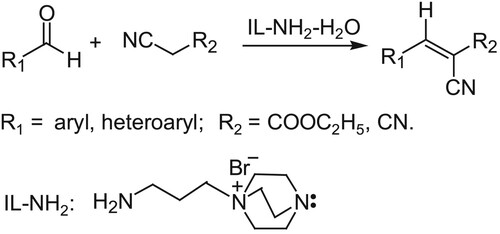
ABSTRACT
Functional ionic liquids have been widely used in the fields of synthetic chemistry, analytical chemistry, electrochemistry and material chemistry owing to their excellent properties such as easy structure modification and adjustable properties. In this work, a series of basic functionalized ionic liquids were prepared from 1, 4-diazabicyclo [2.2.2] octane (DABCO) or hexamethylenetetramine (HMTA) with various halogenated hydrocarbons, then these functional ionic liquids and water were used as solvent-catalyst system in the Knoevenagel reaction of various aldehydes with ethyl cyanoacetate or malononitrile, the influence of the structure of the ionic liquid and the content of water was investigated. The results showed that the composite solvent-catalyst system mixed by the functional ionic liquid N-(3-aminopropyl)-1,4-diazabicyclo [2.2.2] octane bromide with water can effectively promote the Knoevenagel reaction and the alkalinity of amine and hydrogen bond play an important role. Aromatic aldehydes with electron-donating or electron-withdrawing groups can obtain the target product at an excellent yield (79-99%). It is worth noting that the polycyclic aromatic aldehydes with high steric hindrance, 86%-95% yields of the object products were also obtained after 15 h-25 h. Furthermore, the catalyst could be reused for 6 times without any loss in its catalytic activity.
The composite solvent-catalyst system mixed by the Functional ionic liquid N-(3-aminopropyl)-1,4-diazabicyclo[2.2.2]octane bromide with water can effectively promote the Knoevenagel reaction, even for the electron-rich aromatic aldehydes with poor reactivity, high yields of the target products and fast reaction rate were also obtained at room temperature.
1. Introduction
In synthetic chemistry, Knoevenagel reaction is an important reaction for the construction of carbon–carbon double bond, which can provide important intermediates for the synthesis of drugs, cosmetics and advanced materials (Citation1). Knoevenagel reaction was typically catalyzed by weak organic bases such as pyridine and triethylamine (Citation2). In recently, Lewis acids such as ZnCl2(Citation3), CuCl2(Citation4), InCl3 (Citation5), LaCl3 (Citation6), porous materials include acid–base bifunctional mesoporous silica (Citation7), porous CuxMo1–xOy (Citation8), proline-Cu complex(Citation9), MOFs (Citation10, Citation11), magnetic nanoparticles (Citation12), magnesium oxide-stabilized Pickering emulsion (Citation13), molybdenum carbide (Citation14), cage (Citation15), straw ash (Citation16), chitosan nanofibers (Citation17), phenolic quinone binary catalyst (Citation18) and succinate-DABCO (Citation19) have been reported successively in the Knoevenagel reaction. Although these catalysts have achieved a good catalytic effect, however, both homogeneous and heterogeneous catalysts often suffer from long reaction time, complicated treatment, using toxic organic solvents or poor recycling. To solve these problems and to develop a simple and green method, ionic liquids were introduced in the Knoevenagel reaction as an environmentally friendly solvent (Citation20). The ionic liquids [BPy][NO3] (Citation21), [Hmim]PF6 (Citation22), [Bmim]BF4 (Citation23), [Et3NH][HSO4] (Citation24), [Bmim]OH(Citation25), especially some functional ionic liquids such as [C4DABCO][BF4] (Citation26), [DABCO-H][AcO] (Citation27), [DBU][Lac] (Citation28), [DABCO-PDO][PF6] (Citation29) were used one after another in the Knoevenagel reaction and the great improvement was indeed achieved. However, it is still a challenge for the Knoevenagel reaction with electron-rich aromatic aldehydes, poor reaction yields and long reaction time have to be faced frequently. In 2016, Hilvert reported the Knoevenagel reaction catalyzed by enzyme (Citation30), under the optimum reaction conditions, the Knoevenagel reaction of electron-rich aromatic aldehydes achieved relatively good effect, various evidences showed that the reaction was prompted by the amino group in the enzyme. Based on the beneficial effect of enzyme catalyst and the versatility of functional ionic liquid used in the fields of synthesis and catalysis (Citation31–40), extraction (Citation41), electrochemistry (Citation42,Citation43), analytics (Citation44), biotechnology (Citation45), etc., we speculate that the functional ionic liquid containing amine unit can promote the Knoevenagel reaction more efficiently. In order to verify our predication, a series of amine functionalized ionic liquids containing amino, hydroxyl and different carbon chain structures were prepared and applied in the Knoevenagel reaction. It was found that the Knoevenagel reaction was significantly promoted by the composite solvent-catalyst system consisting of N-aminopropyl-1, 4-diazabicyclic [2.2.2] octane bromide salt (IL-NH2) and water, even for the Knoevenagel reaction of electron-rich aromatic aldehydes with poor activity and high steric hindrance, the target products can be also obtained with excellent yields in short reaction time (Scheme 1). Further studies showed that some Knoevenagel reaction products prepared by this method are D-π-A strong fluorescent molecules with good pH response.
2. Materials and methods
2.1. Materials and products
All chemicals were purchased from commercial suppliers and were used without further purification. For the NMR spectra of all products, see Supplementary Materials.
2.2. Methods
1H and 13C NMR were recorded on the Bruker Avance NEO 600M, Bruker Avance NEO 500M or Bruker Avance DPX 400 spectrometer in DMSO-d6 or CDCl3 respectively. Chemical shifts were reported in parts per million (δ), relative to the internal standard of tetramethylsilane (TMS). The elemental analyses were performed in the Institute of Chemistry, Chinese Academy of Sciences. Sonication was performed in Kunshan KQ-400KDE ultrasonic cleaner (with a frequency 40 kHz and a nominal power 400 W), Melting points were determined using WRS-1B digital melting point meter and were not corrected.
2.3. Preparation and characterization of the ionic liquids
2.3.1. Preparation of ionic liquid [DABCO-CH2CH2OH]Br
To a solution of 1,4-diazabicyclo [2.2.2] octane (DABCO) (11.2 g, 0.1 mol) in ethyl acetate (30 mL) was added 2-bromoethanol (7 mL, 0.1 mol), and the mixture was refluxed for 3 h, the crude product [DABCO-CH2CH2OH]Br was formed. Then the crude product was purified by the recrystallization with 15 mL ethyl acetate, and the residual solvent was removed in vacuum to give the product [DABCO-CH2CH2OH]Br (22.5 g, 95%) as a white viscous product. 1H NMR (400 MHz, DMSO-d6) δ: 4.77 (s, 2H), 4.16 (s, 1H), 3.82 (s, 2H), 3.43 (s, 6H), 3.01 (s, 6H); 13C NMR (101 MHz, DMSO-d6) δ: 65.68, 54.44, 52.76, 45.01. Anal. Calcd for C8H17BrN2O: C, 40.52; H, 7.23; N, 11.81. Found: C, 40.26; H, 7.15; N, 11.89.
2.3.2. Preparation of ionic liquid [DABCO-CH2CH2CH2NH2]Br
The same procedure as that described above for [DABCO-CH2CH2OH]Br was followed, after vacuum drying, 23.50 g gray solid was obtained with a yield of 94%. m. p. 180.5°C; 1H NMR (DMSO-d6, 400 MHz) δ: 3.98 (s, 2H), 3.30 (s, 6H), 3.02 (s, 8H), 2.90 (s, 2H), 2.02 (s, 2H); 13C NMR (DMSO-d6, 151 MHz) δ: 52.12, 51.12, 45.09, 44.49, 20.15; Anal. calcd for C9H20BrN3: C 43.21, H 8.06; N 16.80; found C 43.28, H 8.21, N 16.84.
