Abstract
The effect of grape seed oil (GSO) on the animal performance, blood metabolites, meat oxidation stability and muscle fatty acids of lambs was evaluated. Eighteen Baluchi lambs, 196 ± 14 days of age and 39.8 ± 1.7 kg body weight were randomly allocated to three dietary treatments: (1) diet without GSO (Control), (2) diet containing 2% GSO (GSO2) and (3) diet containing 4% GSO (GSO4). Results showed that dry matter intake and animal performance were not affected by treatment (p > .05). Addition of 4% GSO to diet increased plasma total cholesterol and triglyceride (p < .01). Inclusion of GSO to the diets increased (p < .001) the proportions of trans-9 C18:1, vaccenic acid (VA), rumenic acid (RA), C18:2 n-6 and polyunsaturated fatty acid (PUFA), while decreased (p = .01) the concentration of saturated fatty acid in longissimus lumborum (LL) muscle of lambs. At the same time, dietary GSO increased oxidative stability of LL muscle during the 12 days of storage time (p < .0001). In conclusion, these results showed that GSO up to 4% in the lamb diet can improve meat quality by increasing VA, RA, PUFA and oxidative stability in LL muscle lambs without affecting animal performance.
Grape seed oil (GSO) is rich source of linoleic acid and phenolic content and can consider as a natural antioxidant.
GSO increased proportions of Polyunsaturated fatty acid, vaccenic and rumenic acids.
GSO increased oxidative stability of muscle during the storage time.
Highlights
Introduction
Fats are often looked at as the harmful fraction of foods for human consumption because of the possibility of inducing health problems such as obesity, coronary heart disease and tumours. Some fatty acids (FAs) are essential and play a vital role in the diets of human and animals (Williams et al. Citation2011). The supplementation of ruminant diets with lipid sources rich in poly unsaturated fatty acid (PUFA) is an effective strategy to improve the nutritional value of meat fat, decreasing saturated fatty acid (SFA) and enrichment of PUFA, including the health-enhancing FA, such as conjugated linoleic acid (CLA) and n-3 PUFA (Sinclair Citation2007). Although many experiments have shown the effect of using various oils (such as: soybean oil, sunflower oil, etc.) on manipulate meat FA profile, to the best our knowledge, to date, information available in the literature about the use of grape seed oil (GSO) in the ruminant diets is very scarce. Jerónimo et al. (Citation2012) showed that dietary supplementation of grape seed extract had no effect on PUFA and SFA of m. Longissimus lumborum (LL) muscle in lambs when compared to the control group. Recently, Correddu et al. (Citation2016) reported that the feeding of 300 g/d per head of grape seed increased PUFA and decreased SFA in milk of dairy sheep. However, increased PUFA level may limit the shelf-life of meat, because they are more prone to oxidation (Williams et al. Citation2011). Antioxidants can be used to prevent or inhibit oxidation and they are categorised into natural and synthesised types. Most of the antioxidants in food industry belong to synthesised category. The trend is to decrease the use of synthetic antioxidants due to consumer concerns over safety and toxicity and increase of natural antioxidants which derive from eatable materials (Ito et al. Citation1985).
