Abstract
The current study was taken up to examine the role of bioagent (Trichoderma hamatum) in mitigating the deleterious effects of NaCl stress in Ochradenus baccatus. Varying concentrations of salt (0, 75, and 150 mM) were used to observe the effect on growth, pigments, some key metabolic attributes, antioxidant enzymes, and elemental accumulation in O. baccatus. The results indicated significant decrease in seed germination, plant growth, pigment content, membrane stability index, tissue water content, and total lipid content with salt stress. Lipid peroxidation increases with the increasing concentration of NaCl. Moreover, salinity stimulated the biosynthesis of phenols, diacylglycerol, sterol esters, nonesterified fatty acids, and enzymatic antioxidants like superoxide dismutase, catalase, peroxidase, ascorbate peroxidase, glutathione reductase. The Na+ content in shoot increases with elevated levels of NaCl concentration, accompanied with significant decreases in K+, Mg2+, and Ca2+. Application of bioagent (T. hamatum) has been observed to alleviate the antagonistic effect of salt stress on plant growth and metabolic processes. In absence and presence of salt stress, the bioagent stimulated the plant growth and alter the plant metabolism through the modification of the above parameters.
| Abbreviations | ||
| TL | = | total lipid |
| DG | = | diacylglycerol |
| TG | = | triacylglycerol |
| S | = | sterol |
| SE | = | sterol ester |
| FAA | = | nonesterified fatty acids |
| ROS | = | reactive oxygen species |
Introduction
Ochradenus baccatus Del., belongs to family Resedaceae, is widely distributed in South-West and central regions of Saudi Arabia (SA; Al Qurainy et al. Citation2013). O. baccatus (Del.) is a semi-deciduous shrubby plant and grows in dry deserts as bushes (Bronstein et al. Citation2007). It is a very important medicinal plant as it contains high contents of antioxidants and anti-inflammatory agents (Alqasoumi et al. Citation2012).
Nowadays, rehabilitation of rangelands became national target in SA, so the main focus is to maintain range plants from extinction. Ecological factors affect the vegetation of O. baccatus so much that this plant is near to extinction (Al-Abbasi et al. Citation2010). Therefore, the demand of O. baccatus has increased tremendously during the last few decades (Al Qurainy et al. Citation2013). Range plants often experience abiotic stress and among these salt stress is most common that hampers the growth and biomass yield (Alqarawi, Hashem, Abd Allah, Alshahrani, et al. Citation2014). The primary effects of salinity stress are reduced germination percentage, fresh and dry weight of shoot and root, leaf water potential, chlorophyll (Chl) contents, photosynthesis, respiration, and protein synthesis (Neumann Citation2008; Ahmad & Prasad Citation2012a, Citation2012b; Rasool, Ahmad, et al. Citation2013; Rasool, Hameed, et al. Citation2013; Alqarawi, Hashem, Abd Allah, Alshahrani, et al. Citation2014). Salinity also cause nutritional disorders in plants which lead to deficiencies of several nutrients and drastically increasing Na+ levels (Shahid et al. Citation2013). Moreover, the salt stress causes oxidative stress, through the production of variety of reactive oxygen species (ROS) like, singlet oxygen, superoxide ions, hydroxyl radical, H2O2, etc. These ROS are noxious molecules (Ahmad, Sarwat, et al. Citation2008; Ahmad, Umar, & Sharma Citation2010) and causes deleterious effects on mitochondria and chloroplast by disturbing cellular structures (Apel & Hirt Citation2004; Ahmad, Umar, & Sharma Citation2010, Citation2012; Azooz et al. Citation2011; Naz & Bano Citation2013). However plants are equipped with antioxidant machinery which includes superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR; Apel & Hirt Citation2004; Ahmad, Jaleel, Salem, et al. Citation2010; Koyro et al. Citation2012; Rasool, Ahmad, et al. Citation2013). These antioxidants are present in different cellular compartments especially in chloroplast and mitochondria (Apel & Hirt Citation2004; Masood et al. Citation2006) and play a great role in resistance mechanism against salt stress.
Trichoderma spp. are commonly found in soil especially near the roots of plants. Some species form colonizations with the roots and some may live as parasites on other fungi. Interaction of Trichoderma spp. with plants accelerates their growth, crop production, and provides tolerance to different environmental stresses. They also enhance uptake of nutrients (Harman et al. Citation2004), production of many biochemical elicitors including various peroxidases (PODs), chitinases, β-1,3-glucanases, lipoxygenase-pathway hydroperoxide lyase, and compounds like phytoalexins and phenols to promote tolerance against both biotic and abiotic stresses (Gachomo & Kotchoni Citation2008; Shoresh et al. Citation2010). The beneficial effects of Trichoderma species on alleviation of adverse effects of salinity stress have been well documented (Hermosa et al. Citation2012; Rawat et al. Citation2013). The present study has been designed to observe the ability of T. hamatum to alleviate the negative effects of salinity stress.
Materials and methods
The experimental mold and its formulation
The bioagent (Trichoderma hamatum [Bonord.] Bainier) used in present study was isolated previously from tomato root grown in Sharkia governorate, Egypt (Abd-Allah & Ezzat Citation2005). T. hamatum was grown in potato dextrose broth (DIFCO) in flasks and was kept for seven days at 30°C and then shaked at 150 rpm. After the incubation period, the mycelium was lyophilized under vacuum and the lyophilized powder was mixed with talc powder and 1.0% carboxy methyl cellulose as an adhesive agent to give a final concentration of 3.2 × 106 cfu g−1 of carrier material. The formulated T. hamatum was added to the soil before sowing at a rate of 10 g/kg soil.
