ABSTRACT
Carbohydrate supplementation during endurance exercise is known to improve performance, but the effects of food-based approaches in running exercise are understudied. Therefore, this study investigated the performance and gastrointestinal (GI) effects of a carbohydrate supplement containing a natural fructose source compared with a highly processed fructose source in a combined glucose-fructose supplement, during a half-marathon. Eleven trained runners (9 males, 2 females; age 32 ± 8 y, 89:53 ± 13:28 min half-marathon personal record) completed a familiarisation (8 miles) and two experimental trials (13.1 miles) on an outdoor running course, with blood and urine samples collected before and after the run. Subjective GI measures were made throughout the run. Carbohydrate was provided as a natural fructose source in the form of apple puree (AP) or highly processed crystalline fructose (GF) in a 2:1 glucose-to-fructose ratio (additional required glucose was provided through maltodextrin). Half-marathon performance was not different between carbohydrate sources (AP 89:52 ± 09:33 min, GF 88:44 ± 10:09 min; P = 0.684). There were no interaction effects for GI comfort (P = 0.305) or other GI symptoms (P ≥ 0.211). There were no differences between carbohydrate sources in ad libitum fluid intake (AP 409 ± 206 mL; GF 294 ± 149 mL; P = 0.094) or any other urinary (P ≥ 0.724), blood-based (P ≥ 0.215) or subjective (P ≥ 0.421) measures. Apple puree as a natural fructose source was equivalent to crystalline fructose in supporting half-marathon running performance without increasing GI symptoms.
Highlights
Research examining food-first and food-based approaches to carbohydrate supplementation and endurance running performance are limited. Therefore, this study aimed to compare carbohydrate supplements either containing a natural or highly processed fructose source as part of a glucose-fructose supplement on half-marathon running performance and gastrointestinal comfort in trained runners.
Running performance (apple puree 89:52 ± 09:33 min vs. crystalline fructose 88:44 ± 10:09 min), gastrointestinal comfort and symptoms were not different between the two fructose sources.
Apple puree can be effectively used as a carbohydrate source to fuel half-marathon running performance.
Introduction
Exogenous carbohydrate intake during endurance sport has been shown to improve performance (Burke et al., Citation2011). This has led to the formation of carbohydrate intake recommendations for endurance exercise; detailing information on intake based on duration, dose and sugar sources (Burke et al., Citation2011). For example, for exercise lasting 1–2.5 h 30–60 g/h is recommended and >2.5–3 h 90 g/h from multiple sugar sources (i.e. glucose and fructose) is recommended. Typically, sport products use highly processed fructose sources (i.e. fructose corn syrup, crystalline fructose or sucrose), which might elevate biomarkers of renal injury during exercise (Chapman et al., Citation2019). Alternatively, fructose, as well as small amounts of glucose/sucrose, is naturally found in fruit. It is possible that natural fructose sources may not have the same negative health implications (Hyson, Citation2015), whilst still providing an effective option for carbohydrate sports products (Reynolds et al., Citation2022a).
Ingestion of natural carbohydrate sources during exercise is usually in a whole food format (i.e. bananas – Nieman et al., Citation2012; potatoes – Salvador et al., Citation2019; raisins – Rietschier et al., Citation2011) with our recent review suggesting no performance differences during cycling and running protocols compared to processed carbohydrate sources (Reynolds et al., Citation2022a). Using whole foods can be beneficial in terms of accessibility and cost but can provide challenges in terms of storage (i.e. shelf-life and temperature), mastication (particularly challenging during high intensity exercise) and damage (i.e. squashed bananas). Furthermore, a common issue with carbohydrate ingestion during exercise is an increase in gastrointestinal (GI) symptoms (Pfeiffer et al., Citation2012), especially with whole foods (Reynolds et al., Citation2022a). The volume of carbohydrate required from natural sources tends be higher to meet requirements (e.g. 23 g of a carbohydrate gel delivered the same amount of carbohydrate as 128.5 g of potatoes [Salvador et al., Citation2019]), which could contribute to increased GI symptoms. Additionally, GI symptoms may be caused by additional constituents in foods, such as fibre, fat and protein. A fruit puree may provide a natural, “healthier” fructose (and to a lesser extent glucose) source and may be a viable strategy for food-based ingestion during exercise as carbohydrate could be provided in a small volume, easily consumed format that mimics the consistency of a carbohydrate gel, potentially reducing GI symptoms.
We have recently shown that apple puree (plus small amounts of maltodextrin) ingested during laboratory cycling resulted in similar performance and GI symptoms compared to a highly processed fructose and glucose product (Reynolds et al., Citation2022b). However, when another natural source (watermelon puree), with similar in carbohydrate composition was ingested during a 75 km cycling time trial there was no difference in performance, but the relatively high total volumes (∼2.9 L) required to meet carbohydrate recommendations resulted in increased stomach fullness in the watermelon trial (Shanely et al., Citation2016). Laboratory studies typically involve prolonged steady state exercise at a low/moderate intensity followed by a short high-intensity performance test, a design with relatively low ecological validity. Additionally, cycling will often result in reduced GI symptoms compared to running, with runners more prone to lower-GI symptoms including cramps, urge to defecate, and diarrhoea especially with high carbohydrate intakes (Pfeiffer et al., Citation2012; Pugh et al., Citation2018). In race settings, running intensity can be high from the start, therefore there is a longer period of stress compared to typical laboratory-based protocols, a factor that can affect splanchnic blood flow, as well as intestinal absorption rates and permeability (de Oliveira et al., Citation2014; van Wijck et al., Citation2012). This is of particular importance at exercise intensities above 70% V̇O2max, where delayed gastric emptying is observed, meaning even with a low carbohydrate ingestion rate there is the potential for food/chyme to accumulate in the GI tract.
