?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Rice (Oryza sativa L.) stands out as the world's most vital staple food crop, yet is susceptible to UV-B radiation stress. This study investigates the physiological and transcriptome responses in rice exposed to titanium dioxide nanoparticles (TiO2 NPs) under UV-B radiation. Results demonstrate that TiO2 NPs, applied alone (TN) or in combination with UV-B stress (UV+TN), significantly enhance rice plant growth and physiological parameters. Reactive oxygen species (ROS) levels, elevated under UV-B stress, are significantly reduced by TN and UV+TN treatments, thereby regulating antioxidants particularly involved in ascorbate-glutathione pathway. Transcriptomics analysis of identified DEGs in UV+TN, utilizing KEGG pathway analysis, reveals significant enrichment in various pathways. These pathways include glutathione metabolism, pyruvate metabolism, starch and sucrose metabolism, regulation of basal transcription factors, plant hormonal signal transduction pathways, cellular processes associated with energy, and the MAPK signaling pathway. Overall, TiO2 NPs application modulates diverse biological and metabolic pathways, enhancing UV-B stress tolerance in rice.
1. Introduction
Over the last few decades, anthropogenic pollutants have significantly reduced stratospheric ozone layer, which could lead to a considerable increase in ultraviolet (UV) radiation (Manisalidis et al. Citation2020). The depletion of the ozone layer is a concerning condition from the point of view of environmental safety since it poses a threat to human communities as well as forests and vegetation. Living organisms absorb varying amounts of UV radiation depending on factors such as the direction of the light and the amount of ozone present in the atmosphere. Solar UV radiation is composed of three main categories: UV-A (wavelengths between 315 and 400 nm), UV-B (ranging from 280 to 315 nm), and UV-C (below 280 nm) (Williamson et al. Citation2014). In contrast, the photosynthetically active radiation (PAR) is light with a wavelength between 400 and 700 nm (Zhen and Bugbee Citation2020).
Anthropogenic chlorofluorocarbons have depleted the ozone layer in the stratosphere, leading to an increase in UV-B radiation. Even though ultraviolet-B (280–315 nm) light only accounts for 0.5% of the total light energy reaching the Earth's surface, it is the most essential form of light because of the significant effect it has on sessile plants (Ling et al. Citation2022). Moderate levels of UV-B radiation, which are typically found in natural environments, serve as a crucial environmental signal. They trigger responses in plants that enhance their UV-B protection mechanisms and help mitigate UV-B damage. Importantly, these responses do not necessarily hinder plant growth and development processes (Neale et al. Citation2021). On the other hand, elevated levels of UV-B radiation have significant negative impacts on plants and other organisms at a biological level. UV-B radiation can cause damage to cells in multiple ways, such as modifying DNA, proteins, and lipids (Rastogi et al. Citation2010). Plants can also experience a reduction in their photosynthetic efficiency due to the negative impact of UV-B on photosynthetic pigments, as these pigments can absorb the UV spectrum (Piccini et al. Citation2020). In photosynthetic organisms, including plants, UV-B radiation primarily impacts the PSII complex. This is because high-energy UV-B light can disrupt the functioning of the photosynthetic machinery, and PSII is a major focal point of this inhibition (Kreslavski et al. Citation2020). UV-B radiation can also influence stomatal conductance, which, in turn, can change the rate of transpiration (water loss) and the net CO2 assimilation rate in plants (Piccini et al. Citation2020). Furthermore, overexposure to UV radiation can cause certain photosynthesis-related proteins to be downregulated such as Chl a/b binding protein, while increasing the expression of other proteins which induce innate resistance in plants such as pathogen-related protein-1 (PR-1) (Sah et al. Citation2016).
In addition, UV-B radiation is one of the principal causes of oxidative stress. This is due to the formation of reactive oxygen species (ROS) in large quantities, particularly within mitochondria and chloroplasts (Foyer and Hanke Citation2022). The prominent ROS molecules, including hydrogen peroxide (H2O2) and superoxide (O2•−) anions, react rapidly with macromolecules in their vicinity, resulting in oxidation, often referred to as ‘oxidative damage.’ Peroxidation of lipids in thylakoid membranes rich in polyunsaturated fatty acids yields lipid hydroperoxides, aldehydes, and reactive electrophiles. If these compounds were to accumulate in large quantities in different compartments of plant cells, it could systematically result in cellular toxicity (Foyer and Hanke Citation2022). Numerous studies have highlighted the dual role of ROS. They respond to stress by either triggering cell death or acting as signaling molecules, depending on the specific nature and length of the stress (Huang et al. Citation2019). Evidence from previous studies demonstrates that ROS control epigenetic changes, including histone protein alterations and DNA chemical modifications (Jing et al. Citation2022). These mechanisms play a pivotal role in fine-tuning gene expression within specific redox pathways. In short, plants that are subjected to excessive doses of UV-B radiation may undergo changes in the composition of numerous biochemical molecules and modifications in the transcription of a significant number of genes, as well as alterations in UV-B-specific regulatory pathways (Haskirli et al. Citation2021).
To counteract oxidative damage and regulate cellular ROS levels, plants have evolved intricate antioxidant defense systems. These systems are categorized into non-enzymatic and enzymatic antioxidants. Small molecules like ascorbate, glutathione, β-carotene, α-tocopherol, and others are considered as non-enzymatic antioxidants (Dumanović et al. Citation2021). Enzymatic antioxidants, on the other hand, include specialized enzymes like superoxide dismutase (SOD), catalase (CAT), peroxidases (POX), and ascorbate peroxidase (APX). These enzymes work collaboratively to neutralize O2•− or H2O2 (Dumanović et al. Citation2021).
Rice (Oryza sativa L.) holds a position of paramount significance as a staple food crop, catering to the dietary needs of millions of individuals across the globe (Mohidem et al. Citation2022). Additionally, it serves as a pivotal model plant in the realm of genetic and molecular research, contributing to our understanding of fundamental biological processes. Rice is more than just a staple food in most Asian countries; it also plays an important part in maintaining a sustainable food supply and maintaining a healthy national economy (Muthayya et al. Citation2014). Rice provides 76% of the caloric intake to the population of Southeast Asia region (Mohidem et al. Citation2022). Unfortunately, Southeast Asia receives high levels of ultraviolet radiation due to its proximity to the equator and high levels of solar radiation (Tan et al. Citation2018). The degree to which crop plants are affected by UV-B radiation exhibits significant intra- and interspecific variability, with a wide range of sensitivity observed across different species and cultivars, as evidenced by previous research (Hidema and Kumagai Citation2006). Furthermore, research has revealed that rice cultivars from areas with elevated ambient UV-B radiation levels may not display enhanced tolerance to UVB radiation (Mmbando et al. Citation2020). Recent research has demonstrated that the primary focus of efforts to enhance rice resistance to UV-B stress largely revolves around the application of transgenic techniques (Peng et al. Citation2017; Zhang et al. Citation2020a). Nonetheless, developing transgenic plants is a lengthy and resource-intensive process. It requires meticulous laboratory work and rigorous testing to ensure the success of the genetic modifications. Moreover, the involvement of regulatory procedures and public apprehensions regarding genetically edited plants (Spök et al. Citation2022), can significantly extend the time required to bring these crops to realization. In light of the challenges associated with transgenic methods, there is a growing need to explore more neutral and sustainable approaches to mitigate stress and enhance plant growth and development.
The utilization of nanoparticles (NPs) has witnessed an extraordinary global growth, particularly over the past decade, driven by their wide array of applications (Mittal et al. Citation2020; Pandit et al. Citation2022). Nanoparticles, usually measuring between 1 and 100 nanometers, display unique characteristics because of their small size and their significant surface area relative to their volume (Al-Khayri et al. Citation2023). Their use has garnered significant interest in various scientific and industrial domains, including their potential application in ameliorating abiotic stress tolerance in plants (Sun et al. Citation2020; Taha et al. Citation2021). The application and positive impact of various NPs have been evaluated under various abiotic stress conditions, including drought (Tawfik et al. Citation2021), salinity (Rakgotho et al. Citation2022), high temperature (Djanaguiraman et al. Citation2018), low temperature (Wang et al. Citation2021a), and heavy metals (Nazir et al. Citation2022). However, research on the protective role of NPs against UV-B stress remains limited. For example, studies investigating the protective effects of different NPs against UV-B stress have been conducted on wheat (Tripathi et al. Citation2017), spinach (Lei et al. Citation2008), saffron crocus (Moradi Rikabad et al. Citation2019), garden thyme (Azadi et al. Citation2021), and Arabidopsis (Wang et al. Citation2021b). Importantly, most of these studies have primarily focused on the morpho-physiological aspects, with only a few addressing the biochemical aspects. Additionally, there is a scarcity of reports demonstrating the regulation of NPs at the transcriptomics level, particularly in rice plants during UV-B stress. However, the impact of NPs on plants is not uniform, and it can vary based on multiple factors such as the concentration of NPs used, the physiochemical properties of the NPs, the specific plant species, and the duration and intensity of UV-B exposure. Therefore, the response of plants to NPs is complex and cannot be generalized. It is essential to consider all these factors when studying the potential effects of NPs on plants to obtain more accurate and reliable results.
In recent years, there has been a significant increase in the application of titanium dioxide (TiO2) NPs in agriculture (Šebesta et al. Citation2021). This interest extends to the globally important rice crop, where TiO2 NPs have been studied in various environmental stress conditions, including phosphorus deficiency (Zahra et al. Citation2017), cadmium toxicity (Lai et al. Citation2023; Zhang et al. Citation2020b), arsenic stress (Kiany et al. Citation2022), and lead toxicity (Ghouri et al. Citation2024). However, the potential of TiO2 NPs in mitigating UV-B stress in rice has not yet been investigated. This study aims to investigate the impact of TiO2 NPs on rice plants subjected to long-term UV-B radiation. The study hypothesizes that TiO2 NPs can help alleviate the harmful effects of UV-B stress on rice by regulating various physiological and biochemical responses, and modulating gene expressions related to essential biological pathways. To achieve our objective, we investigated the effects of UV-B and TiO2 NP treatments on rice plants through the assessment of various parameters, including (a) plant biomass, (b) pigment content, (c) photosynthesis rate and efficiency, (d) activities of Rubisco and FBPase, (e) accumulation of ROS molecules, (f) levels of antioxidants and osmoregulatory substances, and (g) conducting transcriptomics analysis to identify differentially expressed genes (DEGs) and associated pathways. The findings from this study are anticipated to contribute valuable insights into the potential applications of TiO2 NPs as a means to enhance crop resilience under UV-B stress, ultimately benefiting agricultural practices and food security.
2. Material and methods
2.1 Plant materials and growth conditions
In this study, standard viability testing procedures recommended by ISTA (International Seed Testing Association) were used to test the quality of rice (Oryza sativa L.) seeds. To ensure that the seeds were free from any microbial contamination, surface sterilization was performed on the seeds using a 0.5% (w/v) sodium hypochlorite solution. The seeds were subjected to thorough washing with distilled water to get rid of any residue of the solution that was remained on the seeds. After being sterilized and washed, the seeds were soaked in sterilized water to initiate the germination process. The germination process was initiated in an incubator at a temperature of 28°C. The germination of the seeds was monitored for about 2 days. After germination, healthy and uniform seedlings were then transplanted into planting containers for hydroponic culturing and nutrient solution was maintained throughout the experimental period. The rice seedlings were grown in a climate chamber with 16 h of white light at an intensity of 120 µmol m−2 s−1 during the day and 8 h of darkness, maintaining a constant temperature of 28°C. Throughout the course of the experiment, the seedlings were kept under similar controlled conditions.
2.2 Treatments and experimental design
To examine the effects of TiO2 NPs on the physiological and transcriptomics aspects of rice, a solution was prepared containing 100 mg/L of TiO2 nano-powder (purchased from Sigma-Aldrich, Cat. No. 718467) with a main particle size of 21 nm and a purity level of 99.5%. The TiO2 NPs suspension at the specified concentration (100 mg/L) was prepared in deionized water. It was then agitated by sonication for 30 min (80 W, 25 kHz) in an ice-water bath with occasional stirring for optimal dispersion. This concentration was followed according to recommendations from previous reports, where it was found that a moderate level of TiO2 NPs concentration (100 mg/L) has been shown to produce significant growth stimulatory effects in plants, especially at earlier growth stages (Hu et al. Citation2020; Tighe-Neira et al. Citation2020). The rice plants were given half strength Hoagland’s nutrient solution for two weeks in the growth chamber under similar conditions as mentioned above. After two weeks, rice seedlings were divided into four main experimental groups namely; Control (CK), UV-B stress (UV), titanium dioxide nanoparticles treatment (TN; 100 mg/L), and UV-B + titanium dioxide nanoparticles treatment (UV+TN). For UV-B stress (UV) treatment, the rice seedlings were placed in UV chambers installed with two 15-watt UV-B lamps. The rice seedlings were subjected to a high dose of UV-B radiation (12.8 μW cm−2) daily for four hours, between 10:00 am and 2:00 pm, continuously for 28 days. Every alternate day, the UV-B radiation was measured by using a portable UV radiometer (Handy, Beijing, China). Foliar application of TiO2 NPs was carried out by evenly spraying solution on the plants every 7th day, starting simultaneously with the UV-B treatment, and continued for four weeks during the UV-B stress. The UV-B fluorescent lamps (UV-B313EL, Beijing, China) were positioned at a specific distance (0.5 m) above the rice seedlings to provide consistent UV exposure, while using filters for the effective transmission of light spectrum except UV-C, as previously described in our report (Shahzad et al. Citation2021a). In the context of this study, we established an experimental design based on a randomized complete block design framework, incorporating three replicates for each experimental treatment group, with a total of 15 plants in each treatment. Subsequent to the completion of the UV-B stress exposure, samples were collected from all treatment groups and immediately stored for the downstream physiological, biochemical, and transcriptomics analyses.
2.3 Determination of chlorophylls and carotenoids contents
Samples from fresh leaves (0.4 g) were collected from all the experimental groups (CK, UV, TN, UV+TN). The leaves were first cut into small pieces, after which they were placed in a 5 mL solution of 95% (v/v) ethanol. Samples were kept in darkness at 26°C for 24 h. To determine the contents of chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (TChl) and carotenoids (Cart), the samples were analyzed according to a previously described method (Shahzad et al. Citation2021b). Briefly, a 1 ml solution extracted from each sample was utilized to quantify the concentration of Chla at 663 nm, Chlb at 645 nm, and Cart at 470 nm. This quantification was performed using an ultraviolet spectrophotometer (DU800, Beckman, USA). The calculation of the pigment content was conducted based on the equations provided in a previously mentioned report (Shahzad et al. Citation2021b) as follows:
(1)
(1)
(2)
(2)
(3)
(3)
(4)
(4)
2.4 Determination of gas exchange and chlorophyll fluorescence parameters
The fully expanded leaves from control and treated plants were collected for the analysis of net photosynthetic rate (Pn) and stomatal conductance (Gs). Measurements were conducted using a portable photosynthesis system (Li-6400; LI-COR Biosciences, Lincoln, NE, USA). The test was performed in a clean chamber which was maintained at a constant temperature of 25 °C, air velocity at 500 mol/s, a CO2 supply concentration of 400 μmol/mol, and a fixed LED light source emitting 600 μmol photons m m−2 s−1. To measure fluorescence-related parameters, seedlings were subjected to 30 min of dark incubation prior to measurement. Analysis of chlorophyll fluorescence parameters including the maximum efficiency of PSII (Fv/Fm), and non-photochemical quenching (NPQ) was carried out on leaves using a portable fluorometer MINIPAM (Walz, Effeltrich, Germany) under room temperature and a saturating pulse. The detailed protocol used for this analysis has been described in the previous report (Zhang et al. Citation2019).
2.5 Measurement of Rubisco and FBPase activities
Rubisco (Ribulose-1,5-bisphosphate carboxylase/oxygenase) activity was assessed using the method described by previous researchers (Wishnick and Daniel Lane Citation1971). Briefly, about 0.4 grams of leaf tissues from rice seedlings were macerated in a 2 mL solution containing 1% (w/w) polyvinylpyrrolidone. Subsequently, the macerated material was subjected to extraction using 3 mL of a cold extraction buffer. The buffer used in this experiment consisted of the following components: 50 mM Tris HCl (pH 7.5), 1 mM EDTA, 10 mM MgCl2, 12% (v/v) glycerol, 0.1% (v/v) b-mercaptoethanol, and 1% (w/w) polyvinylpyrrolidone. After preparing the buffer, the mixture was then subjected to centrifugation at 4°C for 15 min at 15,000×g. Following centrifugation, the resulting supernatant was collected and used for the Rubisco activity assay.
Following the method described by Scheibe et al. (Citation1986), FBPase activity was determined spectrophotometrically by monitoring the change in absorbance at 340 nm (Scheibe et al. Citation1986). Briefly, rice leaf tissues were harvested from control and UV stressed plants, and finely ground into a powder using liquid nitrogen and a pre-cooled grinding system. To process the powdered leaf tissues, they were first homogenized using a reaction mixture. This mixture was composed of the following components: 100 mM Tris-HCl buffer (pH 8.0), 16 mM MgCl2, 1 mM EDTA, 20 mM dithiothreitol (DTT), 2% (w/v) insoluble polyvinylpyrrolidone, 0.05% Triton X-100, and 2 mM phenylmethylsulfonyl fluoride. Following homogenization, the mixture underwent centrifugation for 10 min at 15,000×g, and the resulting supernatant was utilized for the FBPase activity assay.