Experimental plant and seed germination test
Dry mature fruits of O. baccatus (Del.) were randomly collected from different rangelands in Riyadh region in 2013 (). Healthy seeds were taken from the fruits and surface sterilized with sodium hypochlorite (5%, v/v) for two minutes at room temperature (+25°C), washed thoroughly with distilled water. After that the seeds were sown in plastic pots with different concentrations of NaCl (0, 75, and 150 mM). Another set was prepared with same concentration of NaCl but the soil in the pots was also supplemented with T. hamatum. The germination percentage is calculated on the basis of total seed percent:
Pots experiment
Healthy plantlets from germinated seeds were selected and sown in separate plastic pots with 1.50 kg soil and sand (one plant/pot). Hoagland's solution (Hoagland & Arnon Citation1950) supplemented with sodium chloride to get concentration of 0, 75, and 150 mM was used for irrigation in pots with and without T. hamatum. The rate of irrigation was 100 ml for each treatment every three days for eight weeks at alternative temperature rate of (27°C day and 18°C night) with a photosynthetic photon flux density of 1500 µmol m−2 s−1 (18 h light and 6 h dark period). At the end of experiment period, (eight weeks) the plants were harvested for growth and biochemical analyses.
The composition of the Hoagland solution is (mg l−1): 270 N (KNO3), 31 P (KH2PO4), 234 K (KNO3), 200 Ca (Ca(NO3)2.4H2O), 64 S (MgSO4.7H2O), 48 Mg (MgSO4.7H2O), 2.8 Fe (Fe-EDTA), 0.5 Mn (MnCl2.4H2O), 0.5 B (H3BO3), 0.02 Cu (CuSO4), 0.05 Zn (ZnSO4.7H2O), and 0.01 Mo (H3MoO4.H2O; Hoagland & Arnon Citation1950).
Photosynthetic pigments
The quantitative analysis of photosynthetic pigments was done by the method of Lichtenthaler and Wellburn (Citation1983). The pigments were extracted from fresh leaves (100 mg) by acetone and the absorbance was read spectrophotometrically at 622, 664, and 440 nm wavelength.
Tissue water content (TWC)
The estimation of TWC was according to Smart and Bingham (Citation1974) and described by Ahmad et al. (Citation2012). TWC is calculated by:
Membrane stability index (MSI)
MSI was estimated according to the method of Sairam et al. (Citation1997). The MSI was calculated by the following formula:
Lipid peroxidation
Lipid peroxidation in terms of concentration of malondialdehyde (MDA) was recorded by the method of Heath and Packer (Citation1968). Absorbance at 532 and 600 nm were used for calculation of MDA equivalents. Blank sample was used as reference. MDA equivalent was calculated by the following equation:
Determination of total phenolics
The total phenolics in the fresh leaves were extracted with 80% (v/v) acetone and estimated using (20%, w/v) sodium carbonate (Na2CO3) and Folin Ciocalteau's phenol reagent following Julkunen-Tiitto (Citation1985). The optical density of the mixtures were read at 750 nm. Standard curve of pyrogallol was used as reference.
Estimation of ion accumulation
A known weight of oven dry leaf sample was digested and Na+, K+, Mg2+, and Ca2+ were estimated according to the method of Wolf (Citation1982) using a flame photometer Jenway Flame Photometer, Bibby Scientific Ltd-Stone-Staffs-St15 0SA–UK. Standard curve of each mineral (10–100 µg ml−1) used as reference.
Lipid analysis
Total lipids (TLs) were extracted by using chloroform:methanol (2:1, v/v), with 0.05% (w/v) of butylated hydroxytoluence (2.6 di-tert-butyl-p-cresol) and were estimated using the charring method of Marsh and Weinstein (Citation1966), with stearic acid (Sigma) as the standard. The neutral lipids in the extracts were separated on thin layer chromatography (TLC) plates (chloroform:methanol:water, 65:35:3; v/v/v, used as mobile phase). The qualitative estimation was carried by reaction of TLC plates with acid dichromate for clarification. The quantitative estimation was carried out spectrophotometrically according to Amenta (Citation1964).
Antioxidant enzyme assays
Fresh leaves of 500 mg were ground in sodium phosphate buffer (50 mM, pH 7.0) containing polyvinylpyrrolidone (1%, PVP). After 20 min of centrifugation (4°C) at 15,000 rpm the supernatant was collected and used to determine the activities of SOD, POD, CAT, and APX).
A method of Samantary (Citation2002) was used to assay the activity of CAT (EC 1.11.1.6). Enzyme activity was expressed in M H2O2 destroyed mg−1 protein−1 min−1.
POD (EC 1.11.1.7) activity was assayed spectrophotometrically according to the method of Kar and Mishra (Citation1976). The amount of purpurogallin formed was estimated by measuring the absorbance at 420 nm. The enzyme activity was expressed as EU mg−1 protein.
The method of Nakano and Asada (Citation1981) was used for the assay of APX (EC 1.11.1.11) activity. APX was assayed as a decrease in absorbance at 290 nm of ascorbate. One unit of enzyme was considered as the amount necessary to decompose 1 mol of substrate min−1 at 25°C.
The activity of GR (EC 1.6.4.2) was evaluated by the method of Carlberg and Mannervik (Citation1985). The decrease in absorbance was read for 2 min at 340 nm. The activity of GR was calculated using the extinction coefficient of nicotinamide adenine dinucleotide phosphate (NADPH) of 6.2 mM−1 cm−1 and expressed as EU mg−l protein.
SOD (EC 1.15.1.1) was determined by photoreduction of nitroblue tetrazolium (NBT) at 540 nm (Beyer & Fridovich Citation1987). One unit of SOD activity equaled to the amount required to inhibit photoreduction of NBT by 50%. The results of antioxidant enzyme assays were expressed as EU.
Statistical analysis
Data collected were analyzed by two-way analysis of variance followed by Duncan's Multiple range Test. The values obtained were the mean ± SE for five replicates in each group. P value at 0.05 was considered as significant.