2.3.3. Preparation of ionic liquid [DABCO-CH2CH2CH2CH3]Br
The same procedure as that described above for [DABCO-CH2CH2OH]Br was followed, after vacuum drying, 23.90 g white solid was obtained with a yield of 96%. m. p. 101.5°C (lit. [Citation46] m. p. 100°C); 1H NMR (400 MHz, CDCl3) δ: 3.69 (t, J = 7.4 Hz, 6H), 3.52 (J = 7.9 Hz, 2H), 3.28 (t, J = 7.4 Hz, 6H), 1.79 (dt, J = 16.1, 7.9 Hz, 2H), 1.43 (dd, J = 14.8, 7.4 Hz, 2H), 1.00 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ: 64.36, 52.47, 45.42, 23.94, 19.74, 13.70; Anal. calcd for C10H21BrN2: C 48.20, H 8.49; N 11.24; found C 48.32, H 8.45, N 11.08.
2.3.4. Preparation of ionic liquid [DABCO-CH2CH3]Br
The same procedure as that described above for [DABCO-CH2CH2OH]Br was followed, after vacuum drying, 21.66 g white solid was obtained with a yield of 98%. m. p. 35.5°C (lit. [Citation46] m.p. 35°C); 1H NMR (400 MHz, CDCl3) δ: 3.68 (dd, J = 16.3, 9.7 Hz, 8H), 3.28 (t, J = 6.7 Hz, 6H), 1.44 (t, J = 6.6 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 59.50, 51.70, 45.18, 7.91; Anal. calcd for C8H17BrN2: C 43.45, H 7.75; N 12.67; found C 43.29, H 7.82, N 12.78.
2.3.5. Preparation of ionic liquid [DABCO-CH2C6H5]Br
The same procedure as that described above for [DABCO-CH2CH2OH]Br was followed, after vacuum drying, 28.02 g white solid was obtained with a yield of 99%. m. p. 263.9°C (lit. [Citation46] m. p. 263°C); 1H NMR (400 MHz, CDCl3) δ: 7.66 (d, J = 7.7 Hz, 2H), 7.42 (t, J = 7.3 Hz, 3H), 5.03 (s, 2H), 3.73 (t, J = 7.4 Hz, 6H), 3.17 (t, J = 7.4 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ: 133.44, 130.63, 129.25, 126.49, 67.05, 51.89, 45.37; Anal. calcd for C13H9BrN2: C 55.13, H 6.76; N 9.89; found C 55.24, H 6.81, N 9.94.
2.3.6. Preparation of ionic liquid [HMTA-CH2CH2CH2NH2]Br
The same procedure as that described above for [DABCO-CH2CH2OH]Br was followed, after vacuum drying, 25.02 g white solid was obtained with a yield of 90%. m. p. 196.8°C; 1H NMR (DMSO-d6, 400 MHz) δ: 5.09 (s, 6H), 4.60 (d, J = 12.5 Hz, 3H), 4.47 (d, J = 12.5 Hz, 3H), 3.98 (t, J = 7.9 Hz, 2H), 2.98 (s, 2H), 2.77 (t, J = 7.9 Hz, 2H), 1.97∼2.02 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ: 78.18, 74.27, 56.00, 43.23, 21.54. Anal. calcd for C9H20BrN5: C 38.86, H 7.25, N 25.17; found C 38.78, H 7.21, N 25.24.
2.3.7. Preparation of ionic liquid [HMTA-CH2CH2OH]Br
The same procedure as that described above for [DABCO-CH2CH2OH]Br was followed, after vacuum drying, 25.18 g white solid was obtained with a yield of 95%. m. p. 206.6°C (lit. [Citation46] m. p. 206°C); 1H NMR (400 MHz, DMSO-d6) δ: 5.37 (s, 1H), 5.24 (s, 6H), 4.62 (d, J = 12.5 Hz, 3H), 4.53 (d, J = 12.5 Hz, 3H), 3.81 (s, 2H), 2.93 (s, 2H); 13C NMR (101 MHz, DMSO-d6) δ: 78.86, 71.47, 58.69, 53.48; Anal. calcd for C8H17BrN4O: C 36.24, H 6.46, N 21.13; found C 37.31, H 6.39, N 21.22.
2.3.8. Preparation of ionic liquid [HMTA-CH2CH3]Br
The same procedure as that described above for [DABCO-CH2CH2OH]Br was followed, after vacuum drying, 24.40 g white solid was obtained with a yield of 98%. m. p. 217.2°C (lit. [Citation46] m. p. 217°C); 1H NMR (400 MHz, DMSO-d6) δ: 5.12 (s, 6H), 4.61 (d, J = 12.4 Hz, 3H), 4.49 (d, J = 12.4 Hz, 3H), 2.89 (q, J = 7.4 Hz, 2H), 1.20 (t, J = 7.4 Hz, 3H). 13C NMR (101 MHz, DMSO-d6) δ: 77.64, 74.27, 51.46, 5.82; Anal. calcd for C8H17BrN4: C 38.57, H 6.88, N 22.49; found C 38.49, H 6.90, N 22.41.
2.4 General procedure for the Knoevenagel reaction
To a stirred mixture of 3 g (12 mmol) [DABCO-CH2CH2CH2NH2]Br with 1 ml H2O at room temperature was added 5 mmol aldehyde and 1.1 mL (1.13 g, 5 mmol) ethyl cyanoacetate, the reaction was monitored by thin layer chromatography (TLC). Upon completion, the saturated brine (10 mL) was added and the object product solidified from the reaction mixture. The crude product was purified by recrystallization from 95% ethanol and the desired products were further characterized by melting point determination and NMR spectroscopy. Compared with the reported NMR data and the melting point data of products (Citation1), the reaction is highly stereoselective with only an E-geometry. The spectral data of all products are listed as follows.
2.4.1. Ethyl-2-cyano-3-anthracenylacrylate (, entry 1)
Yellow solid; m. p. 188.2–188.8°C (lit. [Citation47] m. p. 187°C); 1H NMR (400 MHz, DMSO-d6) δ: 9.41 (s, 1H,CH = ), 8.89 (s, 1H, ArH), 8.27 (d, J = 7.9 Hz, 2H, ArH), 8.60 (d, J = 8.2 Hz, 2H, ArH), 7.70 (t, J = 7.3 Hz, 4H, ArH), 4.49 (q, J = 6.9 Hz, 2H, CH2), 1.45 (t, J = 6.9 Hz, 3H, CH3). 13C NMR (151 MHz, DMSO-d6) δ: 161.17, 155.79, 130.80, 130.44, 129.43, 128.67, 127.78, 126.44, 125.33, 123.82, 114.82, 113.90, 62.98, 14.45.
2.4.2. Ethyl-2-cyano-3-(4-diphenylamino-phenyl)acrylate (, entry 2)
Light yellow solid; m. p. 100.2–100.9°C; 1H NMR (400 MHz, DMSO-d6) δ: 8.24 (s, 1H, CH = ), 8.00 (d, J = 8.5 Hz, 2H, ArH), 7.78 (d, J = 8.5 Hz, 2H, ArH), 7.29 (dt, J = 13.9, 7.9 Hz, 10H, Ph), 4.34 (q, J = 7.0 Hz, 2H, CH2), 1.34 (t, J = 7.0 Hz, 3H, CH3); 13C NMR (151 MHz, DMSO-d6) δ: 163.18, 154.24, 145.98, 145.49, 130.46, 129.00, 126.83, 123.04, 118.59, 118.31, 117.03, 96.85, 62.32, 14.50. IR (KBr) ν: 2210, 1715, 1586, 1260, 1200,1180, 832 cm−1; Anal. calcd for C24H20N2O2: C 78.24, H 5.47, N 7.60; found C 78.28, H 5.51, N 7.69.