2.6 Measurement of soluble sugar and soluble protein
To determine the total soluble sugar content, we started by grinding 0.4 grams of leaf tissues using a grinding system. Subsequently, these ground tissues were combined with an extraction buffer. The extraction buffer was carefully prepared and consisted of the following components: phosphate buffer (50 mM, pH 7), glycerol (10% v/v), ascorbate (1 mM), KCl (100 mM), and b-mercaptoethanol (5 mM). Following a 15-minute centrifugation at 12,000 g of the homogenate, the total soluble sugar content was measured using the phenol-sulfuric acid assay, as described in a previous study (Basit et al. Citation2022).
The total soluble protein content was measured using the Bradford reagent method. In brief, 100 µL of sample enzyme extract was mixed with 5 mL of the Bradford reagent, and the resulting mixture was used to record the absorbance at a wavelength of 595 nm, as previously described (Hanif et al. Citation2021).
2.7 Determination of hydrogen peroxide (H2O2) and superoxide (O2•−)
The contents of hydrogen peroxide (H2O2) and superoxide (O2•−) anions in the rice leaves were determined to measure the level of reactive oxygen species (ROS) in the samples. A spectrophotometric method, with absorbance measured at 390 nm, was employed to quantify the concentration of H2O2 in fresh leaves following the procedure outlined by other report (Velikova et al. Citation2000). Briefly, leaf samples weighing 0.4 g were ground in a 3 mL solution of 5% (w/v) trichloroacetic acid (TCA), supplemented with 0.1 g of activated charcoal, all maintained at a chilling temperature. After homogenizing the mixture, we carried out centrifugation at 14,000 g for 10 min. Following centrifugation, we collected the resulting supernatant in new conical tubes. Next, we mixed this 0.5 mL of supernatant with 0.5 mL of 10 mM potassium phosphate buffer (pH: 7) and 0.7 mL of 1 M potassium iodide. To assess the samples, we measured the absorbance of each at 390 nm. A standard curve line method using the known concentration range was utilized to measure the final H2O2 concentration, expressed as µmol g−¹ FW (fresh weight).
The concentration of superoxide radical ion (O2•−) was determined as follows: Initially, 0.4 grams of fresh leaf tissues were homogenized in 2.5 mL of potassium phosphate buffer prepared at the concentration of 65 mM (pH 7.8), and then centrifuged at 5000 rpm. After that, we mixed 1 mL of the supernatant with 0.9 mL of a 65 mM potassium phosphate buffer and 0.1 mL of a 10 mM hydroxylamine hydrochloride solution. This mixture was allowed to incubate at 25°C for 20 min. After 20 min, a mixture solution containing 300 µl of 17 mM sulfanilamide and 7 mM naphthylamine were added to the solution, and the whole mixture was incubated again. The incubation time at 25°C was 20 min, and the absorbance was measured at 530 nm. The ultimate O2•− concentration was determined using the standard curve line method described in our earlier study (Shahzad et al. Citation2023), and presented as nmol g−1 FW (fresh weight).
2.8 Measurement of antioxidants content
The ascorbate (AsA) content in fresh leaves was determined by homogenizing 0.4 g of leaves in 3 mL of pre-chilled 5% metaphosphoric acid that contains 1 mM EDTA. After the mixture was homogenized, it was centrifuged at 11,500 × g for 15 min at 4°C. We assessed the absorbance at a wavelength of 525 nm. To estimate the total ascorbate content, we employed a standard curve method. In order to quantify the glutathione (GSH) and its oxidized form, glutathione disulfide (GSSG), we initially homogenized 0.4 grams of plant leaf tissue in 2 mL of 2% metaphosphoric acid, which also contained 2 mM EDTA. Following homogenization, the mixture was subjected to centrifugation at 4°C for 10 min at 14,000 g. The resulting supernatant was then neutralized with 0.5 M phosphate buffer (pH 7.5). Afterwards, 0.1 mL of the pH-neutralized supernatant was added to a solution containing 0.2 mM NADPH, 100 mM phosphate buffer (pH 7.5), 5 mM EDTA, and 0.6 mM 5,5′-dithio-bis (2-nitrobenzoic acid). After adding 3 U of glutathione reductase (GR) to this mixture solution, we measured the shift in absorbance at 412 nm over the course of 1 min to track the enzymatic process. For the measurement of GSSG content, 20 μL of 2-vinylpyridine was added to remove GSH by derivatization, whereas 20 μL of water was added for the total GSH level. The quantification of GSH was determined by subtracting the GSSG levels from the total measured, as reported by others with little modification (Mu et al. Citation2022).
2.9 RNA extraction, library construction and sequencing
The rice leaf samples were collected from control and treated plants, followed by total RNA extraction for subsequent RNA-seq analysis. Briefly, leaf samples were used to extract total RNA using the Trizol (Invitrogen, USA) extraction method. Agarose gel electrophoresis was used to evaluate RNA integrity. In line with this purpose, we employed the Agilent Bioanalyzer 2100 (Agilent Technologies, USA) to determine the concentration and purity of the RNA. For library construction, each sample required 1 g RNA with an OD260/280 between 1.9 and 2.2, and OD260/230 ≥ 2.0, and a RIN ≥ 8.0. In this study, we used the Illumina HiSeqTM 2500 platform (Illumina Inc, USA) to perform paired-end sequencing. To prepare the raw reads for analysis, the FastQC method was applied, which identified and removed adapter, poly-N, and low-quality reads from the sequencing data. The clean reads were then mapped to the rice reference genome (MSU Rice Genome Annotation Project Release 7) using Hisat2 v2.0.5 with default parameters. By employing the default settings of prcomp function in the R program, we conducted a principal component analysis (PCA) on individual samples, considering their expression levels. The objective was to gain insight into the relationships among replicates within both the control and treatment groups.
2.10 Data filtering and assembly
Prior to transcriptome assembly, a rigorous data filtering procedure was employed. Data filtration was executed through the application of Cutadapt. During this filtration process, the 3’ end of the adaptor sequence was excised using Cutadapt, ensuring a minimum 10-base pair overlap between the excised portion and the known linker. This step effectively eliminated the adapter sequences present in the raw reads. Additionally, sequences of lower quality, specifically reads containing ambiguous bases denoted as ‘N,’ and those with more than 20% of bases with quality scores less than 20 (Q < 20), were excluded from the dataset.
2.11 Differentially expressed genes and functional annotation
The identification of differentially expressed genes was achieved using the R package DESeq2, which applies a statistical model based on the negative binomial distribution to detect differential expression within datasets, as validated in prior studies (Li et al. Citation2018; Sun et al. Citation2019). The gene expression levels were assessed using the FPKM metric, and a threshold of P < 0.05 and |log2FoldChange| > 2 was applied. Gene function annotation involved utilizing BlastX with an e-value cutoff of less than 10−5, against both the Kyoto Encyclopedia of Genes and Genomes (KEGG, available at http://www.genome.jp/kegg/) and the Gene Ontology (GO, accessible through http://www.geneontology.org/).
2.12 Quantitative real-time-PCR (qRT-PCR)
To perform quantitative real-time (qRT-PCR), RNA extraction from all the treated samples using TRIzol reagent (Invitrogen) was carried out following the guidelines provided by the manufacturer (Takara, China). cDNA synthesis was carried out utilizing 5 μg of total RNA derived from three biological replicates. In the subsequent PCR reaction, a 10 mL reaction volume was prepared, comprising 1 μg cDNA, 5 mL of SYBR Premix Ex Taq II (Takara, China), and 200 nM of primers. To ensure the accuracy and reproducibility of the Illumina RNA-Seq outcomes, we have selected 12 representative genes and assess their expression levels in control and treatment groups using CFX96 TouchTM Real-Time PCR Detection System (Bio-Rad, Hercules, CA, United States). qRT-PCR reactions for all samples were conducted in triplicate, and the expression levels of each gene were determined using the 2-ΔΔCt method (Livak and Schmittgen Citation2001).
2.13 Statistical analysis
The presented data represent the mean values along with their corresponding standard deviations (SD), which were computed from three distinct biological replicates. To ascertain statistical significance (p < 0.05) concerning differences among the values observed in the treated samples in comparison to the control group, a one-way ANOVA analysis was performed, and differences among treatment means were analyzed using the Duncan’s multiple range test. This statistical analysis was conducted employing SPSS 21.0 for Windows (SPSS Inc., Chicago, IL, USA). Data visualization was performed using ggplot2 function of the open source statistical program R (v. 4.1.0) for Windows (http://CRAN.R-project. org/).
3. Results
3.1 Effect of UV-B and TiO2 NPs on the biomass of rice seedlings
In this study, we investigated the effect of UV and TiO2 nanoparticle treatments on the plant biomass measured in terms of shoot fresh weight, shoot dry weight, root fresh weight, and root dry weight (A–D). The results indicated that there were significant (p < 0.05) differences in all treatment groups for the measured parameters. In comparison to the control (CK) group, UV treatment led to a 20.04% reduction in shoot fresh weight, a 26.05% reduction in shoot dry weight, an 18.14% reduction in root fresh weight, and an impressive 28.72% reduction in root dry weight. These findings highlight the efficacy of UV treatment in reducing the biomass of both shoot and root in rice seedlings.
Figure 1. The individual and combined effects of TiO2 NPs and UV-B irradiation on (A) shoot fresh weight, (B) shoot dry weight, (C) root fresh weight, and (D) root dry weight in rice seedlings. Data presented are the means ± SE (n = 8), and significant differences between the means were calculated at p ≤ 0.05 using Duncan’s multiple range test.
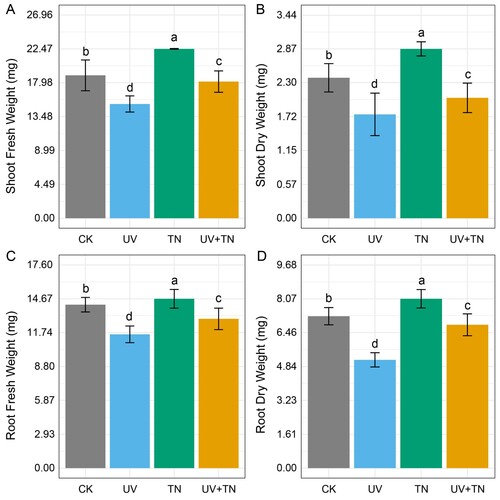
Similarly, TiO2 nanoparticle (TN) treatment exhibited substantial effects on the plant biomass. When compared to the control (CK) group, TN treatment resulted in an 18.51% increase in shoot fresh weight, a 20.58% increase in shoot dry weight, a 3.60% increase in root fresh weight, and an 11.46% increase in root dry weight (A–D). These results indicate that TN treatment promoted biomass growth of shoot and root in rice seedlings.
When comparing the combined UV and TiO2 nanoparticle treatment (UV+TN) with the untreated control (CK), our results showed a reduction in shoot and root biomass values. However, when we examined the combined UV+TN treatment against UV treatment alone, we observed positive impact on seedlings biomass. The UV+TN treatment led to a 19.59% increase in shoot fresh weight, a 15.90% increase in shoot dry weight, an 11.56% increase in root fresh weight, and a remarkable 32.36% increase in root dry weight when compared to UV treatment alone (A–D). These results emphasize that the combination of UV and TN treatments has the potential to substantially boost both shoot and root biomass, surpassing the negative effects of UV treatment alone.
3.2 Effect of UV-B and TiO2 NPs on the pigment contents of rice seedlings
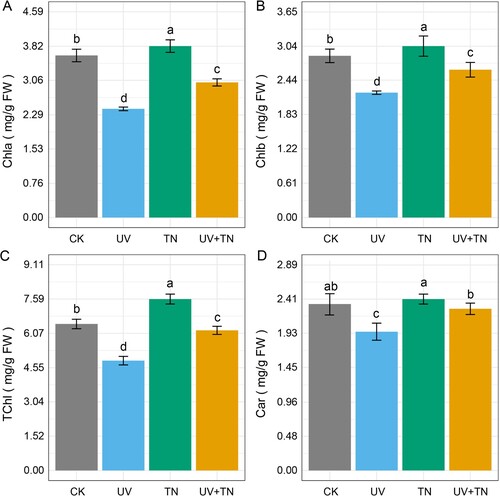
To examine the effect of UV-B stress and supplementation of TiO2 NPs on plant pigments, we measured changes in Chla, Chlb, TChl, and carotenoids in all treatment groups (A–D). The contents of Chla, Chlb, TChl, and carotenoids were significantly reduced (32.94%, 22.77%, 25.03%, 17.83%, respectively) in the UV-B stress group compared to the control group. The Chla, Chlb, and carotenoids (Car) were slightly increased in the TN treatment group compared to the control group, while total chlorophyll was significantly enhanced (17.02%) (D). In the case of the UV+TN treatment group, although the contents of these parameters were lower than in the control group, they were markedly enhanced compared to the UV treatment group. The contents of Chla, Chlb, TChl, and carotenoids were significantly higher (24.33%, 18.35%, 27.60%, and 16.49%, respectively) in the UV+TN group compared to the UV treatment group (A–D). In summary, these results indicate that UV-B stress negatively impacts plant pigments, while the application of TiO2 NPs, either alone or in combination with UV exposure, can have a positive influence on specific pigment levels.
Figure 2. The individual and combined effects of TiO2 NPs and UV-B irradiation on (A) chlorophyll a (Chla), (B) chlorophyll b (Chlb), (C) total chlorophyll (TChl), and (D) carotenoids (Car) in rice seedlings. Data presented are the means ± SE (n = 8), and significant differences between the means were calculated at p ≤ 0.05 using Duncan’s multiple range test.
3.3 Effect of UV-B and TiO2 NPs on photosynthetic parameters
In our study, we investigated the effects of UV and TiO2 nanoparticle treatments on various photosynthetic parameters over a five-day period. The parameters assessed included photosynthesis rate (Pn), stomatal conductance (Gs), maximum quantum yield of photosystem II (Fv/Fm), and non-photochemical quenching (NPQ) (A–D).
Figure 3. The individual and combined effects of TiO2 NPs and UV-B irradiation on key photosynthetic parameters including (A) net photosynthetic rate (Pn), (B) stomatal conductance (Gs), (C) maximum efficiency of photosystem II (Fv/Fm), and (D) non-photochemical quenching (NPQ) in rice plants. Data represented are the means ± SE (n = 8), and significant differences between the means were calculated at p ≤ 0.05 using Duncan’s multiple range test.
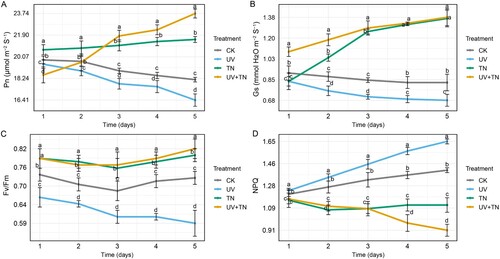
In response to UV-B stress, the Pn exhibited a declining trend with a maximum reduction of 9.52% on day 5 compared to the control (A). The Gs values were consistently lower in the UV group, showing a significant reduction ranging from approximately 7.69% to 18.07% compared to the control (B). The Fv/Fm indicated reduced photosystem II efficiency, with reductions spanning from approximately 9.46% to 19.18% during the five-day period (C). However, the NPQ values increased compared to the control, suggesting higher thermal dissipation of excess energy, with an increase percentage range of approximately 2.48% to 17.02% (D). These results highlight that UV treatment had a consistent negative impact on photosynthetic activity, stomatal conductance, photosystem II efficiency, and energy utilization compared to the control.
In response to TN treatment, the rate of Pn consistently increased, with a range of 4.34% to 18.72% higher values than the control. The Gs values showed a sharp increasing trend in the TN group compared to CK, with the highest percentage increase of 65.06% on day 5. Similarly, TN treatment have been shown to increase the maximum quantum yield of Photosystem II, with the most prominent rise of 9.59% observed on day 5 compared to CK. On the other hand, the NPQ values showed a decreasing trend with percentage reduction ranging from 3.41% to 20.57% (D). These findings indicate that TN treatment had subtle effects on energy dissipation, leading to better capture and utilization of light energy for photosynthesis compared to the control.