Results
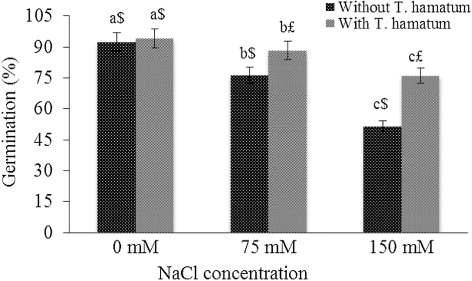
The seed germination decreases with increasing concentration of NaCl and the results are presented in . The maximum decrease of 44.24% in seed germination was observed at 150 mM NaCl stress. Plants treated with NaCl in combination with T. hamatum showed minimum decrease in seed germination. The decrease of only 6.30% and 19.24% was observed at 75 mM and 150 mM NaCl supplemented with T. hamatum, respectively, as compared to control.
Salinity significantly decreased shoot and root length as well as shoot and root dry weight in the present study (). Maximum decline in length of shoot and root was 59.70% and 62.21%, respectively, at 150 mM NaCl. Shoot dry weight decreased to 75.00% and root dry weight to 76.56% at 150 mM NaCl. The application of T. hamatum mitigates the deleterious effect of NaCl stress and enhances the dry weight of root (48.4%) and shoot (125%) significantly as compared with control.
Table 1. Effect of NaCl in presence and absence of T. hamatum on growth and biomass yield of O. baccatus seedlings.
The results pertaining to the effect of salt stress and T. hamatum on Chl is depicted in . Significant decrease of 59.32%, 46.80%, 56.44%, was recorded in Chl a, Chl b, and total Chl, respectively, in O. baccatus at 150 mM NaCl. However, the soil amended with T. hamatum mitigated the antagonistic effect of NaCl stress on pigment content as compared to those treated with NaCl alone. Moreover, the application of T. hamatum alone (in absence of salt stress) caused significant increase in Chl a, Chl b, carotenoids, and total Chl as compared to control plants.
Table 2. Effect of NaCl in presence and absence of T. hamatum on pigment content (mg g−1 fresh weight) of O. baccatus seedlings.
TWC of O. baccatus significantly decreased to 20.44% and 41.36% at 75mM and 150mM NaCl, respectively, as compared to control (). The pretreatment with T. hamatum could alleviate the negative effect of salt stress by decreasing TWC to only 7.92% at 75 mM and 30.25% at 150 mM NaCl stress as compared to the plants treated with NaCl alone.
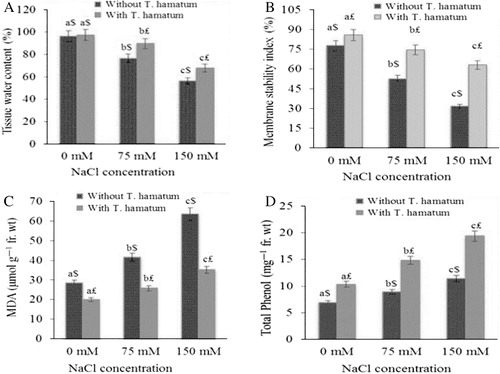
MSI showed significant variation at all stress levels and the results are depicted in . Salt concentrations 75 and 150 mM caused significant decrease of 32.34% and 59.42%, respectively, in MSI as compared to control. The effect of T. hamatum was very effective in decreasing the deleterious effect of salinity stress. Salt stress 75 and 150 mM in combination with T. hamatum showed a decrease of only 12.91% and 26.43% in MSI, respectively, as compared to control.
The results pertaining to the effect of salt stress on MDA contents in presence and absence of T. hamatum is shown in . MDA content was significantly increased to 31.26% and 54.98% at 75 and 150 mM NaCl, respectively, as compared to control. Application of T. hamatum significantly decreased the MDA content to 22.75% at 75 mM and 43.15% at 150 mM NaCl. Hence the bioagent was much effective in decreasing MDA content as compared to salt-treated plants without bioagent.
The data presented in indicated that salinity stimulated the biosynthesis of phenols to 22.55% and 39.47% at 75 and 150 mM NaCl, respectively, as compared to control. Supplementation of T. hamatum further increased the total phenols to 30.09% at 75 mM and 46.51% at 150 mM NaCl. Moreover, in absence of salt stress, T. hamatum caused significant increase in total phenol content in control plants.
The results pertaining to the effect of salt stress and T. hamatum on TLs and neutral lipids are presented in . It was observed that 75mM NaCl caused significant decrease of 32.37%, 18.00%, and 10.20%, in TLs, triacylglycerol (TG), and sterol (S), respectively. However the higher concentration of NaCl (150mM) caused more decrease of 50.20%, in TL, 27.86% in TG, and 47.69% in S as compared to control. Application of T. hamatum in presence of NaCl (150 mM) decreased TL, TG, and S to 40.16%, 11.45%, and 23.43%, respectively, as compared to control. On the other hand salt stress caused remarkable rise in concentrations of diacylglycerol (DG), sterol ester (SE), and nonesterified fatty acids (FAA) and the increase is directly proportional to increasing salt concentrations. Supplementation of T. hamatum with 150 mM NaCl showed minimum increase of only 14.95% in DG as compared to control. The SE and FAA also showed less increase of 15.18% and 15.59%, respectively, at 150 mM NaCl supplemented with T. hamatum.
Table 3. Effect of NaCl in presence and absence of T. hamatum on TLs and neutral lipids of O. baccatus seedlings.
