [Citation36]. Oligopeptides are rapidly absorbed [Citation22,Citation37]; however, the efficiencies of absorption, transportation, and utilization, especially in dipeptides and tripeptides, are higher compared with those of a free amino acid mixture [Citation38]. Existing evidence shows that peptides affect intestinal absorption, enterohepatic bile acid reabsorption, lipogenesis, and lipolysis in hepatocytes and adipocytes by regulating the expression of lipogenic transcription factors [Citation23]. Our data demonstrated that the ingestion of 7 g oligopeptides (similar to the dosage of 9 g per participant reported by Ejima [Citation22]) led to significant increases in five GAA species within 20 min during SS, as well as a mild increase in total plasma FAAs (). In Ejima’s report, some active dipeptides/tripeptides started to emerge in the bloodstream within 30 min and significantly increased 60 min after ingestion of a corn/wheat gluten hydrolyzate. Unfortunately, blood dipeptides/tripeptide levels were not measured in our study; however, we speculated that some dipeptides/tripeptides were simultaneously absorbed as reported by Ejima [Citation24]. FOPS-induced neuroendocrinological factors can activate hormone-sensitive lipase and lipoprotein lipase to rapidly break down TG in adipose tissues and liver (), promote fat oxidation in muscle () during rest, subsequently sustain significant effects including muscle FFA uptake () with the supplementation until next morning (; ), meanwhile to inhibit the breakdown of liver glycogen, and promote the resynthesis of liver glycogen (). However, the fluctuations in circulating hormones in our study (Fig. S1) were inconsistent with those reported in a previous study, in which 3 g Phe stimulated the secretion of glucagon [Citation34]. Nonetheless, our results are similar to the previous finding that a 3 g mixture of Arg, Ala, and Phe did not change the circulating glucagon level [Citation11]. Therefore, it is possible that elevated circulating Arg, Tyr, and Orn (Table S2) trigger the release of growth hormones, epinephrine and norepinephrine, or dopamine [Citation39].
Second, constant FOPS during PE improved lipid metabolism, as shown by the breakdown of plasma TG (), uptake of plasma free fatty acids (NEFA) () from the circulation, and oxidization of fatty acids during the preceding 85 min of PE (). In contrast, NEFAs were not taken up by exercising skeletal muscles in the control condition. The effect lasted until the next morning, as illustrated by NEFA and β-HBA (incomplete production of fatty acids via β-oxidation) levels () and omics analysis of TGs ( and Table S3). This persistent effect may be attributed to two factors of both increased TG breakdown and elevated uptake/oxidation of fatty acids mediated by elevated intracellular FAAs substrate flux, which might accelerate TCA cycle for energy production and drive more FFAs into the TCA cycle (②).
An acute bout of exercise, especially during high-intensity or PE [Citation40,Citation41], greatly increases energy expenditure and the oxidation of BCAAs [Citation42,Citation43]. The large increase in FAAs due to continuous FOPS (14 g per participant, 21 g in total) during PE implies that such supplementation may meet the demand on amino acids as fuel for prolonged exercise (). Amino acids transported to muscle cells may be directly oxidized or indirectly transaminated with the intermediates of TCA cycle to supply more metabolic substrates, as indicated by increased BU and Arg metabolism/biosynthesis (). Decreases in levels of seven species of FAAs following TT in the T group () suggest that some circulating FAAs are taken up by skeletal muscles and metabolized for energy production during high-intensity exercise or used to form other amino acids, such as AAAs and BCAAs, which were observed even after the ingestion of another 10 g oligopeptides (total of 31.5 g oligopeptides ingested) (). These FAAs might have been used for glycogen resynthesis via gluconeogenesis during the 60-min recovery period, as illustrated by BG levels after 60 min of recovery and post TT, respectively (), as well as the result of the enrichment pathway analysis (). Post TT, FAA levels were higher in the experimental condition than in the control condition, indicating that constant FOPS can meet the demand for the FAA supply during the whole course. In contrast, FAA levels were reduced in the control condition (), despite the body’s attempt to increase the supply of FAAs via muscle proteolysis (indicated by an increased BU level with time in the C group) () [Citation43,Citation44].