The UV+TN treatment exhibited a decrease in photosynthesis rate compared to the CK group for the first 2 days. However, by the third day, the UV+TN group displayed a significant increase in photosynthesis rate compared to CK, with a percentage difference of 15.70%. This positive trend continued, with a 20.91% increase on the fourth day and a substantial 30.78% increase on the fifth day (A). Stomatal conductance in the UV+TN group showed a steady increase compared to the CK group throughout the experiment. On the first day, there was a 19.78% increase, which continued to rise to 35.23% on the second day, 51.76% on the third day, 60.24% on the fourth day, and peaked at 66.27% on the fifth day (B). Similarly, Fv/Fm values in the UV+TN group increased gradually and become more prominent (12.33% increase) on day 5 (C). The NPQ values showed a consistent decrease during the five-day stress period with a reduction range of approximately 3.21% to 35.46% in the UV+TN group compared to CK (D). In conclusion, our study reveals distinct responses of photosynthetic parameters to UV and TiO2 nanoparticle (TN) treatments, with TN demonstrating a subtle enhancement in photosynthesis while UV-induced stress consistently led to negative impacts on these parameters. Interestingly, the combined UV+TN treatment displayed a remarkable recovery in photosynthesis and stomatal conductance over time, highlighting its potential for mitigating UV-induced stress in plants.
3.4 Effect of UV-B and TiO2 NPs on photosynthetic enzymes (Rubisco, and FBPase), soluble sugar, and soluble protein
Exposing plants to different treatments had distinct effects on photosynthetic enzyme activities, namely Rubisco and fructose-1,6-bisphosphatase (FBPase). In terms of Rubisco activity (measured in U min−1 mg−1 Protein), the UV treatment resulted in a decrease of 15.78% compared to the control, while the TN treatment led to a significant increase of 17.64% relative to CK. Interestingly, the combined UV and TN treatment (UV+TN) also resulted in a moderate increase of 8.57% compared to CK (A).
Figure 4. The individual and combined effects of TiO2 NPs and UV-B irradiation on important biochemical parameters including (A) Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), (B) fructose-1,6-bisphosphatase (FBPase), (C) soluble sugar, and (D) soluble protein in rice plants. The values represented are the means ± SE (n = 8), and significant differences between the means were calculated at p ≤ 0.05 using Duncan’s multiple range test.
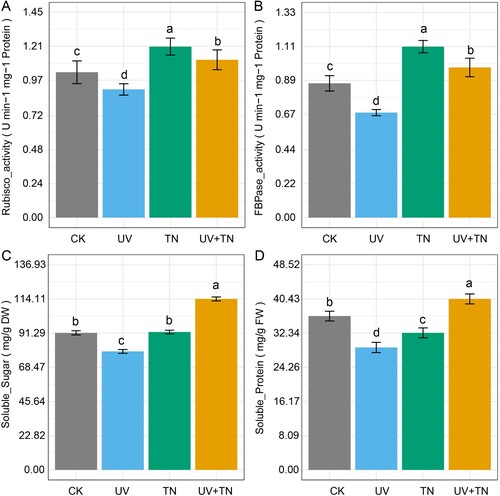
For FBPase activity (measured in U min−1 mg−1 Protein), the UV treatment showed a substantial reduction of 21.81% when compared to CK. In contrast, the TN treatment led to a notable increase of 27.32% compared to CK. When UV and TN were applied together (UV+TN), there was a modest increase of 11.82% relative to CK (B).
Regarding soluble sugar content (measured in mg/g DW), the UV treatment resulted in a decrease of 13.58% compared to CK. The TN treatment showed almost no change in soluble sugar content relative to CK. Remarkably, the combination of UV and TN (UV+TN) led to a substantial increase of 24.72% in soluble sugar content compared to CK (C).
Lastly, when examining soluble protein levels (measured in mg/g FW), the UV treatment exhibited a significant decrease of 20.41% compared to CK. The TN treatment also led to a decrease, though less pronounced, with a reduction of 10.90% relative to CK. Interestingly, the combination of UV and TN (UV+TN) resulted in a modest increase of 11.14% in soluble protein content compared to CK. These findings highlight the diverse effects of these treatments on plant biochemical responses (D).
3.5 Effect of UV-B and TiO2 NPs on ROS generation and antioxidant contents
Next, we investigated the impact of UV and TiO2 nanoparticle treatments on ROS generation over a 5-day period. UV treatment consistently led to higher H2O2 levels compared to the control group at all-time points (A). The average percentage increase in H2O2 levels ranged from approximately 13.6% on day 1 to a substantial 390.45% on day 5. Similarly, UV treatment resulted in higher O2•− levels compared to the control group at all-time points (B). The average percentage increase in O2•− levels ranged from around 13.93% on day 1 up to 93.98% on day 5. This indicates that UV-B radiation induces oxidative stress in plants, resulting in elevated ROS production over time.
Figure 5. The individual and combined effects of TiO2 NPs and UV-B irradiation on oxidative stress, and antioxidant system in rice seedlings. (A) The contents of hydrogen peroxide (H2O2), (B) superoxide anion (O2•−), (C) AsA, (D) GSH, (E) GSSG, and (F) GSH/GSSG ratio in rice plants. The values represented are the means ± SE (n = 8), and significant differences between the means were calculated at p ≤ 0.05 using Duncan’s multiple range test.
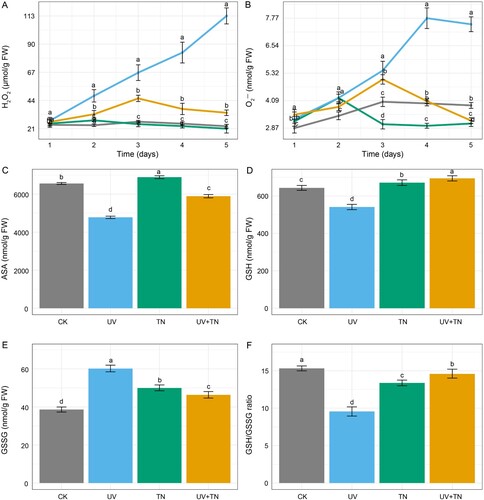
In response to the TN treatment, H2O2 levels did not changed significantly compared to the control group at all-time points. The maximum percentage increase of only 4.11% in H2O2 levels was observed on day 1, and later it showed a normal trend same as in the control (A). TN treatment resulted in higher O2•− levels compared to the control group on day 1 and day 2, with average percentage increases of 10.46% and 23.41%, respectively. However, on day 3, day 4, and day 5, O2•− levels continuously decreased compared to the control group (B). This potentially indicating an adaptive response of the plants to TN exposure.
The combined treatment of UV+TN had a significant impact on H2O2 levels compared to the control group. In general, the UV+TN treatment showed increased H2O2 levels at all-time points compared to the control. However, when comparing H2O2 levels in UV+TN treatment with those in UV, it showed a significant lower value during the five-day period (A). On the first day, the combination of UV+TN resulted in a slight decrease of approximately 3.61% in H2O2 levels compared to UV treatment alone. On subsequent days, the trend shifted dramatically. On day 2 and 3, there was a significant reduction of 31.25% and 32.06%, respectively. The most substantial difference was observed on day 4 and 5, with a remarkable reduction of 55.30% and 69.94%, respectively for H2O2 levels in the UV+TN compared to the UV group (A).
Similarly, the combined treatment of UV+TN had a significant impact on O2•− levels compared to the control group. In general, the UV+TN treatment showed increased O2•− levels for the first 3 days, later, it sharply decreased compared to the control (B). However, when comparing O2•− levels in UV+TN treatment with those in UV, it showed a notable reduction at all-time points. A substantial decrease in O2•− levels of approximately 47.54% and 56.91% on day 4 and 5, respectively, was noticed in UV+TN treatment compared to UV (B). Overall, this result suggests that the combined treatment may suppress ROS production over time.
The contents of ascorbate (AsA), glutathione (GSH), glutathione disulphide (GSSG), and the GSH/GSSG ratio, which are key components of the ascorbate-glutathione cycle and play an important role in redox homeostasis, were analyzed in both control and treatment groups (C–F). Compared to the control group, UV treatment resulted in a notable decrease of AsA levels by approximately 27.12% (C). Similarly, UV treatment led to a decrease in GSH levels by approximately 15.86% compared to the control (D). However, the GSSG pool increased by 57.68%, resulting in the lowest values for the GSH/GSSG ratio in the UV treatment (E–F). In contrast to UV, TN treatment had a positive effect on AsA, GSH, and GSSG levels but reduced the GSH/GSSG ratio compared to the control. With the combined UV and TN treatment (UV+TN), AsA levels decreased by approximately 10.15% compared to the control (C). Notably, GSH and GSSG levels increased by 7.93% and 23.74%, respectively, in the UV+TN group compared to the control, resulting in a decreased GSH/GSSG ratio in the UV+TN group (D–F). Notably, when comparing the combined treatment to UV treatment alone (UV+TN vs. UV), AsA and GSH levels increased significantly by 23.27% and 28.34%, respectively (C–D). Meanwhile, the GSSG pool decreased, resulting in the highest GSH/GSSG ratio observed in the UV+TN group compared to the UV treatment (E–F). These findings suggest that the combination of UV and TN may have a synergistic effect on the AsA-GSH cycle, potentially strengthening the plant's ability to counteract oxidative stress more effectively.
3.6 Overview and quality verification of transcriptome sequencing
The goal of this study was to gain a deeper understanding of the UV-B tolerance mechanism in TiO2 nanoparticles treated rice plants at the molecular level. In order to achieve this goal, RNA sequencing approach was employed to examine differential gene expression in rice seedlings subjected to various treatments, including control (CK), TiO2 nanoparticles treatment (TN), UV-B stress treatment (UV), and UV-B in conjunction with TiO2 nanoparticles treatment (UV+TN). In total, twelve cDNA libraries were created for high throughput RNA-sequencing where each treatment was replicated three times for biological accuracy. The cDNA libraries were sequenced on an Illumina HiseqTM 2500 platform. Twelve samples were filtered with RNA-seq data ranging from 44022218-58083754, and the percentages of Q20 bases and Q30 bases of all samples > 94% and 92%, respectively (). The clean reads of each sample were compared with the reference genome of the Oryza sativa v.70 reference genome (MSU Rice Genome Annotation Project Release 7), and the alignment efficiency > 92%, indicating that the transcriptome data obtained was of good quality (). The gene expression profiles of the biological replicates exhibited a strong correlation, as indicated by the Pearson's correlation coefficients (R2) exceeding 0.85 (A). The utilization of principal component analysis (PCA) enabled the confirmation of the clustering of two biological replicates of each treatment (B). The results not only confirmed the experimental design but also featured the high reliability and repeatability of the samples, and thus used for further downstream analysis.
Figure 6. The overview of transcriptome analysis in different comparison groups. (A) Sample to sample clustering analysis for checking batch effects and their similarity, (B) Principle component analysis (PCA) for gene expression pattern, (C) the number of up-regulated and down-regulated genes among the different comparison groups (UV vs. CK, TN vs. CK, UV+TN vs. CK) (D) Volcano plots for all the expressed genes in the three comparison groups, (E) Venn diagram of the identified up-regulated DEGs in three comparison groups describing unique and common DEGs in different treatments, (F) Venn diagram of the identified down-regulated DEGs in three comparison groups describing unique and common DEGs in different treatments.
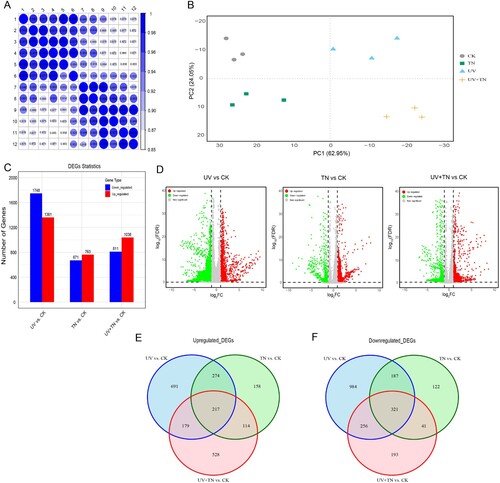
Table 1. The sequencing statistics of 12 RNA libraries in this study.
3.7 Identification of differentially expressed genes (DEGs) by RNA-Seq
To further reveal the difference in response to TiO2 NPs and UV-B stress, the comparative transcriptome analysis was performed using three pairwise comparisons as follows: UV-B stress and control (UV vs. CK), TiO2 NPs and control (TN vs. CK), and UV-B in combination with TiO2 NPs and control (UV+TN vs. CK). We used a false discovery rate (FDR) cutoff of 0.05 and an absolute fold change (Log2FC) threshold ≥ 2 for the assembled genes to determine which ones showed differential expression.
In the UV vs. CK group, a total of 3109 DEGs were identified (C–D), with 1361 being up-regulated and 1748 being down-regulated. In comparison, there were 671 down-regulated and 763 up-regulated DEGs in the TN vs. CK group, for a total of 1434 DEGs. A total of 1849 DEGs were identified in the UV+TN vs. CK comparison group, with 1038 genes showing elevated expression and 811 showing downregulated expression (C–D). The discrepancies in the frequency of upregulated and downregulated DEGs across treatments groups reflect unique regulatory mechanisms associated with each treatment.
Further, the transcripts that overlap either exclusively or commonly between the upregulated and downregulated DEGs in all treatments were plotted as a Venn diagram (E and F). The results revealed that a total of 217 upregulated DEGs were expressed in common across all comparison groups. Additionally, 691, 158, and 528 upregulated DEGs were uniquely expressed in the UV vs. CK, TN vs. CK, and UV+TN vs. CK comparisons, respectively (E). Among downregulated DEGs, a total of 321 DEGs were commonly detected in all comparison groups, while 984, 122, and 193 DEGs were exclusively expressed in the UV vs. CK, TN vs. CK, and UV+TN vs. CK comparison groups, respectively (F). The large number of unique DEGs were identified only in specific comparison groups, indicating a complex transcriptional response pattern that highlight the specific mechanisms and pathways influenced by individual treatments.
3.8 GO term enrichment analysis of DEGs
To better understand the functions associated with up- and downregulated DEGs, the gene ontology (GO) enrichment analysis was carried out mainly in three comparison groups: UV vs. CK (UV vs. CK_up, UV vs. CK_down), TN vs. CK (TN vs. CK_up, TN vs. CK_down), and UV+TN vs. CK (UV+TN vs. CK_up, UV+TN vs. CK_down). display enrichment results for the top 20 terms for each comparison group, generated using the GO enrichment analysis of DEGs. The numbers of genes and GO terms related to cellular components, biological processes, and molecular functions were different across the three groups ( and Supplementary Table S2). In the UV vs. CK_up comparison, the DEGs were enriched in six biological processes (188 DEGs), eight cellular components (134 DEGs), and six molecular functions (216 DEGs). In the UV vs. CK_down comparison group, the DEGs were enriched in eight biological processes (281 DEGs), six cellular components (176 DEGs), and six molecular functions (137 DEGs) (A–B and Supplementary Table S1). In the TN vs. CK_up, the DEGs were enriched in seven biological processes (148 DEGs), seven cellular components (114 DEGs), and six molecular functions (196 DEGs). In the TN vs. CK_down, the DEGs were enriched in nine biological processes (152 DEGs), six cellular components (96 DEGs), and five molecular functions (177 DEGs) (C–D and Supplementary Table S1).
Figure 7. Gene Ontology (GO) enrichment analysis of up- and down-regulated DEGs involved in biological processes, cellular components, and molecular functions in different comparison groups. (A-B) top 20 GO enrichment terms of upregulated and downregulated DEGs in the UV vs. CK comparison group, (C-D) top 20 GO enrichment terms of upregulated and downregulated DEGs in the TN vs. CK comparison group, (E-F) top 20 GO enrichment terms of upregulated and downregulated DEGs in the UV+TN vs. CK comparison group.
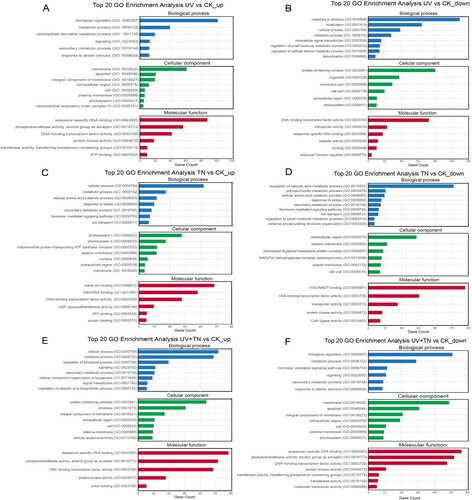
In the UV+TN vs. CK_up comparison group, the DEGs were enriched in eight biological processes (161 DEGs), seven cellular components (129 DEGs), and five molecular functions (180 DEGs). In the UTN vs. CK_down, the DEGs were enriched in six biological processes (117 DEGs), seven cellular components (146 DEGs), and seven molecular functions (181 DEGs) (E–F and Supplementary Table S1). Notably, under the molecular functions’ domain, several of the DEGs in UV vs. CK group were enriched in phosphotransferase activity, alcohol group as acceptor, DNA-binding transcription factor activity, sequence-specific DNA binding, protein kinase activity, transporter activity, ATP binding, catalytic activity, and molecular function regulator. In TN vs. CK comparison, DEGs under molecular functions category were enriched in metal ion binding, NAD/NADP binding, protein binding, UDP glucosyltransferases activity, DNA/RNA binding, transporter activity, ATP binding, DNA-binding transcription factor activity, CoA ligase activity, and protein kinase activity. For molecular functions domain in UV+TN vs. CK comparison, several DEGs were enriched in sequence-specific DNA binding, DNA-binding transcription factor activity, phosphotransferase activity, alcohol group as acceptor, protein kinase activity, transferase activity, anion binding, and molecular transducer activity (A–F and Supplementary Table S1).