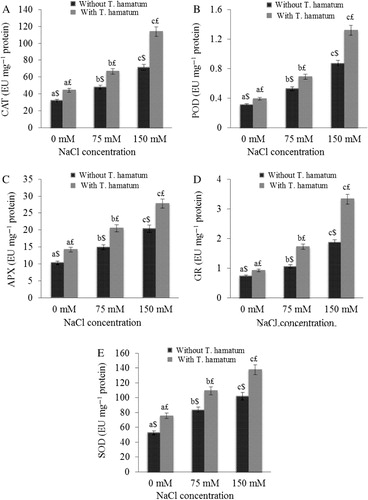
The results pertaining to the effect of salt stress on antioxidant enzymes are reported in . A significant increase in activity of enzymatic antioxidant has been reported in present study. The CAT, POD, APX, GR, and SOD increased to 32.92%, 41.50%, 30.69%, 30.18%, and 40.43%, respectively, at 75 mM NaCl as compared to control. Further increase in the antioxidant enzymes has been reported at 150 mM as compared to 75 mM NaCl and control. Application of T. hamatum further increased the antioxidant enzyme activity at both concentrations of salt stress. This suggests that these enzymes are still supporting the O. baccatus to withstand the NaCl stress. Moreover, it is clear that the application of T. hamatum alone (in the absence of salt stress) caused a significant increase in activities of CAT, POD, APX, GR, and SOD.
The plants under salinity stress showed maximum accumulation of sodium ions and less uptake of potassium ions (). The present study also reported that Na/K ratio is directly proportional to elevated NaCl concentrations. Salt stress (150 mM) caused significant decrease of 58.51% and 52.38% in Mg2+ and Ca2+ as compared to control. The pretreatment of soil with T. hamatum was very effective in alleviating the deleterious effects of salinity stress by increasing K/Na ratio in plants. It also mitigates the deficiency of K+, Mg2+, and Ca2+. Furthermore, the data indicated clearly that T. hamatum alone (in the absence of salt stress) could improve nutrient status of O. baccatus.
Table 4. Effect of NaCl in presence and absence of T. hamatum on elements accumulation of O. baccatus seedlings.
Discussion
Salt stress restricts the plant growth and therefore causes major threat to plant productivity. Seeds of O. baccatus subjected to different concentrations of NaCl stress showed decrease in germination at all stress levels. The results obtained in present study are in concurrence with those in wheat (Iqbal & Ashraf Citation2013) and tomato (Mastouri et al. Citation2010). Application of T. hamatum to the salt stressed seeds showed increased germination in present study. Delay in seed germination under saline conditions was attributed to decreased metabolism of seed reserves due to lower respiration rates (Azam et al. Citation2005), reduced water potential, and imbalance in plant nutrient uptake, which subsequently affected germination rate and plant growth. Specific ion toxicity also plays an important role in decreasing seed germination under salt stress (Iqbal & Ashraf Citation2013). T. hamatum increases the uptake of nutrients and also absorbs water from deep soil to increase the water potential (Martínez-Medina et al. Citation2014). This may also reduce the ion toxicity due to NaCl stress.
In the present study the length of shoot and root and dry weight decreased significantly under NaCl stress which may be attributed to increased osmotic stress, deficiency of nutrients, and disturbance of various physiological and biochemical mechanisms (Iqbal & Ashraf Citation2013; Rasool, Hameed, et al. Citation2013; Alqarawi, Hashem, & Abd Allah. Citation2014). Present study indicated that the application of T. hamatum efficiently increased growth parameters and could be an effective and easily adaptive strategy to alleviate the negative impact of salt stress. Our results corroborated with the findings of Mastouri et al. (Citation2010) and Rawat et al. (Citation2013) who reported that Trichoderma isolates alleviates the adverse effects of salt stress in different plants. Trichoderma associated with plants produces plant growth hormones like cytokinins-like molecules, e.g. zeatin and gibberellin GA3 or GA3 related (Zhang et al. Citation2013; Rawat et al. Citation2013) that have beneficial effects on plant growth under salt stress (Iqbal & Ashraf Citation2013). Moreover Trichoderma association increases root length thus helps the plant to absorb nutrients and water from the soil and thus enhancing the plant's efficiency to counteract salt stress (Arora et al. Citation1992). In many studies Trichoderma spp. could improve the growth of some medicinal plants by producing phytohormones such as gibberellins, auxin, and cytokinins (Sofo et al. Citation2011; Hanefat et al. Citation2012; Martínez-Medina et al. Citation2014; Resende et al. Citation2014).
Our results related to decrease in photosynthetic pigments of O. baccatus plants under NaCl are in agreement with Rasool, Ahmad, et al. (2Citation013) for Cicer arietinum; and Alqarawi, Hashem, Abd Allah, Alshahrani, et al. (Citation2014) for Ephedra alata. The reduction in the pigment content is attributed to the negative effect of salt stress on chloroplast (Zörb et al. Citation2009), increased activity of Chl-degrading enzymes such as chlorophyllase (Sultana et al. Citation1999) hence reduced synthesis of Chl and the instability of the pigment protein complex (Levitt Citation1980). Decrease in β-carotene and zeaxanthin formation is attributed to decrease in carotenoids due to NaCl stress thus increases photoinhibition (Sultana et al. Citation1999). The results also indicated the efficiency of T. hamatum to alleviate the negative effects of NaCl on pigment system and caused significant increase in Chl content in salt-treated plants as well as in control. The mitigation of negative effects of NaCl and improvement in the pigment system by Trichoderma spp. has also been reported by Rawat et al. (Citation2011) and Zhang et al. (Citation2013). T. hamatum associated plants showed improvement in photosynthetic pigments compared with control. The production of phytohormones such as gibberellins, auxin, and cytokinins plays an important role in stimulation of Chl content (Martínez-Medina et al. Citation2014; Resende et al. Citation2014).
Pretreatment with T. hamatum maintains the TWC that was decreased due to salt stress in the present study. Similar to our finding, the decrease in TWC caused by salinity stress is also reported by Josine et al. (Citation2011) and Eisa et al. (Citation2012) in Rosa chinensis and Chenopodium quinoa, respectively. Bae et al. (Citation2009) also reported that T. hamatum increased TWC and improved water status, allowing seedlings to tolerate abiotic stress. One more reason may be the mycorrhizal fungi spreads their mycelium deep in to the soil to absorb water for the host plant.