The TBARS value was strongly affected by the time of storage (p < .01), with values overall increasing across time of storage. However, an effect of the dietary treatment (p < .01), as well as a strong Time × Diet interaction (p < .01) indicated a different trend of development of TBARS across storage duration depending on the dietary treatment. In agreement with our study, Botsoglou et al (Citation2010) reported that diet supplementation with olive leaves (as an antioxidant source) decreased MDA content of breast of turkey after 12 days compared to the control, while had no effect on diets after 4 days. Results obtained in this study are difficult to compare with the previous reports since there have not been pertinent in vivo studies with GSO. The inhibition of lipid oxidation in LL muscle after diet supplementation with GSO is probably the result of various constituents with antioxidant activity that entered the circulatory system, and distributed and retained in lamb tissues. Grape production is widespread throughout the world, exceeding 68 million tons (FAO Citation2013). In 2013, Iran produced more than two million metric tons of grapes. As grape seeds comprise about 5% of the fruit weight (Choi and Lee Citation2009). The amount of discarded grape seeds is more than three million tons annually worldwide and around 100 thousand kton in the particular case of Iran. GSO is produced from the seeds in the pomace left over from juice and wine production by cold pressing method, and thus adds value to the industry. This also helps to reduce waste disposal problems. It is gaining popularity as a culinary oil, and has been studied as a possible source of specialty lipids. It is a rich source of linoleic acid (C18:2 n-6) (Beveridge et al. Citation2005), which is associated with promotion of cardiovascular health by down-regulating low-density lipoprotein cholesterol (LDL-C) production, and enhancing its clearance (Wijendran and Hayes Citation2004). It is reported that grape seed is a good source of natural antioxidants (Lutterodt et al. Citation2011) and during cold pressing process, these compounds are extracted into the cold pressed oil in significant quantities (Parry and Yu Citation2004). Therefore, the aim of this study was to investigate the effect of different level of GSO on animal performance, blood metabolites, oxidative stability and FA profile of lamb meat.
Materials and methods
Animals and diets
Eighteen Baluchi lambs, 196 ± 14 days of age and 39.8 ± 1.7 kg body weight (BW) were randomly assigned to three experimental diets (n = 6 per group): (1) diet without GSO (control), (2) diet containing 2% of GSO (GSO2) and (3) diet containing 4% of GSO (GSO4) (DM basis). Cold-pressed GSO was obtained from the ‘Zait-Kerman’ factory. Experimental diets were formulated to meet the requirements according to NRC (Citation2007). The ingredients and chemical composition of the experimental diets and FA profile, total phenolic compounds (TPC) and antioxidant activity of GSO are given in Tables and . Diets were fed as a total mixed ration (TMR) with 63:37 forage to concentrate ratio. Diets were offered twice daily (08.00 and 16.00 h) ad libitum in TMR form and orts were recorded daily. Before the beginning of the experimental period (42 d), animals were kept in individual pens and adapted to the experimental diets for 14 d. Individual dry matter intake (DMI) was calculated using daily feed offered and feed refuse averaged over the interval of the trial. Animals were weighed after a 16 h fast before the first feeding in the morning at the beginning and the end of the experimental period to determine the average daily gain (ADG). Feed conversion ratio (FCR) was calculated according to: FCR = [DMI (kg/d)/ADG (kg/d)]. Animals involved in this study were cared according to the guidelines of the Iranian Council of Animal Care (Citation1995).
Table 1. Ingredients and chemical composition of experimental diets.
Table 2. Fatty acid composition, total phenolic compounds and antioxidant activity of grape seed oil.
Feedstuffs analysis
Crude protein (CP) (Kjeldahl N × 6.25) was determined by the block digestion method using copper catalyst and steam distillation into boric acid on 2100 Kjeltec distillation unit (Foss, Hillerød, Denmark) according to AOAC (Citation2005, method 2001.11). Content of neutral detergent fibre (NDF) inclusive of residual ash was determined without sodium sulphite and with the inclusion of heat-stable α-amylase (100 mL neutral detergent solution/0.5 g of sample) by method described by Van Soest et al. (Citation1991). Ether extract content was determined by Soxthlet device (model 2050, Foss, Hillerød, Denmark) using procedures of AOAC (Citation2005). Non fibrous carbohydrate (NFC) content was estimated by difference of mean values, that is, 100 − (sum of percentages of moisture, NDF, ash, protein and fats). Total phenolic content and total tannin (TT) were determined by the Folin–Ciocalteu described by Makkar (Citation2003). Antioxidant activity of cold-pressed GSO was determined by 2,2-diphenyl-1-picryhydrazyl (DPPH) method (Cheng et al. Citation2006).