Third, intracellular metabolite level changes initiated local regulation of CHO and lipid catabolic pathways to satisfy energy supply (③). An elevated metabolite, acetyl coenzyme A, that was converted from increased cellular KAAs and simultaneously derived from fatty acid β-oxidation as illustrated by pathway enrichment analysis (), can inhibit pyruvate dehydrogenation (a rate-limiting step) to slow down CHO metabolism [Citation45], consequently facilitating more fatty acids for the TCA cycle for sufficient energy production. In this study, both conditions were performed at the same exercise intensity and energy expenditure level (indicated by VO2) (), with the TCA data suggesting that improving fat oxidation can help to spare the body’s glycogen reserves during exercise or increase glycogen resynthesis during rest after exercise [Citation46]. Our results suggest that FOPS improved glycogen resynthesis during the 60-min recovery period, consequently maintaining a higher BG level in the next 20-min high-intensity TT period (), given an increased availability of muscle/liver glycogen for energy substrate ().
Although increased FAAs may consistently act on lipid metabolism until it returns to baseline, some products may be fed into their own subsequent metabolic pathways, such as NEFAs and glycerol for the resynthesis of lipids, as well as FAAs for protein synthesis/glycogenesis through gluconeogenesis. We observed significantly increased synthesis of LDL-C and TCH () in the morning after the test session, which was likely due to the excessive circulating level of NEFA following the end of the exercise session. However, our findings support the notion that 3 h of constant FOPS in combination with PE, even exercise alone (control without the supplementation of oligopeptides) (; Table S3), can persistently improve lipid metabolism almost throughout the day, which was inconsistent with the report that indicated exercise does not increase 24-h fat oxidation, as though fat metabolism in muscle is improved [Citation47].
The effect of protein and amino acid supplementation on exercise performance is unclear [Citation31]. We did not observe the expected positive effect of FOPS on exercise performance (C: 12.81 ± 0.66 vs T: 12.87 ± 0.64 km during TT). Previous related studies concerning protein supplementation have arrived at similar conclusions [Citation48–51]. A systematic review indicated that, when CHO is delivered at optimal rates during or after endurance exercise, protein supplements appear to have no direct effect on enhancing endurance performance [Citation52]. In this regard, we offered frequent ingestion of sport drink (containing CHO) throughout the trial to maintain the hydration status and BG level. There were no cases of hypoglycemia or exercise fatigue in either group. In addition, sport performance can also be affected by some subjective factors.
In summary, our results strongly suggest that constant FOPS can exert beneficial effects on lipid metabolism in young male cyclists that emerge at diverse physiological states, during both rest periods (SS and recovery after exercise till the next morning) and prolonged aerobic exercise. The temporal and spatial variations in serum biochemistry lipid profiles, plasma TG omics, and fat oxidation were closely in line with levels of plasma FAAs. The mechanisms underlying these effects might be linked to globally rapid and sustained modulation, increased substrate flux, and intracellular local regulation of metabolic pathways, as summarized in .
At present, health scientists are trying to find effective strategies to prevent and treat accumulated fat, obesity, and dyslipidemia. Exercise-induced skeletal muscle damage, fatigue, and impaired exercise performance are undoubtedly a concern for athletes and fitness enthusiasts. Our findings may help these potential consumers to rationally select food-derived healthy products to address lipid metabolic related issues or disorders. Most importantly, food-derived oligopeptides demonstrated a rapidly and sustained improved effect on lipid metabolism, both at rest and during prolonged exercise. Probably, food-derived oligopeptides can become a popular product in the field of sports nutrition in the future.