Salinity stress is also having negative effect on MSI. Decrease in MSI content due to salinity stress in the present study is in accordance with Kafi et al. (Citation2011), Rawat et al. (Citation2013), and Rao et al. (Citation2013) who reported that MSI decreases under salinity stress in sorghum, chickpea, and wheat, respectively. The cell injury by salt stress firstly appears in cell membranes (Ashraf & Ali Citation2008), hence MSI is considered one of the useful parameters to plant resistance against salinity stress (Farooq & Azam Citation2006). The positive impact of Trichoderma spp. on MSI is in accordance with Rawat et al. (Citation2013). Lipid peroxidation expressed as MDA content significantly increased under salt stress. Such accumulation of MDA contents under salt stress is also reported by Hejazi Mehrizi et al. (Citation2012) in Rosmarinus officinalis and Rasool, Ahmad, et al. (Citation2013) in C. arietinum. The pretreatment with T. hamatum caused significant decrease in MDA content in the present study. Salinity stress causes generation of ROS that attack the biomolecules including plasma membranes thus causing injuries and lipid peroxidation, hence the stability of the membranes is lost resulting in higher leakage of solutes. H2O2 generated through oxidative stress is responsible for higher leakage of solutes (Dionisio-Sese & Tobita Citation1998). The use of T. hamatum enhanced the antioxidant enzymes that quenches these ROS so the plasma membranes are affected least. T. hamatum-treated plants showed decrease in MDA content, which could be due to the expression of stress-related proteins like glutathione S-transferase, glutathione dependent formaldehyde dehydrogenase, and POD (Alqarawi, Hashem, & Abd Allah Citation2014).
These proteins scavenges ROS and protects the cell from damage. Reduced contents of MDA is an important indicator of stress tolerance as reported in mulberry (Ahmad, Jaleel, & Sharma Citation2010), chickpea (Rasool, Ahmad, et al. Citation2013), and mustard (Ahmad et al. Citation2012).
Phenolic compounds are important class of plant secondary metabolites, produced typically to enhance reproduction and give protection to plants against biotic and abiotic stresses (Pohjala & Tammela Citation2012). Plants respond to abiotic stresses by synthesizing and accumulating phytoalexins, flavonoids, terpenoids, phenolic derivatives, aglycones, etc. The synthesis of phenolic compounds increased resistance against salt stress as reported by Wehner et al. (Citation2010). Such increase in phenolic compounds reported due to NaCl in the present study are in agreement with the reports of Mehr et al. (Citation2012) and Petridis et al. (Citation2012) in Anethum graveolens and Olea europaea, respectively. The application of T. hamatum caused further increase in phenolic content as compared to control and salt-treated plants alone. Plants are stimulated by Trichoderma strains to manufacture some novel compounds, which are defensive in nature. The phenolic compounds possess antifungal, antibacterial, and antiviral as well as antioxidant properties that quenches the ROS generated through oxidative stress.
In the present study TL, TG, and S decreased significantly, however DG, SE, and FAA increased with increasing salt concentrations. Such inhibitory effect of salinity on lipid content (TL, TG, and S) might be attributed to destructive and negative effects of salt on chloroplasts and membrane permeability (Ahmad et al. Citation2012; Naz & Bano Citation2013). Sterols play an important role in maintenance of membrane permeability and fluidity (Hosono Citation1992), but are affected with abiotic stress including salinity (Kaya et al. Citation2001). Salinity induced oxidative stress resulting in enhanced membrane damage and electrolyte leakage (Ahmad, Sarwat, et al. Citation2008; Ahmad, Umar, & Sharma Citation2010; Rasool, Ahmad, et al. Citation2013). The scavenging nature of the antioxidants is one of the reasons to maintain the integrity of the membrane lipids under salt stress. Another reason might be the efficient absorption of nutrients through T. hamatum that maintains the growth and functioning of organelles related to photosynthesis. Through these mechanisms we may conclude that application of T. hamatum alleviates the deleterious effects of salinity on total and neutral lipids.
Salt stress increased the activity of antioxidant enzymes like CAT, POD, APX, GR, and SOD and the activities are directly proportional with increasing concentrations of salt. Similar results have also been observed in Sesamum indicum (Koca et al. Citation2007), Pisum sativum (Ahmad, John, et al. Citation2008), Vicia faba (Azooz et al. Citation2011), Brassica juncea (Ahmad et al. Citation2012), and C. arietinum (Rasool, Ahmad, et al. Citation2013). The application of T. hamatum further increased the antioxidant enzymes hence decreased the effects of salt stress in the present study. The potential of T. hamatum in alleviating the salt stress may be through an auxin-dependent mechanism (Zhang et al. Citation2013), which may depend on plant producing signals (Athar & Ashraf Citation2009). Iqbal and Ashraf (Citation2013) also reported the pretreatment of wheat with auxin showed consistent promotive and beneficial effects on plant metabolism under salt stress. Increase in antioxidant enzymes denotes that more and more ROS must be scavenged and the effects of these ROS are minimized. SOD catalyzes the dismutation of O2 ·− to H2O2 and O2. It protects the cells by removing O2·− that increases the risk of OH· formation. CAT plays an important role in the removal of H2O2 from the different organelles of the cell. One molecule of CAT can convert approximately six million molecules of H2O2 to H2O and O2 each minute. APX and POD is also involved in scavenging of H2O2 and plays an important role in stress tolerance. GR is a flavoprotein oxidoreductase and is a potential enzyme of the ascorbate-glutathione system.
Membrane proteins play a significant role in selective distribution of ions within the plant or cell (Ashraf & Harris Citation2004). In our study, salinity stress increased shoot Na+ content however significant decrease in K+, Mg2+, and Ca2+ were observed. Our results are in accordance with the findings of Pandolfi et al. (Citation2012) in pea, Kanwal et al. (Citation2013) in wheat, and Iqbal and Ashraf (Citation2013) in wheat. T. hamatum was very effective in increasing the accumulation of Ca2+ and K+ instead of Na+ content in the present study. The tolerance to salinity has been related with the accumulation of Ca2+ and K+ in cereals (Iqbal & Ashraf Citation2013). The production of exogenous plant growth regulators by T. hamatum (Zhang et al. Citation2013) could explain the alleviation of salt stress via the biological impact of α-naphthaleneacetic acid, indole-3-acetic acid, and indole-3-butyric acid as reported by Iqbal and Ashraf (Citation2013).