The aim of the present study was to examine half-marathon running performance, gastrointestinal comfort, and acute kidney injury markers of two different fructose sources (fruit and highly processed fructose) ingested during exercise. A half-marathon was selected as exercise intensity is high for a prolonged duration and carbohydrate supplementation would be recommended, meaning if the fruit puree were to influence GI symptoms (and/or performance), it would be from the combined effect of duration and intensity (Costa et al., Citation2017). It was hypothesised that running performance would be similar between fructose sources, but GI symptoms would increase in the fruit puree-based condition.
Materials and methods
Participants
Sixteen trained runners who had a minimum of one year of running experience, running ≥2-3 h/week, were healthy and injury free were recruited to the study. Of the 16 participants recruited, 11 (2 females, 9 males) completed the study (mean ± standard deviation) age (32 ± 8 years), height (1.75 ± 0.8 m; SECA 216, Hamburg, Germany), mass (67.1 ± 7.9 kg; Adam CFW-150, Milton Keynes, UK). Reasons for withdrawal included illness (1), injury (1), fatigue (1), did not meet study criteria (1), unknown (1). Participants on average ran 42 ± 20 miles/week and competed at various distances including 5 km (personal record: 18:19 ± 01:51 min, n = 10), 10 km (38:10 ± 04:14 min, n = 10), half-marathon (89:16 ± 14:22 min, n = 11) and marathon (186:19 ± 28:42 min, n = 5). Seven participants had experience of consuming carbohydrate during competition and five consumed carbohydrate during longer training sessions. One female had a regular menstrual cycle, and the other was amenorrhoeic. Menstrual cycle phase was not controlled, as it does not appear to influence endurance exercise performance (McNulty et al., Citation2020). The study gained Institutional Ethical Approval from Loughborough University Ethics Approvals (Human Participants) Sub-Committee and participants provided written informed consent and completed a medical screening questionnaire. Based on previous unpublished data from our laboratory using a similar protocol for a repeated 10-mile run setting an α = 0.05 and a correlation of 0.95 it was determined 11 participants were required to detect a 3% difference in performance (G-Power; Dusseldorf; Germany).
Pre-trial standardisation
Participants were asked to prepare as if they were racing a half-marathon and record their behaviour in an exercise and food diary (including caffeine consumption) over the 24 h pre-trial and then replicated these diet and exercise patterns before the second trial. They abstained from alcohol and strenuous exercise over this time. Participants consumed a standardised breakfast of ∼1.5 g carbohydrate/kg body mass and 8 mL water/kg body mass (consisting of cereal bars; Nutrigrain, Kellogg’s, UK, orange juice; Tesco, UK, and water) 90 min before arrival at the laboratory (i.e. ∼2 h before starting exercise). No caffeine was consumed on the morning of experimental trials. Compliance with pre-trial standardisation procedures was verbally confirmed before trials. All trials commenced between 09:00 and 12:00, with each participant’s specific time standardised between trials to control for circadian rhythm and diurnal variation in performance (Drust et al., Citation2005). Participants wore the same footwear and clothing in both experimental trials.
Experimental design
Familiarisation
Visit one was a familiarisation trial, identical to the experimental trials, but over a shorter distance (8 miles). Carbohydrate was provided as maltodextrin only (maltodextrin; MyProtein, Northwich, UK): 24 g at 0, and 12 g at 3.1 and 6.1 miles.
Experimental trials
Participants completed the two experimental trials (AP: apple puree providing a natural fructose source; GF: crystalline fructose) in a randomised order separated by ≥14 days. Participants were given 10 min to complete a self-selecting, standardised warm-up which was recorded and replicated for both trials. The course consisted of a one-mile out and back flat L-shaped loop, on a wide tarmac footpath, with no road crossings. The additional 0.1 mile (to make 13.1 miles) was completed initially, followed by 13 laps and participants were asked to complete the distance as fast as possible. At 3.1, 6.1 and 9.1 miles, participants stopped running for 60 s to consume the carbohydrate. In both trials 60 g carbohydrate (24 g immediately pre-run, 12 g at 3.1, 6.1 and 9.1 miles) was provided via 50 mL syringe, in a 2:1 glucose:fructose ratio. This fuelling strategy was based on current carbohydrate guidelines (Burke et al., Citation2011) and race set-up where feed/drink stations are positioned every ∼5 km as recommended by World Athletics In AP, apple puree (BIONA ORGANIC; Surrey, UK) provided a natural fructose and glucose source (per 100 g of puree, energy: 237 kcal, glucose: 3.28 g, fructose: 7.08 g, sucrose: 2.15 g, fibre: 1.3 g, fat: 0.1 g, protein: 0.2 g). The puree had been previously analysed for nutritional composition by Campden BRI (Campden, UK). Per serving, 5.86 g maltodextrin (MyProtein, Northwich, UK) was added to 49.05 g apple puree to create a 2:1 glucose:fructose ratio. In GF, volume and concentration were matched to AP, with the same glucose:fructose ratio achieved by combining maltodextrin with crystalline fructose (Bulk Powders, Colchester, UK). Apple flavouring (0.01 g per serving; MyProtein, Northwich, UK) was added to both carbohydrate sources. At 0, 3.1, 6.1 and 9.1 miles participants were provided with ad libitum water access, with intake recorded.
