Abstract
Introduction: The Lipid Emulsion Therapy workgroup, organized by the American Academy of Clinical Toxicology, recently conducted a systematic review, which subjectively evaluated lipid emulsion as a treatment for local anesthetic toxicity. We re-extracted data and conducted a meta-analysis of survival in animal models.
Methods: We extracted survival data from 26 publications and conducted a random-effect meta-analysis based on odds ratio weighted by inverse variance. We assessed the benefit of lipid emulsion as an independent variable in resuscitative models (16 studies). We measured Cochran’s Q for heterogeneity and I2 to determine variance contributed by heterogeneity. Finally, we conducted a funnel plot analysis and Egger’s test to assess for publication bias in studies.
Results: Lipid emulsion reduced the odds of death in resuscitative models (OR =0.24; 95%CI: 0.1–0.56, p = .0012). Heterogeneity analysis indicated a homogenous distribution. Funnel plot analysis did not indicate publication bias in experimental models.
Discussion: Meta-analysis of animal data supports the use of lipid emulsion (in combination with other resuscitative measures) for the treatment of local anesthetic toxicity, specifically from bupivacaine. Our conclusion differed from the original review. Analysis of outliers reinforced the need for good life support measures (securement of airway and chest compressions) along with prompt treatment with lipid.
Introduction
The use of intravenous lipid emulsion (ILE) as a treatment for local anesthetic systemic toxicity (LAST) arose in the past 10 years based on animal studies, case reports and mechanistic data [Citation1]. Since LAST is a rare event [Citation2], randomized clinical trials to assess the efficacy of ILE in LAST are impractical. As such, practitioners base the efficacy of ILE, as a treatment for cardiac toxicity, on case studies and animal models. Recently, the American Academy of Clinical Toxicology (AACT) organized a working group (referred to hereafter as “the AACT ILE workgroup” or “workgroup”) to conduct a qualitative systematic review of the available literature to assess the efficacy of ILE for LAST and for non-local anesthetic poisonings, along with laboratory interference and complications associated with ILE [Citation3].
In the AACT ILE workgroup paper regarding LAST, they reported a survival percentage of 98% in humans treated with ILE for LAST, but concluded that “…there is currently no consistent evidence that ILE is more effective that vasopressors” [Citation4]. The workgroup asserted that the high survival percentage in human case reports reflected sole-reporting of positive outcomes (i.e., positive-publication bias). As such, they based their conclusion on animal studies. The AACT ILE workgroup used a subjective measure of “supports therapeutic effect of ILE alone” without reporting the quantitative analysis of survival data in the animal studies. As the workgroup stipulated survival as the primary outcome in their methodology paper [Citation3], we re-extracted and quantitatively analyzed survival data from the animal studies included in the original review. We performed a meta-analysis to test whether ILE provides a quantitative survival benefit in animal models of LAST.
Methods
Data extraction
We extracted dichotomous survival outcomes (number surviving, number dying) from the randomized animal studies listed in the AACT lipid emulsion workgroup’s paper [Citation5–30]. Criteria for inclusion or exclusion, rationale for inclusion along with other PRISMA reporting checklist items are reported in the original methodology paper [Citation3]. Both authors read the publications (abstracts and articles) and independently extracted survival data and other associated data. Two manuscripts presented multiple interpretations of survival and a third party (see acknowledgements) provided tie-breaking on these manuscripts [Citation22,Citation23]. For datasets presented as both a conference abstract and a published article, we only included data from the peer-reviewed journal article [Citation9,Citation22,Citation29]. We tabulated survival based on reported survival along with surrogates for survival defined in the original manuscripts. These surrogates included inference from cardiovascular parameters, return of spontaneous circulation (ROSC) and threshold of cardiac function (e.g., 50% of baseline) based on blood pressure and rate pressure product (RPP). We further identified interventions and differences in methods that may have led to bias in individual studies.