2.4.3. Ethyl-2-cyano-3-(6-methyl-2-oxo-2H-chromen-4-yl)acrylate (, entry 3)
Red brown oil; 1H NMR (400 MHz, DMSO-d6) δ: 9.02 (s, 1H, CH = ), 8.74 (s, 1H), 8.62 (t, J = 7.5 Hz, 1H), 8.18 (d, J = 7.5 Hz, 1H), 8.00 (s, 1H), 4.42–4.32 (q, J = 20.0 Hz, 2H), 2.42 (s, 3H), 1.25 (t, J = 20 Hz, 3H); 13C NMR (101 MHz, CDCl3) δ: 167.05, 166.48, 158.79, 147.62, 145.61, 134.70, 130.28, 128.07, 126.81, 122.39, 117.67, 115.45, 109.78, 60.47, 20.96, 13.96. IR (KBr) ν: 3068, 2924, 2193, 1734, 1701, 1597, 1456, 1421, 1259 cm−1; Anal. calcd for C16H13NO4: C 67.84, H 4.63, N 4.94; found C 67.92, H 4.58, N 5.09.
2.4.4. Ethyl-2-cyano-3-[6-(2-cyano-2-ethoxycarbonyl-vinyl)-9-ethyl-9H-3-carbazolyl] acrylate (, entry 4)
Yellow solid; m. p. 198.4–199.5°C; 1H NMR (400 MHz, DMSO-d6) δ: 8.90 (s, 2H), 8.59 (s, 2H), 8.38 (d, J = 8.7 Hz, 2H), 7.98 (d, J = 8.7 Hz, 2H), 4.62 (q, J = 6.7 Hz, 2H), 4.41 (q, J = 6.7 Hz, 4H), 1.45–1.38 (m, 9H); 13C NMR (151 MHz, DMSO-d6) δ: 165.59, 158.05, 133.16, 129.77, 120.63, 118.55, 118.35, 117.41, 116.51, 102.13, 59.49, 47.45, 14.72, 12.83. IR (KBr) ν: 2923, 2219, 1718, 1579, 1480, 1229, 808 cm−1; Anal. calcd for C26H23N3O4: C 70.73, H 5.25; N 9.52; found C 70.78, H 5.39, N 9.49.
2.4.5. Ethyl-2-cyano-3-pyrenyl acrylate (, entry 5)
Yellow solid; m. p. 139.4–140.2°C; 1H NMR (400 MHz, DMSO-d6) δ: 9.33 (s, 1H), 8.68 (d, J = 8.2 Hz, 1H), 8.40 (s, 6H), 8.26 (d, J = 9.0 Hz, 1H), 8.18 (t, J = 7.6 Hz, 1H), 4.43 (q, J = 7.0 Hz, 2H), 1.40 (t, J = 7.0 Hz, 3H); 13C NMR (151 MHz, DMSO-d6) δ: 162.33, 152.81, 134.35, 130.74, 130.09, 127.62, 127.33, 126.47, 125.30, 124.00, 123.61, 122.73, 116.33, 105.41, 62.90, 14.51. IR (KBr) ν: 3008, 2998, 2222, 1716, 1605, 1265 cm−1; Anal. calcd for C22H15NO2: C 81.21; H 4.65; N 4.30; found C 81.28, H 4.59, N 4.39.
2.4.6. Ethyl-2-cyano-3-(4-phenylphenyl)acrylate (, entry 6)
Green solid; m. p. 121.6–122.4°C (lit. [Citation39] m. p. 121.8°C); 1H NMR (400 MHz, DMSO-d6) δ: 8.17 (d, J = 8.0 Hz, 2H), 7.93 (d, J = 8.0 Hz, 2H), 7.80 (d, J = 7.6 Hz, 2H), 7.54–7.45 (m, 3H), 4.34 (q, J = 7.0 Hz, 2H), 1.32 (t, J = 7.0 Hz, 3H); 13C NMR (151 MHz, DMSO-d6) δ: 162.34, 154.87, 145.13, 138.91, 132.03, 130.78, 129.57, 129.11, 127.80, 127.43, 116.22, 102.40, 62.80, 14.44.
2.4.7. Ethyl-2-cyano-3-(4-hydroxyphenyl)acrylate (, entry 7)
Yellow solid; m. p. 170.8–171.2°C (lit. [Citation49] m. p. 170–171°C); 1H NMR (400 MHz, DMSO-d6) δ: 10.83 (s, 1H), 8.24 (s, 1H), 8.00 (d, J = 8.3 Hz, 2H), 6.95 (d, J = 8.3 Hz, 2H), 4.29 (q, J = 7.0 Hz, 2H), 1.29 (t, J = 7.0 Hz, 3H); 13C NMR (151 MHz, DMSO-d6) δ: 163.32, 163.03, 155.10, 134.40, 122.95, 116.88,116.81, 97.51, 62.38, 14.48.
2.4.8. Ethyl-2-cyano-3-(4-ammoniaphenyl)acrylate (, entry 8)
Yellow solid; m. p. 162.5–162.9°C (lit. [Citation48] m. p. 162.5°C); 1H NMR (400 MHz, DMSO-d6) δ: 8.03 (s, 1H), 7.84 (d, J = 8.3 Hz, 2H), 6.73 (s, 2H), 6.66 (d, J = 8.3 Hz, 2H), 4.25 (q, J = 7.0 Hz, 2H), 1.27 (t, J = 7.0 Hz, 3H); 13C NMR(151 MHz, DMSO-d6) δ: 164.01, 155.51, 154.83, 134.88, 118.82, 118.03, 113.97, 91.67, 61.82, 14.58.
2.4.9. Ethyl-2-cyano-3-(3-methoxy-4-hydroxyphenyl)acrylate (, entry 9)
Yellow solid; m. p. 107.8–108.4°C (lit. [Citation50] m. p. 108–109°C); 1H NMR (400 MHz, DMSO-d6) δ: 8.23 (s, 1H), 7.77 (s, 1H), 7.63 (d, J = 8.0 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 4.29 (d, J = 6.9 Hz, 2H), 3.82 (s, 3H), 2.99 (s, 1H), 1.30 (t, J = 6.9 Hz, 3H); 13C NMR (151 MHz, DMSO-d6) δ: 163.12, 155.25, 153.71, 148.32, 127.78, 129.00, 122.97, 117.13, 116.53, 114.38, 96.98, 55.97, 44.68, 14.48.
2.4.10. Ethyl-2-cyano-3-(2-methoxyphenyl)acrylate (, entry 10)
Yellow solid; m. p. 74.0–74.7°C (lit. [Citation51] m. p. 72–74°C); 1H NMR (500 MHz, CDCl3) δ: 8.75 (s, 1H), 8.28 (d, J = 7.9 Hz, 1H), 7.51 (t, J = 7.9 Hz, 1H), 7.05 (t, J = 7.6 Hz, 1H), 6.96 (d, J = 8.4 Hz, 1H), 4.38 (q, J = 7.1 Hz, 2H), 3.90 (s, 3H), 1.39 (t, J = 7.1 Hz, 3H). 13C NMR(126 MHz, CDCl3) δ: 162.82, 159.23, 149.76, 134.97, 129.35, 120.95, 120.73, 115.89, 111.19, 102.40, 62.48, 55.77, 14.19.
2.4.11. Ethyl-2-cyano-3-(4-methoxyphenyl)acrylate (, entry 11)
Yellow solid; m. p. 82.1–82.8°C (lit. [Citation51] m. p. 81–82°C); 1H NMR (400 MHz, CDCl3) δ: 8.17 (s, 1H), 8.01 (d, J = 8.8 Hz, 2H), 7.00 (d, J = 8.9 Hz, 2H), 4.37 (q, J = 7.1 Hz, 2H), 3.90 (s, 3H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR(126 MHz, CDCl3) δ: 163.79, 163.10, 154.34, 133.62, 124.39, 116.19, 114.78, 99.41, 62.41, 55.62, 14.19.
2.4.12. Ethyl-2-cyano-3-(2, 4-dimethoxyphenyl)acrylate (, entry 12)
Yellow solid; m. p. 140.5–140.9°C (lit. [Citation48] m. p. 140.5°C); 1H NMR (400 MHz, DMSO-d6) δ: 8.52 (s, 1H), 8.22 (d, J = 9.0 Hz, 1H), 6.77 (d, J = 9.0 Hz, 1H), 6.73 (s, 1H), 4.29 (q, J = 7.0 Hz, 2H), 3.91 (d, J = 11.2 Hz, 6H), 1.29 (t, J = 7.0 Hz, 3H). 13C NMR(151 MHz, DMSO-d6) δ: 166.32, 163.12, 161.79, 148.15, 130.48, 116.94, 113.16, 107.74, 98.76, 97.81, 62.41, 56.78, 56.40, 14.48.