In conclusion, although the mechanisms explaining how Trichoderma spp. stimulates plant growth is not fully understood, the increase in total and neutral lipids and antioxidants by T. hamatum treatment decreases the negative effect of NaCl. Scavenging of ROS by antioxidants is the main defense mechanism involved in the sustainability of O. baccatus plants. The beneficial impact of Trichoderma spp. to plants provides new strategies to mitigate salt stress and also develop new ways to enhance the tolerance capacity against salinity.
Acknowledgment
Deanship of Scientific Research at King Saud University is highly acknowledged for funding the research Group Project no. RGP-271.
References
- Abd-Allah EF, Ezzat SM. 2005. Natural occurrence of citrinin in rice grains and its biocontrol by Trichoderma hamatum. Phytoparasitica. 33:73–84. 10.1007/BF02980928
- Ahmad P, Hakeem KR, Kumar A, Ashraf M, Akram NA. 2012. Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr J Biotechnol. 11:2694–2703.
- Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S. 2010. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol. 30:161–175. 10.3109/07388550903524243
- Ahmad P, Jaleel CA, Sharma S. 2010. Antioxidant defense system, lipid peroxidation, proline-metabolizing enzymes, and biochemical activities in two Morus alba genotypes subjected to NaCl stress. Russian J Plant Physiol. 57:509–517. 10.1134/S1021443710040084
- Ahmad P, John R, Sarwat M, Umar S. 2008. Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int J Plant Prod. 2:353–366.
- Ahmad P, Prasad MNV. 2012a. Abiotic stress responses in plants: metabolism, productivity and sustainability. New York (NY): Springer.
- Ahmad P, Prasad MNV. 2012b. Environmental adaptations and stress tolerance in plants in the era of climate change. New York (NY): Springer Science+Business Media.
- Ahmad P, Sarwat M, Sharma S. 2008. Reactive oxygen species, antioxidants and signaling in plants. J Plant Biol. 51:167–173. 10.1007/BF03030694
- Ahmad P, Umar S, Sharma S. 2010. Mechanism of free radical scavenging and role of phytohormones during abiotic stress in plants. In: Ashraf M, Ozturk M, Ahmad MSA, editors. Plant adaptation and phytoremediation. Dordrecht: Springer; p. 99–108.
- Al-Abbasi T, Al-Farhan A, Al-Khulaidi AW, Hall M, Llewellyn OA, Miller AG, Patzelt A. 2010. Important plant areas in the Arabian Peninsula. Edinb J Bot. 67:25–35. 10.1017/S0960428609990217
- Alqarawi AA, Hashem A, Abd Allah EF, Alshahrani TS, Huqail AA. 2014. Effect of salinity on moisture content, pigment system, and lipid composition in Ephedra alata Decne. Acta Biol Hung. 65(1): 61–71. 10.1556/ABiol.65.2014.1.6
- Alqarawi AA, Hashem A, Abd Allah EF. 2014. Alleviation of salt-induced adverse impact via mycorrhizal fungi in Ephedra aphylla Forssk. J Plant Interact. 9:802–810. 10.1080/17429145.2014.949886
- Alqasoumi SI, Soliman GAEH, Awaad AS, Donia AERM. 2012. Antiinflammatory activity, safety and protective effects of Leptadenia pyrotechnica, Haloxylon salicornicum and Ochradenus baccatus in ulcerative colitis. Phytopharmacol. 2:58–71.
- Al Qurainy F, Nadeem M, Khan S, Alansi S, Tarroum M. 2013. Efficient regeneration of a potential medicinal plant Ochradenus baccatus Delile from cotyledon and shoot axis. Pak J Bot. 45:501–505.
- Amenta JS. 1964. A rapid method for quantification of lipids separated by thin layer chromatography. J Lipid Res. 5:270–272.
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 55:373–399. 10.1146/annurev.arplant.55.031903.141701
- Arora DK, Elander RP, Mukherji KG. 1992. Fungal biotechnology. Handbook of applied mycology. New York (NY): Markel Dekker; p. 4.
- Ashraf M, Ali Q. 2008. Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ Exp Bot. 63:266–273. 10.1016/j.envexpbot.2007.11.008
- Ashraf M, Harris PJC. 2004. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 166:3–16. 10.1016/j.plantsci.2003.10.024
- Athar HR, Ashraf M. 2009. Strategies for crop improvement against salinity and drought stress: an overview. In: Ashraf M, Ozturk M, Athar HR, editors. Salinity and water stress. The Netherlands: Springer Verlag; p. 1–16.
- Azam F, Lodhi A, Farooq S, Harry-Ókuru R, Imam SH. 2005. Seed treatment with phytohormones and crop productivity. III. Physiological/biochemical changes in germinating seeds and rooting characteristics of wheat (Triticum aestivum L.) following exposure to 2,4 D. Pak J Bot. 37:865–874.
- Azooz MM, Youssef AM, Ahmad P. 2011. Evaluation of salicylic acid (SA) application on growth, osmotic solutes and antioxidant enzyme activities on broad bean seedlings grown under diluted seawater. Inter J Plant Physiol Biochem. 3:253–264.
- Bae H, Sicher R, Kim M, Kim S, Strem M, Melnick R, Bailey B. 2009. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J Exp Bot. 60:3279–3295. 10.1093/jxb/erp165
- Beyer WF, Fridovich I. 1987. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 161:559–566. 10.1016/0003-2697(87)90489-1
- Bronstein JL, Izhaki I, Nathan R, Tewksbury JJ, Spiegel O, Lotan A, Altstein O. 2007. Fleshy-fruited plants and frugivores in desert ecosystems. In: Dennis AJ, Schupp EW, Green R, Westcott DA, editors. Seed dispersal: theory and its application in a changing world. Wallingford (UK): CAB International; p. 148–177.