Laboratory samples analysis
Individual blood samples were taken before the morning feedings from the jugular vein at the end of experiment, and centrifuged at 3000 rpm × g for 20 min. Plasma was stored at −20 °C until analysis. Plasma samples were analysed for glucose, triglyceride, total cholesterol, albumin and total protein concentrations by using a commercially assay kits (Pars-azmon Co., Tehran, Iran).
At the end of the trial, lambs were weighed after 16 h of feed deprivation and then slaughtered. After the slaughter, non-carcase components were removed, and then carcase was chilled at 4 °C for 24 h. The LL muscle (between the 12th thoracic and 5th lumbar vertebrae) of the left half carcase was collected. Fresh LL muscle samples were divided into two portions. One portion was divided randomly into four sub-samples, vacuum-packed and used to evaluate the lipid stability during refrigerated storage in darkness at 4 °C, using one sub-sample for each day of storage. The remaining portion was stored at −20 °C for determining the FA profile.
Fatty acid composition of the LL muscle was determined after extraction of total lipids in accordance to the method described by Folch et al. (Citation1957). Briefly, a sample of each (0.5 g, two replicates) was placed in a 5 mL screw-top test tube followed by addition of 2 mL of methanolic potassium hydroxide (2 N). The tube cap was then tightened and the tube was vigorously shaken for 2 s. Next, 2 mL of hexane were added into the tube and the tube was shaken for 5 s before being placed in an ultrasonic bath at 35 °C for 15 min full stop subsequently, the upper layer was separated and passed through a filter (0.45 µm) containing sodium sulphate anhydrous. Finally, 1 µL of the obtained filtrate was injected to a gas chromatograph (GC) (YL6100 GC, YOUNG LIN INSTRUMENT, Anyang, Korea). The GC was equipped with a J&W CP-Sil 88 fused silica capillary column (100 m × 0.25 mm, 0.20 µm film thickness, Agilent Technologies, USA). The temperatures of injector and detector ports were set at 270 and 300 °C, respectively. The FA composition was analysed by isotherm programme. The column temperature was held at 175 °C for 60 min. The identification of individual FAME (fatty acid methyl esters) was based on a standard mixture of 37 Component FAME Mix (FAME Mix C4-24, 18919-1AMP, Supelco, Sigma-Aldrich, Bellefonte, PA, USA) and 60 individual FAME standards (Sigma-Aldrich, USA). The identification of CLA isomers was based on co-injection with commercial standard mixtures (Sigma Aldrich, Bellefonte, PA, USA). Concentration of FAs were expressed as g/100 g of total FAME.
Lipid oxidation of ground samples of LL muscle during 1, 4, 8 and 12 days of storage at 4 °C was assessed by measuring thiobarbituric acid reactive substances (TBARS) according to the method described by Esterbauer and Cheeseman (Citation1990). Concentration of TBARS was expressed as mg malondialdehyde (MDA)/kg fresh meat.
Statistical analysis
A completely randomised design with three treatments (diets) and six replicates (lambs) was used for the study. Data were analysed using a GLM procedure of SAS (Citation2002) with treatment (diet) as the fixed effect. Data of LL muscle lipid oxidation (TBARS values) were analysed using a completely randomised design with repeated-measures based on values at 1, 4, 8 and 12 days of aging. The fixed effects in the model were: the dietary treatment (Diet), the time of storage (Time) and their interaction (Diet × Time), while individual animal was included as a random factor. Significance was declared at p ≤ .05 and trends at p < .10 and p > .05 using Tukey’s multiple comparison tests. In the figure, results are presented as means ± standard error (s.e.).