2.4.13. Ethyl-2-cyano-3-(4-dimethylamino-phenyl) acrylate (, entry 13)
Yellow solid; m. p. 125.1–125.7°C (lit. [Citation48] m. p. 125.3°C);1H NMR (400 MHz, DMSO-d6) δ: 8.11 (s, 1H), 7.96 (d, J = 8.6 Hz, 2H), 6.84 (d, J = 8.6 Hz, 2H), 4.26 (q, J = 7.0 Hz, 2H), 3.09 (s, 6H), 1.28 (t, J = 7.0 Hz, 3H). 13C NMR(151 MHz, DMSO-d6) δ: 163.90, 154.57, 154.14, 134.21, 118.72, 117.98, 112.11, 92.45, 61.87, 43.29, 14.57.
2.4.14. Ethyl-2-cyano-3-phenylacrylate (, entry 14)
White solid; m. p. 51.2–51.9°C (lit. [Citation49] m. p. 50–52°C); 1H NMR (400 MHz, DMSO-d6) δ: 8.25 (s, 1H), 7.99 (d, J = 7.2 Hz, 2H), 7.58–7.49 (m, 3H), 4.39 (q, J = 7.1 Hz, 2H), 1.40 (t, J = 7.1 Hz, 3H). 13C NMR(126 MHz, CDCl3) δ: 162.82, 155.37, 133.52, 131.83, 131.43, 129.35, 115.89, 102.40, 62.48, 14.19.
2.4.15. Ethyl-2-cyano-3-(4-chlorophenyl)acrylate (, entry 15)
White solid; m. p. 92.6–93.2°C (lit. [Citation52] m. p. 92–94°C); 1H NMR (400 MHz, CDCl3) δ: 8.18 (s, 1H, CH = ), 7.92 (d, J = 8.4 Hz, 2H, ArH), 7.46 (d, J = 8.4 Hz, 2H, ArH), 4.38 (q, J = 7.1 Hz, 2H, CH2), 1.39 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3) δ: 162.15, 153.27, 139.50, 132.18, 129.89, 129.63, 115.22, 103.53, 62.83, 14.13.
2.4.16. Ethyl-2-cyano-3-(4-fluorophenyl)acrylate (, entry 16)
White solid; m. p. 96.8–97.5°C (lit. [Citation51] m. p. 94–96°C); 1H NMR (400 MHz, CDCl3) δ: 8.22 (s, 1H), 8.04 (t, J = 8.6 Hz, 2H), 7.20 (t, J = 8.6 Hz, 2H), 4.38 (q, J = 7.1 Hz, 2H), 1.41 (t, J = 7.1 Hz, 3H). 13C NMR(126 MHz, CDCl3) δ: 164.40, 162.40, 153.47, 133.62, 127.90, 116.81, 115.43, 102.64, 62.80, 14.15.
2.4.17. Ethyl-2-cyano-3-(2, 4-dichlorophenyl)acrylate (, entry 17)
Yellow solid; m. p. 81.5–82.1°C (lit. [Citation53] m. p. 81–82°C); 1H NMR (400 MHz, CDCl3) δ: 8.63 (s, 1H), 8.22 (d, J = 9.0 Hz, 1H), 7.55 (s, 1H), 7.42 (d, J = 9.0 Hz, 1H), 4.43 (q, J = 7.1 Hz, 2H), 1.43 (t, J = 7.1 Hz, 3H). 13C NMR(101 MHz, CDCl3) δ: 162.40, 149.43, 139.34, 137.61, 130.59, 130.06, 128.34, 127.87, 114.16, 106.26, 62.80, 14.15.
2.4.18. Ethyl-2-cyano-3-(4-nitrophenyl)acrylate (, entry 18)
Yellow solid; m. p. 169.8–171.5°C (lit. [Citation53] m. p. 168–171°C); 1H NMR (400 MHz, CDCl3) δ: 8.37 (d, J = 8.8 Hz, 2H), 8.33 (s, 1H), 8.16 (d, J = 8.8 Hz, 2H), 4.45 (q, J = 7.1 Hz, 2H), 1.44 (t, J = 7.1 Hz, 3H). 13C NMR(126 MHz, CDCl3) δ: 161.41, 151.70, 149.76, 136.92, 131.51, 124.33, 114.51, 107.44, 63.35, 14.10.
2.4.19. Ethyl-2-cyano-3-(4-cyanophenyl)acrylate (, entry 19)
White solid; m. p. 168.6–169.2°C (lit. [Citation48] m. p. 168.7°C); 1H NMR (500 MHz, CDCl3) δ: 8.25 (s, 1H), 8.07 (d, J = 8.4 Hz, 2H), 7.80 (d, J = 8.3 Hz, 2H), 4.42 (q, J = 6.8 Hz, 2H), 1.42 (t, J = 7.5 Hz, 3H). 13C NMR(126 MHz, CDCl3) δ: 161.52, 152.21, 135.26, 132.86, 131.00, 117.68, 116.03, 114.60, 106.83, 63.27, 14.10.
2.4.20. Ethyl-2-cyano-3-(3-nitrophenyl)acrylate (, entry 20)
Yellow solid; m. p. 129.7–130.5°C (lit. [Citation51] m. p. 130–131°C); 1H NMR (400 MHz, CDCl3) δ: 8.72 (s, 1H), 8.42 (t, J = 6.6 Hz, 2H), 8.33 (s, 1H), 7.76 (t, J = 8.1 Hz, 1H), 4.43 (q, J = 7.1 Hz, 2H), 1.42 (t, J = 7.1 Hz, 3H). 13C NMR(126 MHz, CDCl3) δ: 161.51, 151.78, 149.76, 135.13, 132.85, 130.54, 127.00, 125.91, 114.54, 106.69, 63.24, 14.06.
2.4.21. Diethyl-3, 3'-(1, 4-phenylene)-bis(2-cyanoacrylate) (, entry 21)
White crystalline solid; m. p. 200.1–200.5°C (lit. [Citation54] m. p. 199–200°C); 1H NMR (400 MHz, CDCl3) δ: 8.26 (s, 2H), 8.10 (s, 4H), 4.41 (q, J = 7.1 Hz, 4H), 1.42 (t, J = 7.1 Hz, 6H); 13C NMR (101 MHz, CDCl3) δ: 161.41, 152.60, 135.16, 131.51, 114.51, 105.78, 63.35, 14.10.
2.4.22. 2-Benzylidene-malononitrile (, entry 22)
Light yellow solid; m. p. 80.6–81.2°C (lit. [Citation53] m. p. 79–80°C); 1H NMR (400 MHz, DMSO-d6) δ: 8.57 (s, 1H), 7.96 (d, J = 7.6 Hz, 2H), 7.61–7.71 (m, 3H). 13C NMR(151 MHz, DMSO-d6) δ: 162.01, 134.81, 131.72, 130.92, 129.96, 114.62, 113.64, 82.06.
2.4.23. 2-(4-Dimethylamino-benzylidene)-malononitrile (, entry 23)
Light yellow solid; m. p. 180.6–181.2°C (lit. [Citation55] m. p. 180.0–181.0°C); 1H NMR (400 MHz, DMSO-d6) δ: 8.06 (s, 1H), 7.85 (d, J = 8.6 Hz, 2H), 6.87 (d, J = 8.7 Hz, 2H), 3.11 (s, 6H). 13C NMR(151 MHz, DMSO-d6) δ: 159.29, 154.77, 134.03, 119.17, 116.70, 115.96, 112.24, 69.08, 43.00.
2.4.24. 2-(4-Hydroxy-benzylidene)-malononitrile (, entry 24)
Light yellow solid; m. p. 186.8–187.6°C (lit. [Citation55] m. p. 187.5–188.0°C); 1H NMR (400 MHz, DMSO-d6) δ: 11.07 (s, 1H), 8.32 (s, 1H), 7.90 (d, J = 8.4 Hz, 2H), 6.98 (d, J = 8.4 Hz, 2H). 13C NMR(151 MHz, DMSO-d6) δ: 164.31, 160.95, 134.30, 123.22, 117.05, 115.51, 114.61, 75.54.