- Carlberg I, Mannervik B. 1985. Glutathione-reductase. Methods Enzymol. 113:484–490. 10.1016/S0076-6879(85)13062-4
- Dionisio-Sese ML, Tobita S. 1998. Antioxidant responses of rice seedlings to salinity stress. Plant Sci Limerick. 135:1–9. 10.1016/S0168-9452(98)00025-9
- Eisa S, Hussin S, Geissler N, Koyro HW. 2012. Effect of NaCl salinity on water relations, photosynthesis and chemical composition of Quinoa (Chenopodium quinoa Willd.) as a potential cash crop halophyte. Aust J Crop Sci. 6:357–368.
- Farooq S, Azam F. 2006. The use of cell membrane stability (CMS) technique to screen for salt tolerant wheat varieties. J Plant Physiol. 163:629–637. 10.1016/j.jplph.2005.06.006
- Gachomo EW, Kotchoni SO. 2008. The use of Trichoderma harzianum and T. viride as potential biocontrol agents against peanut microflora and their effectiveness in reducing aflatoxin contamination of infected kernels. Biotechnol. 7:439–447. 10.3923/biotech.2008.439.447
- Hanefat OE, Sobowale AA, Ilusanya OAF, Feyisola RT. 2012. The influence of Glomus moseae and Trichoderma harzianum on phytohormone production in soybeans (Glycine max L. Mer) planted in sterilzed and unsterilzed soils. Am J Exp Agric. 2:516–524. 10.9734/AJEA/2012/910
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M. 2004. Trichoderma species — opportunistic, avirulent plant symbionts. Nature Rev Microbiol. 2:43–56. 10.1038/nrmicro797
- Heath RL, Packer L. 1968. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 125:189–198. 10.1016/0003-9861(68)90654-1
- Hejazi Mehrizi M, Shariatmadari H, Khoshgoftarmanesh AH, Dehghani F. 2012. Copper effects on growth, lipid peroxidation, and total phenolic content of rosemary leaves under salinity stress. J Agr Sci Tech. 14:205–212.
- Hermosa R, Viterbo A, Chet I, Monte E. 2012. Plant-beneficial effects of Trichoderma and of its genes. Microbiol. 158:17–25. 10.1099/mic.0.052274-0
- Hoagland DR, Arnon DI. 1950. The water-culture method for growing plants without soil. Berkeley (CA): California Agricultural Experiment Station, University of California, Circular 347; 32 p.
- Hosono K. 1992. Effect of salt stress on lipid composition and membrane fluidity of the salt tolerant yeast Zygosaccharomyces rouxii. J Gen Microbiol. 138:91–96. 10.1099/00221287-138-1-91
- Iqbal M, Ashraf M. 2013. Alleviation of salinity-induced perturbations in ionic and hormonal concentrations in spring wheat through seed preconditioning in synthetic auxins. Acta Physiol Plant. 35:1093–1112. 10.1007/s11738-012-1147-z
- Josine TL, Ji J, Wang G, Wu J. 2011. Salinity stress tissue-regenerated Rosa chinensis Jacq. improves water and proline content. Afr J Agri Res. 6:3409–3418.
- Julkunen-Tiitto R. 1985. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem. 33:213–217. 10.1021/jf00062a013
- Kafi M, Nabati J, Masoumi A, Mehrgerdi MZ. 2011. Effect of salinity and silicon application on oxidative damage of sorghum (Sorghum bicolor L. Moench.). Pak J Bot. 43:2457–2462.
- Kanwal H, Ashraf M, Hameed M. 2013. Water relations and ionic composition in the seedlings of some newly developed and candidate cultivars of wheat (Triticum aestivum L.) under saline conditions. Pak J Bot. 45:1221–1227.
- Kar M, Mishra D. 1976. Catalase, peroxidase, and polyphenoloxidase activities during rice leaf senescence. Plant Physiol. 57:315–319. 10.1104/pp.57.2.315
- Kaya C, Higgs D, Kirnak H. 2001. The effects of high salinity (NaCl) and supplementary phosphorus and potassium on physiology and nutrition development of spinach. Bulg J Plant Physiol. 27:47–59.
- Koca H, Bor M, Özdemir F, Türkan I. 2007. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ Exp Bot. 60:344–351. 10.1016/j.envexpbot.2006.12.005
- Koyro HW, Ahmad P, Geissler N. 2012. Abiotic stress responses in plants: an overview. In: Ahmad P, Prasad MNV, editors. Environmental adaptations and stress tolerance of plants in the era of climate change. New York (NY): Springer Science and Business Media; p. 1–28.
- Levitt J. 1980. Response of plants to environmental stresses. Vol. II: water, radiation, salt and other stresses. 2nd ed. New York: New York Academic Press; 606 p.
- Lichtenthaler HK, Wellburn AR. 1983. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans. 11:591–592.
- Marsh JB, Weinstein DB. 1966. Simple charring method for determination of lipids. J Lipid Res. 7:574–576.
- Martínez-Medina A, Alguacil MDM, Pascual JA, van Wees SCM. 2014. Phytohormone profiles induced by Trichoderma isolates correspond with their biocontrol and plant growth-promoting activity on melon plants. J Chem Ecol. 40:804–815.
- Masood A, Shah NA, Zeeshan M, Abraham G. 2006. Differential response of antioxidant enzymes to salinity stress in two varieties of Azolla (Azolla pinnata and Azolla filiculoides). Env Exp Bot. 58:216–222. 10.1016/j.envexpbot.2005.08.002
- Mastouri F, Björkman T, Harman GE. 2010. Seed treatment with Trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathol. 100:1213–1221. 10.1094/PHYTO-03-10-0091
- Mehr ZS, khajeh H, Bahabadi SE, Sabbagh SK. 2012. Changes on proline, phenolic compounds and activity of antioxidant enzymes in Anethum graveolens L. under salt stress. Int J Agron Plant Prod. 3:710–715.
