Abstract
Introduction. Recurrent coagulopathy has been observed in patients after rattlesnake envenomation treated with Crotalidae Polyvalent Immune Fab (ovine) [FabAV]. While recurrent coagulopathy is well documented in the literature, clinically significant sequelae have not been reported. We present a case of recurrent thrombocytopenia after western diamondback envenomation treated with FabAV, resulting in an extensive recurrent local hemorrhage. Case Report. A 24-year-old male presented to our emergency department several hours after western diamondback envenomation. He sustained bites to both hands and the right flank by leaning over his pet “snake enclosure.” On presentation, the patient was hypotensive, tachycardic, and thrombocytopenic with a platelet count of 17/nl. Antivenom therapy was initiated according to the standard FabAV protocol. However, sixteen hours after completion of the recommended FabAV infusion, the patient experienced a recurrent thrombocytopenia with a dramatic seventeen point drop in hematocrit. The source of bleeding was clinically attributed to an expanding hematoma at the site of envenomation. Discussion. FabAV has become the standard treatment for symptomatic crotalid envenomation. However, the pharmacokinetics of this drug predispose it to recurrent coagulopathies. While studies have shown persistent and recurrent coagulopathic derangements after FabAV therapy, no clinically significant sequelae have been reported. This report highlights the potential for recurrent local hemorrhagic complications following rattlesnake envenomation, even after treatment guided by the current FabAV protocol. Conclusions. Recurrent coagulopathy following FabAV therapy can result in clinically significant hemorrhage, supporting the observation that extended repeat dosing may be necessary to adequately treat subjects of rattlesnake envenomation.
Introduction
The annual worldwide incidence of snakebites is estimated at 2.5 million, with estimates of as many as 100,000 deaths (Citation1). Local tissue injury and coagulopathy are frequently documented causes of morbidity and mortality (Citation2,Citation3). Treatment primarily relies on the administration of immunoglobulin antivenoms. Currently, Crotalidae Polyvalent Immune Fab (ovine) [FabAV] is the only FDA approved antivenom commercially available in the United States for the treatment of North American crotalid envenomation. It is formulated using venoms from the following species: Crotalus atrox (Western diamondback rattlesnake), C. adamanteus (Eastern diamondback rattlesnake), C. scutulatus (Mojave rattlesnake), and Agkistrodon piscivorus (cottonmouth or water moccasin) (Citation4). Recurrence of coagulapathy, including hypofibrinogenemia, thrombocytopenia and increased prothrombin time, has been documented with the use of FabAV (Citation5). Both the management and clinical significance of recurrent coagulopathy are not well defined in the literature. In the following case report, we present a case of serious recurrent local hemorrhage associated with thrombocytopenia after rattlesnake envenomation treated with FabAV.
Case report
A 24-year-old male, past medical history significant for a childhood heart murmur requiring porcine mitral valve repair on no medications, presented to a community hospital after being bitten by two rattlesnakes. The patient evidently leaned into his snake “enclosure” while intoxicated with no report of trauma other than the snakebites. He was bitten on both hands and his right flank by at least two snakes that he identified as western diamondback rattlesnakes. He was seen at a local community hospital within eighty minutes of envenomation, where initial laboratory values were as follows: platelet count 108/nl [nl 150–440/nl] two hours after envenomation followed by a repeat platelet count of 17/nl approximately four hours after envenomation.