2.4.25. 2-(4-Chlorobenzylidene) malononitrile (, entry 25)
White solid; m. p. 161.9–162.7°C (lit. [Citation55] m. p. 162–163°C); 1H NMR (400 MHz, DMSO-d6) δ: 8.56 (s, 1H), 7.96 (d, J = 8.2 Hz, 2H), 7.73 (d, J = 8.1 Hz, 2H). 13C NMR(151 MHz, DMSO-d6) δ: 160.54, 139.46, 132.56, 130.51, 130.15, 114.47, 113.44, 82.69.
3. Results and discussion
Firstly, according to the literature method (Citation46), a series of functional ionic liquids containing amine unit were prepared by mixing bromo hydrocarbons with DABCO or HMTA (). The structure of the target products was confirmed by nuclear magnetic resonance.
Table 1. Synthesis of amine ionic liquids.
With these basic functional ionic liquids in hand, then they were used in the Knoevenagel reaction between p-chlorobenzaldehyde and ethyl cyanoacetate, the effect of amine ionic liquids, water and ultrasonic wave were carefully explored and the experimental results are summarized in . When N-aminopropyl-1, 4-diazabicyclic [2.2.2] octane bromide was used alone to catalyze Knoevenagel reaction in solvent-free condition, the yield of the target product reached 85% after 3 h (, entry 1). Although a good acceleration effect has been achieved, we are not very satisfied with the result. After careful observation of the reaction phenomenon, it was found that under the condition of magnetic stirring without solvent, the reaction had insoluble substance, and the wrapping of product and raw material hinders the reaction to a certain extent. In our previous development of green organic reactions, it was found that using water alone or aqueous mixtures as solvent could significantly promote some specific organic reactions (Citation56). Based on this, we tried to use water in the Knoevenagel reaction catalyzed by N-aminopropyl -1, 4-diazabicyclic [2.2.2] octane bromide salt. When 0.5 mL water was added into the reaction system, after 40 min of reaction, TLC monitoring showed that the raw material aldehyde has been consumed. Comparing with the reaction of entry 1, the reaction rate and yield were indeed improved. When 1 mL water was added, TLC showed that the aldehyde disappeared after 15 min of reaction, and a large number of solid products appeared. After simple filtration and water washing, the target product was obtained with 99% yield. However, when more water was added to the reaction system (, Entries 4, 5), the reaction time and reaction yield did not increase but decreased to some extent. The reasons for the above experimental results may be as follows: (I) The mixture of ionic liquid N-aminopropyl-1, 4-diazabicyclic [2.2.2] octane bromide with water provides a favorable polar environment and hydrogen bond network, which had a good stabilizing effect on the intermediates of the reaction, and thus showed a significant promoting effect on the reaction. (II) The addition of excessive water will reduce the solubility of reaction materials, which is not benefit to the interaction between molecular, and further affect the reaction rate and product yield.
Table 2. Effect of ionic liquids with different structures on the Knoevenagel reactiona.
In order to further investigate the mechanism of catalyst N-aminopropyl 1, 4-diazabicyclic [2.2.2] octane bromide, seven other ionic liquids with similar structures were used in the Knoevenagel reaction of p-chlorobenzaldehyde and ethyl cyanoacetate (, entries 6–12), The yield of the Knoevenagel reaction catalyzed by N-aminopropyl hexamethylenetetramine bromide with weak alkalinity was 92% after 50 min reaction, which indicate that the catalytic activity of the catalyst was related to the alkalinity of tertiary amine structure, and the strongly basic ionic liquids are more beneficial to the reaction. When ionic liquid catalysts [HMTA-CH2CH2OH]Br and [DABCO-CH2CH2OH]Br containing hydroxyl group were used in Knoevenagel reaction, although the target product was obtained with better yield within 1 h, it was obvious that the effect of ionic liquid catalyst containing hydroxyl group was not as significant as that of amino group. When ionic catalysts [DABCO-CH2CH2CH2CH2CH3]Br, [DABCO-CH2CH3]Br and [DABCO-CH2C6H5]Br containing tertiary amine unit but without amino and hydroxyl groups were used for Knoevenagel reaction, although the reaction yield reached 91%, 90% and 88% respectively (, entries 10–12), the disappearance time of the raw material was significantly prolonged, and reaching 3 h. These results further indicate that the primary and tertiary amine structural units of catalysts are important catalytic sites. Based on the case that ultrasound can promote some specific organic reactions (Citation57), ultrasound was further applied to the Knoevenagel reaction catalyzed by [DABCO-CH2CH2CH2NH2]Br, and it was found that ultrasound had almost no promoting effect on the reaction (, entry 13). For the pure aqueous Knoevenagel reaction with no ionic liquid, only trace product is produced for 24 h (, entry 14). In order to better optimize the reaction, the amount of catalyst was reduced from 12 mmol (3 g) to 10 mmol (2.5 g), it was found that the reaction time was extended to 25 min and the yield was 98%(, entry 15). When the amount of catalyst was increased to 14 mmol (3.5 g), there is little change in reaction time and yield (, entry 16). Based on the above experimental results, the optimal reaction conditions of the model reaction are as follows: reactant p-chlorobenzaldehyde 5 mmol, ethyl cyanoacetate 5 mmol, catalyst [DABCO-CH2CH2CH2NH2]Br 12 mmol (3 g), water 1 mL, at room temperature.
In order to further explore the versatility of the composite system composed of functional ionic liquid [DABCO-CH2CH2CH2NH2]Br with water for different Knoevenagel reaction substrates, various polycyclic aromatic aldehydes, hetero-aromatic aldehydes and some electron-rich aromatic aldehydes with poor activity reported in literature were used in this reaction, the results are shown in . Aromatic aldehydes bearing substituent groups such as nitro, chloro, fluoro and cyano groups, the excellent yield 99% was obtained in short reaction times (8∼18 min), the aromatic aldehydes with electron-giving groups such as amino, hydroxyl and methoxyl groups have a slightly longer reaction time (50 min–24 h), but still have a good yield (79–92%) of the target product. For polycyclic aromatic aldehydes, such as 1-pyrene formaldehyde, the target product was obtained with 95% yield after 24 h. For heteraromatic aldehydes such as 6-methyl-2-oxo-2H-chromene-4-carbaldehyde (, entry 3) and N-ethylcarbazole-3, 6-diformaldehyde (, entry 4), 86% and 92% yields were respectively obtained after 15 and 20 h. Electron-rich aromatic aldehyde 4-diphenylaminobenzaldehyde (, entry 2) was reacted for 25 h to obtain the target product with 98% yield. On the basis of this success, active methylene malonitrile was also used in Knoevenagel reaction (, entries 22–25), and the yields of the target products were 88–99% within 18 min–10 h, and the activity of malonitrile was better than that of ethyl cyanoacetate.
Table 3. Knoevenagel reaction accelerated by the composite solvent-catalyst systemTable Footnotea.
To evaluate the possibility of recycling of the composite solvent-catalyst system composed of [DABCO-CH2CH2CH2NH2]Br and water, the Knoevenagel reaction of p-chlorobenzaldehyde and ethyl cyanoacetate was used as the model reaction and the cyclic reaction test was carried out (). At the end of the first reaction, the product was extracted with ethyl acetate, and the remaining solvent-catalyst system was directly used for the next cycle. The cycle results showed that each reaction time was nearly 15 min, and the yield almost remained at about 98%. Moreover, after the reaction was repeated for 6 times, the composite solvent-catalyst system could still maintain good catalytic effect. In order to further explore the stability of the catalyst, the recycled catalyst was washed with ethyl acetate, vacuum drying and NMR spectroscopy characterization (see Supplementary Materials). It was found that the structure of the catalyst was consistent with the original structure and did not change.
Table 4. Reusability of the composite solvent-catalyst.