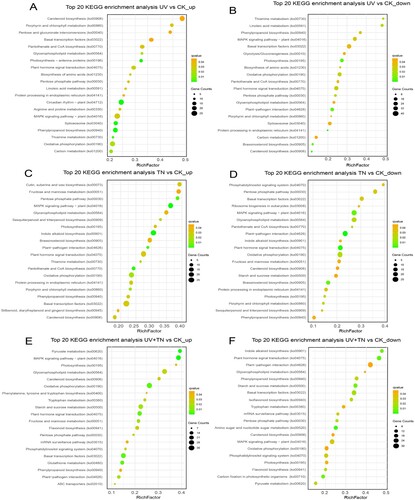
3.9 KEGG pathway enrichment analysis of DEGs
To gain a better understanding of metabolic processes, we performed the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis of the identified DEGs. To assess whether there are significant differences in the pathways of differentially expressed genes (DEGs), we conducted enrichment analyses specifically focusing on the top 20 KEGG pathways with the most statistically significant q-values (A–F). In the UV vs. CK_up, the top most significantly enriched pathways include circadian rhythm–plant (ko04712), phenylpropanoid biosynthesis (ko00940), spliceosome (ko03040), photosynthetic-antenna proteins (ko00196), and thiamine metabolism (ko00730). In the UV vs. CK_down comparison group, the top most significantly enriched pathways include photosynthesis (ko00195), carotenoid biosynthesis (ko00906), plant-pathogen interaction (ko04626), protein processing in endoplasmic reticulum (ko04141), and brassinosteroid biosynthesis (ko00905)(A–B and Supplementary Table S2). In the TN vs. CK_up comparison group, DEGs were significantly enriched in pathways including pantothenate and CoA biosynthesis (ko00770), pentose phosphate pathway (ko00030), plant-pathogen interaction (ko04626), MAPK signaling pathway–plant (ko04016), and indole alkaloid biosynthesis (ko00901). Similarly, in the TN vs. CK_down group, most of the DEGs were primarily enriched in plant-pathogen interaction (ko04626), MAPK signaling pathway–plant (ko04016), brassinosteroid biosynthesis (ko00905), and plant hormone signal transduction (ko04075)(C–D and Supplementary Table S3). In the UV+TN vs. CK_up group the top most significantly enriched pathways involved in pyruvate metabolism (ko00620), MAPK signaling pathway–plant (ko04016), oxidative phosphorylation (ko00190), plant hormone signal transduction (ko04075), basal transcription factors (ko03022), and glutathione metabolism (ko00480) (E and Supplementary Table S2). In the UV+TN vs. CK_down comparison group, the top most significantly enriched pathways include carbon fixation in photosynthetic organisms (ko00710), indole alkaloid biosynthesis (ko00901), plant hormone signal transduction (ko04075), amino sugar and nucleotide sugar metabolism (ko00520), and starch and sucrose metabolism (ko00500) (F and Supplementary Table S2). These findings suggest that the enrichment pathways with the highest significance varied significantly across the three comparison groups, and these pathways likely to have a crucial impact on UV-B tolerance in rice plants.
Figure 8. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways annotation and top 20 KEGG enrichment terms that reflects the involvement of number of DEGs (up- and down-regulated) in orchestrating various functions in rice. (A-B) top 20 KEGG enrichment terms of upregulated and downregulated DEGs in the UV vs. CK comparison group, (C-D) top 20 KEGG enrichment terms of upregulated and downregulated DEGs in the TN vs. CK comparison group, (E-F) top 20 KEGG enrichment terms of upregulated and downregulated DEGs in the UV+TN vs. CK comparison group.
3.10 Regulation of DEGs related to plant transcription factors
Transcription factors (TFs) are a diverse group of proteins that play a crucial role in regulating the expression of several other genes during perception and signaling of UV-B stress. Based on the transcriptomic analysis, we identify a total of 56 commonly expressed transcription factors belonging to MYB (myeloblastosis), bHLH (basic helix–loop–helix), DOF (Dof-type domain-containing TF), EREBP (ethylene response element-binding protein), HSF (heat shock transcription factor), bZIP (basic leucine zipper), NAC (NAC domain containing TF), and WRKY (WRKY domain containing TF) in the main three comparison groups (A and Supplementary Table S3).
Figure 9. Heatmap visualization of DEGs in the three comparison groups (UV vs. CK, TN vs. CK, UV+TN vs. CK) associated with (A) transcription factors, (B) oxidative stress and antioxidant system, (C) cell wall biosynthesis, (D) hormone regulation and (E) MAPK signaling. A decrease and an increase in transcript abundance relative to control samples were shown in red and blue signals, respectively.
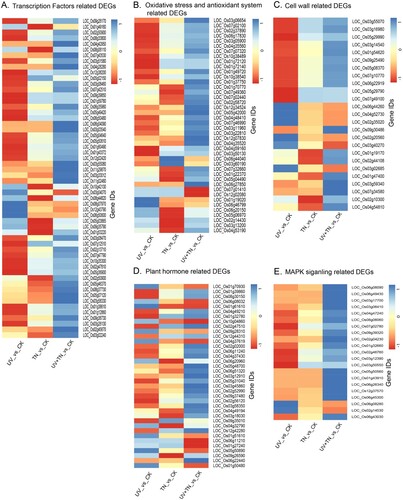
Interestingly, most of the TFs were characterized to be downregulated in response to UV-B stress (UV vs. CK), whereas these TFs were significantly upregulated in UV-B stressed plants supplied with TiO2 NPs (UV+TN vs. CK). Out of 11 MYB TFs, 8 were found to be downregulated in UV vs. CK, however, 5 NAC TFs (LOC_Os10g42130, LOC_Os03g04070, LOC_Os08g44820, LOC_Os06g04090, LOC_Os03g03540), and 3 HSF TFs (LOC_Os06g35960, LOC_Os09g35790, LOC_Os01g53220) were upregulated in the same treatment (A and Supplementary Table S3). Similarly, 7 bHLH TFs (out of 9), 5 Dof (out of 5), 2 bZIP (out of 6), 8 EREBP (out of 8), 9 WRKY (out of 9) were all downregulated in UV vs. CK treatment. In TN vs. CK treatment, out of 56 commonly expressed TFs, 24 TFs were downregulated while 32 TFs were upregulated. In case of UV+TN vs. CK treatment, out of 56 commonly expressed TFs, only 7 were downregulated (3 bZIP, 2 Dof, 1 NAC, and 1 MYB), while 49 were all upregulated in the same treatment (A and Supplementary Table S3). Further, we also found that 10, 4, and 5 TFs were uniquely upregulated in UV vs. CK, TN vs. CK, and UV+TN vs. CK groups, respectively (Supplementary Table S3). There were four TFs (LOC_Os04g51320, LOC_Os01g13000, LOC_Os10g41330, LOC_Os01g17000), which were specifically downregulated in UV vs. CK but upregulated in UV+TN vs. CK treatment. One WRKY TF (LOC_Os02g36595) showed downregulated in TN vs. CK, but it displayed notably elevated expression in UV+TN vs. CK treatment (Supplementary Table S3). These results clearly indicate a higher proportion of different members of the corresponding TF family in the UV+TN treatment in rice, thus revealing a distinct regulatory mechanism for coping with UV-B stress compared to the other comparison groups.
3.11 Regulation of DEGs related to oxidative stress and antioxidant defense system
The activation of both enzymatic and non-enzymatic antioxidants is a significant mechanism that plants employ to enhance their tolerance to UV-B stress. According to the above enrichment results of DEGs for GO terms and KEGG pathways, we further analyzed the response of different oxidative stress and antioxidant defense related DEGs in three comparison groups at the transcriptional level. A total of 42 common DEGs which belong to oxidases, glutathione-s-transferases (GSTs), peroxidases, superoxide dismutase, and ascorbate peroxidase were respectively predicted in up- and downregulated DEGs in three comparison groups (B and Supplementary Table S4). Among these DEGs, several oxidases such as, indole-3-pyruvate monooxygenase (LOC_Os03g06654), flavin-containing monooxygenase (LOC_Os07g02100), and oxoglutarate-dependent dioxygenase (LOC_Os02g37890) were significantly downregulated in UV vs. CK comparison group, while markedly upregulated in UV-B stressed plants supplied with TiO2 NPs (UV+TN vs. CK treatment group) (B and Supplementary Table S4). Similarly, we noticed that out of 10 DEGs belonging to glutathione-s-transferases, 9 were downregulated in UV vs. CK comparison group, while all of these DEGs were significantly upregulated in UV+TN vs. CK treatment group. For instance, glutathione-s-transferase related genes which were significantly downregulated with lowest expression (LOC_Os04g35560, LOC_Os07g07320, LOC_Os10g38489) in UV vs. CK, while markedly upregulated in UV+TN vs. CK treatment group (Supplementary Table S4). In TN vs. CK comparison group, all of the glutathione-s-transferase related genes were slightly upregulated except for glutathione s-transferase 3 (LOC_Os01g70770).
Among 20 DEGs related to peroxidases, several of them were upregulated, however, some of them were highly expressed (LOC_Os12g34524, LOC_Os02g58720, and LOC_Os05g42000) in the UV+TN vs. CK treatment group as compared to UV vs. CK group (B and Supplementary Table S4). Interestingly, 4 superoxide dismutase related genes (LOC_Os04g48410, LOC_Os07g46990, LOC_Os03g11960, LOC_Os03g22810), and 2 ascorbate peroxidase genes (LOC_Os12g07830, LOC_Os04g35520) were all downregulated in UV vs. CK comparison group, while these genes were significantly upregulated in UV+TN vs. CK treatment group (B and Supplementary Table S4). Additionally, there were four uniquely upregulated genes (LOC_Os04g29210, LOC_Os07g02100, LOC_Os04g59190, LOC_Os02g14440) and one uniquely downregulated gene (LOC_Os03g22010) found in the UV vs. CK group (Supplementary Table S4). Further, three genes belonging to oxidases (LOC_Os06g23114, LOC_Os06g01490, LOC_Os10g36390), and three GST-related genes (LOC_Os01g72130, LOC_Os03g57200, LOC_Os01g49710) were found to be upregulated both in TN vs. CK and UV+TN vs. CK treatment groups (Supplementary Table S4). Furthermore, there were eight antioxidant related genes which showed opposite expression pattern in the same treatment groups (downregulated in TN vs. CK, and upregulated in UV+TN vs. CK treatment) (Supplementary Table S4). These results indicate a vital role of antioxidants in maintaining cellular redox homeostasis by eliminating reactive oxygen species, thus primarily preventing oxidative stress in the UV+TN treatment.
3.12 Regulation of DEGs related to cell wall metabolism
In response to stress, specifically UV irradiation, alterations in cell wall structure and composition can serve a protective role by providing mechanical barriers and producing associated protective substances. These mechanisms can help attenuate radiation, ultimately safeguarding plant tissues and organs from the harmful effects of UV-B radiation. In our study, a total of 25 common and 34 unique DEGs have been identified which belong to cell wall modification and metabolism, such as UDP-glucuronic acid decarboxylase, cellulose synthase, xyloglucan endotransglucosylase/hydrolase, mannan endo-1,4-beta-mannosidase, UDP-glucose 6-dehydrogenase, pectinesterase, expansin, xyloglucan, exopolygalacturonase, and fasciclin-like arabinogalactan (C and Supplementary Table S5). Out of 25 common genes, 11 and 10 genes were downregulated in UV vs. CK and TN vs. CK treatments, respectively (Supplementary Table S5). In case of the UV+TN vs. CK treatment group, only 6 genes were downregulated while 19 common genes were significantly upregulated (Supplementary Table S5). Seven genes were uniquely upregulated in UV vs. CK comparison, while 11 genes were specifically downregulated in TN vs. CK group. There were four genes (LOC_Os09g32670, LOC_Os02g54890, LOC_Os07g24190, LOC_Os07g36690) which were upregulated both in TN vs. CK and UV+TN vs CK treatments (Supplementary Table S5). However, there were 12 genes that showed opposite expression pattern in the same treatment groups (downregulated in TN vs. CK, and upregulated in UV+TN vs. CK treatment) (Supplementary Table S5). These results indicate that the cell wall metabolism was more active in UV+TN treatment and might be one of the key processes of TiO2 NPs-mediated UV-B tolerance.
3.13 Regulation of DEGs related to plant hormone signal transduction pathway
In response to UV-B, regulation of hormones signaling is ubiquitous in the modulation of plant architecture and metabolism, and most often, these phytohormones act synergistically to mitigate the effects of an abiotic stress. As found in the present study, the total of 37 commonly annotated DEGs were involved in the regulation of various hormones which are responsible for signaling mechanisms in rice under UV-B stress and application of TiO2 NPs (D, Supplementary Table S6). Out of 37 common DEGs related to plant hormones, 18 were downregulated and 19 were upregulated in response to UV-B compared to the control. In the TN vs. CK group, 17 genes were downregulated while 20 were upregulated. In case of the UV+TN vs. CK group, 10 were downregulated while 27 genes were upregulated (Supplementary Table S6). In particular, 4 ethylene (ETH), 3 jasmonate (JA), 1 salicylic acid (SA), 2 gibberellic acid (GA), 3 abscisic acid (ABA), 1 benzoic acid (BA), and 4 auxin (IAA) related genes were characterized to be downregulated in UV vs. CK group. While 6 ETH, 4 ABA, 3 SA, 2 GA, and 2 IAA related genes were downregulated in the TN vs. CK treatment group. However, 3 ETH, 4 ABA, 2 JA, and 1 IAA were particularly downregulated in the UV+TN vs. CK treatment group (D, Supplementary Table S6). Notably, some upregulated genes in the UV+TN vs. CK treatment group, such as 3 ethylene-related genes (LOC_Os01g39860, LOC_Os08g30150, LOC_Os04g49210), 2 jasmonate-related genes (LOC_Os02g02000, LOC_Os04g37430), 2 ABA-related genes (LOC_Os02g47510, LOC_Os12g42280), and 2 IAA-related genes (LOC_Os02g52990, LOC_Os03g58350), showed markedly higher expression levels, while significantly suppressed in the UV vs. CK group (D, Supplementary Table S6).
Further, there were 9 uniquely upregulated genes in UV vs. CK treatment. Five phytohormones genes showed upregulation in TN vs. CK, while downregulated in UV+TN vs. CK treatment. Similarly, we found eight phytohormone genes which show opposite expression pattern (downregulated in TN, and upregulated in UV+TN vs. control) (Supplementary Table S6). These results suggest that phytohormone signal transduction contributes significantly in regulating downstream pathways for UV-B stress tolerance in rice, particularly in the UV+TN treatment.
3.14 Regulation of DEGs related to MAPK signaling pathway
In the present research, the bioinformatics analysis revealed that the MAPK signaling pathway was one of the KEGG pathways that had been significantly enriched to the greatest extent. The components of the MAPK pathway comprise a particular set of enzymes that mediate the plant's response to various stress factors, including exposure to high UV-B radiation. The results revealed that 21 DEGs were commonly regulated in all the three comparison groups (E, Supplementary Table S7). Moreover, the higher proportion of DEGs were found upregulated in UV+TN vs. CK (19) followed by TN vs. CK (16) treatment, however, most of the DEGs (12) were downregulated in UV vs. CK, which indicated that kinases responsive genes might play a crucial role to activate the signaling mechanisms in rice by TiO2 NPs under UV-B stress. Another distinction observed was the significantly greater upregulation of MAPK genes in the UV+TN vs. CK comparison, in contrast to their expression levels in the TN vs. CK comparison (Supplementary Table S7). There were five MAPK genes (LOC_Os01g01740, LOC_Os05g44290, LOC_Os01g54480, LOC_Os07g39520), which were specifically downregulated in UV vs. CK treatment. Two MAPK genes, LOC_Os06g43840 and LOC_Os01g50420, were exclusively upregulated, while another two, LOC_Os05g01780 and LOC_Os01g43910, were exclusively suppressed in the TN vs. CK comparison. Five MAPK genes (LOC_Os05g49140, LOC_Os01g47530, LOC_Os10g29540, LOC_Os02g32610) were uniquely upregulated in UV+TN vs. CK treatment (Supplementary Table S7). While two MAPK genes (LOC_Os02g54510, LOC_Os12g41260) showed opposite expression pattern (downregulated in TN, and upregulated in UV+TN vs. control) in the same treatments (Supplementary Table S7). These results clearly indicate that a higher proportion of MAPK genes were involved in the UV+TN treatment group, leading to various extracellular or intracellular responses via targeting downstream signaling targets, such as gene expression changes and stress adaptation.