- Nakano Y, Asada K. 1981. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol. 22:867–880.
- Naz R, Bano A. 2013. Influence of exogenously applied salicylic acid and plant growth promoting rhizobacteria inoculation on the growth and physiology of some sunflower (Helianthus annuus L.). Pak J Bot. 45:367–373.
- Neumann PM. 2008. Coping mechanisms for crop plants in drought-prone environments. Ann Bot. 101:901–907. 10.1093/aob/mcn018
- Pandolfi C, Mancuso S, Shabala S. 2012. Physiology of acclimation to salinity stress in pea (Pisum sativum). Environ Exp Bot. 84:44–51. 10.1016/j.envexpbot.2012.04.015
- Petridis A, Therios I, Samouris G, Koundouras S, Giannakoula A. 2012. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol Biochem. 60:1–11. 10.1016/j.plaphy.2012.07.014
- Pohjala L, Tammela P. 2012. Aggregating behavior of phenolic compounds — a source of false bioassay results? Molecules. 17:10774–10790. 10.3390/molecules170910774
- Rao A, Ahmad SD, Sabir SM, Awan SI, Shah AH, Abbas SR, Shafique S, Khan F, Chaudhary A. 2013. Potential antioxidant activities improve salt tolerance in ten varieties of wheat (Triticum aestivum L.). Am J Plant Sci. 4:69–76. 10.4236/ajps.2013.46A010
- Rasool S, Ahmad A, Siddiqi TO, Ahmad P. 2013. Changes in growth, lipid peroxidation and some key antioxidant enzymes in chickpea genotypes under salt stress. Acta Physiol Plant. 35:1039–1050. 10.1007/s11738-012-1142-4
- Rasool S, Hameed A, Azooz MM, Rehman M, Siddiqi TO, Ahmad P. 2013. Salt stress: causes, types and response of plants. In: Ahmad P, Azooz MM, Prasad MNV, editors. Ecophysiology and response of plants under salt stress. New York (NY): Springer LLC; p. 1–24.
- Rawat L, Singh Y, Shukla N, Kumar J. 2011. Alleviation of the adverse effects of salinity stress in wheat (Triticum aestivum L.) by seed biopriming with salinity tolerant isolates of Trichoderma harzianum. Plant Soil. 347:387–400. 10.1007/s11104-011-0858-z
- Rawat L, Singh Y, Shukla N, Kumar J. 2013. Salinity tolerant Trichoderma harzianum reinforces NaCl tolerance and reduces population dynamics of Fusarium oxysporum f.sp. ciceroin chickpea (Cicer arietinum L.) under salt stress conditions. Arch Phytopathol Plant Protect. 46:1442–1467. 10.1080/03235408.2013.769316
- Resende MP, Jakoby ICMC, dos Santos LCR, Soares MA, Pereira FD, Souchie EL, Silva FG. 2014. Phosphate solubilization and phytohormone production by endophytic and rhizosphere Trichoderma isolates of guanandi (Calophyllum Brasiliense Cambess). Afr J Microbiol Res. 8:2616–2623. 10.5897/AJMR2014.6633
- Sairam RK, Deshmukh PS, Shukla DS. 1997. Tolerance of drought and temperature stress in relation to increased antioxidant enzyme activity in wheat. J Agron Crop Sci. 178:171–178. 10.1111/j.1439-037X.1997.tb00486.x
- Samantary S. 2002. Biochemical responses of Cr-tolerant and Cr-sensitive mung bean cultivars grown on varying levels of chromium. Chemosphere. 47:1065–1072. 10.1016/S0045-6535(02)00091-7
- Shahid MA, Ashraf MY, Pervez MA, Ahmad R, Balal RM, Garcia-Sanchez F. 2013. Impact of salt stress on concentrations of Na+, Cl– and organic solutes in pea cultivars. Pak J Bot. 45:755–761.
- Shoresh M, Mastouri F, Harman GE. 2010. Induced systemic resistance and plant responses to fungal biocontrol agents. Ann Rev Phytopathol. 48:21–43. 10.1146/annurev-phyto-073009-114450
- Smart RE, Bingham GE. 1974. Rapid estimates of relative water content. Plant Physiol. 53:258–260. 10.1104/pp.53.2.258
- Sofo A, Scopa A, Manfra M, de Nisco M, Tenore G, Troisi J, di Fiori R, Novellino E. 2011. Trichoderma harzianum strain T-22 induces changes in phytohormone levels in cherry rootstocks (Prunus cerasus × P. canescens). Plant Growth Regul. 65:421–425. 10.1007/s10725-011-9610-1
- Sultana N, Ikeda T, Itoh R. 1999. Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ Exp Bot. 42:211–220. 10.1016/S0098-8472(99)00035-0
- Wehner J, Antunes PM, Powell JR, Mazukatow J, Rillig MC. 2010. Plant pathogen protection by arbuscular mycorrhizas: a role for fungal diversity? Pedobiologia. 53:197–201. 10.1016/j.pedobi.2009.10.002
- Wolf B. 1982. A comprehensive system of leaf analyses and its use for diagnosing crop nutrient status. Comm Soil Sci Plant Anal. 13:1035–1059. 10.1080/00103628209367332
- Zhang F, Yuan J, Yang X, Cui Y, Chen L, Ran W, Shen Q. 2013. Putative Trichoderma harzianum mutant promotes cucumber growth by enhanced production of indole acetic acid and plant colonization. Plant Soil. 368:433–444. 10.1007/s11104-012-1519-6
- Zörb C, Herbst R, Forreiter C, Schubert S. 2009. Short-term effects of salt exposure on the maize chloroplast protein pattern. Proteomic. 9:4209–4220.