The patient was transferred to our regional Snakebite Treatment Center for antivenom therapy, arriving eight hours after initial envenomation. On arrival, the patient complained of severe pain to the right flank and right hand. He was hypotensive and tachycardic, blood pressure 83/48 mmHg and heart rate 104 beats per minute. Both the hands were edematous and ecchymotic, with a normal neurovascular exam. His right flank revealed an area of erythema and ecchymosis approximately 5 × 5 cm at the bite site. Initial laboratories at our institution were significant for a platelet count of 12/nl, fibrinogen level of 185 mg/dL [nl 200–400 mg/dL], d-dimer of 4 mg/dL [nl 0.43–2.66 mg/dL], and INR of 1.2 [nl 0.9–1.1]. Chest radiograph showed no evidence of other trauma to the thoracic region. Five vials of FabAV were immediately administered over one hour, along with three liters of lactated ringers solution. The patient remained hypotensive, and five additional vials of FabAV were administered over the next hour along with two liters of lactated ringers solution. Blood pressure improved to 114/86 mmHg, and repeat platelet count improved to 206/nl. His other coagulation parameters normalized. The patient was admitted to the Surgical Intensive Care Unit for further management. He received two vials of FabAV every six hours continuously for the next 18 hours, with subsequent repeat platelet counts over 200/nl. On physical exam at this time, the injury to both hands had not changed, and the right flank showed a 10 × 15 cm hematoma inferiorly and a 7 × 7 cm hematoma superiorly.
On hospital day two, sixteen hours after completion of the FabAV infusion, morning laboratories revealed a platelet count of 15/nl. Furthermore, hematocrit decreased from 37.8% on admission to 21.6%, with expansion of the right flank hematoma to involve the entire right side from the level of T3 to the right upper thigh (). Subsequently, four vials of FabAV, two units platelets and two units packed red blood cells were administered over the course of the next 12 hours. In response, platelet count increased to 90/nl and hematocrit increased to 25% (). Of note, other significant laboratories, including fibrinogen level, d-dimer and INR normalized within 24 hours of envenomation and remained normal throughout the hospital course.
Fig. 1. (a) Right flank 2 days after envenomation by Western Diamondback; (b) Right flank 4 days after envenomation.
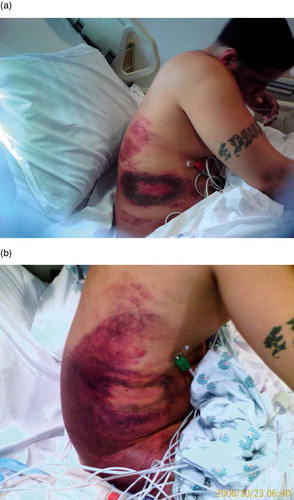
Fig. 2. FabAV treatment course. Arrow 1: 5 vials FabAV + 5 vials FabAV + 6 vial FabAV (2 vials every 6 hours for 18 hours). Arrow 2: 4 vials FabAV + 2U platelets + 2U PRBCs. Arrow 3: 4 vials FabAV. Arrow 4: 2 vials FabAV.
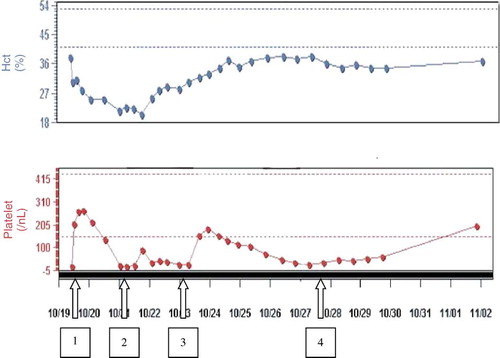
Over the next seven days, the patient was treated with six additional vials of FabAV as needed to maintain a platelet count above 30. His right flank hematoma began to resolve, his hematocrit normalized and the patient was discharged on hospital day eleven. Follow-up laboratories fourteen days after envenomation revealed a platelet count of 198/nl and hematocrit of 37%.
Discussion
Over the past 50 years, the chemical composition of immunoglobulin antivenoms has evolved. Since 1954, Antivenin (Crotalidae) Polyvalent [Equine] (ACP) was the sole treatment for crotalid envenomation in North America. ACP is composed of minimally purified animal sera, containing whole IgG, obtained by fractionating blood from healthy horses immunized with representative snake venoms (Citation6). FabAV has essentially replaced ACP in the treatment of rattlesnake and other crotalid envenomation in the United States. FabAV, composed of Fab fragments, is less immunogenic. It causes fewer hypersensitivity reactions, including anaphylaxis and serum sickness (Citation7).