Results and discussion
The results of DMI and growth performance are presented in Table . Supplementation of GSO had no effect on DMI and metabolisable energy intake which in turn did not alter the growth performance of the lambs (p > .05). In this study, DMI and ADG were not affected by dietary treatment. The effects of oil supplementation in diets on the animal performance of ruminants are variable, and such variability could be associated with basal diets (i.e. energy density and level of grain), level of oil inclusion, oil composition (i.e. contents of free and saturated fatty acids), and whether diets were formulated to be isoenergetic (Awawdeh et al. Citation2009). The results of this study are in agreement with other studies where animal diets were supplemented with soybean oil (Ludden et al. Citation2009), sunflower oil (Mir et al. Citation2003) or a mixture of linseed oil and sunflower oil (He et al. Citation2011). The lack of effects can also be explained by the similar age (196 ± 14 days) and weight (46.3, 45.7 and 45.5 kg for Control, GSO2 and GSO4, respectively) at slaughter, and proximate diet composition across experimental diets.
Table 3. Effect of treatment on dry matter intake and growth performance.
Results from blood metabolites are presented in Table . Lambs fed the GSO4 diet had higher (p < .01) plasma triglyceride and total cholesterol compared with those fed the control and GSO2 diets; however, the concentrations of glucose, albumin and total protein were not affected by dietary treatments (p > .05). Triglyceride and total cholesterol in plasma were both greater for lambs fed the GSO4 diet. Concentrations of glucose, albumin and total protein in plasma were not affected by dietary treatments. The lack of significant variation of plasma glucose levels between the control and GSO supplemented diets is in accordance with the absence of effect of plant oils on plasma glucose in goat (Bernard et al. Citation2005, Citation2009). Several researchers showed that supplementation of diet with soybean oil, cottonseed oil and palm oil increased blood triglyceride and cholesterol contents (Hernandez et al. Citation1978; Garcia et al. Citation2003). Similarly, addition of sunflower oil at levels of 3.7% and 6.1% of diet DM increased triglyceride and total cholesterol levels in the plasma of Alpine (Bernard et al. Citation2009) and Saanen (Razzaghi et al. Citation2015) goats. In the current study, increasing of triglyceride may be due to an increase in dietary fat stimulated intestinal cholesterol synthesis to meet the increased demand, and for absorption and transport of fat in ruminants (Nestel et al. Citation1978).
Table 4. Effect of diets on plasma metabolites in lambs.
The FA composition of intramuscular fat collected from the lambs of the three experimental treatments is reported in Table . The proportions of trans-9 C18:1, trans-11 C18:1 (VA), cis-9 trans-11 C18:2 CLA (RA), total CLA and C18:2 n-6 were increased (p < .001), whereas C16:0 was decreased (p = .002) by GSO feeding. The results showed that the increased proportion of GSO in the diet increased the concentrations of PUFA (p = .001), PUFA/SFA (p < .0001) and PUFA/MUFA ratio (p < .001), and also decreased the concentrations of SFA (p < .05) in intramuscular fat. In current study, the ratio of n-6:n-3 in intramuscular fat increased with level of GSO supplementation (p < .001). In the present study, C16:0 of intramuscular fat was reduced with GSO supplementation. Unsaturated lipid supplementation frequently decreases the C16:0 concentrations (Jerónimo et al. Citation2012) which are desirable because this FA is the major hypercholesterolaemia FA. The concentration of C18:0 in LL muscle for lambs fed GSO was not significantly different compared with those for lambs fed control; these results are in agreement with previous studies (Bolte et al. Citation2002; Lu et al. Citation2008). As expected, meat from GSO-fed lambs had the highest C18:2 n-6 concentrations, this pattern is consistent with other trials where lambs were fed oils rich in C18:2 n-6 (Bessa et al. Citation2005; Boles et al. Citation2005). Consistent with the result of the present study, supplementation of lamb diets with oil rich in C18:2 n-6 increased PUFA, trans-9 C18:1, VA and RA contents in the obtained meat (Bolte et al. Citation2002; Bessa et al. Citation2007; Jerónimo et al. Citation2012). Lu