Study blinding
Participants and investigators directly relating to performance were blinded from the study’s aims until they had completed all visits. They were initially informed the purpose of the study was to investigate the effect of different carbohydrate sources, both providing an optimal dosage. During the runs, participants were not able to see/know their running time and the only interaction between investigators and participants was to record subjective responses and to verbally remind participants (using standard wording) of distance completed every three miles and at the start of the final mile. Participants completed an exit questionnaire upon study completion to determine what they thought the study aim was, whether they could identify the carbohydrate supplements and if they could identify the trial containing apple puree.
Measures
On arrival, participants sat for 15 min before a venous blood sample was collected by venepuncture of an antecubital vein, a urine sample was collected, and body mass (in minimal clothing) measured. Post-run body mass was measured, a urine sample was collected and then participants sat for 15 min before another venous blood sample was collected by venepuncture of an antecubital vein. After thorough cleaning and drying of the skin, a sweat patch was applied to the right scapula (Tegaderm + Pad; 3M Healthcare, Loughborough, UK). Heart rate (T9, Polar H10, Polar Teams Pro; Polar Electro, Kempele, Finland) was measured at 0, 3.1, 6.1, 9.1 and 13.1 miles. Rating of perceived exertion (RPE; Borg, Citation1982, 6–20 Scale) and GI comfort (Jeukendrup et al., Citation2000, 0–10 scale), were recorded at 0, 3.1, 6.1, 9.1 and 13.1 miles. Additional GI scales (Jeukendrup et al., Citation2000, 0 = no symptom, 10 = maximum symptom, scores ≥5 were classed as severe) assessing hunger, thirst, nausea, stomach fullness, stomach bloatedness, stomach cramps, abdominal stitch, flatulence, urge to belch, urge to vomit, urge to urinate and urge to defecate, as well as thermal sensation (Lee et al., Citation2008a; −10 to + 10 Scale) were assessed pre- and post-run. During trials, ambient temperature, wet bulb globe temperature (WBGT), relative humidity and wind speed (Kestrel 4400; Nielsen-Kellerman Co., Philadelphia, USA) were recorded.
Sample processing and analysis
Urine specific gravity (USG) was measured using a hand-held analyser (Ceti, Refractometer), before samples were aliquoted and stored at −80°C until analysis of biomarkers of kidney injury, (kidney injury molecule-1; KIM-1 and neutrophil gelatinase-associated lipocalin; NGAL) by enzyme-linked immunosorbent assay (ELISA) (Human KIM-1, ELISA kit ADI-900-226-001, Enzo Life Sciences, Farmingdale, NY, USA; CV 5.3%, Human, NGAL ELISA kit 036, Bioporto, Diagnostics, Hellerup, Denmark; CV 6.8%). NGAL and KIM-1 data presented is USG corrected. A total of 11 mL of venous blood was collected, with 1 mL dispensed into tubes containing K2-EDTA (1.75 mg/L), 5 mL into pre-chilled lithium heparin tubes and 2.5 mL into pre-chilled K2-EDTA tubes (both left on ice before centrifugation). The final 2.5 mL was dispensed into a tube containing a clotting activator but was not used for analysis in this study. Final analysis of bloods for all variables except plasma volume changes (n = 11) was n = 10 due to inability to collect sufficient sample (only 1 mL was collected) post-run for one male participant. The K2-EDTA whole blood (1 mL) was used to measure haemoglobin (in duplicate by the cyanmethemoglobin method) and haematocrit (in triplicate by microcentrifugation), to estimate changes in blood, red cell, and plasma volume, relative to pre-exercise (Dill & Costill, Citation1974). The remaining blood (1048 g, 10 min, 4°C) and sweat (377 g, 10 min, 4°C) were centrifuged, aliquoted and stored at −80°C until analysis. Sweat sodium was analysed (in duplicate) by flame photometry (Model 410; Sherwood Scientific; Cambridge, UK). Lithium heparin treated plasma was analysed for osmolality (in duplicate) via freezing point depression (Gonotec Osmomat Auto Cryoscopic Osmometer; Gonotec; CV 0.2%), plasma glucose, creatinine, myoglobin, creatine kinase, and uric acid concentrations were analysed using a bench-top analyser (ABX Pentra C400; Horiba Medical, Northampton, UK). K2-EDTA treated plasma was used for analysis of intestinal fatty acid binding protein −1 (IFABP-1) by ELISA (Human FABP2/I-FABP, Quantikine ELISA kit, RnD Systems; CV 12.7%).