In order to further evaluate the catalyst’s reactivity, we also compared the catalyst [DABCO-CH2CH2CH2NH2]Br with some homogeneous or heterogeneous catalysts reported in the literature for the Knoevenagel condensation (, Entries 1–14). As shown in , Although the catalytic activity of organic base DABCO in pure water solvent is relatively weak (, Entries 14), it shows excellent catalytic activity in ionic liquid (, Entries 13), but this method requires a reaction temperature of 50°C, and small molecule catalyst DABCO cannot be recycled. In terms of the reaction solvent, reaction temperature, reaction time, yields, etc., the catalyst [DABCO-CH2CH2CH2NH2]Br has many advantages over reported catalysts.
Table 5. Comparisons of the catalyst [DABCO-CH2CH2CH2NH2]Br with various homogeneous or heterogeneous catalysts in Knoevenagel condensation of 4-chlorobenzaldehyde with ethyl 2-cyanoacetate.
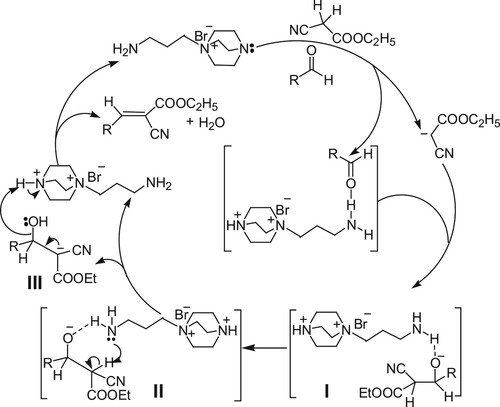
Functional ionic liquid [DABCO-CH2CH2CH2NH2]Br(IL-NH2), as a water-soluble strong polar compound, on one hand, it interacts with water to promote the reaction by stabilizing the reaction intermediates, on the other hand, as shown in Scheme 2, the primary amine and tertiary amine units of the functional ionic liquid interact with the reaction substrate active methylene compound and aldehyde respectively to generate carbanion and activated aldehyde by hydrogen bonding, the carbanion attack the carbonyl carbon of activated aldehyde and an aldol condensation intermediate I was formed. Then, the hydrogen atom of the active methylene interacted with the basic N center in the IL-NH2 through a six-member ring (intermediate II), and intermediate III was formed. In intermediate III, the lone pair electrons on the oxygen atom of the hydroxyl group interact with the acidic N–H of the quaternary ammonium, and subsequent elimination of water resulted in the formation of the olefin. In the whole process, [DABCO-CH2CH2CH2NH2]Br not only as a base to activate the acidic methylene group of cyanoacetic ester and as a hydrogen bond donor to activate the carbonyl group of aldehyde, but also accelerate the Knoevenagel reaction by allowing proton transfer to occur via a six-member ring in which IL-NH2 acted as a shuttle to transfer the proton from the C-position to the alkoxide of intermediate II, and the intermediate II was changed intermediate III. Finally, the intermediate III undergo elimination reaction and the Knoevenagel reaction product was obtained.
To further explore the properties of the Knoevenagel reaction products, the spectral properties of several Knoevenagel reaction products with large conjugated systems and their response to pH value were preliminary investigated (see the Supplementary Materials for the spectra), and it was found that the 3-anthracen-9-yl-2-cyano acrylate, ethyl-2-cyano-3-(4-diphenylamino-phenyl) acrylate and ethyl-2-cyano-3-(6-methyl-2-oxo-2H-chromen-4-yl) acrylate had good fluorescence properties (see the Tables 5–8 in the Supplementary Materials), and the fluorescence intensity of the compounds decreased with the increase of solution pH value (see the Figures 1–10 in the Supplementary Materials). In particular, ethyl-2-cyano-3-(4-diphenylamino-phenyl) acrylate was fluorescence quenched at pH = 11, and ethyl-2-cyano-3-(6-methyl-2-oxo-2H-chromen-4-yl) acrylate was fluorescence quenched at pH = 13.
4. Conclusion
Eight kinds of basic amine ionic liquids were designed and used as catalysts for Knoevenagel reaction. It was found that the solvent-catalyst system composed of [DABCO-CH2CH2CH2NH2]Br and water could effectively promote the reaction. For the Knoevenagel reaction of various aldehydes with ethyl cyanoacetate or malononitrile, most of them can get the target product in a short reaction time with a better yield. Even for the electron-rich and large steric hindrance aromatic aldehydes, better reaction effect has also been achieved. In addition, in the reaction process, the bifunctional ionic liquid [DABCO-CH2CH2CH2NH2]Br keeps good stability and can still maintain good catalytic effect after being recycled for 6 times. 2-cyano-3-(4-diphenylamino-phenyl)-acrylic acid ethyl ester and 2-cyano-3-(6-methyl-2-oxo-2H-chromen-4-yl)-acrylic acid ethyl ester have stable strong fluorescence properties. Moreover, it has good fluorescence response to pH value. In conclusion, a recyclable solvent-catalyst system was developed and used in the Knoevenagel condensation reaction. which offers some significant advantages including simple operation, excellent yield, short reaction time and recyclability, and it is expected to be used in other base-catalyzed organic reactions.
Supplemental Material
Download MS Word (4.3 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhao, S.; Wang, X.; Zhang, L. Rapid and Efficient Knoevenagel Condensation Catalyzed by a Novel Protic Ionic Liquid Under Ultrasonic Irradiation. RSC Adv. 2013, 3, 11691–11696.
- Beurden, K.; Koning, S.; Molendijk, D.; Schijndel, J. The Knoevenagel Reaction: A Review of the Unfinished Treasure Map to Forming Carbon-Carbon Bonds. Green Chem. Lett. Rev. 2020, 13, 349–364.
- Rao, P.S.; Venkatratnam, R.V. Zinc Chloride as a New Catalyst for Knoevenagel Condensation. Tetrahedron Lett. 1991, 32, 5821–5822.
- Attansi, O.; Fillippone, P.; Mei, A. Effect of Metal Ions in Organic Synthesis. Part XVI. Knoevenagel Condensations of Aldehydes and Tosylhydrazones with 2, 4-Pentanedione by Copper (II) Chloride-Catalyzed Reaction. Synth. Commun. 1983, 13, 1203–1208.
- Ogiwara, Y.; Takahashi, K.; Kitazawa, T.; Saka, N. Indium(III)-Catalyzed Knoevenagel Condensation of Aldehydes and Activated Methylenes Using Acetic Anhydride as a Promoter. J. Org. Chem. 2015, 80, 3101–3110.
- Narsaiah, A.V.; Nagaiah, K. An Efficient Knoevenagel Condensation Catalyzed by LaCl3·7H2O in Heterogeneous Medium. Synth. Commun. 2003, 33, 3825–3832.
- Cai, M.; Wang, X.; Fang, Y.; Chen, Y.; Dai, L. Robust Mg(Ca)Zr-Doped Acid-Base Bifunctional Mesoporous Silica and Their Applications in the Deacetalization-Knoevenagel Reaction. Inorg. Chem. 2021, 60, 8924–8935.
- Liu, D.; Hou, S.; Shu, Y.; Zhao, J.; Wang, L.; Zhang, P. Mechanochemical NaCl-Mediated Synthesis of Porous CuxMo1–xOy Catalyst for Knoevenagel Condensation. Ind. Eng. Chem. Res. 2021, 60, 17778–17785.
- Kalantari, F.; Rezayati, S.; Ramazani, A.; Aghahosseini, H.; Ślepokura, K.; Lis, T. Proline-Cu Complex Based 1,3,5-Triazine Coated on Fe3O4 Magnetic Nanoparticles: A Nanocatalyst for the Knoevenagel Condensation of Aldehyde with Malononitrile. ACS Applied Nano Materials 2022, 5, 1783–1797.
- Zhang, T.; Chen, H.; Liu, S.; Lv, H.; Zhang, X.; Li, Q. Highly Robust {Ln4}-Organic Frameworks (Ln = Ho, Yb) for Excellent Catalytic Performance on Cycloaddition Reaction of Epoxides with CO2 and Knoevenagel Condensation. ACS Catal. 2021, 11, 14916–14925.