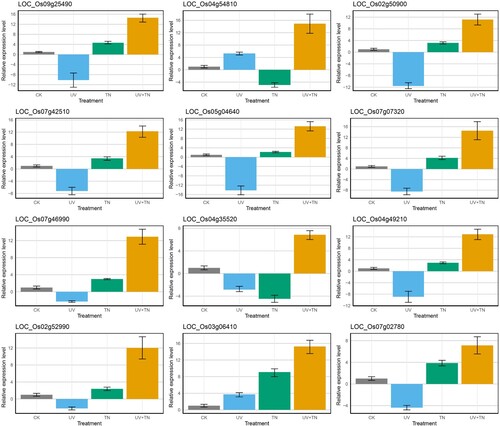
3.15 Validation of selected DEGs by qRT-PCR
To validate the accuracy of our RNA-seq results, we have selected 12 genes based on the variations in expression patterns among the treatment groups. Among them, three genes from transcription factors (LOC_Os02g50900, LOC_Os07g42510, LOC_Os05g04640), three antioxidants-related (LOC_Os07g07320, LOC_Os07g46990, LOC_Os04g35520), two cell wall (LOC_Os09g25490, LOC_Os04g54810), 2 plant hormones (LOC_Os04g49210, LOC_Os02g52990), and 2 MAPK signaling related genes (LOC_Os03g06410, LOC_Os07g02780) were selected across all experimental groups. The qRT-PCR data for these 12 genes showed a similar expression pattern as observed in the RNA-seq data, albeit at different levels ( and Supplementary Table S8). In general, qRT-PCR analysis confirmed the results of RNA-seq data. The identified genes represent promising candidates for further in vivo exploration within rice plants to elucidate their molecular functions, particularly in the context of detoxification mechanisms.
4. Discussion
In recent decades, changes in climate patterns and shifts in stratospheric ozone dynamics have led to modifications in the levels of ultraviolet-B radiation (UV-B) that reach the Earth's surface. UV-B radiation, which falls within the narrow yet biologically potent range (280–320 nm) on the electromagnetic spectrum, possesses the capability to influence plant growth and developmental processes (Yadav et al. Citation2020). Mild exposures to UV-B radiation are recognized for inducing beneficial effects termed as ‘eustress’ in plants, but when plants experience excessive UV-B radiation, it leads to a state of ‘distress.’ This elevated irradiance can trigger various physiological, biochemical, and molecular aberrations in plants (Chen et al. Citation2022; Liaqat et al. Citation2023). Rice, a crucial staple crop, is cultivated globally due to its immense demand and economic significance. It's worth noting that rice plants exhibit a high level of sensitivity to UV-B stress, which can negatively affect several morphological, physiological, and biochemical aspects (Mmbando et al. Citation2020; Shahzad et al. Citation2021a). Consequently, there is a pressing need for increased efforts in discovering methods to enhance rice resistance and sustain its productivity at high levels. Recently, the efficient utilization of natural resources, with a focus on minimizing adverse impacts on the environment, has spurred the adoption of innovative technologies like nanotechnology. Nanotechnology employs nanoparticles (NPs) ranging from 1 to 100 nm, featuring remarkable attributes, including a substantial surface-to-volume ratio and diverse physicochemical and biochemical properties, shaped by their elevated surface energy (Altammar Citation2023). The distinctive characteristics of NPs hold promise for enhancing specific plant traits, particularly in the presence of various abiotic stress factors. In this context, several studies have reported the positive impact of different NPs on plant growth, physiology and biochemical attributes against different abiotic stresses (Song et al. Citation2021; Wang et al. Citation2021b; Bisht et al. Citation2022). Nevertheless, understanding the precise mechanisms by which NPs enhance stress tolerance in various plant species necessitates further in-depth research and use of reliable technologies. RNA-sequencing has drawn attention in plant research due to its ability to provide genome-wide differential RNA expression data (transcriptomics) across different environmental stress conditions (Yang et al. Citation2021). Plants response to stress involves a highly complex process, and transcriptomics can be utilized to quantitatively analyze changes in plant gene expression under specific stress conditions, thereby revealing the intricate regulatory network and alterations in underlying metabolic pathways (Wei et al. Citation2020).
Recent studies demonstrate the positive impact of titanium dioxide (TiO2) nanoparticles in improving several aspects of plant growth, physiological and biochemical aspects against different abiotic stresses such as drought (Karvar et al. Citation2022), salinity (Gohari et al. Citation2020), temperature fluctuations (Amini et al. Citation2017), and heavy metals (Cai et al. Citation2017; Kumar et al. Citation2023; Ghouri et al. Citation2023). Notably, some studies have shown that TiO2 NPs can mitigate the negative effects of UV-B exposure. However, these investigations primarily focused on short-term UV-B treatments, ranging from a few minutes (Lei et al. Citation2008) to a maximum of one day (Wang et al. Citation2021b). To date no studies have demonstrated the potential impact of TiO2 NPs on rice plants, a globally important crop, when subjected to long-term UV-B treatment. In this study, we present a comprehensive analysis of the transcriptional, physiological, and biochemical responses of rice seedlings subjected to long-term UV-B stress and treated with TiO2 NPs. This analysis reveals the involvement of multiple components, including photosynthesis, cellular and regulatory processes, ROS scavenging, stress signaling, and metabolic pathways.
Exposure to high-intensity UV-B radiation has been demonstrated to induce stress in rice plants, resulting in detrimental effects on growth and development at both the seedling and reproductive stages (Yu et al. Citation2013; Shahzad et al. Citation2021a). Similarly, current study also reveal the significant reduction of shoot and root biomass in response to UV-B stress (). Interestingly, TiO2-NP treatment mitigated the negative effects of UV-B stress on rice growth and enhanced plant shoot and root biomass. In general, the increase in plant biomass is the result of improvement in photosynthetic performance (Chen et al. Citation2018).
Photodamage in plants during photosynthesis occurs when the balance between light absorption and utilization is disrupted, leading to the generation of ROS and subsequent damage to photosynthetic components (Zavafer and Mancilla Citation2021). Research investigating the direct impacts of UV-B radiation on the photosynthetic machinery has revealed several detrimental effects. These include the deactivation of photosystem II (PSII), a decline in levels of photosynthetic pigments, disruption of thylakoid and chloroplast structure, diminished Rubisco activity, and the downregulation of transcription of genes associated with photosynthesis (Piccini et al. Citation2020).
In this study, pigment contents were significantly decreased in rice plants subjected to UV-B stress. However, treatment with TiO2 NPs resulted in an enhancement of pigment contents, including chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids, in stressed plants (see ). Furthermore, gas exchange and chlorophyll fluorescence attributes, sensitive indicators of photosynthesis, exhibited a prominent difference with and without TiO2-NP treatments under stress. For instance, the net photosynthetic rate (Pn), stomatal conductance (Gs), and maximum quantum yield of PSII (Fv/Fm) were significantly lower in UV-stressed plants (). Conversely, these parameters exhibited their maximum increase in TiO2-NP treatments, both in the presence (UV+TN) and absence of UV stress (TN). Additionally, a higher NPQ value following UV-B stress, and lower values in TN and UV+TN conditions, indicate the photoprotective effects provided by TiO2-NP treatments in rice plants (). In previous studies, the application of TiO2 NPs has been shown to intricately modulate plant physiology, enhancing photosynthetic attributes in different plant species, such as Dracocephalum moldavica L., a perennial herb (Gohari et al. Citation2020), and wheat plants (Mustafa et al. Citation2021), when subjected to abiotic stress. Under severe stress conditions, the decrease in Fv/Fm indicates that the PSII reaction center has been damaged, which directly disrupts the functionality of the photosynthetic electron transport chain (Stefanov et al. Citation2022). Under stress condition, as Fv/Fm decreases, it is often accompanied by an increase in NPQ (Moustakas et al. Citation2022). The increased NPQ mechanism can reduce the energy transfer to photosynthetic reaction centers, and thus decrease the overall photosynthetic efficiency in plants under stress (Moustakas et al. Citation2022). The enhancement of Fv/Fm and decline of NPQ in TN and UV+TN treatments mainly indicated improvements related to PSII efficiency in rice plants. The observed higher Pn rate in the UV+TN treatment compared to the TN treatment, along with corresponding Fv/Fm values, provides evidence for the effectiveness of TiO2 nanoparticles in preserving photosynthetic efficiency, particularly under UV-B stress conditions. Previous research findings suggest that TiO2 NPs have been shown to boost photosystem II activity in spinach by stimulating oxygen production and facilitating the transfer of energy under UV-B radiation (Lei et al. Citation2008). In this context, a recent study has also established that priming plants with TiO2 nanoparticles was effective in reducing cadmium toxicity and improving photosynthetic efficiency by enhancing Fv/Fm and decreasing NPQ in lettuce plants (Bano et al. Citation2024). Based on the aforementioned evidence, our results confirm that the supplementation of TiO2 nanoparticles can enhance photosynthetic efficiency by optimizing Fv/Fm and minimizing NPQ under UV-B stress. This suggests a potential regulation of the electron transport chain by improving ATP and NADPH production (Gu Citation2023), which could potentially enhance net photosynthesis, particularly under UV-B stress conditions.
The elevated UV-B irradiation induces oxidative stress caused by the accumulation of a variety of intracellular ROS molecules and/or their derivatives, leading to impaired redox homeostasis in plants (Xie et al. Citation2023). Previous reports have also shown that oxidative stress induced by various abiotic factors significantly reduces the activity of Rubisco and FBPase (Altaf et al. Citation2022; Gao et al. Citation2023), which play pivotal roles in the Calvin cycle and are considered the most significant photosynthetic enzymes. In light of this evidence, our results also demonstrated a significant reduction in Rubisco and FBPase activities in plants exposed to UV-B stress (). Conversely, the application of TiO2-NP treatments enhanced the activities of these crucial enzymes, thereby greatly improving the photosynthetic efficiency of rice plants during stress. A recent study highlights the mechanism of enhanced photosynthetic performance by different metal oxide nanoparticles including TiO2 by enhancing the chlorophyll content, PSII function, and increasing the activity of key photosynthetic enzymes (Ghorbanpour et al. Citation2021).
To maintain the intracellular osmotic balance, plants usually accumulate osmoregulatory substances such soluble sugars and soluble proteins for regulating osmotic balance (Wu et al. Citation2022). Moreover, the accumulation of soluble sugars and soluble proteins represents a crucial mechanism for suppressing oxidative stress in plants (Rady et al. Citation2023). In the current study, we observed a significant reduction in contents of soluble sugar and soluble protein during UV-B stress. In contrast, the UV+TN treatment markedly enhanced the levels of soluble sugar and soluble protein (), demonstrating the plant's capacity to detoxify ROS and maintain cellular homeostasis. Similarly, a recent study also demonstrated the accumulation of soluble sugars and soluble proteins after the application of TiO2 NPs to mitigate cellular damage induced by deficit irrigation regimes (Karvar et al. Citation2022).
The ROS consist primarily of free radicals and non-radicals, including hydrogen peroxide (H2O2) and superoxide (O2•−). These entities are highly destructive and can inflict significant damage to proteins, DNA, and lipids, consequently disrupting regular cellular processes in plants (Dvořák et al. Citation2021). The damage to macromolecules caused by ROS is typically reduced through the regulation of the AsA-GSH cycle in different plant species including rice (Singh et al. Citation2020). The two important non-enzymatic antioxidants, such as ascorbate (AsA) and glutathione (GSH) are major players of this cycle and determine a plant's ability to withstand oxidative stress (Huang et al. Citation2023). Additionally, in the context of stress signaling and the regulation of redox potential, the higher GSH/GSSG ratio plays a significant and multifaceted role. When GSH interacts with reactive oxygen species and/or their chemical derivatives, it is converted to GSSG, leading to a reduction in the GSH/GSSG ratio, which, in turn, induces oxidative stress (Jiang et al. Citation2022). In the current study, both H2O2 and O2•− were accumulated in large quantities in rice seedlings during UV-B stress, while TiO2-NP treatments significantly reduced these ROS (). Further, AsA and GSH were declined in response to UV-B stress but greatly improved as a result of TiO2 NP treatments, both in the presence (UV+TN) and absence of UV stress (TN). The GSSG pool increased in all treatments, resulting in the lowest GSH/GSSG ratio in the UV group, while it was restored to higher values in the TN and UV+TN treatments (). Consistent with these results, a prior study indicated that the use of TiO2 NPs substantially improved levels of AsA, GSH, AsA/DHA, and the GSH/GSSG ratio in sunflower plants, thereby activating the antioxidant defense system to potentially mitigate chromium toxicity (Kumar et al. Citation2023).
Under a given stress condition, the downstream secondary effects of nanoparticles are intricate, and there is a growing focus among researchers on obtaining a comprehensive molecular understanding of how different plant species respond to the interplay of stress factors and nanoparticles through whole transcriptome analysis using RNA-Seq approach (Khan et al. Citation2023; Santin et al. Citation2023). In order to better understand the mechanism of TiO2 NPs in conferring UV-B tolerance, we used RNA-Seq technology to reveal the differential gene expression and associated metabolic pathways in relation to their individual and combined effects in rice plants. To the best of our knowledge, this study is the first to explore the transcriptional changes exhibited by rice plants in response to both long-term UV-B exposure and combined treatment with TiO2 NPs. In the current study, our transcriptome data revealed that UV-B stress upregulated and downregulated a large number of differentially expressed genes (DEGs) compared to the control (), indicating a complex and dynamic regulatory response of the rice plant's transcriptome to UV-B stress, potentially involving a wide range of molecular pathways and biological processes (Sun et al. Citation2019; Kong et al. Citation2021). In contrast, a smaller number of DEGs have been observed to be upregulated and downregulated by the application of TiO2 NPs individually (TN) or in combination with UV-B (UV+TN) compared to the control (), indicating a more targeted and selective influence of TiO2 NPs on the rice plant transcriptome under these conditions. This suggests that TiO2 NPs may exert a more specific regulatory impact on certain molecular pathways and genetic responses when compared to the broader and more extensive changes induced by UV-B stress alone. Previous evidence also suggests specific targeted regulatory responses mediated by nanoparticles in different plant species such as Arabidopsis (García-Sánchez et al. Citation2015), wheat (Zhang et al. Citation2018), soybean (Mirakhorli et al. Citation2021), and pearl millet (Khan et al. Citation2023). In this context, our transcriptome analysis revealed a significant number of differentially expressed genes involved in cellular and metabolic processes, the response to stress stimuli, hormone-mediated signal transduction, transcription factor activity, as well as osmoregulation and antioxidant mechanisms ( and ).
Plant transcription factors (TFs) play a pivotal role in governing gene expression, exerting their influence over essential aspects of plant functionality. This includes orchestrating responses to environmental cues and hormonal signals, as well as directing the processes of cell differentiation and organ development (Berry and Argueso Citation2022). Numerous TF families, such as WRKY, MYB, NAC, DOF, bHLH, AP2/ERF, and bZIP, have been linked to UV-B stress responses (Qian et al. Citation2020; Apoorva et al. Citation2021). Furthermore, a wide range of transcription factor genes have been found to be associated with increased stress tolerance in both model organisms and economically significant crop species (Tolosa and Zhang Citation2020; Ewas et al. Citation2022; Saidi et al. Citation2023). In agreement to previous evidence, in this study we have identified several important genes belonging to TF families such as MYB, bHLH, DOF, EREBP, HSF, bZIP, NAC, and WRKYs (). Out of these, some TFs have shown a unique upregulation or downregulation pattern with respect to specific treatments. Further, 56 transcription factors were commonly regulated with varying expression levels across the three main comparison groups (UV vs. CK, TN vs. CK, and UV+TN vs. CK), which strongly suggest their potential association with stress tolerance (). Among them, the MYB, bHLH, and WRKY families were the primary transcription factors that exhibited significant upregulation in response to TiO2 NPs and their combined effect with UV-B stress (UV+TN) when compared to the control. The MYB TF family is a highly diverse group of proteins that can be found in all eukaryotic organisms, serving various functions. The functions of MYB genes, particularly those belonging to the R2R3-MYB class, play a pivotal role in the orchestration of an organism's responses to environmental stresses (Katiyar et al. Citation2012). For example, a recent study shows that MYB73/MYB77 interacts with UV-B photoreceptor to regulate auxin signaling and plant growth (Yang et al. Citation2020). MYB23 was responsive to UV-B and played a pivotal role in initiating trichomes, functioning as both an insect defense mechanism and a protective shield for shaded tissues against UV-B radiation (Khosla et al. Citation2014). In a recent study, researchers have explored the role of MYB pathways in regulating anthocyanin biosynthesis to confer UV-B tolerance in blueberries (Song et al. Citation2023). The bHLH TF family is the second-largest transcription factor superfamily in plants and plays a crucial role in plant growth, tissue development, and environmental adaptation (Guo et al. Citation2021). In a previous report, transcriptomics study revealed a total of 30 differentially expressed bHLH genes, which were involved to function alone or making a complex (MYB–bHLH–WD40) to enhance defense against UV-B stress by regulating phenylpropanoid pathways (Liang et al. Citation2023). Similarly, another intriguing study conducted in Ginkgo biloba using a transcriptomics approach revealed that several members of WRKY and MYB transcription factors were involved in regulating secondary metabolism during UV-B tolerance (Zhao et al. Citation2020). The interaction between UV-B stress and TiO2 NPs has not been clearly elucidated in plant species with respect to the regulation of gene expression by major transcription factors; however, current findings suggest crucial functions played by transcription factors induced by TiO2 NPs in protecting plants from UV damage. Altogether, these findings indicate that TiO2 NPs activate the expression of several transcription factors, ultimately enabling the plant to cope with UV-B stress.