Statistical analysis
Statistical analyses were completed using Statistical Package for Social Sciences (SPSS) for Windows version 25 SPSS; Chicago, IL, USA. Normality of data was assessed using Shapiro-Wilks tests. Statistical significance was set at P < 0.05 and results were presented as mean ± SD, except for gastrointestinal scale responses and renal biomarkers which were median (IQR). Two-way repeated measures ANOVA were used to analyse data containing two factors (trial x time), whilst paired t-tests or Wilcoxon signed rank tests were used, as appropriate, to analyse data containing one factor. Bonferroni corrected post-hoc tests were completed for time effects. To correct for violation of sphericity the degrees of freedom were corrected using Greenhouse-Geisser (ε > 0.75) or Huynh-Felt (ε < 0.75).
Results
Pre-trial measures
There were no pre-trial differences for body mass (P = 0.460), urine specific gravity (P = 0.226), heart rate (P = 0.751), physiological (P ≥ 0.182), or subjective variables (P ≥ 0.211).
Weather
Weather conditions were not different between carbohydrate sources: temperature (AP: 16.7 ± 2.7°C; GF: 16.5 ± 2.7°C; P = 0.917), relative humidity (AP: 77.4 ± 11.0%; GF: 73.3 ± 12.0%; P = 0.388) WBGT (AP: 16.0 ± 2.9°C; GF: 15.3 ± 3.4°C; P = 0.551), and wind speed (GF: 1.4 ± 0.8 m/s; AP: 1.2 ± 1.0 m/s; P = 0.514).
Half-marathon performance
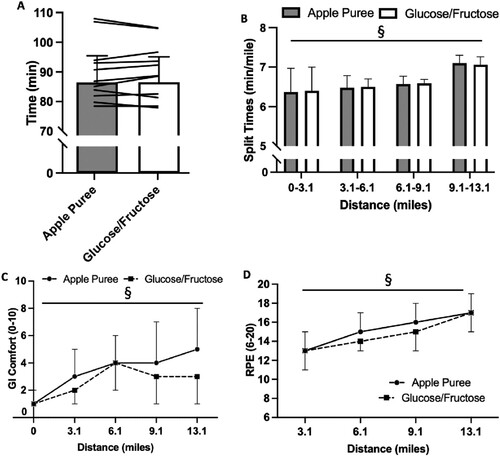
Performance was not different between carbohydrate sources (AP: 89:52 ± 09:33 min, GF: 88:44 ± 10:09 min; P = 0.684; A). There was no influence of source on split times (i.e. pacing; time x trial interaction P = 0.676; B) but split times got progressively slower (P = 0.002). There was also no trial order effect, with time to complete the half-marathon on visit one not different to visit two (trial 1: 88:59 ± 10:40 min, trial 2: 90:25 ± 09:30 min; P = 0.068).
Figure 1. 13.1 mile running performance (min; A) and split times (min/mile; B) over the run for 13.1 miles for Glucose/Fructose (GF) and Apple Puree (AP). Bars are mean ± SD. Lines are individual participant times. Gastrointestinal comfort (0-10; C) and RPE (6-20; D) over 13.1 mile run for Glucose/Fructose (GF) and Apple Puree (AP). Data presented as mean ± SD. § denotes significantly different over time (P < 0.05).
Physiological responses
Heart rate increased from rest to starting exercise (P < 0.001) but was not different between carbohydrate sources (mean heart rate during exercise; AP: 171 ± 12 bpm, GF: 175 ± 10 bpm; trial effect P = 0.375, time x trial interaction: P = 0.277). Plasma albumin, creatine kinase, creatinine, glucose, myoglobin and uric acid concentrations were not different between carbohydrate sources (trial effect P ≥ 0.231, time x trial interaction P ≥ 0.215; ) but increased from pre- to post-run (P ≤ 0.001). IFABP-1 was not different between carbohydrate sources (trial effect P = 0.634, time x trial interaction P = 0.549) but increased from pre- to post-run (P = 0.021).
Table 1. Plasma albumin, creatine kinase, creatinine, glucose, myoglobin and uric acid concentrations (n = 10) pre and post. Data presented as mean ± SD.
Subjective scale responses
RPE (trial effect P = 0.148, time x trial interaction P = 0.404; C) and thermal sensation (trial effect P = 0.487, time x trial interaction effect P = 1.000; ) progressively increased over run duration (P < 0.001), with no difference between carbohydrate sources. GI comfort was not different between carbohydrate sources (trial effect P = 0.305, time x trial interaction P = 0.127; C), but significantly increased from rest after 6.1 miles (P < 0.001). All other GI symptoms were not different between carbohydrate sources (trial effect P ≥ 0.050, time x trial interaction P ≥ 0.232; ). Thirst, bloatedness, stomach cramps, abdominal stitch, flatulence, urge to belch and urge to urinate were higher post-run (P ≥ 0.014; ). All symptoms post-run were classified as severe (≥ 5) by at least one participant.