- Varnaseri, N.; Rouhani, F.; Ramazani, A.; Morsali, A. Size and Function Influence Study on Enhanced Catalytic Performance of a Cooperative MOF for Mild, Green and Fast C-C Bond Formation. Dalton Trans. 2020, 49, 3234–3242.
- Kalantari, F.; Ramazani, A.; Heravi, R.P.M.; Aghahosseini, H.; Ślepokura, K. Magnetic Nanoparticles Functionalized with Copper Hydroxyproline Complexes as an Efficient, Recoverable, and Recyclable Nanocatalyst: Synthesis and Its Catalytic Application in a Tandem Knoevenagel–Michael Cyclocondensation Reaction. Inorg. Chem. 2021, 60, 15010–15023.
- Sadgar, A.L.; Deore, T.S.; Jayaram, R.V. Pickering Interfacial Catalysis-Knoevenagel Condensation in Magnesium Oxide-Stabilized Pickering Emulsion. ACS Omega 2020, 5, 12224–12235.
- Tavakolian, M.; Najafpour, M.M. Molybdenum Carbide as an Efficient and Durable Catalyst for Aqueous Knoevenagel Condensation. New J. Chem. 2019, 43, 16437–16440.
- Murase, T.; Nishijima, Y.; Fujita, M. Cage-Catalyzed Knoevenagel Condensation Under Neutral Conditions in Water. J. Am. Chem. Soc. 2012, 134, 162–164.
- Seckler, D.; Dea, C.M.; Rios, E.A.M.; de Godoi, M.; Rampon, D.S.; D’Oca, M.G.M.; D’Oca, C.D.R.M. Rice Straw Ash Extract/Glycerol: An Efficient Sustainable Approach for Knoevenagel Condensation. New J. Chem. 2022, 46, 4570–4578.
- Hirayama, Y.; Kanomata, K.; Hatakeyama, M.; Kitaoka, T. Chitosan Nanofiber-Catalyzed Highly Selective Knoevenagel Condensation in Aqueous Methanol. RSC Adv. 2020, 10, 26771–26776.
- Takakura, R.; Koyama, K.; Kuwata, M.; Yamada, T.; Sajiki, H.; Sawama, Y. Hydroquinone and Benzoquinone-Catalyzed Aqueous Knoevenagel Condensation. Org. Biomol. Chem. 2020, 18, 6594–6597.
- Jung, K.; Kim, S.; Choi, S.; Kim, I.; Han, G. Development of a DABCO-Succinic Acid Based Catalytic System for the Aza-Michael Addition and Aza-Michael/Knoevenagel Tandem Reaction of Thiazolidine-2, 4-Dione to Electron Deficient Alkenes. J. Org. Chem. 2021, 86, 16785–16794.
- Li, J.; He, D.; Lin, Z.; Cen, L.; Wu, W.; Jiang, H. NHC-Palladium-Catalyzed Ionic Liquid-Accelerated Regioselective Oxyarylation of Alkynes with Diaryl Ethers. Green Chem. 2022, 24, 1983–1988.
- Li, Y.Q.; Xu, X.M.; Zhou, M.Y. n-Butyl Pyridinium Nitrate as a Reusable Ionic Liquid Medium for Knoevenagel Condensation. Chin. Chem. Lett. 2003, 14, 448–450.
- Morison, D.W.; Forbes, D.C.; Davis, J.H. Base-Promoted Reactions in Ionic Liquid Solvents. The Knoevenagel and Robinson Annulation Reactions. Tetrahedron Lett. 2001, 42, 6053–6055.
- Wang, Y.; Shang, Z.C.; Fan, T.X.; Chen, X. Synthetic and Theoretical Study on Proline-Catalyzed Knoevenagel Condensation in Ionic Liquid. J. Mol. Catal. A 2006, 253, 212–221.
- Siddiqui, Z.N.; Khan, K. [Et3NH] [HSO4]-Catalyzed Efficient, Eco-Friendly, and Sustainable Synthesis of Quinoline Derivatives via Knoevenagel Condensation. ACS Sustainable Chem. Eng. 2014, 2, 1187–1194.
- Li, J.; Sun, H.; Cai, X.-C.; Dai, L.-Y. Application of Basic Ionic Liquid [Bmim]OH to Knoevenagel and Perkin Reactions. Chin. J. Org. Chem. 2007, 27, 1296–1299.
- Xu, D.-Z.; Liu, Y.; Shi, S.; Wang, Y. A Simple, Efficient and Green Procedure for Knoevenagel Condensation Catalyzed by [C4dabco][BF4] Ionic Liquid in Water. Green Chem. 2010, 12, 514–517.
- Yang, C.; Su, W.-Q.; Xu, D.-Z. Ionic Liquid [Dabco-H][AcO] as a Highly Efficient and Recyclable Catalyst for the Synthesis of Various Bisenol Derivatives via Domino Knoevenagel-Michael Reaction in Aqueous Media. RSC Adv. 2016, 6, 99656–99663.
- Ying, A.-G.; Liu, L.; Wu, G.-F.; Chen, X.-Z.; Ye, W.-D.; Chen, J.-H.; Zhang, K.-Y. Knoevenagel Condensation Catalyzed by DBU Brönsted Ionic Liquid Without Solvent. Chem. Res. Chin. Univ. 2009, 25, 876–881.
- Ying, A.; Ni, Y.; Xu, S.; Liu, S.; Yang, J.; Li, R. Novel DABCO Based Ionic Liquids: Green and Efficient Catalysts with Dual Catalytic Roles for Aqueous Knoevenagel Condensation. Ind. Eng. Chem. Res. 2014, 53, 5678–5682.
- Garrabou, X.; Wicky, B.I.M.; Hilvert, D. Fast Knoevenagel Condensations Catalyzed by an Artificial Schiff-Base-Forming Enzyme. J. Am. Chem. Soc. 2016, 138, 6972–6974.
- Islam, M.J.; Kumer, A.; Sarker, M.N.; Paul, S. The Activity of Alkyl Groups in Morpholinium Cation on Chemical Reactivity, and Biological Properties of Morpholinium Tetrafluroborate Ionic Liquid Using the DFT Method. Chem. Methodol. 2020, 4, 130–142.
- Sajjadifar, S.; Amini, I.; Habibzadeh, S.; Mansouri, G.; Ebadi, E. Acidic Ionic Liquid Based Silica-Coated Fe3O4 Nanoparticles as a New Nanomagnetic Catalyst for Preparation of Aryl and Heteroaryl Thiocyanates. Chem. Methodol. 2020, 4, 624–635.
- Zhu, H.-P.; Yang, F.; Tang, J.; He, M.-Y. Brønsted Acidic Ionic Liquid 1-Methylimidazolium Tetrafluoroborate: A Green Catalyst and Recyclable Medium for Esterification. Green Chem. 2003, 5, 38–39.
- Zhang, L.; Xian, M.; He, Y.; Li, L.; Yang, J.; Yu, S.; Xu, X. A Brønsted Acidic Ionic Liquid as an Efficient and Environmentally Benign Catalyst for Biodiesel Synthesis from Free Fatty Acids and Alcohols. Biores. Technol. 2009, 100 (19), 4368–4373.
- Azizi, N.; Abdoli-Senejani, M.; Abbasi, F. An Efficient Brønsted–Lewis Acidic Ionic Liquid Catalyzed Tetrahydropyranylation of Alcohols. Tetrahedron Lett. 2016, 57, 5009–5011.
- Azizi, N.; Shirdel, F. Task Specific Dicationic Acidic Ionic Liquids Catalyzed Efficient and Rapid Synthesis of Benzoxanthenones Derivatives. J. Mol. Liq. 2016, 222, 783–787.
- Cole, A.C.; Jensen, J.L.; Ntai, I.; Tran, K.L.T.; Weaver, K.J.; Forbes, D.C.; Davis, J.H. Novel Brønsted Acidic Ionic Liquids and Their Use as Dual Solvent-Catalysts. J. Am. Chem. Soc. 2002, 124, 5962–5963.
- Amarasekara, A.S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183.