4.1. Limitations of the study
Our findings suggest that constant FOPS may improve intraday lipid metabolism in young male cyclists via diverse pathways. However, the single-blind study design might bring to subjective bias, even though we adopted some objective indicators and operations as many as possible to avoid subjective judgment during the trial. Further, the study did not include control participants who were not engaged in regular exercise and who did not digest proteins alone in the study. Thus, we could not confirm the effects of oligopeptide supplementation under sedentary states and of lower-cost proteins. In addition, our analysis could not verify the effects of long-term oligopeptide supplementation, especially potential health-impairing effect. Further, we did not consistently measure the levels of FAAs, TGs (omics analysis), TCA cycle intermediates or circulating levels of dipeptides/tripeptides, growth hormones, and catecholamines at all time points. Blood outcomes do not completely reflect intracellular metabolic status, thus, we cannot fully explain the mechanisms underlying hypolipidemic effects. Future studies should focus on the effects of whole protein supplementation as a control, and of combined food-derived peptides or oligopeptides with probiotics and functionally healthy food components on lipid metabolism in exercising crowd, especially in physically active participants who are overweight or obeseand with dyslipidemia and metabolic-related disorders, using a more rigorous study design.
5. Conclusion
Continuous ingestion of multi-food-derived oligopeptides (0.05 g every 20–30 min, 0.5 g per kg) promoted whole-body TG breakdown, NEFA uptake, and fat oxidation during relatively sedentary states (SS and recovery period till the next morning) and PE. These effects were closely linked to elevated plasma FAAs and possibly oligopeptides. We speculate that elevated levels of circulating FAAs and oligopeptides may modulate the release of lipolytic neuroendocrinological factors to promote lipid metabolism. Simultaneously, increased circulating FAAs may provide more amino acid substrate flux to accelerate the TCA cycle during exercise and for muscle glycogen resynthesis during recovery post exercise. Moreover, acetyl coenzyme A converted by KAAs and derived from fatty acid β-oxidation may slow CHO breakdown and increase the availability of fatty acids for the TCA cycle. The data obtained in the current study provide insight into new nutrition strategies for athletes and physically active individuals that may enhance adaptions to exercise and/or training, potentially leading to improved health and sports performance through increased fat oxidation. The long-term and possible adverse effects of FOPS should be examined in future studies, which should involve various populations, including those with abnormal lipid metabolism and obesity.
Authorship contribution
Aina Jin, Zhaobo Kan, Qiushi Tang, Jing Shao, and Qi Han completed sample collection and analysis. Aina Jin performed the data analysis and manuscript preparation. Yashan Chang and Nan An were responsible for project administration. Muqing Yi was responsible for study design, funding acquisition, and writing review. All authors approved the final version of the paper.
Supplemental Material
Download Zip (3.5 MB)Acknowledgments
The authors sincerely thank Jianlibao Group Co. Ltd for the donation of test beverages.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/15502783.2023.2254741
Additional information
Funding
References
- Lin, X, Li, H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol. 2021;12:706978. doi: 10.3389/fendo.2021.706978
- Kim, JH, Park, Y. Combined effects of phytochemicals and exercise on fatty acid oxidation. J Exerc Nutr Biochem. 2016;20(4):20–773. doi: 10.20463/jenb.2016.0053