Meta-analysis
We analyzed data in Microsoft Excel (Redmond, WA) and Prism GraphPad 6.0 (San Diego, CA). We conducted a meta-analysis based on the odds ratio of death with weights calculated by the inverse-variance method. Odds-ratio (OR) = (# died ILE/# survived ILE)/(# died control/# survived control). Variance = (1/# died ILE) + (1/# survived ILE) + (1/# died control) + (1/# survived control). Weight =1/variance. Due to a variety of study models (e.g., dog, rat, pig, rabbit) and variety of interventions (e.g., with and without CPR, with and without volume control, with and without epinephrine) we used a random-effects model. For zero-values in odds-ratio calculation, we added 0.5 to all values in the calculation according to convention [Citation31]. We used two-sided t-test with p < .05 for significance. We examined all experimental studies with survival effects (e.g., we excluded studies in which all animals lived or all animals died), and further included studies with ILE as the independent variable. To quantify effect size, we converted the odds ratio to Cohen’s d (d = LogOddsRatio × sqrt(3)/π). To assess heterogeneity, we calculated Cochran’s Q and further calculated I2, which is the percentage of variance accounted for by heterogeneity in studies [Citation32]. Next, we generated a funnel plot of odds ratio against the standard error of natural log of odds ratio. This plot is designed to identify additional heterogeneity or skew indicating possible publication bias in studies that is not picked up by the Cochran’s Q. Finally, we conducted Egger’s test regressing standard normal deviate (SND = odds ratio/standard error) against the precision (inverse of standard error) using the equation: SND = a + b×precision and used an F-test to determine whether the intercept passed through the origin. This test indicates asymmetry in the funnel plot if A ≠ 0. We used p = .1 as a threshold for significance in Egger’s test as recommended since we examined <20 studies [Citation33].
Results
Meta-analysis
We extracted data from 26 original publications [Citation5–30]. Of these manuscripts, 16 used models with an insult in which we could calculate the odds ratio of death/survival, and 13 included ILE as an independent variable (). We extracted data for the remaining 13 publications and report reason for exclusion and basic conclusion in . The odds-ratio quantifies the risk of death (including the surrogates: lack of ROSC and failure to recover 20% RPP) when treated with ILE with an odds-ratio <1 supporting ILE. In 16 studies (from 13 publications), investigators used ILE as an independent variable [Citation7,Citation9,Citation14–18,Citation20–23,Citation28,Citation29]. Heterogeneity analysis refuted a heterogeneous population (Q = 23.67; p > .05; χ2 for df(15) = 25) with I2 = 36% of variance. Random effects model with v0 = 1.1 reduced Q to 15.5 and I2 to 3%. In these studies, treatment with ILE reduced the odds of death (OR =0.24; 95%CI: 0.1–0.56, Z= −3.26, p = .0012). Taken as the inverse odds-ratio, the odds of survival increased to 4.25 when compared to resuscitative measures not including ILE (). The Cohen’s d (0.786) indicated a large effect size.
Figure 1. Forest plot of survival with adjuvant intravenous lipid emulsion. Meta-analysis comparing odds ratio (OR) of death based on treatment with adjuvant intravenous lipid emulsion (ILE) compared to standard resuscitation with accompanying 95% confidence interval (95%CI). Odds-ratio less that 1 favors treatment with ILE while odds-ratio above 1 favors treatment without ILE. bupi: treated with bupivacaine; mepi: treated with mepivacaine; w/epi: comparison with epinephrine in both control and ILE group; no epi: comparisson with no epinephrine in both control and ILE group.
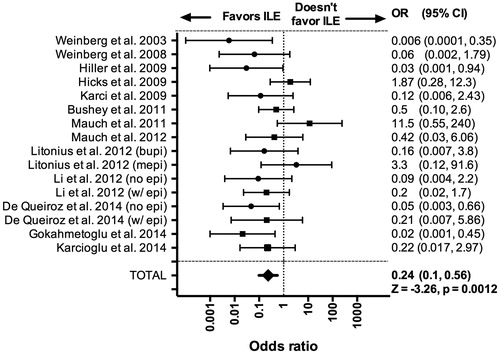
Table 1. Publications analyzed for survival benefit.
Table 2. Publications excluded from meta-analysis.
Funnel plot analysis
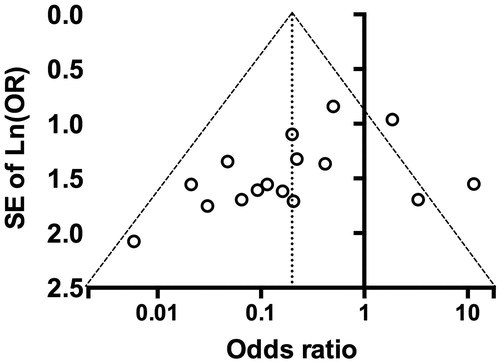
We used funnel plot analysis to assess for publication bias in studies. For ILE as a treatment, funnel plot analysis demonstrated no publication bias in the data with representation of studies in both sides of the funnel (). Two studies fell outside of the 95%CI [Citation21,Citation23]. Egger’s test demonstrated no asymmetry with regression through the origin (90%CI: −1.688 to 0.035; p = .1132).