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of Novel, Moisture-Stable, Lewis-Acidic Ionic Liquids Containing Quaternary Ammonium Salts with Functional Side Chains. Chem. Commun. 2001, 1, 2010–2011.
- Ding, J.; Wang, P.; He, Y.; Cheng, L.; Li, X.; Fang, C.; Li, H.; Wan, H.; Guan, G. Porous Sulfonyl Binuclear Carbonate Poly(Ionic Liquid)s for One-pot Fixation of Diluted CO2 Into Dimethyl Carbonate. Appl. Catal. B Environ. 2023, 324, 122278.
- Ventura, S.P.M.; Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-Liquid-Mediated Extraction and Separation Processes for Bioactive Compounds: Past,: Present, and Future Trends. Chem. Rev. 2017, 117, 6984–7052.
- Aflatoonian, M.R.; Tajik, S.; Aflatoonian, B.; Shoaie, I.S.; Sheikhshoaie, M.; Beitollahi, H. Copper Oxide, Ionic Liquid and Mn(III) Salen Modified Carbon Paste Electrode as Selective Electrochemical Sensor for Determination of Droxidopa in the Presence of Carbidopa. Eurasian Chem. Commun. 2020, 2, 387–397.
- Saghiri, S.; Ebrahimi, M.; Bozorgmehr, M. Electrochemical Amplified Sensor with MgO Nanoparticle and Ionic Liquid: A Powerful Strategy for Methyldopa Analysis. Chem. Methodol. 2021, 5, 234–239.
- Trujillo-Rodríguez, M.J.; Nan, H.; Varona, M.; Emaus, M.N.; Souza, I.D.; Anderson, J.L. Advances of Ionic Liquids in Analytical Chemistry. Anal. Chem. 2019, 91, 505–531.
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem. Rev. 2017, 117, 7132–7189.
- Jia, X.; Wang, D.; Zhao, S. Preparation of Deep Eutectic Solvents and Its Application in Morita-Baylis-Hillman Reaction. Chem. Res. Appl. 2021, 33, 2330–2338.
- Yang, Y.; Yao, H.-F.; Xi, F.-G.; Gao, E.-Q. Amino-Functionalized Zr(IV) Metal-Organic Framework as Bifunctional Acid-Base Catalyst for Knoevenagel Condensation. J. Mol. Catal. A: Chem. 2014, 390, 198–205.
- Zhang, X.; He, X.; Zhao, S. Preparation of a Novel Fe3O4@SiO2@Propyl@DBU Magnetic Core–Shell Nanocatalyst for Knoevenagel Reaction in Aqueous Medium. Green Chem. Lett. Rev. 2021, 14, 85–98.
- Song, A.; Wang, X.; Lam, K.S. A Convenient Synthesis of Coumarin-3-Carboxylic Acids via Knoevenagel Condensation of Meldrum'S Acid with Ortho-Hydroxyaryl Aldehydes or Ketones. Tetrahedron Lett. 2003, 44, 1755–1758.
- Popp, F.D. Notes-Synthesis of 3-Hydroxypyridines. I. Condensation of Aromatic Aldehydes with Ethyl Cyanoacetate. J. Org. Chem. 1960, 25, 646–647.
- Li, G.; Xiao, J.; Zhang, W. Knoevenagel Condensation Catalyzed by a Tertiary-Amine Functionalized Polyacrylonitrile Fiber. Green Chem. 2011, 13, 1828–1836.
- Yue, C.; Mao, A.; Wei, Y.; Lue, M. Knoevenagel Condensation Reaction Catalyzed by Task-Specific Ionic Liquid Under Solvent Free Conditions. Catal. Commun. 2008, 9, 1571–1574.
- Ying, A.-G.; Wang, L.-M.; Wang, L.-L.; Chen, X.-Z.; Ye, W.-D. Green and Efficient Knoevenagel Condensation Catalyzed by a DBU Based Ionic Liquid in Water. J. Chem. Res. 2010, 1, 30–33.
- Zhang, J.; Jiang, T.; Han, B.; Zhu, A.; Ma, X. Knoevenagel Condensation Catalyzed by 1,1,3,3-Tetramethylguanidium Lactate. Synth. Commun. 2006, 36, 3305–3317.
- Ren, Z.; Cao, W.; Tong, W. The Knoevenagel Condensation Reaction of Aromatic Aldehydes with Malononitrile by Grinding in the Absence of Solvents and Catalysts. Synth. Commun. 2002, 32, 3475–3479.
- Zhao, S.; He, M.; Guo, Z.; Zhou, N.; Wang, D.; Li, J.; Zhang, L. [HyEtPy]Cl-H2O: An Efficient and Versatile Solvent System for the DABCO-Catalyzed Morita-Baylis-Hillman Reaction. RSC Adv. 2015, 5, 32839–32845.
- Zhao, S.; Xu, X.; Zheng, L.; Liu, H. An Efficient Ultrasonic-Assisted Synthesis of Imidazolium and Pyridinium Salts Based on the Zincke Reaction. Ultrason. Sonochem. 2010, 17, 685–689.
- Parvin, M.N.; Jin, H.; Ansari, M.B.; Oh, S.-M.; Park, S.-E. Imidazolium Chloride Immobilized SBA-15 as a Heterogenized Organocatalyst for Solvent Free Knoevenagel Condensation Using Microwave. Appl. Catal. A 2012, 413–414, 205–212.
- Postole, G.; Chowdhury, B.; Karmakar, B.; Pinki, K.; Banerji, J.; Auroux, A. Knoevenagel Condensation Reaction Over Acid–Base Bifunctional Nanocrystalline CexZr1−xO2 Solid Solutions. J. Catal. 2010, 269, 110–121.
- Ansari, M.B.; Jin, H.; Parvin, M.N.; Park, S.-E. Mesoporous Carbon Nitride as a Metal-Free Base Catalyst in the Microwave Assisted Knoevenagel Condensation of Ethylcyanoacetate with Aromatic Aldehydes. Catal. Today 2012, 185, 211–216.
- Liu, Y.; Liang, J.; Liu, X.H.; Fan, J.C.; Shang, Z.C. Polyethylene Glycol (PEG) as a Benign Solvent for Knoevenagel Condensation. Chin. Chem. Lett. 2008, 19, 1043–1046.
- Boroujeni, K.P.; Jafarinasa, M. Polystyrene-Supported Chloroaluminate Ionic Liquid as a New Heterogeneous Lewis Acid Catalyst for Knoevenagel Condensation. Chin. Chem. Lett. 2012, 23, 1067–1070.
- Kumar, A.; Dewan, M.; Saxena, A.; De, A.; Mozumdar, S. Knoevenagel Condensation Catalyzed by Chemo-Selective Ni-Nanoparticles in Neutral Medium. Catal. Commun. 2010, 11, 679–683.
- Sugahara, K.; Kimura, T.; Kamata, K.; Yamaguchi, K.; Mizuno, N. A Highly Negatively Charged γ-Keggin Germanodecatungstate Efficient for Knoevenagel Condensation. Chem. Commun. 2012, 48, 8422–8424.
- Elhamifar, D.; Kazempoor, S.; Karimi, B. Amine-Functionalized Ionic Liquid-Based Mesoporous Organosilica as a Highly Efficientnanocatalyst for the Knoevenagel Condensation. Catal. Sci. Technol. 2016, 6, 4318–4326.
- Sayed, M.; Shi, Z.; Gholami, F.; Fatehi, P.; Soliman, A.I.A. Ag@TiO2 Nanocomposite as an Efficient Catalyst for Knoevenagel Condensation. ACS Omega 2022, 7, 32393–32400.
- Jia, X.; Zhang, X.; Wang, Z.; Zhao, S. Tertiary Amine Ionic Liquid Incorporated Fe3O4 Nanoparticles as a Versatile Catalyst for the Knoevenagel Reaction. Synth. Commun. 2022, 52, 774–786.
- Meng, D.; Qiao, Y.; Wang, X.; Wen, W.; Zhao, S. DABCO-Catalyzed Knoevenagel Condensation of Aldehydes with Ethyl Cyanoacetate Using Hydroxy Ionic Liquid as a Promoter. RSC Adv. 2018, 8, 30180–30185.