Table 2. Subjective scale responses (0-10 scale) for thermal comfort and gastrointestinal symptoms. Data presented as median (Q1-Q3).
Fluid balance
There were no difference between carbohydrate sources for voluntary fluid intake (P = 0.094; ), sweat rate (P = 0.707; ), sweat sodium concentration (P = 0.890; ), or body mass loss (P = 0.507; ). All participants lost weight (i.e. accrued dehydration), with three participants exceeding 2% dehydration in the AP trial and six participants exceeding 2% dehydration in the GF trial. Plasma volume change (P = 0.349; ) and plasma osmolality were not different between carbohydrate sources (trial effect P = 0.629, time x trial interaction P = 0.935; ) but plasma osmolality increased from pre- to post-run (P < 0.001). USG was not different between carbohydrate sources (P = 0.371) but increased post run (P < 0.001; ).
Table 3. Urine specific gravity, body mass (kg), sweat loss (L), sweat sodium (mmol/L), body mass loss (%), plasma volume change (%) and ad libitum fluid intake (mL) pre- and post-run. n = 11 for all variables except plasma osmolality where n = 10. Data presented as mean ± SD.
Renal biomarkers
uNGAL and uKIM-1 concentrations increased from pre- to post-run (P ≤ 0.012), but there were no differences between carbohydrate sources (uNGAL AP: pre 3.13 (6.80), post 17.45 (12.24) ng/mL; GF: pre 2.22 (4.00), post 17.07 (17.74) ng/mL; trial effect P = 0.170; time x trial interaction P = 0.467, uKIM-1 AP: Pre 1.52 (2.36), post 13.97 (12.05), GF: Pre 1.29 (1.22), post 5.61 (12.28) ng/mL; trial effect P = 0.174, time x trial interaction P = 0.070).
Study blinding
No participant correctly identified the aim of the study or both carbohydrate supplements. Participants believed the aim was to compare carbohydrate supplements on half-marathon performance (n = 8), investigate the effect of carbohydrate on half-marathon performance (n = 2) or the relationship between running performance and GI comfort with carbohydrate supplementation (n = 1). When informed one trial contained apple puree all participants correctly identified the trial.
Discussion
Established carbohydrate guidelines recommend up to 60 g/h of carbohydrate from glucose or a combination of glucose and fructose for exercise up to 2.5 h (Burke et al., Citation2011) but provide little information on the food/supplement sources of these sugars. This information can be important for athletes exercising for long durations, particularly at high intensities. Thus, the aim of the study was to compare half-marathon performance and GI symptoms between consumption of a natural fructose source (apple puree), and highly processed fructose (crystalline) during exercise when consumed in a 2:1 glucose-to-fructose ratio. The current study design did not account for the different running speeds of the individuals participating and this meant that carbohydrate ingestion rates varied between ∼34-55 g/h, but all met current recommendations (Burke et al., Citation2011). There was no difference in performance or GI symptoms, suggesting that a fruit puree might be used as a fructose source to maximise running performance, providing a more natural easy-to-consume option for runners and increasing the variety of carbohydrate options to fuel prolonged running. The performance findings are consistent with laboratory-based cycling and running trials comparing carbohydrate drinks, gels and chews with other whole food sources, including bananas (Nieman et al., Citation2012, Citation2018), raisins (Kern et al., Citation2007; Rietschier et al., Citation2011; Too et al., Citation2012), watermelon (Shanely et al., Citation2016) and potatoes (Salvador et al., Citation2019), but contrast with increased GI symptoms observed in many of these previous studies (Reynolds et al., Citation2022a). Laboratory-based studies that consist of a preload followed by a short time-trial (∼15 min) are unlikely to replicate pacing strategies in race scenarios, especially for endurance running and may not induce the prolonged physiological strain associated with elevated GI symptoms (van Wijck et al., Citation2012), giving the present data good ecological validity.
There were no differences for physiological, performance or subjective measures (e.g. heart rate, RPE, pacing (min/mile), blood or urine markers) between carbohydrate sources. Although glucose/fructose uptake or use were not measured, plasma glucose concentrations were not different between trials suggesting similar carbohydrate availability between sources. Although the lack of a carbohydrate-free trial did not allow us to examine whether carbohydrate in either source improved performance (Burke et al., Citation2005), the results of previous investigations (Rollo et al., Citation2012) suggest it likely would have, but importantly this was not our aim. For optimal performance during a half-marathon (i.e. > 60 min for the vast majority of athletes), peri-exercise carbohydrate intake is recommended, and we aimed to compare the efficacy of different sources. Therefore, that there was no performance difference between the two trials is important, as it implies that performance can be optimised with more natural carbohydrate supplements without increasing GI discomfort/symptoms, an important finding for runners (Tiller et al., Citation2019). Food-based carbohydrate sources have been recommended for ultra-runners (Tiller et al., Citation2019) to increase the variety of options, reduce taste/texture fatigue and increase adherence to recommendations despite the scarcity of available evidence (Reynolds et al., Citation2022a). This study provides evidence that such strategies might be efficacious for such athletes.