et al. (Citation2008) reported that inclusion of soybean or linseed oil in the diet of lamb increased contents of C18:2 n-6 and C18:3 n-3 in LL muscle, because these oils have high contents of C18:2 n-6 and C18:3 n-3, respectively. Other researchers have reported higher concentrations of unsaturated fatty acids (UFA) in muscle of lambs fed sunflower or safflower seeds (Bolte et al. Citation2002; Rizzi et al. Citation2002; Kott et al. Citation2003). Madron et al. (Citation2002) also reported higher levels of RA in beef meat with increasing levels of full-fat extruded soybeans fed to steers. Correddu et al. (Citation2016) showed that the addition of linseed alone or in combination with grape seed increased VA and RA compared to the control. The higher RA response to sunflower oil supplementation when compared to linseed oil was most probably explained by ruminal metabolism of both C18:2 n-6 and C18:3 n-3 (Harfoot and Hazlewood Citation1998). At least for forage-based diets, the well-established mainstream pathway for C18:2 n-6 ruminal biohydrogenation is straight forward with an initial isomerisation with formation of RA and its reduction to VA. The established pathways for biohydrogenation of C18:3 n-3 results in a greater diversity of products including cis-9 trans-11 cis-15 C18:3, trans-11 cis-15 C18:2, trans-15 C18:1 and VA, but not RA. The increase in PUFA, due to the dietary inclusion of GSO resulted in higher PUFA:SFA ratios in both of the treated groups, as compared to control. The amounts of increase in this ratio, PUFA: SFA (i.e. increase in PUFA and decrease in SFA), in the LL muscle – from GSO groups, when compared against the control group, are very interesting, indicating the fact that, replacing dietary SFA with PUFA is likely to reduce the occurrence of coronary heart disease (Mozaffarian et al. Citation2010). The increase of n6:n3 ratio in LL muscle of GSO, mainly related to the increase of C18:2 n-6, in GSO. This is in agreement with the findings of Correddu et al. (Citation2016) in sheep who showed increased levels of C18:2 n-6 in milk fed residues from grapes. Increasing of C18:2 n-6 in diet lead to increasing of this fatty acid in muscle and n6:n3 ratio.
Table 5. Effect of diets on total fat (mg/100 g muscle) and fatty acid composition (g/100 g total fatty acids) of muscle from lambs.
The change in the TBARS level in the lamb meat stored up to 12 days at refrigerated conditions was found to be affected by dietary treatment (p < .0001), storage time (p < .0001), and the interaction between diet and storage time (p = .003; Figure ). After 12 days of storage, TBARS values measured in meat from lambs in the control group were higher compared to the GSO groups (p < .05), while no differences (p > .05) between dietary treatments on TBARS values were observed at 1, 4 and 8 day of storage. In this study, meat from GSO supplemented lambs had higher PUFA concentration than meat from the control lambs (Table ) and, thus, can be expected to be more prone to lipid oxidation (Morrissey et al. Citation1998). Lipid oxidation results in the production of free radicals, which may lead to the oxidation of meat pigments and generation of rancid odours and flavours (Faustman and Cassens Citation1990). In present trial, the lipid oxidation in LL muscle was measured by the reaction of 2-thiobarbituric acid with MDA (Esterbauer and Cheeseman Citation1990). In the current study, TBARS values were lower after 12 days of storage in the GSO groups compared with the control. To our knowledge, this is the first study showing the positive effect of dietary GSO in reducing lipid oxidation of meat. In agreement with our results, Gladine et al. (Citation2007) found that adding 100 g of grape seed and peel extract/kg of DM of diet directly into the rumen of sheep improved antioxidant levels and reduced susceptibility to lipid oxidation of plasma, as measured by an oxidation induced assay. Also inclusion of 2.5% DM of grape seed extract in the lambs’ diets decreased TBARS values in LL muscle (Jerónimo et al. Citation2012). Clinical data have shown that antioxidant potential of grape seed is twenty and fifty folds as large as those of vitamins E and C, respectively (Shi et al. Citation2003). Emami et al. (Citation2015) showed that TBARS values linearly decreased between 1 and 2 months after slaughter by inclusion of pomegranate seed pulp in the diets.