In spite of the clear benefits of FabAV with respect to safety, its pharmacokinetics predispose it to recurrent coagulopathies. The half-life of FabAV is less than that of its target venom components. Unbound Fab is metabolized more quickly than unneutralized venom components, with an elimination half-life of 15–20 hours (Citation9). This “venom/antivenom kinetic mismatch” allows for the continued and prolonged absorption of venom components into the systemic circulation, likely contributing to the recurrent coagulopathies that have been observed (Citation8,Citation9). Other mechanisms that have been proposed to explain this phenomenon include 1) the presence of a depot of unneutralized venom at the bite site that is slowly released into the systemic circulation (Citation5), 2) the separation of venom/antivenom complexes in the systemic circulation after initial neutralization, 3) the presence of venom components with late onset effects different from those originally active, and 4) development of a host immune response to the antivenom itself (Citation9). Additional factors, other than continued or recurrent venom activity, must also be considered with respect to recurrent thrombocytopenia after snake envenomation. These other causes, specifically for thrombocytopenia, may include increased platelet destruction due to sequestration of platelets in damaged tissue, decreased or dysfunctional bone marrow stores and decreased responsiveness of platelets to deaggregation (Citation10).
Until recently, data on recurrent coagulopathy has been limited. Adverse clinical outcomes related to recurrent coagulopathy following administration of ACP were not reported. Coagulation abnormalities were believed to be short-lived, and patients were typically discharged after initial normalization of serum coagulation studies without follow-up blood work. Since the introduction of FabAV, recurrent coagulopathy has been observed in multiple patients, motivating further investigation. Boyer et al. conducted a multicenter, prospective clinical trial designed to detect recurrent and persistent coagulation abnormalities after FabAV administration. They showed 53% (20 of 38 patients) had recurrent, persistent or late coagulopathy from 2–14 days after envenomation (Citation5). Bogdan et al. then reevaluated the use of ACP with respect to recurrent coagulopathy. In a retrospective analysis of 354 consecutive cases, they found 14 of 31 cases (45%) with adequate follow-up testing had recurrent coagulopathy after ACP administration (Citation11). The obvious limitation of this study is that only 31 of 354 patients received adequate follow-up testing for analysis. While both studies showed a high incidence of persistent coagulopathies, neither observed any clinically significant hemorrhagic sequelae. Furthermore, Boyer et al. noted that local reaction to snake envenomation stabilized in all patients after initial treatment with FabAV (Citation5,Citation9).
This case is an example of clinically significant recurrent local hemorrhage secondary to rattlesnake envenomation, worsening despite the currently recommended treatment regime with FabAV and initial normalization of coagulopathic laboratory abnormalities. The patient's initial coagulopathy included a low platelet count, low fibrinogen level and elevated d-dimer. With FabAV therapy, the patient's laboratory studies normalized, and he appeared to stabilize clinically. However, 16 hours after completion of FabAV treatment and forty-eight hours post envenomation, the thrombocytopenia recurred while fibrinogen, d-dimer and INR remained within normal limits. He developed extensive additional bleeding into the muscles of the right flank, with a further drop in hematocrit. As prior studies have reported stabilization of local injury after FabAV therapy (Citation5,Citation9), it is of particular interest that the patient's clinical course included extensive recurrent hemorrhage at the site of initial envenomation.
It is important to note that certain limitations beyond those inherent to a case report exist in this case. First, the diagnosis of a local hemorrhage into the muscles of the flank was determined clinically, without further imaging like CT scan to search for another possible source of bleeding. Second, venom antigen and antivenom levels were not measured in this patient. We assume that the recurrent coagulopathy was due to dwindling antivenom levels over time. However, as mentioned above, both platelet destruction and dysfunction may also play a role. The marked improvement with repeat FabAV dosing, however, favors decreased antivenom levels as a primary cause.
The current cost of FabAV to our hospital pharmacy is $1,300.00 per vial. This patient required 26 vials throughout his hospital stay. Although our public hospital provides care without regard to a patient's insurance status or ability to pay, we are concerned that the cost of FabAV may be a potential barrier to care in other settings.
