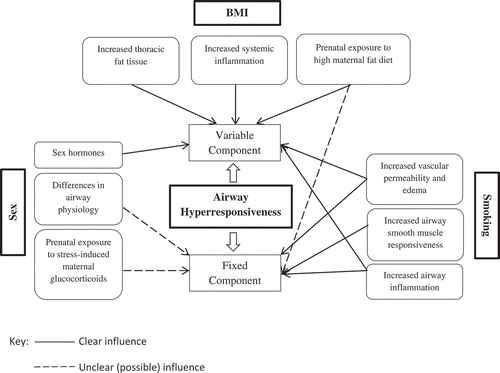
Airway hyperresponsiveness (AHR) is the exaggerated narrowing of the airways in response to physical or chemical stimuli. It is a core characteristic of asthma, but is also present in chronic obstructive pulmonary disease (COPD) patients and the general asymptomatic population. Nowadays, experts agree that AHR has a fixed and a variable component. Generally, the fixed component reflects structural changes of the airways (i.e. airway remodeling) and the variable component reflects airway inflammation [Citation1]. The primary mechanical cause of airway narrowing is contraction of the airway smooth muscle (ASM). The sensitivity of ASM contraction differs between individuals and within the same individual over time. Thus, it is important not to merely focus on differences in ASM contractility, but rather broaden our focus when trying to understand the complexities of AHR by studying various other internal and external factors that contribute to differences in AHR. These internal and external factors can be modifiable: e.g. smoking, body mass index (BMI), and air pollution, or non-modifiable: e.g. sex and age. In the current editorial, we focus on three important risk factors for AHR (i.e. sex, smoking, and BMI), and discuss the existing knowledge on their role in AHR development or severity in relation to understanding the mechanisms underlying AHR.
1. Sex
Many studies have investigated sex-differences in asthma and other respiratory health outcomes. In childhood, asthma is more prevalent in boys whereas it is more common among females in adulthood [Citation2]. Interestingly, this sex-specific shift around puberty is also seen in the non-asthmatic general population [Citation3] and in animal studies [Citation4]. The turning point for this sex-specific shift is puberty, which underlines the role of sex hormones. Indeed, the female sex hormone estrogen is a risk factor for AHR, while protective effects are seen for the male sex hormone testosterone, as it decreases Th2-mediated airway inflammation [Citation2,Citation4]. Estrogen increases goblet cell hyperplasia and the release of IL-13, making females more prone to AHR [Citation5]. Animal studies support this by showing less AHR and airway inflammation in ovariectomized mice and mice deficient in specific estrogen receptors, while lack of testosterone as in castrated male mice increased eosinophil and lymphocyte count and led to airway inflammation [Citation2]. Since sex hormones affect airway inflammation, they likely act on the variable component of AHR. This implies that the effects can be reversible, which is partly reflected by a lower incidence of asthma in post-menopausal women [Citation6]. However, the associations between menopause and AHR have not been studied yet. Another mechanism underlying the sex-specific differences in AHR could be airway physiology, with adult females having lower lung function and narrower airways than males, resulting in increased susceptibility of females to develop AHR [Citation7]. In childhood, lung volumes are not different between boys and girls but boys have lower flow rates than girls indicating narrower airways in boys [Citation8]. A final explanation for the sex-differences in AHR is the different effect of specific environmental factors in males and females. Examples of these are smoking and BMI, with females generally being more susceptible to their effects. Recently, it was shown that prenatal stress also has a sex-differential effect on AHR: female mice subjected to prenatal stress had more severe AHR than males [Citation9]. The high glucocorticoid secretion in mothers as a result of stress may lower the levels of maternal progesterone which can increase the risk of asthma and AHR in female offspring since they have lower levels of progesterone expression in their lungs than males [Citation9,Citation10]. In summary, the association between sex and AHR depends on age, with a higher AHR prevalence in males during childhood and in females during adulthood. Sex-hormones most likely act on the variable component of AHR while differences in airway physiology contribute to the fixed component. Interestingly, airway physiology changes from childhood to adulthood and this indicates that the fixed AHR component might not be entirely stable over a lifetime.