- Hetlelid, KJ, Plews, DJ, Herold, E, et al. Rethinking the role of fat oxidation: substrate utilisation during high-intensity interval training in well-trained and recreationally trained runners. BMJ Open Sport Exerc Med. 2015;1(1):e000047. doi: 10.1136/bmjsem-2015-000047
- Aslankeser, Z, Balci, SS. Re-examination of the contribution of substrates to energy expenditure during high-intensity intermittent exercise in endurance athletes. PeerJ. 2017;5:e3769. doi: 10.7717/peerj.3769
- Muscella, A, Stefàno, E, Lunetti, P. The regulation of fat metabolism during aerobic exercise. Biomolecules. 2020;10(12):1699. doi: 10.3390/biom10121699
- Horowitz, JF, Klein, S. Lipid metabolism during endurance exercise. Am J Clin Nutr. 2000;72(S2):558S–563S. doi: 10.1093/ajcn/72.2.558S
- Kainulainen, H, Hulmi, JJ, Kujala, UM. Potential role of branched-chain amino acid catabolism in regulating fat oxidation. Exerc Sport Sci Rev. 2013;41(4):194–200. doi: 10.1097/JES.0b013e3182a4e6b6
- Chen, CN, Hsu, KJ, Chien, KY, et al. Effects of combined high-protein diet and exercise intervention on cardiometabolic health in middle-aged obese adults: A randomized controlled trial. Front Cardiovasc Med. 2018;8:705282. doi: 10.3389/fcvm.2021.705282
- Oliveira, C, Boulé, NG, Berg, A, et al. Consumption of a high-protein meal replacement leads to higher fat oxidation, suppression of hunger, and improved metabolic profile after an exercise session. Nutrients. 2021;13(1):155. doi: 10.3390/nu13010155
- Kim, DH, Kim, SH, Jeong, WS, et al. Effect of BCAA intake during endurance exercises on fatigue substances, muscle damage substances, and energy metabolism substances. J Exerc Nutr Biochem. 2013;17(4):169–180. doi: 10.5717/jenb.2013.17.4.169
- Ueda, K, Nakamura, Y, Yamaguchi, M, et al. Amino acid mixture enriched with arginine, alanine, and phenylalanine stimulates fat metabolism during exercise. Int J Sport Nutr Exerc Metab. 2016;26(1):46–54. doi: 10.1123/ijsnem.2015-0137
- Kujala, UM, Peltonen, M, Laine, MK, et al. Branched-chain amino acid levels are related with surrogates of disturbed lipid metabolism among older men. Front Med. 2016;3:57. doi: 10.3389/fmed.2016.00057
- Karvinen, S, Fachada, V, Sahinaho, UM, et al. Branched-chain amino acid deprivation decreases lipid oxidation and lipogenesis in C2C12 myotubes. Metabolites. 2022;12(4):328. doi: 10.3390/metabo12040328
- Duan, Y, Zhang, L, Li, F, et al. β-Hydroxy-β-methylbutyrate modulates lipid metabolism in adipose tissues of growing pigs. Food Funct. 2018;9(9):4836–4846. doi: 10.1039/C8FO00898A
- Kamei, Y, Hatazawa, Y, Uchitomi, R, et al. Regulation of skeletal muscle function by amino acids. Nutrients. 2020;12(1):261. doi: 10.3390/nu12010261
- Tam, CS, Johnson, WD, Rood, J, et al. Increased human growth hormone after oral consumption of an amino acid supplement: results of a randomized, placebo-controlled, double-blind, crossover study in healthy subjects. Am J Ther. 2020;27(4):e333–e337. doi: 10.1097/MJT.0000000000000893
- Ruiz Ruiz, JC, Betancur Ancona, DA, Segura Campos, MR. Bioactive vegetable proteins and peptides in lipid-lowering; nutraceutical potential. Nutrición Hospitalaria. 2014;29(4):776–784. doi: 10.3305/nh.2014.29.4.7208
- Chatterjee, C, Gleddie, S, Xiao, CW. Soybean bioactive peptides and their functional properties. Nutrients. 2018;10(9):1211. doi: 10.3390/nu10091211
- Mizushige, T. Neuromodulatory peptides: orally active anxiolytic-like and antidepressant-like peptides derived from dietary plant proteins. Peptides. 2021;142:170569. doi: 10.1016/j.peptides.2021.170569
- Peighambardoust, SH, Karami, Z, Pateiro, M, et al. A review on health-promoting, biological, and functional aspects of bioactive peptides in food applications. Biomolecules. 2021;11(5):631. doi: 10.3390/biom11050631