Figure 1. Effect of diet and storage time on the TBARS values of LL muscle. a,b,cWithin days of storage, different superscripts indicate differences between dietary treatments (p ≤ .05).w,x,y,zWithin each dietary treatment, different superscripts indicate difference between days of storage (p ≤ .05). Values are means, with SD represented by vertical bars.
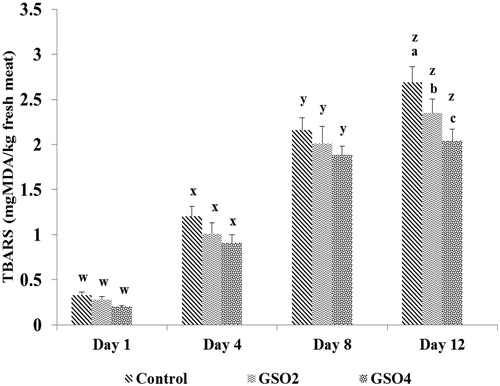
Cold pressing is a method in which high temperatures and chemical treatments are avoided. Consequently, it may preserve healthy components such as natural antioxidants (Yu et al. Citation2005). Polyphenols such as tannins inhibit lipid peroxidation by acting as chain-breaking peroxyl-radical scavengers, and can protect meat from oxidation (Sreelatha and Padma Citation2009). We found that, compared to the control diet, the GSO-supplemented diets increased the concentration of total phenols (Table ). Therefore, the reduction in TBARS content of meat of the lambs fed GSO diets was probably due to the transfer of polyphenolic compounds from GSO to the LL muscle. Nevertheless, previous works have shown that, inclusion of grape extract in rat diets increased significantly the vitamin E content in liver (Gladine et al. Citation2007) and the activity of antioxidant enzymes in kidney and liver (Ananthan et al. Citation2004; Gladine et al. Citation2007).
Conclusions
According to the results of this study, GSO can be included up to 4% in the lamb diet with no adverse effects on the DMI and growth performance. Furthermore, inclusion of GSO to the diets improved the nutritional value of lamb meat by increasing the proportions of linoleic acid, VA, RA and PUFA, while lowering the concentration of SFA. Also, inclusion of GSO to the diet increased n6:n3 ratio in LL muscle. At the same time, dietary GSO increased oxidative stability of LL muscle after 12 days of storage. In conclusion, GSO can be used as a rich source of linoleic acid and phenolic compound with high antioxidant activity at levels up to 4% in the diet of ruminants to improve meat quality.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ananthan R, Latha M, Ramkumar K, Pari L, Baskar C, Bai VN. 2004. Modulatory effects of Gymnema montanum leaf extract on alloxan-induced oxidative stress in Wistar rats. Nutrition. 20:280–285.
- Association of Official Analytical Chemists (AOAC). 2005. Official methods of analysis. 18th ed. Gaithersburg (MA): AOAC International.
- Awawdeh M, Obeidat B, Abdullah A, Hananeh W. 2009. Effects of yellow grease or soybean oil on performance, nutrient digestibility and carcass characteristics of finishing Awassi lambs. Animal Feed Sci Technol. 153:216–227.
- Bernard L, Bonnet M, Leroux C, Shingfield KJ, Chilliard Y. 2009. Effect of sunflower-seed oil and linseed oil on tissue lipid metabolism, gene expression, and milk fatty acid secretion in Alpine goats fed maize silage-based diets. J Dairy Sci. 92:6083–6094.