Finally, the older Wyeth product (ACP) was less prone to cause these re-emergence problems regarding coagulopathy. Reemergence phenomenae were not reported in the many years that ACP was the sole antivenom used for crotalid envenomations in the U.S. Until alternative antivenoms become available (Anti-Vipmyn from Bioclon S.A., for example, currently the subject of a randomized controlled trial), there remains an important role for ACP either as an alternative to FabAV or when administered in combination with it. The decision by Wyeth to continue production of ACP would be a valuable contribution to the armamentarium available to the clinician treating rattlesnake bites.
In conclusion, this case supports the observation that extended repeat dosing of FabAV may be necessary to adequately treat subjects of rattlesnake envenomation (Citation2,Citation5,Citation7,Citation9,Citation12,Citation13). Based on clinical data from Dart et al, the manufacturer currently recommends administering 4–6 vials FabAV over one hour (repeated if control of envenomation is not achieved after the first dose) followed by 2 vials every 6 hours for a total of 18 hours (Citation2,Citation4). Beyond this period of treatment, the management of North American crotalid envenomation is not well defined. Recurrent coagulopathy may be found as late as two weeks after envenomation, particularly in those individuals with initial coagulopathic derangements (Citation5,Citation11). While the clinical significance of this phenomenon has not been well studied, this case highlights the potential for dangerous delayed hemorrhagic complications following FabAV therapy.
References
- JP Chippaux. Snake bites: Appraisal of the global situation. Bull World Health Organ 1998; 76 (5):515–524.
- BS Gold, RC Dart, and RA Barrish. Bites and Venomous Snakes. NEJM 2002; 347:347–356.
- J White. Snake venoms and coagulopathy. Toxicon 2005; 45:951–967.
- Crotalidae Polyvalent Immune Fab (Ovine). AHFS Drug Information® (2006).
- LV Boyer, SA Seifert, RF Clark, JT McNally, SR Williams, SP Nordt, FG Walter, and RC Dart. Recurrent and persistent coagulopathy following pit viper envenomation. Arch Intern Med 1999; 159:706–710.
- Antivenin (Crotalidae) Polyvalent. Mosby's Drug Consult™ - 16th ed. (2006).
- RC Dart, and J McNally. Efficacy, safety, and use of snake antivenoms in the United States. Ann Emerg Med 2001; 37:181–8.
- SA Seifert, and LV Boyer. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 1. Pharmacokinetics and pharmacodynamics of immunoglobulin antivenoms and related antibodies. Ann Emerg Med 2001; 37:189–195.
- LV Boyer, SA Seifert, and JS Cain. Recurrence phenomena after immunoglobulin therapy for snake envenomations: Part 2. Guidelines for clinical management with Crotaline Fab antivenom. Ann Emerg Med 2001; 37:196–210.
- SA Seifert, LV Boyer, RC Dart, RS Porter, and L Sjostrom. Relationship of Venom Effects to Venom Antigen and Antivenom Serum Concentrations in a Patient with Crotalus atrox Envenomation Treated with Fab Antivenom. Ann Emerg Med 1997; 30:49–53.
- GM Bogdan, RC Dart, SC Falbo, J McNally, and D Spaite. Recurrent coagulopathy after antivenom treatment of crotalid snakebite. South Med J 2000; 93:562–566.
- RC Dart, SA Seifert, LV Boyer, RC Clark, E Hall, P McKinney, J McNally, CS Kitchems, SC Curry, GM Bogdan, SB Ward, and RS Porter. A randomized multicenter trial of crotalinae polyvalent immune Fab (ovine) antivenom for the treatment for crotaline snakebite in the United States. Arch Intern Med 2001; 161:2030–6.
- RC Dart, SA Seifert, L Carroll, RF Clark, E Hall, LV Boyer-Hassen, SC Curry, CS Kitchens, and RA Garcia. Affinity-purified, mixed monospecific crotalid antivenom ovine Fab for the treatment of crotalid venom poisoning. Ann Emerg Med 1997; 30:33–9.