- Yuan, H, Luo, Z, Ban, Z, et al. Bioactive peptides of plant origin: distribution, functionality, and evidence of benefits in food and health. Food Funct. 2022;13(6):3133–3158. doi: 10.1039/D1FO04077D
- Ejima, A, Nakamura, M, Suzuki YA, et al. Identification of food-derived peptides in human blood after ingestion of corn and wheat gluten hydrolysates. J Food Bioact. 2018;2:104–111. doi: 10.31665/JFB.2018.2145
- Udenigwe, CC, Rouvinen-Watt, K. The role of food peptides in lipid metabolism during dyslipidemia and associated health conditions. Int J Mol Sci. 2015;16(5):9303–9313. doi: 10.3390/ijms16059303
- Sidorova, YS, Mazo, VK, Kochetkova, AA. Experimental evaluation of hypolipidemic properties of soy and rice proteins and their enzyme hydrolysates. A brief review. Vopr Pitan. 2018;87(2):77–84. doi: 10.24411/0042-8833-2018-10021
- Nagaoka, S. Structure-function properties of hypolipidemic peptides. J Food Biochem. 2019;43(1):e12539. doi: 10.1111/jfbc.12539
- Nagaoka, S, Takeuchi, A, Banno, A. Plant-derived peptides improving lipid and glucose metabolism. Peptides. 2021;142:170577. doi: 10.1016/j.peptides.2021.170577
- Pan, X, Hu, Y, Gu, R, et al. Preventive effect of wheat peptides supplementation on the occurrence of overtraining in Sanda athletes (in Chinese). Chin J Sports Med. 2015;34(2):170–174.
- Li, M, Yin, M, Ling, K, et al. Experimental study on the antifatigue function of soybean peptide and wheat peptide (in Chinese). Food Sci Technol. 2019;44(9):303–307.
- Li, H, Li, Y, Wang, W. Effects of food-derived oligopeptides with different levels of purine on hyperuricemia (in Chinese). Chin J Convalescent Med. 2023;32(3):225–230.
- Huang, M, Xu, S. Research progress on digestion and absorption mechanism and nutritional function of food derived oligopeptides (in Chinese). Food And Nutr In China. 2021;27(10):55–58.
- Jäger, R, Kerksick, CM, Campbell, BI, et al. International society of sports nutrition position stand: protein and exercise. J Int Soc Sport Nutr. 2017;14:20. doi: 10.1186/s12970-017-0177-8
- Han, X, Luo, R, Wang, L, et al. Potential predictive value of serum targeted metabolites and concurrently mutated genes for EGFR-TKI therapeutic efficacy in lung adenocarcinoma patients with EGFR sensitizing mutations. Am J Cancer Res. 2020;10(12):4266–4286.
- Zhang, P, Zhang, X, Huang, Y, et al. Atorvastatin alleviates microglia-mediated neuroinflammation via modulating the microbial composition and the intestinal barrier function in ischemic stroke mice. Free Radic Biol Med. 2021;162:104–117. doi: 10.1016/j.freeradbiomed.2020.11.032
- Ueda, K, Sanbongi, C, Yamaguchi, M, et al. The effects of phenylalanine on exercise-induced fat oxidation: a preliminary, double-blind, placebo-controlled, crossover trial. J Int Soc Sports Nutr. 2017;14(1):34. doi: 10.1186/s12970-017-0191-x
- Caponio, GR, Wang, DQ, Di Ciaula, A, et al. Regulation of cholesterol metabolism by bioactive components of soy proteins: novel translational evidence. Int J Mol Sci. 2020;22(1):227. doi: 10.3390/ijms22010227
- Rutherfurd-Markwick, KJ. Food proteins as a source of bioactive peptides with diverse functions. Br J Nutr. 2012;108(S2):S149–S157. doi: 10.1017/S000711451200253X
- Chakrabarti, S, Guha, S, Majumder, K. Food-derived bioactive peptides in human health: challenges and opportunities. Nutrients. 2018;10(11):1738. doi: 10.3390/nu10111738
- Nakayama, K, Sanbongi, C, Ikegami, S. Effects of whey protein hydrolysate ingestion on postprandial aminoacidemia compared with a free amino acid mixture in young men. Nutrients. 2018;10(4):507. doi: 10.3390/nu10040507
- Jeukendrup, A, Gleeson, M. Sports nutrition: an introduction to energy production and performance. 2nd ed. Champaign, IL: Human Kinetics; 2010. p. 82-91&p170–193.