Figure 2. Funnel plot of meta-analysis for intravenous lipid emulsion. Funnel plot accompanying data from to examine for graphical evidence of bias in survival numbers. Odds-ratio of death when treated with intravenous lipid emulsion (ILE) versus control plotted against standard error of natural log of odds-ratio (SE of ln(OR)). Funnel is centered at overall median weighted odds ratio with 95% confidence interval within the funnel
Discussion
Meta-analysis of the randomized animal studies from the AACT ILE workgroup demonstrated that ILE provides a survival benefit as a treatment for LAST, specifically in models of cardiac arrest from bupivacaine and when coupled with other resuscitative measures. Funnel plot analysis indicated no evidence of publication bias. The animal data agree with the survival benefit seen in human case reports examined by the AACT ILE workgroup study.
Meta-analysis of adjuvant lipid
Lipid emulsion as a treatment for LAST arose in the anesthesia community because it addressed the medical problem that LAST does not respond well to traditional resuscitation drugs (e.g., vasopressors) in both humans [Citation34,Citation35] and animals [Citation36]. Practitioners rapidly adopted the use of ILE for LAST and professional anesthesia societies recommended the use of ILE [Citation37,Citation38]. However, some authors posit that lipid is ineffective at treating LAST so it is important to know what the body of literature says as a whole. Our meta-analysis confirmed that ILE, when used as a treatment for LAST (in conjunction with other resuscitative measures), reduced the odds of death (OR =0.24; 95%CI: 0.1–0.56) with a large effect size (Cohen’s d = 0.786). Cochran’s Q indicated a homogeneous population and funnel plot analysis did not indicate publication bias in the distribution of studies. Two studies fell outside of the 95% confidence interval in the funnel plot. Each of these studies employed experimental designs that reflect the outlier status and provide insight into future questions about ILE. In one outlier, Litonius et al. investigated ILE for mepivacaine toxicity in contrast to all the other studies which investigated bupivacaine [Citation21]. Based on lipophilic partitioning, it is possible that different local anesthetics (i.e., mepivacaine vs ropivacaine vs bupivacaine) respond differently to lipid resuscitation and the result may reflect the less effective reversal of mepivacaine (LogP = 2.04) toxicity compared to bupivacaine (LogP = 3.64) toxicity. The second outlier, Mauch et al. included a number of confounders (e.g., lack of chest compressions as part of the common treatment, see ). Additionally, the investigators treated animals with additional doses of epinephrine if mean arterial pressure dropped below 75% of baseline and for one animal in the ILE treated group, “the epinephrine rescue dose given at 5 min after cessation of bupivacaine immediately caused short-term ventricular tachycardia followed by ventricular fibrillation and death” [Citation23]. As illustrated by this point, the paper included a mixture of un-timed independent variables, which limited interpretation. Due to the study design of the paper by Mauch et al., we required the input of the third party to define outcome. We agreed to define it as an overall outcome, instead of outcome prior to these additional independent interventions.
Studies without a quantitative difference
Our meta-analysis only included studies with a survival difference and ILE as the independent variable. Of the remaining 13 publications, 10 used study designs not intended to evaluate survival benefit. Of these 10, six indicated that ILE exerted benefits on hemodynamics [Citation5,Citation10,Citation12,Citation13], pharmacokinetics [Citation25] or resolution of electrocardiogram abnormalities [Citation8]. We could not analyze the remaining four for benefit because of lack of a control group [Citation20,Citation27], intentional dosing to death [Citation6], or lack of comparison [Citation30]. None of these studies argued against the use of lipid or supported vasopressors over lipid. Of the remaining three studies, one favored ILE in direct comparison with epinephrine and vasopressin. The other two studies favored epinephrine over ILE [Citation24,Citation26]. However, these two studies employed methods that do not match the other studies. In order to simulate a tonic–clonic seizure secondary to local anesthetic in Mayr et al., the investigators subjected animals to a simultaneous local anesthetic insult and asphyxial arrest (by mechanically clamping the trachea). Further, the animals received a pancuronium infusion [Citation24]. Lipid is detrimental in asphyxial arrest [Citation39], so it is impossible to know whether the poor outcome with ILE was related to the asphyxia, the pancuronium or failure to reverse bupivacaine toxicity. In the next study, Wat et al. attempted to simulate the delay to administration of ILE. They delivered epinephrine immediately after cardiac arrest, but postponed treatment with ILE by 10 minutes [Citation26]. As such, the study design does not match the other studies. In an attempt to mimic clinical situations, both studies included numerous uncontrolled variables, which makes interpretation difficult. From our perspective, instead of arguing against ILE, they just reinforce core principles in the management of LAST, including securement of the airway, good basic life support, and preventing delay until administration of lipid.