Unlike previous studies (Guillochon & Rowlands, Citation2017; Ishihara et al., Citation2020; Lee et al., Citation2008b; Nieman et al., Citation2012, Citation2018; Salvador et al., Citation2019), GI symptoms were not different between trials. IFABP-1 concentrations increased over the run, but were also not different between carbohydrate sources, suggesting an increase in GI permeability caused by the exercise, but no effect of carbohydrate source. The increased fibre content (∼3 g) and plant components present in apple puree did not impact GI comfort/symptoms despite current guidelines recommending a low fibre intake during exercise (Jeukendrup, Citation2014). Fructose malabsorption has been related to GI symptoms such as bloating and diarrhoea in doses as small as 5 g (DiNicolantonio & Lucan, Citation2015), potentially via mechanisms involving delayed GI absorption, blood flow redistribution or fibre content increasing GI motility. In the current study, participants consumed a 20 g of fructose, suggesting the fruit puree had little effect on fructose malabsorption, at least in these participants. Future studies should examine longer duration exercise and/or higher carbohydrate ingestion rates, but it seems unlikely the fruit puree will influence these effects since ingestion of more than double this amount did not produce affects during cycling (Reynolds et al., Citation2022b). However, care should be taken in selecting an appropriate fruit/food source of carbohydrate as other sources may have a higher fibre content.
Sweat rate and hydration responses (assessed through fluid intake, dehydration accrued, plasma volume change, serum osmolality, urine specific gravity) were not different between supplements. Importantly, ad-libitum fluid intake was not different between carbohydrate sources, which could have increased GI volume and symptoms. Three participants in the AP trial and six participants in the GF trial exceeded 2% body mass loss, which is a level of dehydration that might impair performance in many athletes (Funnell et al., Citation2019; James et al., Citation2019). Dehydration accrued (∼1.8% on average) was similar to other studies in running (Cheuvront and Haymes, Citation2001; Rollo et al., Citation2012), with the source of carbohydrate supplement not appearing to influence the response. Elevation of urine and serum kidney injury biomarkers have been reported post exercise (Juett et al., Citation2020) and may be amplified with fructose consumption (Chapman et al., Citation2019) or dehydration (Juett et al., Citation2021). In this study, both uNGAL and uKIM-1 concentrations increased from rest to post race implying that running exercise is associated with renal disturbance and aligns with previous research (Juett et al., Citation2020). However, the fructose source did not influence uNGAL or uKIM-1 concentrations, potentially due to the low amount of fructose consumed. These results are supported by recent findings that the fructose source (apple puree or crystalline fructose) did not influence uNGAL or uKIM-1 concentrations following >2 h cycling (Reynolds et al., Citation2022b).
In the current study females were included, however, menstrual cycle was not controlled, as recent meta-analyses showed a trivial effect for both menstrual cycle phase and contraceptive use on performance (Elliott-Sale et al., Citation2020; McNulty et al., Citation2020). This approach may not have the same scientific rigour as studies that control for menstrual cycle phase, but it is in keeping with the ecological validity reflecting daily life.
Conclusion
Apple puree as a natural fructose (and to a lesser extent glucose) source provided an alternative to a highly processed fructose source to fuel prolonged running performance in trained runners. There were no elevated GI symptoms experienced in the apple puree trial compared to a highly processed fructose source and no differences in physiological parameters. Natural carbohydrate sources based on a fruit puree may provide a suitable alternative to traditional carbohydrate supplements for running distance of half-marathon or longer. Future research may need to investigate the impact of higher ingestion rates and/or longer durations, or more intense running exercise.
Acknowledgements
The study was designed by KMR, LAJ, SAM and LJJ; KMR, LAJ, MPF, IA, JS, AB, WH, CG, GM, YL and SAM collected the data; KMR, LAJ and MPF performed biochemical/data analysis; KMR wrote the manuscript with assistance from LAJ, MPF, IA, JS, AB, WH, CG, GM, YL, SAM and LJJ. All authors approved the final version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Borg, G. A. (1982). Psychophysical bases of perceived exertion. Medicine and Science in Sports and Exercise, 14, 377–381.