The cellular machinery for cell wall synthesis and metabolism in plant species is encoded by members of large multi-gene families. Both dicot and monocot plants rely on genes involved in the cell wall for structural integrity and stress tolerance (Houston et al. Citation2016). The plant cell wall is made up of a framework of cellulose microfibrils surrounded by several types of non-cellulosic polysaccharides. Pectins, such as homogalacturonan and rhamnogalacturonan, serve as a versatile adhesive structure that can impart either flexibility or rigidity depending on chemical modifications (Gigli-Bisceglia et al. Citation2020). Xyloglucan is thought to serve as the primary adhesive material, interconnecting the cellulose microfibrils. Polymers such as 1,3–glucan, 1,3;1,4–glucan, mannan, arabinan, xylan, and phenolic compounds like lignin are also involved and play different roles depending on the cell type, species, and developmental stage (Gigli-Bisceglia et al. Citation2020). The interaction between UV-B stress and TiO2 NPs has not been clearly elucidated in plant species with respect to the regulation of cell-wall related gene expression. However, a recent report demonstrated the effect of gold nanoparticles on the chemical modifications of the cell wall polysaccharides in barley root tissues (Milewska-Hendel et al. Citation2021). In this study, we reported differential expression of several cell wall associated genes such as UDP-glucuronic acid decarboxylase, UDP-glucose 6-dehydrogenase, cellulose synthase, pectinesterase, expansin, xyloglucan, xyloglucan endotransglucosylase/hydrolase, mannan endo-1,4-beta-mannosidase, exopolygalacturonase, and fasciclin-like arabinogalactan in response to UV-B stress and TiO2 NPs treatments (). There were two candidate genes, namely LOC_Os09g32670 (annotated as UDP-glucuronate 4-epimerase 6) and LOC_Os02g54890 (annotated as UDP-glucuronate 4-epimerase 1), which exhibited unique expression when TiO2 NPs were applied individually (TN) or in combination with UV-B (UV+TN), in comparison to the control. Similarly, two other genes, LOC_Os07g24190 (annotated as cellulose synthase A) and LOC_Os07g36690 (annotated as glucan synthase 2), showed unique expression patterns in TN and UV+TN treatments. The unique expression of these genes in treatment groups suggests their potential involvement in specific molecular pathways or responses that are activated in response to the application of TiO2 NPs individually (TN) or in combination with UV-B (UV+TN), highlighting their roles in cellular processes and stress tolerance. Altogether, the distinct expressions of cell wall metabolism related genes in the TN and UV+TN treatments emphasize their vital roles in plant stress tolerance.
Plant hormones act as essential signaling molecules, regulating cellular processes at the molecular level, and ultimately enhancing stress tolerance in plants by modifying stress-responsive genes (Ahmad et al. Citation2022). Hormones exert control over transcription and translation patterns in plants by regulating an array of cell signaling components, including MAPK, G-proteins, and transcription factors, while the regulation of various cellular receptors and transporters related to hormones such as ABC, COI1, GID1, NPF, MATE, GTR1, LHT1, and PINs is also a crucial element in the plant's response to various stressors (Zhang et al. Citation2023). In this study, the TiO2 NP treatment affected the transcription levels of 37 common genes related to ethylene (ETH), jasmonate (JA), salicylic acid (SA), gibberellic acid (GA), benzoic acid (BA), auxin (IAA), cytokinin (CK), and abscisic acid (ABA) signal transduction pathways under UV-B stress (). Among them, ETH, ABA, and IAA were the primary classes of hormones that showed significant changes in response to TiO2 NPs and their combined effect with UV-B stress (UV+TN) compared to the control. Ethylene, a versatile plant hormone, plays crucial roles in both plant growth and development, while also acting as a mediator in responding to various abiotic stresses, including UV-B stress (He et al. Citation2011). In our study, three ethylene-responsive genes associated with senescence (LOC_Os01g70930, LOC_Os06g08032, LOC_Os01g61610) were all upregulated in response to UV-B stress. Interestingly, the expression of these three genes significantly declined in response to TN and UV+TN treatments. Previous reports have also demonstrated the induction of senescence-related genes in response to UV-B stress (Sztatelman et al. Citation2015). Leaf senescence, the final stage of plant development, is governed by a complex interplay of genetic and environmental factors, orchestrating the gradual aging and deterioration of leaves. In rice, the knockdown of several genes that promote senescence increased the stress tolerance of plants by preserving leaf chlorophyll levels and maintaining an efficient photosynthetic capacity (Lee and Masclaux-Daubresse Citation2021). Abscisic acid (ABA) is another important carotenoid-derived phytohormone, playing indispensable roles in facilitating plant adaptation and enhancing tolerance to diverse forms of abiotic stress (Vishwakarma et al. Citation2017). Carotenoids play a dual role in plants, functioning as both supplementary photosynthetic pigments and integral components of the photosynthetic machinery. The signaling cascade initiated by ABA results in significant alterations in gene expression. These modifications encompass variations in transcription, processing of transcripts, and the stability of RNA molecules (Sah et al. Citation2016). The process of ABA biosynthesis commences with the conversion of zeaxanthin via the ABA deficient 1 (ABA1) enzyme, resulting in the formation of epoxycarotenoids such as violaxanthin. Carotenoids can undergo cleavage either through ROS or by the action of cleavage enzymes: carotenoid cleavage dioxygenases (CCDs) and 9-cis-epoxycarotenoid dioxygenases (NCEDs). This cleavage gives rise to a diverse family of metabolites known as apocarotenoids, some of which serve as precursors to important phytohormones like abscisic acid (e.g. xanthoxin) and strigolactones (Jia et al. Citation2022). In this study, genes that code for CCD and NCED (LOC_Os02g47510, LOC_Os12g42280, LOC_Os12g44310) were downregulated in response to UV-B, however, these genes were significantly upregulated both in the TN and UV+TN treatments. This suggests active regulation of CCD and NCED genes by TiO2 NP treatments, either individually or in combination with UV-B, revealing a potential mechanism for ABA biosynthesis and plant stress response. Auxins, another key phytohormone, play a pivotal role in a wide range of developmental processes, including cell elongation, leaf and stem development, root growth, and phototropic and gravitropic responses, with their primary biosynthesis originating from the amino acid tryptophan (Korver et al. Citation2018). The connection between auxins and UV-B-regulated growth is evident through transcriptomic studies, which have described alterations in the expression of auxin-related genes such as small auxin-up RNA (SAUR), Aux/IAA, auxin importer AUX1, and auxin exporter PIN7 in plants (Pontin et al. Citation2010). In the present study, three SAUR genes exhibited differential expression across the comparison groups. Notably, two SAUR genes (LOC_Os02g52990 and LOC_Os09g37480) were downregulated in response to UV-B stress when compared to the control. Conversely, all three SAUR genes (LOC_Os02g52990, LOC_Os09g37480, and LOC_Os03g45860) were upregulated in both the TN and UV+TN treatments, with the UV+TN group demonstrating a notably pronounced level of expression. Likewise, two Aux/IAA genes (LOC_Os02g56120 and LOC_Os03g58350) were downregulated in response to UV-B, displaying moderate upregulation in the TN treatment and notably higher expression in the UV+TN treatment.
MAPK cascades are crucial signaling pathways in eukaryotic organisms, governing cellular responses that shape growth, development, and environmental adaptation. These cascades consist of MAPK, MAPKK, and MAPKKK components, often with overlapping functions. Stimulation prompts the activation of top-tier MAPKKK(s), initiating the activation of downstream MAPKK(s), which subsequently activate MAPK(s). These activated MAPKs phosphorylate a range of substrates, such as transcription factors, enzymes, and structural proteins, triggering cellular responses (Lin et al. Citation2021). Further, MAPK cascades assume a pivotal role in mediating protein phosphorylation and transmitting signals essential for plant hormone pathways, consequently exerting significant regulatory control over developmental processes, senescence, stress response, and acclimation (Jagodzik et al. Citation2018). In the present study, out of the 21 kinase-related genes commonly identified in the comparison groups, 19 and 16 genes were upregulated in the UV+TN and TN conditions, respectively, whereas most of these genes were downregulated in response to UV-B stress compared to the control (). Furthermore, five uniquely expressed MAPK genes were also found in the UV+TN treatment. These commonly and uniquely upregulated genes suggest that protein kinases serve an essential role in the signal transduction of a plant's response to UV-B stress. Additionally, the UV+TN treatment exhibited a higher number of upregulated kinase-related genes, indicating the active involvement of MAPK signaling in stress tolerance. This evidence also aligns with prior research that showed changes in MAPK gene transcripts in rice seedlings exposed to extended UV-B radiation, suggesting the pathway's crucial involvement in regulating the diverse cellular processes (Banerjee et al. Citation2023). These changes collectively contribute to shaping the overall transcript landscape, ultimately enhancing the plant's tolerance to UV-B stress. Taken together, these findings imply that TiO2 NP can influence the expression of genes related to hormonal pathways in rice seedlings, triggering a series of signaling networks that enhance the plant's ability to withstand stress.
The process of plant acclimation to UV-induced oxidative stress involves signaling in a feedback manner, which activates the antioxidant system. As a result, various antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), glutathione peroxidase (GPX), and glutathione S-transferase (GST), as well as non-enzymatic antioxidants such as ascorbic acid (AsA), glutathione (GSH), phenolic acids, alkaloids, flavonoids, carotenoids, α-tocopherol, and other nonprotein amino acids, become key markers for assessing stress tolerance in plants (Dumanović et al. Citation2021). In the current study, a total of 42 common DEGs, which are part of the antioxidant defense system (including SOD, APX, GST, PRX, polyamine oxidases, diamine oxidases, etc.) were characterized as significantly upregulated and/or downregulated in the three comparison groups (). Interestingly, expression of most oxidases (7 out of 9) was suppressed in response to UV-B, while transcripts of most of these genes were upregulated in TN (5), and UV+TN (7) treatments. Glutathione S-transferases (GSTs), a particular class of enzymes encoded by a large gene family that are ubiquitously distributed in animals, plants, and even in some prokaryotes. GSTs primarily catalyze the conjugation of reduced glutathione (GSH) to a range of hydrophobic and electrophilic compounds, enabling the detoxification of various endogenous or xenobiotic compounds (Zhuge et al. Citation2020). Apart from their detoxification role, GSTs possess the remarkable capability to govern cellular redox balance, ensuring protection against the detrimental effects of UV radiation and oxidative stress (Herrera-Vásquez et al. Citation2021). In the case of UV-B stress, transcripts of all the GSTs were downregulated (except LOC_Os01g70770). Meanwhile, the expression of most of the GST genes was significantly upregulated in the TN group (9 out of 10), with all the GST genes being highly expressed (10 out of 10) in the UV+TN treatment group. Similarly, DEGs related to SOD and APX were all downregulated in response to UV-B stress, while they were highly expressed in the UV+TN treatment. However, in the TN treatment, we observed that the transcripts related to SOD genes were upregulated, while the expression of APX genes was downregulated. Previous study also demonstrate that foliar application of TiO2 NP could significantly decrease the cadmium toxicity via regulation of SOD and GST activities and consequently activate several metabolic pathways (Lian et al. Citation2020). The regulation of the expression of several genes related to the antioxidant system, specifically GST, SOD, APX, oxidases, and peroxidases, as demonstrated by our transcriptomics data, strongly suggests that the exposure to TiO2 NPs during UV-B stress could have a profound and intricate impact on the plant's adaptive response to oxidative stress, thus shedding light on the underlying molecular mechanisms involved in stress tolerance.
5. Conclusion
To sum it up, this study shows that TiO2 NPs play a significant role in controlling the expression of multiple genes (DEGs) associated with the activation of diverse metabolic and signaling pathways in rice plants when subjected to UV-B stress. The observed improvements in growth, photosynthetic efficiency, and increase in the content of antioxidants highlight the positive role of TiO2 NPs in mitigating UV-B stress. It is worth noting that these responses are dependent on nanoparticle concentration, plant species, and the type of stress, as observed in other studies. Notably, our transcriptomics analysis emphasizes the profound impact of TiO2 NPs on gene regulation, particularly through the MYB, bHLH, and WRKY transcription factor families. These regulatory networks shape the overall transcriptome landscape, enhancing the plant's adaptability to UV-B stress. Further, the study highlights significant changes in the primary hormonal classes, including IAA, ETH, and ABA, in response to TiO2 NPs and their combined effect with UV-B stress (UV+TN), as compared to the control. This intricate interplay reveals the dynamic nature of plant hormone signaling in stress response. Our transcriptomics data strongly support the notion that TiO2 NPs have a profound impact on the plant's adaptive response to oxidative stress via regulating several genes related to the antioxidant system, including GST, SOD, APX, oxidases, and peroxidases. The enrichment of DEGs in the MAPK signaling pathway reflect the plant's rapid perception of stress signals, leading to enhanced tolerance to UV-B stress. In conclusion, this research unveils the multifaceted mechanisms through which TiO2 NPs enhance UV-B stress tolerance in rice plants, encompassing ROS control, transcriptional regulation, hormone signaling, and antioxidant defense. Further field-based investigation is necessary to uncover the precise complex interactions between nanoparticles and the natural environment. Moreover, it will allow for an evaluation of the long-term ecological impact, thus ensuring the safety and sustainability of these approaches.
Supplemental Material
Download Zip (167.9 KB)Acknowledgements
Authors are thankful for the full financial support for APC provided by Universitas Padjadjaran. The author (Fohad Mabood Husain) would like to thank the Researchers Supporting Project (RSPD2024R729), King Saud University, Riyadh, Saudi Arabia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Additional information
Funding
Notes on contributors
Raheel Shahzad
Raheel Shahzad is a postdoctoral research fellow at Universitas Padjadjaran, Indonesia. His work focuses on physiological, cellular and molecular mechanisms of plant adaptations to various environmental stress factors.
Putri Widyanti Harlina
Putri Widyanti Harlina is assistant professor at department of food technology, Universitas Padjadjaran, Indonesia. She focuses on lipidomics and antioxidation functions in foods.
Shahid Ullah Khan
Shahid Ullah Khan works as assistant professor at college of agronomy and biotechnology, Southwest University, Chongqing, China.
Muhammad Ihtisham
Muhammad Ihtisham works as assistant professor at school of agriculture, forestry and food engineering, Yibin University, China.
Aamir Hamid Khan
Aamir Hamid Khan works at faculty of biology, department of biogeography, paleoecology and environmental protection, University of Lodz, Lodz, Poland.
Fohad Mabood Husain
Fohad Mabood Husain works as professor at department of food science and nutrition, King Saud University, Riyadh, Saudi Arabia.
Agung Karuniawan
Agung Karuniawan, full professor, working at faculty of agriculture, Universitas Padjadjaran, Indonesia. His expertise is in plant genetic resources/breeding, focuses on grain, legume and tuber crops
References
- Ahmad I, Zhu G, Zhou G, Song X, Hussein Ibrahim ME, Ibrahim Salih EG, Hussain S, Younas MU. 2022. Pivotal role of phytohormones and their responsive genes in plant growth and their signaling and transduction pathway under salt stress in cotton. Int J Mol Sci. 23(13):7339. doi:10.3390/ijms23137339.
- Al-Khayri JM, Rashmi R, Surya Ulhas R, Sudheer WN, Banadka A, Nagella P, Aldaej MI, Rezk AA, Shehata WF, Almaghasla MI. 2023. The role of nanoparticles in response of plants to abiotic stress at physiological, biochemical, and molecular levels. Plants. 12(2):292. doi:10.3390/plants12020292.
- Altaf MA, Shu H, Hao Y, Mumtaz MA, Lu X, Wang Z. 2022. Melatonin affects the photosynthetic performance of pepper (Capsicum annuum L.) seedlings under cold stress. Antioxidants. 11(12):2414. doi:10.3390/antiox11122414.
- Altammar KA. 2023. A review on nanoparticles: characteristics, synthesis, applications, and challenges. Front Microbiol. 14:1155622. doi:10.3389/fmicb.2023.1155622.
- Amini S, Maali-Amiri R, Mohammadi R, Kazemi-Shahandashti SS. 2017. cDNA-AFLP analysis of transcripts induced in chickpea plants by TiO2 nanoparticles during cold stress. Plant Physiol Biochem. 111:39–49. doi:10.1016/j.plaphy.2016.11.011.
- Apoorva JD, Pandey-Rai S, Agrawal SB. 2021. Untangling the UV-B radiation-induced transcriptional network regulating plant morphogenesis and secondary metabolite production. Environ Exp Bot. 192:104655. doi:10.1016/j.envexpbot.2021.104655.
- Azadi M, Siavash Moghaddam S, Rahimi A, Pourakbar L, Popović-Djordjević J. 2021. Biosynthesized silver nanoparticles ameliorate yield, leaf photosynthetic pigments, and essential oil composition of garden thyme (Thymus vulgaris L.) exposed to UV-B stress. Journal of Environmental Chemical Engineering. 9:105919. doi:10.1016/j.jece.2021.105919.