- Bernard L, Rouel J, Leroux C, Ferlay A, Faulconnier Y, Legrand P, Chilliard Y. 2005. Mammary lipid metabolism and milk fatty acid secretion in alpine goats fed plantlipids. J Dairy Sci. 88:1478–1489.
- Bessa RJ, Alves SP, Jerónimo E, Alfaia CM, Prates JA, Santos-Silva J. 2007. Effect of lipid supplements on ruminal biohydrogenation intermediates and muscle fatty acids in lambs. Eur J Lipid Sci Technol. 109:868–878.
- Bessa RJB, Portugal P, Mendes I, Santos-Silva J. 2005. Effect of lipid supplementation on growth performance, carcass and meat quality and fatty acid composition of intramuscular lipids of lambs fed dehydrated lucerne or concentrate. Livest Prod Sci. 96:185–194.
- Beveridge TH, Girard B, Kopp T, Drover JC. 2005. Yield and composition of grape seed oils extracted by supercritical carbon dioxide and petroleum ether: varietal effects. J Agric Food Chem. 53:1799–1804.
- Botsoglou E, Govaris A, Christaki E, Botsoglou N. 2010. Effect of dietary olive leaves and/or a-tocopheryl acetate supplementation on microbial growth and lipid oxidation of turkey breast fillets during refrigerated storage. Food Chem. 121:17–22.
- Boles J, Kott R, Hatfield P, Bergman J, Flynn C. 2005. Supplemental safflower oil affects the fatty acid profile, including conjugated linoleic acid, of lamb. J Animal Sci. 83:2175–2181.
- Bolte M, Hess B, Means W, Moss G, Rule D. 2002. Feeding lambs high-oleate or high-linoleate safflower seeds differentially influences carcass fatty acid composition. J Animal Sci. 80:609–616.
- Cheng Z, Moore J, Yu L. 2006. High-throughput relative DPPH radical scavenging capacity assay. J Agric Food Chem. 54:7429–7436.
- Choi Y, Lee J. 2009. Antioxidant and antiproliferative properties of a tocotrienol-rich fraction from grape seeds. Food Chem. 114:1386–1390.
- Correddu F, Gaspa G, Pulina G, Nudda A. 2016. Grape seed and linseed, alone and in combination, enhance unsaturated fatty acids in the milk of Sarda dairy sheep. J Dairy Sci. 99:1725–1735.
- Emami A, Ganjkhanlou M, Fathi Nasri MH, Zali A, Rashidi L. 2015. Pomegranate seed pulp as a novel replacement of dietary cereal grains for kids. Small Ruminant Res. 123:238–245.
- Esterbauer H, Cheeseman KH. 1990. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 186:407–421.
- FAO. 2013. Statistical databases. FAO, Rome; [accessed 12 Jan 2017]. Available from: http://faostat.fao.org.
- Faustman C, Cassens R. 1990. The biochemical basis for discoloration in fresh meat: a review. J Muscle Foods. 1:217–243.
- Folch J, Lees M, Sloane-Stanley G. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 226:497–509.
- Garcia MR, Amstalden M, Morrison CD, Keisler DH, Williams GL. 2003. Age at puberty, total fat and conjugated linoleic acid content of carcass, and circulating metabolic hormones in beef heifers fed a diet high in linoleic acid beginning at four months of age. J Animal Sci. 81:261–268.
- Gladine C, Rock E, Morand C, Bauchart D, Durand D. 2007. Bioavailability and antioxidant capacity of plant extracts rich in polyphenols, given as a single acute dose, in sheep made highly susceptible to lipoperoxidation. Br J Nutr. 98:691–701.
- Harfoot C, Hazlewood G. 1998. Lipid metabolism in the rumen. In The rumen microbial ecosystem. London and New York: Elsevier Applied Science.