2. Cigarette smoking
A higher number of pack-years and a longer duration of cigarette smoking are both established risk factors for the development of AHR. Smoking contributes to both the variable AHR component, mainly by inducing airway inflammation, and the fixed AHR component, since excessive and prolonged smoking may lead to airway remodeling. Studies have shown higher levels of airway inflammation in smokers compared to non-smokers [Citation11]. Additionally, smoking increases airway vascular permeability and causes mucosal edema, which further contribute to AHR [Citation12]. One possible mechanism underlying the association between smoking and the fixed component of AHR might be the increase in ASM responsiveness via increased release and entry of Ca2+. Calcium regulating proteins like CD38 and TRPC3 have increased expression in smokers, adversely affecting the role of Ca2+ in ASM function [Citation13]. Studies on both animal and humans have shown that cigarette smoking induces the production of pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α alongside a surge in inflammatory cells [Citation14]. Specifically, cigarette smoke has been shown to affect polarization (activation) of M1 macrophages, making these macrophages, which are abundant in small airways, increase the release of IL-23 and IL-1β and promote cellular responses resulting in AHR [Citation15]. Chronic smoking can also increase the excitability of the vagal bronchopulmonary sensory nerves causing severe bronchoconstriction [Citation16]. Secondhand smoking can potentially contribute to AHR. Animal studies show that secondhand smoking from mothers increased airway resistance and activated inflammatory mechanisms in the offspring [Citation17]. Interestingly, quitting smoking leads to a lower AHR severity over time, but it does not entirely normalize AHR in all subjects, indicating that the extensive structural alterations to the airways in some individuals are irreversible [Citation18].
3. Body mass index
Studies have shown that higher BMI is associated with a higher prevalence of AHR, although not all studies agree on this. One postulated mechanical mechanism relating higher BMI to AHR is the accumulation of fat tissue in the thoracic cavity, which is associated with lower lung volumes and with a higher vulnerability to small airway closure, both important risk factors for AHR [Citation19]. In addition, overweight and obesity are associated with higher levels of systemic inflammation, and this might contribute to AHR. However, it is unclear whether obesity causes AHR or whether AHR leads to weight gain due to respiratory symptoms that limit physical activity. Longitudinal studies on adolescents and adults showed that obesity was associated with the development of AHR after 4–6 years [Citation20,Citation21], but a recent study on obese children did not find this higher risk of AHR in adulthood [Citation22]. Studies on weight loss in asthmatics showed that weight-loss is associated with a decrease in AHR [Citation23], which supports the association between higher BMI and AHR. However, research on the effect of weight loss on the severity or prevalence of AHR in the general population is generally lacking. Overall, the evidence suggests that high BMI does not contribute to the fixed component of AHR since no irreversible changes in AHR are observed. In light of this, it is interesting to note that a recent murine study showed that offspring of mothers on a high-fat diet during pregnancy had a higher prevalence of AHR compared to offspring of mothers on a normal fat diet [Citation24]. Whether this higher AHR-prevalence is reversible over time remains to be established.
By reviewing the evidence on three well-established risk factors for AHR (i.e. sex, smoking, and BMI, see for a schematic presentation of the evidence), we found support for the notion that AHR consists of a fixed component, reflecting airway remodeling, and a variable component, reflecting airway inflammation. Both sex and smoking exert their effects on AHR via both components whereas BMI mainly targets the variable component of AHR. Interestingly, the sex-specific differences in the fixed component of AHR indicate there might be changes in this fixed component over time. Further research is warranted to determine the exact sex-specific changes over time in lung physiology as this may identify targets for preventing or treating AHR. Both smoking and high BMI are risk factors that can be modified and this editorial indicates that, especially for smoking, it is important to quit as early as possible so that irreversible airway damage will be prevented. Research on these key risk factors for AHR can help in unraveling AHR further, as the current level of evidence is not lucid enough. For example, further research is warranted to pinpoint exact sex-specific changes over time in lung physiology and if this contributes to permanent changes in airway structure. The influence of novel factors such as maternal diet and prenatal exposure to stress and whether these have an enduring effect on lung physiology and AHR, specifically the fixed component, should also be further investigated.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewers disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006;118(3):551–559.
- Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep. 2017 Mar 23;17(3):19.
- Uekert SJ, Akan G, Evans MD, et al. Sex-related differences in immune development and the expression of atopy in early childhood. J Allergy Clin Immunol. 2006 Dec;118(6):1375–1381.
- McKenzie R, Burton MD, Royce SG, et al. Age and sex influences on airway hyperresponsiveness. J Asthma. 2010;47(6):651–654.