- Banerjee G, Singh D, Pandey C, Jonwal S, Basu U, Parida SK, Pandey A, Sinha AK. 2023. Rice mitogen-activated protein kinase regulates serotonin accumulation and interacts with cell cycle regulators under prolonged UV-B exposure. Plant Physiol Biochem. 203:108078. doi:10.1016/j.plaphy.2023.108078.
- Bano N, Khan S, Hamid Y, Bano F, Khan AG, Ullah MA, Li T, Ullah H, Rinklebe J, Shaheen SM. 2024. Seed nano-priming with multiple nanoparticles enhanced the growth parameters of lettuce and mitigated cadmium (Cd) bio-toxicity: an advanced technique for remediation of Cd contaminated environments. Environ Pollut. 344:123300. doi:10.1016/j.envpol.2024.123300.
- Basit F, Bhat J, Han J, Guan Y, Jan B, Shakoor A, Alansi S. 2022. Screening of rice cultivars for Cr-stress response by using the parameters of seed germination, morpho-physiological and antioxidant analysis. Saudi J Biol Sci. 29(5):3918–3928. doi:10.1016/j.sjbs.2022.02.038.
- Berry HM, Argueso CT. 2022. More than growth: phytohormone-regulated transcription factors controlling plant immunity, plant development and plant architecture. Curr Opin Plant Biol. 70:102309. doi:10.1016/j.pbi.2022.102309.
- Bisht S, Sharma V, Kumari N. 2022. Biosynthesized magnetite nanoparticles from Polyalthia longifolia leaves improve photosynthetic performance and yield of Trigonella foenum-graecum under drought stress. Plant Stress. 5:100090. doi:10.1016/j.stress.2022.100090.
- Cai F, Wu X, Zhang H, Shen X, Zhang M, Chen W, Gao Q, White JC, Tao S, Wang X. 2017. Impact of TiO2 nanoparticles on lead uptake and bioaccumulation in rice (Oryza sativa L.). NanoImpact. 5:101–108. doi:10.1016/j.impact.2017.01.006.
- Chen Z, Dong Y, Huang X. 2022. Plant responses to UV-B radiation: signaling, acclimation and stress tolerance. Stress Biology. 2:51. doi:10.1007/s44154-022-00076-9.
- Chen Z, Tao X, Khan A, Tan DKY, Luo H. 2018. Biomass accumulation, photosynthetic traits and root development of cotton as affected by irrigation and nitrogen-fertilization. Front Plant Sci. 9:173. doi:10.3389/fpls.2018.00173.
- Djanaguiraman M, Belliraj N, Bossmann SH, Prasad PVV. 2018. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega. 3:2479–2491. doi:10.1021/acsomega.7b01934.
- Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V. 2021. The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Front Plant Sci. 11:552969. doi:10.3389/fpls.2020.552969.
- Dvořák P, Krasylenko Y, Zeiner A, Šamaj J, Takáč T. 2021. Signaling toward reactive oxygen species-scavenging enzymes in plants. Front Plant Sci. 11:618835. doi:10.3389/fpls.2020.618835.
- Ewas M, Harlina PW, Shahzad R, Khames E, Ali F, Nishawy E, Elsafty N, Ibrahim HM, Gallego PP.. 2022. Constitutive expression of SlMX1 gene improves fruit yield and quality, health-promoting compounds, fungal resistance and delays ripening in transgenic tomato plants. J Plant Interact. 17(1):517–536. doi:10.1080/17429145.2022.2066730.
- Foyer CH, Hanke G. 2022. ROS production and signalling in chloroplasts: cornerstones and evolving concepts. Plant J. 111:642–661. doi:10.1111/tpj.15856.
- Gao Y, Zhang J, Wang C, Han K, Hu L, Niu T, Yang Y, Chang Y, Xie J. 2023. Exogenous proline enhances systemic defense against salt stress in celery by regulating photosystem, phenolic compounds, and antioxidant system. Plants. 12(4):928. doi:10.3390/plants12040928.
- García-Sánchez S, Bernales I, Cristobal S. 2015. Early response to nanoparticles in the arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genomics. 16:341. doi:10.1186/s12864-015-1530-4.
- Ghorbanpour M, Movahedi A, Hatami M, Kariman K, Bovand F, Shahid MA. 2021. Insights into nanoparticle-induced changes in plant photosynthesis. Photosynthetica. 59:570–586. doi:10.32615/ps.2021.049.
- Ghouri F, Shahid MJ, Liu J, Sun L, Riaz M, Imran M, Ali S, Liu X, Shahid MQ. 2023. The protective role of tetraploidy and nanoparticles in arsenic-stressed rice: Evidence from RNA sequencing, ultrastructural and physiological studies. J Hazard Mater. 458:132019. doi:10.1016/j.jhazmat.2023.132019.
- Ghouri F, Shahid MJ, Zhong M, Zia MA, Alomrani SO, Liu J, Sun L, Ali S, Liu X, Shahid MQ. 2024. Alleviated lead toxicity in rice plant by co-augmented action of genome doubling and TiO2 nanoparticles on gene expression, cytological and physiological changes. Sci Total Environ. 911:168709. doi:10.1016/j.scitotenv.2023.168709.
- Gigli-Bisceglia N, Engelsdorf T, Hamann T. 2020. Plant cell wall integrity maintenance in model plants and crop species-relevant cell wall components and underlying guiding principles. Cell Mol Life Sci. 77:2049–2077. doi:10.1007/s00018-019-03388-8.
- Gohari G, Mohammadi A, Akbari A, Panahirad S, Dadpour MR, Fotopoulos V, Kimura S. 2020. Titanium dioxide nanoparticles (TiO2 NPs) promote growth and ameliorate salinity stress effects on essential oil profile and biochemical attributes of Dracocephalum moldavica. Sci Rep. 10:912. doi:10.1038/s41598-020-57794-1.
- Gu L. 2023. Optimizing the electron transport chain to sustainably improve photosynthesis. Plant Physiol. 193(4):2398–2412. doi:10.1093/plphys/kiad490.
- Guo J, Sun B, He H, Zhang Y, Tian H, Wang B. 2021. Current understanding of bHLH transcription factors in plant abiotic stress tolerance. Int J Mol Sci. 22(9):4921. doi:10.3390/ijms22094921.
- Hanif S, Saleem MF, Sarwar M, Irshad M, Shakoor A, Wahid MA, Khan HZ. 2021. Biochemically triggered heat and drought stress tolerance in rice by proline application. J Plant Growth Regul. 40:305–312. doi:10.1007/s00344-020-10095-3.
- Haskirli H, Yilmaz O, Ozgur R, Uzilday B, Turkan I. 2021. Melatonin mitigates UV-B stress via regulating oxidative stress response, cellular redox and alternative electron sinks in Arabidopsis thaliana. Phytochemistry. 182:112592. doi:10.1016/j.phytochem.2020.112592.
- He J, Yue X, Wang R, Zhang Y. 2011. Ethylene mediates UV-B-induced stomatal closure via peroxidase-dependent hydrogen peroxide synthesis in Vicia faba L. J Exp Bot. 62:2657–2666. doi:10.1093/jxb/erq431.
- Herrera-Vásquez A, Fonseca A, Ugalde JM, Lamig L, Seguel A, Moyano TC, Gutiérrez RA, Salinas P, Vidal EA, Holuigue L. 2021. TGA class II transcription factors are essential to restrict oxidative stress in response to UV-B stress in Arabidopsis. J Exp Bot. 72:1891–1905. doi:10.1093/jxb/eraa534.
- Hidema J, Kumagai T. 2006. Sensitivity of rice to ultraviolet-B radiation. Ann Bot. 97:933–942. doi:10.1093/aob/mcl044.
- Houston K, Tucker MR, Chowdhury J, Shirley N, Little A. 2016. The plant cell wall: a complex and dynamic structure as revealed by the responses of genes under stress conditions. Front Plant Sci. 7:984. doi:10.3389/fpls.2016.00984.
- Hu J, Wu X, Wu F, Chen W, White JC, Yang Y, Wang B, Xing B, Tao S, Wang X. 2020. Potential application of titanium dioxide nanoparticles to improve the nutritional quality of coriander (Coriandrum sativum L.). J Hazard Mater. 389:121837. doi:10.1016/j.jhazmat.2019.121837.
- Huang H, Ullah F, Zhou DX, Yi M, Zhao Y. 2019. Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci. 10:800. doi:10.3389/fpls.2019.00800.
- Huang X, Zheng D, Feng N, Huang A, Zhang R, Meng F, Jie Y, Mu B, Mu D, Zhou H. 2023. Effects of prohexadione calcium spraying during the booting stage on panicle traits, yield, and related physiological characteristics of rice under salt stress. PeerJ. 11:e14673. doi:10.7717/peerj.14673.
- Jagodzik P, Tajdel-Zielinska M, Ciesla A, Marczak M, Ludwikow A. 2018. Mitogen-activated protein kinase cascades in plant hormone signaling. Front Plant Sci. 9:1387. doi:10.3389/fpls.2018.01387.
- Jia KP, Mi J, Ali S, Ohyanagi H, Moreno JC, Ablazov A, Balakrishna A, Berqdar L, Fiore A, Diretto G, et al. 2022. An alternative, zeaxanthin epoxidase-independent abscisic acid biosynthetic pathway in plants. Mol Plant. 15:151–166. doi:10.1016/j.molp.2021.09.008.
- Jiang Z, Zhu H, Zhu H, Tao Y, Liu C, Liu J, Yang F, Li M. 2022. Exogenous ABA Enhances the Antioxidant Defense system of maize by regulating the AsA-GSH cycle under drought stress. Sustainability. 14(5):3071. doi:10.3390/su14053071.
- Jing M, Zhang H, Wei M, Tang Y, Xia Y, Chen Y, Shen Z, Chen C. 2022. Reactive oxygen species partly mediate DNA methylation in responses to different heavy metals in pokeweed. Front Plant Sci. 13:845108. doi:10.3389/fpls.2022.845108.
- Karvar M, Azari A, Rahimi A, Maddah-Hosseini S, Ahmadi-Lahijani MJ. 2022. Titanium dioxide nanoparticles (TiO2-NPs) enhance drought tolerance and grain yield of sweet corn (Zea mays L.) under deficit irrigation regimes. Acta Physiol Plant. 44:14. doi:10.1007/s11738-021-03349-4.
- Katiyar A, Smita S, Lenka SK, Rajwanshi R, Chinnusamy V, Bansal KC. 2012. Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis. BMC Genomics. 13:544. doi:10.1186/1471-2164-13-544.
- Khan I, Awan SA, Rizwan M, Akram MA, Zia-ur-Rehman M, Wang X, Zhang X, Huang L. 2023. Physiological and transcriptome analyses demonstrate the silver nanoparticles mediated alleviation of salt stress in pearl millet (Pennisetum glaucum L). Environ Pollut. 318:120863. doi:10.1016/j.envpol.2022.120863.
- Khosla A, Paper JM, Boehler AP, Bradley AM, Neumann TR, Schrick K. 2014. HD-Zip proteins GL2 and HDG11 have redundant functions in ArabidopsisTrichomes, and GL2 activates a positive feedback loop via MYB23. Plant Cell. 26:2184–2200. doi:10.1105/tpc.113.120360.
- Kiany T, Pishkar L, Sartipnia N, Iranbakhsh A, Barzin G. 2022. Effects of silicon and titanium dioxide nanoparticles on arsenic accumulation, phytochelatin metabolism, and antioxidant system by rice under arsenic toxicity. Environ Sci Pollut Res. 29:34725–34737. doi:10.1007/s11356-021-17927-z.
- Kong W, Sun T, Zhang C, Deng X, Li Y. 2021. Comparative transcriptome analysis reveals the mechanisms underlying differences in salt tolerance between indica and japonica rice at seedling stage. Front Plant Sci. 12:725436. doi:10.3389/fpls.2021.725436.
- Korver RA, Koevoets IT, Testerink C. 2018. Out of shape during stress: a key role for Auxin. Trends Plant Sci. 23:783–793. doi:10.1016/j.tplants.2018.05.011.
- Kreslavski VD, Huang X, Semenova G, Khudyakova A, Shirshikova G, Hummatov N, Zharmukhamedov SK, Li X, Allakhverdiev SI, Nie C, Shabala S. 2020. Linking sensitivity of photosystem II to UV-B with chloroplast ultrastructure and UV-B absorbing pigments contents in A. thaliana L. phyAphyB double mutants. Plant Growth Regul. 91:13–21. doi:10.1007/s10725-020-00584-6.
- Kumar D, Dhankher OP, Tripathi RD, Seth CS. 2023. Titanium dioxide nanoparticles potentially regulate the mechanism(s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in Helianthus annuus L. J Hazard Mater. 454:131418. doi:10.1016/j.jhazmat.2023.131418.
- Lai M, Ghouri F, Sarwar S, alomrani SO, Riaz M, Haider FU, Liu J, Imran M, Ali S, Liu X, Shahid MQ. 2023. Modulation of metal transporters, oxidative stress and cell abnormalities by synergistic application of silicon and titanium oxide nanoparticles: A strategy for cadmium tolerance in rice. Chemosphere. 345:140439. doi:10.1016/j.chemosphere.2023.140439.
- Lee S, Masclaux-Daubresse C. 2021. Current understanding of leaf senescence in rice. Int J Mol Sci. 22(9):4515. doi:10.3390/ijms22094515.
- Lei Z, Mingyu S, Xiao W, Chao L, Chunxiang Q, Liang C, Hao H, Xiaoqing L, Fashui H. 2008. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol Trace Elem Res. 121:69–79. doi:10.1007/s12011-007-8028-0.
- Li N, Liu H, Sun J, Zheng H, Wang J, Yang L, Zhao H, Zou D. 2018. Transcriptome analysis of two contrasting rice cultivars during alkaline stress. Sci Rep. 8:9586. doi:10.1038/s41598-018-27940-x.
- Lian J, Zhao L, Wu J, Xiong H, Bao Y, Zeb A, Tang J, Liu W. 2020. Foliar spray of TiO2 nanoparticles prevails over root application in reducing Cd accumulation and mitigating Cd-induced phytotoxicity in maize (Zea mays L.). Chemosphere. 239: 124794. doi:10.1016/j.chemosphere.2019.124794.
- Liang YE, Zhang H, Zhu J, Wang H, Mei W, Jiang B, Ding X, Dai H. 2023. Transcriptomic analysis reveals the involvement of flavonoids synthesis genes and transcription factors in dracaena cambodiana response to ultraviolet-B radiation. Forests. 14(5):979. doi:10.3390/f14050979.
- Liaqat W, Altaf MT, Barutçular C, Nawaz H, Ullah I, Basit A, Mohamed HI. 2023. Ultraviolet-B radiation in relation to agriculture in the context of climate change: a review. Cereal Res Commun. 1:24.
- Lin L, Wu J, Jiang M, Wang Y. 2021. Plant mitogen-activated protein kinase cascades in environmental stresses. Int J Mol Sci. 22(4):1543. doi:10.3390/ijms22041543.
- Ling C, Wang X, Li Z, He Y, Li Y. 2022. Effects and mechanism of enhanced UV-B radiation on the flag leaf angle of rice. Int J Mol Sci. 23(21):12776. doi:10.3390/ijms232112776.
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 25:402–408. doi:10.1006/meth.2001.1262.
- Manisalidis I, Stavropoulou E, Stavropoulos A, Bezirtzoglou E. 2020. Environmental and health impacts of air pollution: a review. Front Public Health. 8:14. doi:10.3389/fpubh.2020.00014.
- Milewska-Hendel A, Sala K, Gepfert W, Kurczyńska E. 2021. Gold nanoparticles-induced modifications in cell wall composition in barley roots. Cells. 10(8):1965. doi:10.3390/cells10081965.
- Mirakhorli T, Ardebili ZO, Ladan-Moghadam A, Danaee E. 2021. Bulk and nanoparticles of zinc oxide exerted their beneficial effects by conferring modifications in transcription factors, histone deacetylase, carbon and nitrogen assimilation, antioxidant biomarkers, and secondary metabolism in soybean. PLoS One. 16:e0256905. doi:10.1371/journal.pone.0256905.
- Mittal D, Kaur G, Singh P, Yadav K, Ali SA. 2020. Nanoparticle-based sustainable agriculture and food science: recent advances and future outlook. Front Nanotech. 2:579954. doi:10.3389/fnano.2020.579954.
- Mmbando GS, Teranishi M, Hidema J. 2020. Very high sensitivity of African rice to artificial ultraviolet-B radiation caused by genotype and quantity of cyclobutane pyrimidine dimer photolyase. Sci Rep. 10:3158. doi:10.1038/s41598-020-59720-x.
- Mohidem NA, Hashim N, Shamsudin R, Che Man H. 2022. Rice for food security: revisiting its production, diversity, rice milling process and nutrient content. Agriculture. 12(6):741. doi:10.3390/agriculture12060741.