Discrepancies with original review
Our conclusion differed from the original AACT ILE workgroup’s conclusion. This difference may arise from our different methodologies and associated methods. The original workgroup used a subjective and qualitative criterion of “favors use of ILE alone”. In contrast, we used a quantitative evaluation of survival benefit limited to models of cardiac arrest with ILE as an independent variable (13 out of 29 randomized animal publications). Of the remaining 16 publications, the original workgroup reported three as both abstract and manuscript while we only included the manuscript [Citation6,Citation16,Citation23]. Of the remaining 13 publications, only two argued against ILE but both contained experimental designs that limited comparability with other studies (as discussed in the Studies without a quantitative difference section). During our data extraction, we found a number of discrepancies between survival numbers in the original manuscripts and those reported by AACT ILE workgroup paper. We presume these discrepancies arose as transcription errors in the extraction of data from such a large number of papers. Additionally, we disagreed with the subjective reading of a number of manuscripts. As the authors did not provide a strict definition of how they evaluated “favors use of ILE alone”, we could not deduce how our difference in interpretation arose. It is possible that the differences in subjective interpretation and/or transcription errors contributed to our different conclusion.
Considerations of mechanistic benefit
In contrast to other treatments, ILE combats LAST through a known mechanistic benefit that includes a cardiotonic effect and a redistribution benefit, shuttling drug from cardiac tissue to the liver for processing and muscle for storage [Citation40,Citation41]. Two published human trials (unpublished at the time of the original review) support this mechanistic effect. Both investigated whether ILE modified onset of subjective neurological symptoms following a low dose of intravenous local anesthetic (lidocaine, ropivacaine & levobupivacaine). While neither met the primary endpoint, both confirmed a pharmacokinetic benefit to ILE [Citation42,Citation43]. Further, analysis by Dureau et al. indicated that ILE could substantially modify bupivacaine pharmacokinetics when bupivacaine doses were elevated and rising. Both these trials confirm a redistribution benefit provided by ILE in human models, comporting with the animal data. As clinical trials of higher doses are both impractical and unethical, future human questions should focus on retrospective cohort analysis (with propensity score matching) or registry-based studies.
Conclusions
In summary, meta-analysis of the animal data from studies cited by the AACT ILE workgroup found that ILE reduced odds of death from LAST (based on survival data and survival surrogates). Lipid emulsion failed in the context of asphyxial arrest [Citation24], lack of CPR [Citation23] and delay in treatment [Citation26]. As such, the randomized animal data presented in the AACT ILE workgroup paper support the use of ILE in LAST, in combination with good resuscitative measures (i.e., intubation and chest compressions). Further, as neurological symptoms and hypotension often precede cardiovascular arrest in LAST, it follows that practitioners should consider ILE as a preventative agent in these situations to abate progression to cardiac arrest.
Acknowledgements
We would like to thank Leon Gussow for providing a third party to mediate discrepancies in data extraction and interpretation. We would like to thank Guy Weinberg, Marina Gitman, Robert Fettiplace, and Chris Wu for helpful discussion and comments regarding the manuscript. We provided Guy Weinberg an early set of figures to present on our behalf at the 36th International Congress of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) 24–27 May, 2016, Madrid, Spain.
Disclosure statement
Neither Mr. Fettiplace or Dr. McCabe receive any grant funding or have any conflicts of interest. Neither Mr. Fettiplace or Dr. McCabe has a financial or academic conflict of interest preventing neutral assessment of the literature (i.e., no committee member’s livelihood or academic career is depending on a grant studying lipid emulsion in poisoning).
Additional information
Funding
References
- Fettiplace MR, Weinberg G. Past, present, and future of lipid resuscitation therapy. JPEN J Parenter Enteral Nutr. 2015;39:72S–83S.