- Burke, L. M., Hawley, J. A., Wong, S. H., & Jeukendrup, A. E. (2011). Carbohydrates for training and competition. Journal of Sports Sciences, 29(S1), S17–S27. https://doi.org/10.1080/02640414.2011.585473
- Burke, L. M., Wood, C., Pyne, D. B., Telford, R. D., & Saunders, P. U. (2005). Effect of carbohydrate intake on half-marathon performance of well-trained runners. International Journal of Sport Nutrition and Exercise Metabolism, 15(6), 573–589. https://doi.org/10.1123/ijsnem.15.6.573
- Chapman, C. L., Johnson, B. D., Sackett, J. R., Parker, M. D., & Schlader, Z. J. (2019). Soft drink consumption during and following exercise in the heat elevates biomarkers of acute kidney injury. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 316(3), R189–R198. https://doi.org/10.1152/ajpregu.00351.2018
- Cheuvront, S. N., & Haymes, E. M. (2001). Thermoregulation and marathon running. Sports Medicine, 31(10), 743–762. http://doi.org/10.2165/00007256-200131100-00004
- Costa, R. J. S., Spine, R. M. J., Kitic, C. M., & Gibson, P. R. (2017). Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Alimentary Pharmacology & Therapeutics, 46(3), 246–265. https://doi.org/10.1111/apt.14157
- de Oliveira, E. P., Burini, R. C., & Jeukendrup, A. E. (2014). Gastrointestinal complaints during exercise: Prevalence, etiology, and nutritional recommendations. Sports Medicine, 44(1), 79–85. https://doi.org/10.1007/s40279-014-0153-2
- Dill, D. B., & Costill, D. L. (1974). Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. Journal of Applied Physiology, 37(2), 247–248. https://doi.org/10.1152/jappl.1974.37.2.247
- DiNicolantonio, J. J., & Lucan, S. C. (2015). Is fructose malabsorption a cause of irritable bowel syndrome? Medical Hypotheses, 85(3), 295–297. https://doi.org/10.1016/j.mehy.2015.05.019
- Drust, B., Waterhouse, J., Atkinson, G., Edwards, B., & Reilly, T. (2005). Circadian rhythms in sports performance—an update. Chronobiology International, 22(1), 21–44. https://doi.org/10.1081/CBI-200041039
- Elliott-Sale, K. J., McNulty, K. L., Ansdell, P., Goodall, S., Hicks, K. M., Thomas, K., … Dolan, E. (2020). The effects of oral contraceptives on exercise performance in women: A systematic review and meta-analysis. Sports Medicine, 1–28. https://doi.org/10.1007/s40279-020-01317-5.
- Funnell, M. P., Mears, S. A., Bergin-Taylor, K., & James, L. J. (2019). Blinded and unblinded hypohydration similarly impair cycling time trial performance in the heat in trained cyclists. Journal of Applied Physiology, 126(4), 870–879. https://doi.org/10.1152/japplphysiol.01026.2018
- Guillochon, M., & Rowlands, D. S. (2017). Solid, gel, and liquid carbohydrate format effects on gut comfort and performance. International Journal of Sport Nutrition And Exercise Metabolism, 27(3), 247–254. https://doi.org/10.1123/ijsnem.2016-0211
- Hyson, D. A. (2015). A review and critical analysis of the scientific literature related to 100% fruit juice and human health. Advances in Nutrition, 6(1), 37–51. https://doi.org/10.3945/an.114.005728
- Ishihara, K., Taniguchi, H., Akiyama, N., & Asami, Y. (2020). Easy to swallow rice cake as a carbohydrate source during endurance exercise suppressed feelings of thirst And hunger without changing exercise performance. Journal of Nutritional Science and Vitaminology, 66(2), 128–135. https://doi.org/10.3177/jnsv.66.128
- James, L. J., Funnell, M. P., James, R. M., & Mears, S. A. (2019). Does hypohydration really impair endurance performance? Methodological considerations for interpreting hydration research. Sports Medicine, 49(2), 103–114. https://doi.org/10.1007/s40279-019-01188-5
- Jeukendrup, A. E. (2014). A step towards personalized sports nutrition: Carbohydrate intake during exercise. Sports Medicine, 44(1), 25–33. https://doi.org/10.1007/s40279-014-0148-z
- Jeukendrup, A. E., Vet-Joop, K., Sturk, A., Stegen, J. H. J. C., Senden, J., Saris, W. H. M., & Wagenmakers, A. J. M. (2000). Relationship between gastro-intestinal complaints and endotoxaemia, cytokine release and the acute-phase reaction during and after a long-distance triathlon in highly trained men. Clinical Science, 98(1), 47–55. https://doi.org/10.1042/CS19990258
- Juett, L. A., James, L. J., & Mears, S. A. (2020). Effects of exercise on acute kidney injury biomarkers and the potential influence of fluid intake. Annals of Nutrition and Metabolism, 76(Suppl. 1), 53–59. https://doi.org/10.1159/000515022
- Juett, L. A., Midwood, K. L., Funnell, M. P., James, L. J., & Mears, S. A. (2021). Hypohydration produced by high-intensity intermittent running increases biomarkers of renal injury in males. European Journal of Applied Physiology, 121(12), 3485–3497. https://doi.org/10.1007/s00421-021-04804-3
- Kern, M., Heslin, C. J., & Rezende, R. S. (2007). Metabolic and performance effects of raisins versus sports gel as pre-exercise feedings in cyclists. The Journal of Strength & Conditioning Research, 21(4), 1204–1207. https://doi.org/10.1519/R-21226.1.
- Lee, J. K. W., Maughan, R. J., & Shirreffs, S. M. (2008a). The influence of serial feeding of drinks at different temperatures on thermoregulatory responses during cycling. Journal of Sports Sciences, 26(6), 583–590. https://doi.org/10.1080/02640410701697388
- Lee, J. K. W., Maughan, R. J., Shirreffs, S. M., & Watson, P. (2008b). Effects of milk ingestion on prolonged exercise capacity in young, healthy men. Nutrition, 24(4), 340–347. https://doi.org/10.1016/j.nut.2008.01.001
- McNulty, K. L., Elliott-Sale, K. J., Dolan, E., Swinton, P. A., Ansdell, P., Goodall, S., … Hicks, K. M. (2020). The effects of menstrual cycle phase on exercise performance in eumenorrheic women: A systematic review and meta-analysis. Sports Medicine, 1–15. https://doi.org/10.1007/s40279-020-01319-3.