- Moradi Rikabad M, Pourakbar L, Siavash Moghaddam S, Popović-Djordjević J. 2019. Agrobiological, chemical and antioxidant properties of saffron (Crocus sativus L.) exposed to TiO2 nanoparticles and ultraviolet-B stress. Ind Crops Prod. 137:137–143. doi:10.1016/j.indcrop.2019.05.017.
- Moustakas M, Sperdouli I, Adamakis IS, Moustaka J, İşgören S, Şaş B. 2022. Harnessing the role of foliar applied salicylic acid in decreasing chlorophyll content to reassess photosystem II photoprotection in crop plants. Int J Mol Sci. 23(13):7038. doi:10.3390/ijms23137038.
- Mu DW, Feng NJ, Zheng DF, Zhou H, Liu L, Chen GJ, Mu B. 2022. Physiological mechanism of exogenous brassinolide alleviating salt stress injury in rice seedlings. Sci Rep. 12:20439. doi:10.1038/s41598-022-24747-9.
- Mustafa N, Raja NI, Ilyas N, Ikram M, Mashwani ZUR, Ehsan M. 2021. Foliar applications of plant-based titanium dioxide nanoparticles to improve agronomic and physiological attributes of wheat (Triticum aestivum L.) plants under salinity stress. Green Process Synth. 10:246–257. doi:10.1515/gps-2021-0025.
- Muthayya S, Sugimoto JD, Montgomery S, Maberly GF. 2014. An overview of global rice production, supply, trade, and consumption. Ann N Y Acad Sci. 1324:7–14. doi:10.1111/nyas.12540.
- Nazir MM, Li Q, Noman M, Ulhassan Z, Ali S, Ahmed T, Zeng F, Zhang G. 2022. Calcium Oxide nanoparticles have the role of alleviating arsenic toxicity of barley. Front Plant Sci. 13:843795. doi:10.3389/fpls.2022.843795.
- Neale RE, Barnes PW, Robson TM, Neale PJ, Williamson CE, Zepp RG, Wilson SR, Madronich S, Andrady AL, Heikkilä AM, et al. 2021. Environmental effects of stratospheric ozone depletion, UV radiation, and interactions with climate change: UNEP environmental effects assessment panel, update 2020. Photochem Photobiol Sci. 20:1–67. doi:10.1007/s43630-020-00001-x.
- Pandit C, Roy A, Ghotekar S, Khusro A, Islam MN, Emran TB, Lam SE, Khandaker MU, Bradley DA. 2022. Biological agents for synthesis of nanoparticles and their applications. J King Saud University - Sci. 34:101869. doi:10.1016/j.jksus.2022.101869.
- Peng M, Shahzad R, Gul A, Subthain H, Shen S, Lei L, Zheng Z, Zhou J, Lu D, Wang S, et al. 2017. Differentially evolved glucosyltransferases determine natural variation of rice flavone accumulation and UV-tolerance. Nat Commun. 8:1975. doi:10.1038/s41467-017-02168-x.
- Piccini C, Cai G, Dias MC, Romi M, Longo R, Cantini C. 2020. UV-B radiation affects photosynthesis-related processes of two Italian Olea europaea (L.) varieties differently. Plants. 9(12):1712. doi:10.3390/plants9121712.
- Pontin MA, Piccoli PN, Francisco R, Bottini R, Martinez-Zapater JM, Lijavetzky D. 2010. Transcriptome changes in grapevine (Vitis vinifera L.) cv. Malbec leaves induced by ultraviolet-B radiation. BMC Plant Biol. 10:224. doi:10.1186/1471-2229-10-224.
- Qian C, Chen Z, Liu Q, Mao W, Chen Y, Tian W, Liu Y, Han J, Ouyang X, Huang X. 2020. Coordinated transcriptional regulation by the UV-B photoreceptor and multiple transcription factors for plant UV-B responses. Mol Plant. 13:777–792. doi:10.1016/j.molp.2020.02.015.
- Rady MM, Salama MMM, Kuşvuran S, Kuşvuran A, Ahmed AF, Ali EF, Farouk HA, Osman AS, Selim KA, Mahmoud AEM. 2023. Exploring the role of novel biostimulators in suppressing oxidative stress and reinforcing the antioxidant defense systems in Cucurbita pepo plants exposed to cadmium and lead toxicity. Agronomy. 13(7):1916. doi:10.3390/agronomy13071916.
- Rakgotho T, Ndou N, Mulaudzi T, Iwuoha E, Mayedwa N, Ajayi RF. 2022. Green-synthesized zinc oxide nanoparticles mitigate salt stress in Sorghum bicolor. Agriculture. 12(5):597. doi:10.3390/agriculture12050597.
- Rastogi RP, Richa Kumar A, Tyagi MB, Sinha RP. 2010. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J Nucleic Acids. 2010:1. doi:10.4061/2010/592980.
- Sah SK, Reddy KR, Li J. 2016. Abscisic acid and abiotic stress tolerance in crop plants. Front Plant Sci. 7:571.
- Saidi MN, Mahjoubi H, Yacoubi I. 2023. Transcriptome meta-analysis of abiotic stresses-responsive genes and identification of candidate transcription factors for broad stress tolerance in wheat. Protoplasma. 260:707–721. doi:10.1007/s00709-022-01807-5.
- Santin M, Simoni S, Vangelisti A, Giordani T, Cavallini A, Mannucci A, Ranieri A, Castagna A. 2023. Transcriptomic analysis on the peel of UV-B-exposed peach fruit reveals an upregulation of phenolic- and UVR8-related pathways. Plants. 12(9):1818. doi:10.3390/plants12091818.
- Scheibe R, Fickenscher K, Ashton AR. 1986. Studies on the mechanism of the reductive activation of NADP-malate dehydrogenase by thioredoxin m and low-molecular-weight thiols. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 870:191–197. doi:10.1016/0167-4838(86)90221-9.
- Šebesta M, Kolenčík M, Sunil BR, Illa R, Mosnáček J, Ingle AP, Urík M. 2021. Field application of ZnO and TiO2 nanoparticles on agricultural plants. Agronomy. 11:2281. doi:10.3390/agronomy11112281.
- Shahzad R, Ewas M, Harlina PW, Khan SU, Zhenyuan P, Nie X, Nishawy E. 2021a. β-Sitosterol differentially regulates key metabolites for growth improvement and stress tolerance in rice plants during prolonged UV-B stress. J Gen Eng Biotech. 19(1):79. doi:10.1186/s43141-021-00183-6.
- Shahzad R, Harlina PW, Ewas M, Zhenyuan P, Nie X, Gallego PP, Ullah Khan S, Nishawy E, Khan AH, Jia H. 2021b. Foliar applied 24-epibrassinolide alleviates salt stress in rice (Oryza sativa L.) by suppression of ABA levels and upregulation of secondary metabolites. J Plant Int. 16:533–549. doi:10.1080/17429145.2021.2002444.
- Shahzad R, Harlina PW, Gallego PP, Flexas J, Ewas M, Leiwen X, Karuniawan A. 2023. The seaweed Ascophyllum nodosum -based biostimulant enhances salt stress tolerance in rice ( Oryza sativa L.) by remodeling physiological, biochemical, and metabolic responses. J Plant Int. 18(1). doi:10.1080/17429145.2023.2266514.
- Singh P, Singh I, Shah K. 2020. Alterations in antioxidative machinery and growth parameters upon application of nitric oxide donor that reduces detrimental effects of cadmium in rice seedlings with increasing days of growth. S Afr J Bot. 131:283–294. doi:10.1016/j.sajb.2020.02.022.
- Song Y, Jiang M, Zhang H, Li R. 2021. Zinc oxide nanoparticles alleviate chilling stress in rice (Oryza Sativa L.) by regulating antioxidative system and chilling response transcription factors. Molecules. 26(8):2196. doi:10.3390/molecules26082196.
- Song Y, Ma B, Guo Q, Zhou L, Zhou X, Ming Z, You H, Zhang C. 2023. MYB pathways that regulate UV-B-induced anthocyanin biosynthesis in blueberry (Vaccinium corymbosum). Front Plant Sci. 14:1125382. doi:10.3389/fpls.2023.1125382.
- Spök A, Sprink T, Allan AC, Yamaguchi T, Dayé C. 2022. Towards social acceptability of genome-edited plants in industrialised countries? Emerging evidence from Europe, United States, Canada, Australia, New Zealand, and Japan. Frontiers in Genome Editing. 4:899331. doi:10.3389/fgeed.2022.899331.
- Stefanov MA, Rashkov GD, Apostolova EL. 2022. Assessment of the photosynthetic apparatus functions by chlorophyll fluorescence and P700 absorbance in C3 and C4 plants under physiological conditions and under salt stress. Int J Mol Sci. 23:3768. doi:10.3390/ijms23073768.
- Sun L, Song F, Guo J, Zhu X, Liu S, Liu F, Li X. 2020. Nano-ZnO-induced drought tolerance is associated with melatonin synthesis and metabolism in maize. Int J Mol Sci. 21(3):782. doi:10.3390/ijms21030782.
- Sun L, Wang J, Song K, Sun Y, Qin Q, Xue Y. 2019. Transcriptome analysis of rice (Oryza sativa L.) shoots responsive to cadmium stress. Sci Rep. 9:10177. doi:10.1038/s41598-019-46684-w.
- Sztatelman O, Grzyb J, Gabryś H, Banaś AK. 2015. The effect of UV-B on arabidopsis leaves depends on light conditions after treatment. BMC Plant Biol. 15:281. doi:10.1186/s12870-015-0667-2.
- Taha RS, Seleiman MF, Shami A, Alhammad BA, Mahdi AHA. 2021. Integrated application of selenium and silicon enhances growth and anatomical structure, antioxidant defense system and yield of wheat grown in salt-stressed soil. Plants. 10:1040. doi:10.3390/plants10061040.
- Tan KC, Lim HS, Jafri M, Z M. 2018. Study on solar ultraviolet erythemal dose distribution over peninsular Malaysia using ozone monitoring instrument. Egypt J Remote Sens Space Sci. 21:105–110.
- Tawfik MM, Mohamed MH, Sadak MS, Thalooth AT. 2021. Iron oxide nanoparticles effect on growth, physiological traits and nutritional contents of Moringa oleifera grown in saline environment. Bull Nat Res Cent. 45:177. doi:10.1186/s42269-021-00624-9.
- Tighe-Neira R, Reyes-Díaz M, Nunes-Nesi A, Recio G, Carmona E, Corgne A, Rengel Z, Inostroza-Blancheteau C. 2020. Titanium dioxide nanoparticles provoke transient increase in photosynthetic performance and differential response in antioxidant system in Raphanus sativus L. Sci Hortic. 269:109418. doi:10.1016/j.scienta.2020.109418.
- Tolosa LN, Zhang Z. 2020. The role of major transcription factors in Solanaceous food crops under different stress conditions: current and future perspectives. Plants. 9:56. doi:10.3390/plants9010056.
- Tripathi DK, Singh S, Singh VP, Prasad SM, Dubey NK, Chauhan DK. 2017. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem. 110:70–81. doi:10.1016/j.plaphy.2016.06.026.
- Velikova V, Yordanov I, Edreva A. 2000. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 151:59–66. doi:10.1016/S0168-9452(99)00197-1.
- Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S. 2017. Abscisic acid signaling and abiotic stress tolerance in plants. A review on current knowledge and future prospects. Front Plant Sci. 8:161.
- Wang A, Li J, Al-Huqail AA, Al-Harbi MS, Ali EF, Wang J, Ding Z, Rekaby SA, Ghoneim AM, Eissa MA. 2021a. Mechanisms of chitosan nanoparticles in the regulation of cold stress resistance in banana plants. Nanomaterials. 11(10):2670. doi:10.3390/nano11102670.
- Wang J, Li M, Feng J, Yan X, Chen H, Han R. 2021b. Effects of TiO2-NPs pretreatment on UV-B stress tolerance in Arabidopsis thaliana. Chemosphere. 281:130809. doi:10.1016/j.chemosphere.2021.130809.
- Wei B, Hou K, Zhang H, Wang X, Wu W. 2020. Integrating transcriptomics and metabolomics to studies key metabolism, pathways and candidate genes associated with drought-tolerance in Carthamus tinctorius L. Under drought stress. Ind Crops Prod. 151:112465. doi:10.1016/j.indcrop.2020.112465.
- Williamson CE, Zepp RG, Lucas RM, Madronich S, Austin AT, Ballaré CL, Norval M, Sulzberger B, Bais AF, McKenzie RL, et al. 2014. Solar ultraviolet radiation in a changing climate. Nat Clim Change. 4:434–441. doi:10.1038/nclimate2225.
- Wishnick M, Daniel Lane M. 1971. Methods in enzymology. Meth Enzymol. 23:570–577. doi:10.1016/S0076-6879(71)23129-3.
- Wu S, Tian J, Ren T, Wang Y. 2022. Osmotic adjustment and antioxidant system regulated by nitrogen deposition improve photosynthetic and growth performance and alleviate oxidative damage in dwarf bamboo under drought stress. Front Plant Sci. 13:819071. doi:10.3389/fpls.2022.819071.
- Xie C, Wang P, Gu Z, Yang R. 2023. Spermidine alleviates oxidative damage and enhances phenolic compounds accumulation in barley seedlings under UV-B stress. J Sci Food Agric. 103:648–656. doi:10.1002/jsfa.12176.
- Yadav A, Singh D, Lingwan M, Yadukrishnan P, Masakapalli SK, Datta S. 2020. Light signaling and UV-B-mediated plant growth regulation. J Integr Plant Biol. 62:1270–1292. doi:10.1111/jipb.12932.
- Yang Y, Saand MA, Huang L, Abdelaal WB, Zhang J, Wu Y, Li J, Sirohi MH, Wang F. 2021. Applications of multi-omics technologies for crop improvement. Front Plant Sci. 12:563953. doi:10.3389/fpls.2021.563953.
- Yang Y, Zhang L, Chen P, Liang T, Li X, Liu H. 2020. UV-B photoreceptor UVR8 interacts with MYB73/MYB77 to regulate auxin responses and lateral root development. EMBO J. 39:e101928. doi:10.15252/embj.2019101928.
- Yu GH, Li W, Yuan ZY, Cui HY, Lv CG, Gao ZP, Han B, Gong YZ, Chen GX. 2013. The effects of enhanced UV-B radiation on photosynthetic and biochemical activities in super-high-yield hybrid rice Liangyoupeijiu at the reproductive stage. Photosynthetica. 51:33–44. doi:10.1007/s11099-012-0081-z.
- Zahra Z, Waseem N, Zahra R, Lee H, Badshah MA, Mehmood A, Choi HK, Arshad M. 2017. Growth and metabolic responses of rice (Oryza sativa L.) cultivated in phosphorus-deficient soil amended with TiO2 nanoparticles. J Agric Food Chem. 65:5598–5606. doi:10.1021/acs.jafc.7b01843.
- Zavafer A, Mancilla C. 2021. Concepts of photochemical damage of photosystem II and the role of excessive excitation. J Photochem Photobiol C. 47:100421. doi:10.1016/j.jphotochemrev.2021.100421.
- Zhang F, Guo H, Huang J, Yang C, Li Y, Wang X, Qu L, Liu X, Luo J. 2020a. A UV-B-responsive glycosyltransferase, OsUGT706C2, modulates flavonoid metabolism in rice. Sci China Life Sci. 63(7):1037–1052. doi:10.1007/s11427-019-1604-3.
- Zhang M, He S, Zhan Y, Qin B, Jin X, Wang M, Zhang Y, Hu G, Teng Z, Wu Y. 2019. Exogenous melatonin reduces the inhibitory effect of osmotic stress on photosynthesis in soybean. PLoS One. 14:e0226542.
- Zhang W, Long J, Geng J, Li J, Wei Z. 2020b. Impact of Titanium Dioxide Nanoparticles on Cd Phytotoxicity and Bioaccumulation in Rice (Oryza sativa L.). Int J Environ Res Public Health. 17(9):2979. doi:10.3390/ijerph17092979.
- Zhang Y, Berman A, Shani E. 2023. Plant hormone transport and localization: signaling molecules on the move. Annu Rev Plant Biol. 74:453–479. doi:10.1146/annurev-arplant-070722-015329.
- Zhang Z, Ke M, Qu Q, Peijnenburg WJGM, Lu T, Zhang Q, Ye Y, Xu P, Du B, Sun L, Qian H. 2018. Impact of copper nanoparticles and ionic copper exposure on wheat (Triticum aestivum L.) root morphology and antioxidant response. Environ Pollut. 239:689–697. doi:10.1016/j.envpol.2018.04.066.
- Zhao B, Wang L, Pang S, Jia Z, Wang L, Li W, Jin B. 2020. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind Crops Prod. 151:112483. doi:10.1016/j.indcrop.2020.112483.
- Zhen S, Bugbee B. 2020. Far-red photons have equivalent efficiency to traditional photosynthetic photons: Implications for redefining photosynthetically active radiation. Plant Cell Environ. 43:1259–1272. doi:10.1111/pce.13730.
- Zhuge XL, Xu H, Xiu ZJ, Yang HL. 2020. Biochemical functions of glutathione S-Transferase family of Salix babylonica. Front Plant Sci. 11:364. doi:10.3389/fpls.2020.00364.