- Barrington MJ, Kluger R. Ultrasound guidance reduces the risk of local anesthetic systemic toxicity following peripheral nerve blockade. Reg Anesth Pain Med. 2013;38:289–297.
- Gosselin S, Morris M, Miller-Nesbitt A, et al. Methodology for AACT evidence-based recommendations on the use of intravenous lipid emulsion therapy in poisoning. Clin Toxicol. 2015;53:557–564.
- Hoegberg L, Bania T, Lavergne V, et al. Systematic review of the effect of intravenous lipid emulsion therapy for local anesthetic toxicity. Clin Toxicol. 2016;54:167–193.
- Bonfim MR, De Simone Melo M, Dreyer E, et al. Lipid therapy with two agents in ropivacaine-induced toxicity: experimental study in swine. Rev Bras Anestesiol. 2012;62:685–695.
- Buckenmaier CC, Capacchione J, Mielke AR, et al. The effect of lipid emulsion infusion on postmortem ropivacaine concentrations in swine: endeavoring to comprehend a soldier's death. Anesth Analg. 2012;114:894–900.
- Bushey BA, Auld VH, Volk JE, et al. Combined lipid emulsion and ACLS resuscitation following bupivacaine- and hypoxia-induced cardiovascular collapse in unanesthetized swine. AANA J. 2011;79:129–138.
- Candela D, Louart G, Bousquet PJ, et al. Reversal of bupivacaine-induced cardiac electrophysiologic changes by two lipid emulsions in anesthetized and mechanically ventilated piglets. Anesth Analg. 2010;110:1473–1479.
- De Queiroz Siqueira M, Chassard D, Musard H, et al. Resuscitation with lipid, epinephrine, or both in levobupivacaine-induced cardiac toxicity in newborn piglets. Br J Anaesth. 2014;112:729–734.
- De Simone Melo M, Bonfim MR, Dreyer E, et al. Hemodynamic changes in lipid emulsion therapy (SMOFlipid) for bupivacaine toxicity in swines. Acta Cir Bras. 2012;27:318–324.
- Di Gregorio G, Schwartz D, Ripper R, et al. Lipid emulsion is superior to vasopressin in a rodent model of resuscitation from toxin-induced cardiac arrest. Crit Care Med. 2009;37:993–999.
- Fettiplace MR, Akpa BS, Ripper R, et al. Resuscitation with lipid emulsion: dose-dependent recovery from cardiac pharmacotoxicity requires a cardiotonic effect. Anesthesiology. 2014;120:915–925.
- Fettiplace MR, Ripper R, Lis K, et al. Intraosseous lipid emulsion: an effective alternative to IV delivery in emergency situations. Crit Care Med. 2014;42:e157–e160.
- Gokahmetoglu G, Aksu R, Bicer C, et al. The effect of levosimendan combined with 20% lipid emulsion treatment on survival from bupivacaine induced toxicity in experiment. Bratisl Lek Listy. 2014;115:275–297.
- Hicks SD, Salcido DD, Logue ES, et al. Lipid emulsion combined with epinephrine and vasopressin does not improve survival in a swine model of bupivacaine-induced cardiac arrest. Anesthesiology. 2009;111:138–146.
- Hiller DB, Di Gregorio G, Ripper R, et al. Epinephrine impairs lipid resuscitation from bupivacaine overdose: a threshold effect. Anesthesiology. 2009;111:498–505.
- Karci A, AykaC Ibisoglu A, Oransay K, et al. The effects of lipid treatment on levobupivacaine induced cardiotoxicity in rats [abstract]. Eur J Anaesthesiol. 2009;108.
- Karcıoğlu M, Tuzcu K, Sefil F, et al. Efficacy of resuscitation with Intralipid in a levobupivacaine-induced cardiac arrest model. Turk J Med Sci. 2014;44:330–336.
- Li Z, Xia Y, Dong X, et al. Lipid resuscitation of bupivacaine toxicity: long-chain triglyceride emulsion provides benefits over long- and medium-chain triglyceride emulsion. Anesthesiology. 2011;115:1219–1228.
- Li B, Yan J, Shen Y, et al. Association of sustained cardiovascular recovery with epinephrine in the delayed lipid-based resuscitation from cardiac arrest induced by bupivacaine overdose in rats. Br J Anaesth. 2012;108:857–863.
- Litonius ES, Niiya T, Neuvonen PJ, et al. Intravenous lipid emulsion only minimally influences bupivacaine and mepivacaine distribution in plasma and does not enhance recovery from intoxication in pigs. Anesth Analg. 2012;114:901–906.