- Morris, C, Grada, CO, Ryan, M, et al. The relationship between aerobic fitness level and metabolic profiles in healthy adults. Mol Nutr Food Res. 2013;57(7):1246–1254. doi: 10.1002/mnfr.201200629
- Moore, DR, Camera, DM, Areta, JL. Beyond muscle hypertrophy: why dietary protein is important for endurance athletes. Appl Physiol Nutr Metab. 2014;39(9):987–997. doi: 10.1139/apnm-2013-0591
- Gumus Balikcioglu, P, Ramaker, ME, Mason, KA, et al. Branched-chain amino acid catabolism and cardiopulmonary function following acute maximal exercise testing in adolescents. Front Cardiovasc Med. 2021;8:721354. doi: 10.3389/fcvm.2021.721354
- Mikulski, T, Dabrowski, J, Hilgier, W. Effects of supplementation with branched chain amino acids and ornithine aspartate on plasma ammonia and central fatigue during exercise in healthy men. Folia Neuropathol. 2015;53(4):377–386. doi: 10.5114/fn.2015.56552
- Contrepois, K, Wu, S, Moneghetti, KJ, et al. Molecular choreography of acute exercise. Cell. 2020;181(5):1112–1130.e16. doi: 10.1016/j.cell.2020.04.043
- Schranner, D, Kastenmüller, G, Schönfelder, M, et al. Metabolite concentration changes in humans after a bout of exercise: a systematic review of exercise metabolomics studies. Sports Med - Open. 2020;6(1):11. doi: 10.1186/s40798-020-0238-4
- Cermak, NM, van Loon, LJ. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013;43(11):1139–1155. doi: 10.1007/s40279-013-0079-0
- Melanson, EL, Maclean, PS, Hill, JO. Exercise improves fat metabolism in muscle but does not increase 24-h fat oxidation. Exerc Sport Sci Rev. 2009;37(2):93–101. doi: 10.1097/JES.0b013e31819c2f0b
- Jonvik, KL, Paulussen, KJM, Danen, SL, et al. Protein supplementation does not augment adaptations to endurance exercise training. Med Sci Sports Exercise. 2019;51(10):2041–2049. doi: 10.1249/MSS.0000000000002028
- Naclerio, F, Larumbe-Zabala, E, Cooper, R, et al. Effect of a carbohydrate-protein multi-ingredient supplement on intermittent sprint performance and muscle damage in recreational athletes. Appl Physiol Nutr Metab. 2014;39(10):1151–1158. doi: 10.1139/apnm-2013-0556
- Greer, BK, Price, A, Jones, B. Timing influence of carbohydrate-protein ingestion on muscle soreness and next-day running performance. J Diet Suppl. 2014;11(2):166–174. doi: 10.3109/19390211.2013.859215
- Coletta, A, Thompson, DL, Raynor, HA. The influence of commercially-available carbohydrate and carbohydrate-protein supplements on endurance running performance in recreational athletes during a field trial. J Int Soc Sports Nutr. 2013;10(1):17–24. doi: 10.1186/1550-2783-10-17
- McLellan, TM, Pasiakos, SM, Lieberman, HR. Effects of protein in combination with carbohydrate supplements on acute or repeat endurance exercise performance: a systematic review. Sports Med. 2014;44(4):535–550. doi: 10.1007/s40279-013-0133-y