- Cai Y, Zhou J, Webb DC. Estrogen stimulates Th2 cytokine production and regulates the compartmentalisation of eosinophils during allergen challenge in a mouse model of asthma. Int Arch Allergy Immunol. 2012;158(3):252–260.
- Troisi RJ, Speizer FE, Willett WC, et al. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am J Respir Crit Care Med. 1995 Oct 20;152(4 Pt 1):1183–1188.
- Leynaert B, Bousquet J, Henry C, et al. Is bronchial hyperresponsiveness more frequent in women than in men? A population-based study. Am J Respir Crit Care Med. 1997 Nov;156(5):1413–1420.
- Hibbert ME, Couriel JM, Landau LI. Changes in lung, airway, and chest wall function in boys and girls between 8 and 12 yr. J Appl Physiol. 1984 Aug;57(2):304–308.
- Zazara DE, Perani CV, Solano ME, et al. Prenatal stress challenge impairs fetal lung development and asthma severity sex-specifically in mice. J Reprod Immunol. 2018 Feb;125:100–105.
- Hartwig IRV, Bruenahl CA, Ramisch K, et al. Reduced levels of maternal progesterone during pregnancy increase the risk for allergic airway diseases in females only. J Mol Med. 2014;92(10):1093–1104.
- Shiels MS, Katki HA, Freedman ND, et al. Cigarette smoking and variations in systemic immune and inflammation markers. JNCI J Natl Cancer Inst. 2014 Nov;106(11).
- Daffonchio L, Hernandez A, Melillo G, et al. Effectiveness of levodropropizine against cigarette smoke-induced airway hyperreactivity: possible mechanism. Eur J Pharmacol. 1993 Apr;228(5–6):257–261.
- Wylam ME, Sathish V, VanOosten SK, et al. Mechanisms of cigarette smoke effects on human airway smooth muscle. PLoS One. 2015;10(6):e0128778.
- Moerloose KB, Robays LJ, Maes T, et al. Cigarette smoke exposure facilitates allergic sensitization in mice. Respir Res. 2006 Mar;7:49.
- Strzelak A, Ratajczak A, Adamiec A, et al. Tobacco smoke induces and alters immune responses in the lung triggering inflammation, allergy, asthma and other lung diseases: a mechanistic review. Int J Environ Res Public Health. 2018 May 21;15(5):1033.
- Kou YR, Kwong K, Lee L-Y. Airway inflammation and hypersensitivity induced by chronic smoking. Respir Physiol Neurobiol. 2011 Sep;178(3):395–405.
- Singh SP, Gundavarapu S, Pena-Philippides JC, et al. Prenatal secondhand cigarette smoke promotes Th2 polarization and impairs goblet cell differentiation and airway mucus formation. J Immunol. 2011 Nov 1;187(9):4542–4552.
- Paoletti P, Carrozzi L, Viegi G, et al. Distribution of bronchial responsiveness in a general population: effect of sex, age, smoking, and level of pulmonary function. Am J Respir Crit Care Med. 1995 Jun;151(6):1770–1777.
- Burgess JA, Matheson MC, Diao F, et al. Bronchial hyperresponsiveness and obesity in middle age: insights from an Australian cohort. Eur Respir J. 2017 Sep;50(3):1602181.
- Litonjua AA, Sparrow D, Celedon JC, et al. Association of body mass index with the development of methacholine airway hyperresponsiveness in men: the normative aging study. Thorax. 2002 Jul;57(7):581–585.
- Rasmussen F, Hancox RJ, Nair P, et al. Associations between airway hyperresponsiveness, obesity and lipoproteins in a longitudinal cohort. Clin Respir J. 2013 Jul;7(3):268–275.
- Toennesen LL, Bjerregaard A, Porsbjerg C, et al. Overweight in childhood and adolescence: does it lead to airway hyperresponsiveness in adulthood? J Asthma. 2018;55(2):137–144.
- Pakhale S, Baron J, Dent R, et al. Effects of weight loss on airway responsiveness in obese adults with asthma. Chest. 2015 Jun;147(6):1582–1590.
- MacDonald KD, Moran AR, Scherman AJ, et al. Maternal high-fat diet in mice leads to innate airway hyperresponsiveness in the adult offspring. Physiol Rep. 2017 Mar;5(5):e13082.