- Mauch J, Jurado OM, Spielmann N, et al. Resuscitation strategies from bupivacaine-induced cardiac arrest. Paediatr Anaesth. 2012;22:124–129.
- Mauch J, Martin Jurado O, Spielmann N, et al. Comparison of epinephrine vs lipid rescue to treat severe local anesthetic toxicity - an experimental study in piglets. Paediatr Anaesth. 2011;21:1103–1108.
- Mayr VD, Mitterschiffthaler L, Neurauter A, et al. A comparison of the combination of epinephrine and vasopressin with lipid emulsion in a porcine model of asphyxial cardiac arrest after intravenous injection of bupivacaine. Anesth Analg. 2008;106:1566–1571.
- Shi K, Xia Y, Wang Q, et al. The effect of lipid emulsion on pharmacokinetics and tissue distribution of bupivacaine in rats. Anesth Analg. 2013;116:804–809.
- Wat C, Yuen M, Li R, et al. Lipid emulsion infusion vs standard cardiac resuscitation in managemnet of ropivacaine cardiac toxicity in pigs [abstract]. Anaesth Intensive Care. 2009;37:661–662.
- Weinberg G, Paisanthasan C, Feinstein D, et al. The effect of bupivacaine on myocardial tissue hypoxia and acidosis during ventricular fibrillation. Anesth Analg. 2004;98:790–795.
- Weinberg G, Ripper R, Feinstein DL, et al. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg Anesth Pain Med. 2003;28:198–202.
- Weinberg GL, Di Gregorio G, Ripper R, et al. Resuscitation with lipid versus epinephrine in a rat model of bupivacaine overdose. Anesthesiology. 2008;108:907–913.
- Yoshimoto M, Horiguchi T, Nishikawa T. Eddicacy of lipid resuscitation in cardiac arrest induced by bupivacaine-glucose mixture in rats [abstract]. Eur J Anaesthesiol. 2014;31:210.
- Deeks JJ, Higgins JPT. Statistical algorithms in Review Manager 5 on behalf of the Statistical Methods Group of The Cochrane Collaboration. Event (London). 2010;2010:1–11.
- Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, et al. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. 2006;11:193–206.
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 2011;315:629–634.
- Albright G. Cardiac arrest following regional anesthesia with etidocaine or bupivacaine. Anesthesiology. 1979;51:285–287.
- Mayer E. The toxic effects following the use of local anesthetics: an analysis of the reports of forty-three deaths submitted to the committee for the study of toxic effects of local anesthetics of the american medical association, and the recommendations of the C. JAMA. 1924;82:876–885.
- Groban L, Deal DD, Vernon JC, et al. Cardiac resuscitation after incremental overdosage with lidocaine, bupivacaine, levobupivacaine, and ropivacaine in anesthetized dogs. Anesth Analg. 2001;92:37–43.
- Cave G, Harrop-Griffiths W, Harbey M, et al. AAGBI safety guideline: management of severse local anaesthetic toxicity. AAGBI. 2010.
- Neal JM, Bernards CM, Butterworth JF, et al. ASRA practice advisory on local anesthetic systemic toxicity. Reg Anesth Pain Med. 2010;35:152–161.
- Harvey M, Cave G, Kazemi A. Intralipid infusion diminishes return of spontaneous circulation after hypoxic cardiac arrest in rabbits. Anesth Analg. 2009;108:1163–1168.
- Fettiplace MR, Lis K, Ripper R, et al. Multi-modal contributions to detoxification of acute pharmacotoxicity by a triglyceride micro-emulsion. J Control Release. 2015;198:62–70.
- Fettiplace MR, Kowal K, Ripper R, et al. Insulin signaling in bupivacaine-induced cardiac toxicity: sensitization during recovery and potentiation by lipid emulsion. Anesthesiology. 2016;124:428–442.
- Heinonen JA, Litonius E, Salmi T, et al. Intravenous lipid emulsion given to volunteers does not affect symptoms of lidocaine brain toxicity. Basic Clin Pharmacol Toxicol. 2015;116:378–383.
- Dureau P, Charbit B, Nicolas N, et al. Effect of intralipid® on the dose of ropivacaine or levobupivacaine tolerated by volunteers: a clinical and pharmacokinetic study. Anesthesiology. 2016;125:474–483.