- Nieman, D. C., Gillitt, N. D., Henson, D. A., Sha, W., Shanely, R. A., Knab, A. M., Cialdella-Kam, L., & Jin, F. (2012). Bananas as an energy source during exercise: A metabolomics approach. Plos One, 7(5), E37479. https://doi.org/10.1371/journal.pone.0037479
- Nieman, D. C., Gillitt, N. D., Sha, W., Esposito, D., & Ramamoorthy, S. (2018). Metabolic recovery from heavy exertion following banana compared to sugar beverage or water only ingestion: A randomized, crossover trial. Plos One, 13, E0194843. https://doi.org/10.1371/journal.pone.0194843.
- Pfeiffer, B., Stellingwerff, T., Hodgson, A. B., Randell, R., Pöttgen, K., Res, P., … Jeukendrup, A. E. (2012). Nutritional intake and gastrointestinal problems during competitive endurance events. Medicine and Science in Sports and Exercise, 44, 344–351. https://doi.org/10.1249/MSS.0b013e31822dc809.
- Pugh, J., Kirk, B., Fearn, R., Morton, J., & Close, G. (2018). Prevalence, severity and potential nutritional causes of gastrointestinal symptoms during a marathon In recreational runners. Nutrients, 10(7), 811. https://doi.org/10.3390/nu10070811
- Reynolds, K. M., Clifford, T., Mears, S. A., & James, L. J. (2022a). A food first approach to carbohydrate supplementation in endurance exercise: A systematic review. International Journal of Sport Nutrition and Exercise Metabolism, 32(4), 296–310. https://doi.org/10.1123/ijsnem.2021-0261
- Reynolds, K. M., Juett, L. A., Cobb, J., Hulston, C. J., Mears, S. A., & James, L. J. (2022b). Apple puree as a natural fructose source provides an effective alternative to artificial fructose sources for fuelling endurance cycling performance in males. Nutraceuticals, 2(3), 205–217. https://doi.org/10.3390/nutraceuticals2030015
- Rietschier, H. L., Henagan, T. M., Earnest, C. P., Baker, B. L., Cortez, C. C., & Stewart, L. K. (2011). Sun-dried raisins are a cost-effective alternative to sports jelly beans in prolonged cycling. Journal of Strength and Conditioning Research, 25(11), 3150–3156. https://doi.org/10.1519/JSC.0b013e31820f5089
- Rollo, I., James, L., Croft, L., & Williams, C. (2012). The effect of carbohydrate-electrolyte beverage drinking strategy on 10-mile running performance. International Journal of Sport Nutrition and Exercise Metabolism, 22(5), 338–346. https://doi.org/10.1123/ijsnem.22.5.338
- Salvador, A. F., Mckenna, C. F., Alamilla, R. A., Cloud, R. M., Keeble, A. R., Miltko, A., Scaroni, S. E., Beals, J. W., Ulanov, A. V., Dilger, R. N., & Bauer, L. L. (2019). Potato ingestion is as effective as carbohydrate gels To support prolonged cycling performance. Journal of Applied Physiology, 127(6), 1651–1659. https://doi.org/10.1152/japplphysiol.00567.2019
- Shanely, R., Nieman, D., Perkins-Veazie, P., Henson, D., Meaney, M., Knab, A., & Cialdell-Kam, L. (2016). Comparison of watermelon and carbohydrate beverage on exercise-induced alterations in systemic inflammation, immune dysfunction, and plasma antioxidant capacity. Nutrients, 8(8), 518. https://doi.org/10.3390/nu8080518
- Tiller, N. B., Roberts, J. D., Beasley, L., Chapman, S., Pinto, J. M., Smith, L., Wiffin, M., Russell, M., Sparks, S. A., Duckworth, L., & O’Hara, J. (2019). International society Of sports nutrition position stand: Nutritional considerations For single-stage ultra-marathon training And racing. Journal of the International Society of Sports Nutrition, 16(1), 1–23. https://doi.org/10.1186/s12970-019-0312-9
- Too, B. W., Cicai, S., Hockett, K. R., Applegate, E., Davis, B. A., & Casazza, G. A. (2012). Comparison of coconut water and a carbohydrate-electrolyte sport drink on measures of hydration and physical performance in exercise-trained men. Journal of the International Society of Sports Nutrition, 9(1), 1–9. https://doi.org/10.1186/1550-2783-9-1
- van Wijck, K., Lenaerts, K., Grootjans, J., Wijnands, K. A., Poeze, M., Van Loon, L. J., Dejong, C. H., & Buurman, W. A. (2012). Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. American Journal of Physiology-Gastrointestinal and Liver Physiology, 303(2), G155–G168. https://doi.org/10.1152/ajpgi.00066.2012