- He M, Mir P, Sharma R, Schwartzkopf-Genswein K, Entz T, Travis G, Dugan M, Rolland D, Okine E, Dodson M. 2011. Effect of supplementation of beef steer diets with oil containing n6 and n3 fatty acids and 48 h feed withdrawal treatments on animal productivity, carcass characteristics and fatty acid composition. Livest Sci. 142:253–263.
- Hernandez AM, Dryden FD, Marchello JA, Shell LA. 1978. Protein protected fat for ruminants. iv. Plasma lipid, insulin and depot fat composition of lambs. J Animal Sci. 46:1338–1345.
- Iranian Council of Animal Care. 1995. Guide to the care and use of experimental animals, Vol. 1. Isfahan, Iran: Isfahan University of Technology.
- Ito N, Fukushima S, Tsuda H. 1985. Carcinogenicity and modification of the carcinogenic response by BHA, BHT, and other antioxidants. CRC Crit Rev Toxicol. 15:109–150.
- Jerónimo E, Alfaia CM, Alves SP, Dentinho MT, Prates JA, Vasta V, Santos-Silva J, Bessa RJ. 2012. Effect of dietary grape seed extract and Cistus ladanifer L. in combination with vegetable oil supplementation on lamb meat quality. Meat Sci. 92:841–847.
- Kott R, Hatfield P, Bergman J, Flynn C, Van Wagoner H, Boles J. 2003. Feedlot performance, carcass composition, and muscle and fat CLA concentrations of lambs fed diets supplemented with safflower seeds. Small Ruminant Res. 49:11–17.
- Lu P, Zhang LY, Yin JD, Everts AK, Li DF. 2008. Effects of soybean oil and linseed oil on fatty acid compositions of muscle lipids and cooked pork flavour. Meat Sci. 80:910–918.
- Ludden PA, Kucuk O, Rule D, Hess B. 2009. Growth and carcass fatty acid composition of beef steers fed soybean oil for increasing duration before slaughter. Meat Sci. 82:185–192.
- Lutterodt H, Slavin M, Whent M, Turner E, Yu LL. 2011. Fatty acid composition, oxidative stability, antioxidant and antiproliferative properties of selected cold-pressed grape seed oils and flours. Food Chem. 128:391–399.
- Madron M, Peterson D, Dwyer D, Corl B, Baumgard L, Beermann D, Bauman D. 2002. Effect of extruded full-fat soybeans on conjugated linoleic acid content of intramuscular, intermuscular, and subcutaneous fat in beef steers. J Animal Sci. 80:1135–1143.
- Makkar H. 2003. Effects and fate of tannins in ruminant animals, adaptation to tannins, and strategies to overcome detrimental effects of feeding tannin-rich feeds. Small Ruminant Res. 49:241–256.
- Mir P, McAllister T, Zaman S, Morgan Jones S, He M, Aalhus J, Jeremiah L, Goonewardene L, Weselake R, Mir Z. 2003. Effect of dietary sunflower oil and vitamin E on beef cattle performance, carcass characteristics and meat quality. Can J Animal Sci. 83:53–66.
- Morrissey P, Sheehy P, Galvin K, Kerry J, Buckley D. 1998. Lipid stability in meat and meat products. Meat Sci. 49:S73–S86.
- Mozaffarian D, Micha R, Wallace S. 2010. Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 7:e1000252.
- Nestel PJ, Poyser A, Hood RL, Mills SC, Willis MR, Cook LJ, Scott TW. 1978. The effect of dietary fat supplements on cholesterol metabolism in ruminants. J Lipid Res. 19:899–908.
- NRC. 2007. Nutrient requirements of small ruminants: sheep, goats, cervids, and new world camelids. Washington, DC: National Academy Press.
- Parry J, Yu L. 2004. Fatty acid content and antioxidant properties of cold‐pressed black raspberry seed oil and meal. J Food Sci. 69:189–193.
- Razzaghi A, Valizadeh R, Naserian AA, Mesgaran MD, Rashidi L. 2015. Effects of sucrose and sunflower oil addition to diet of Saanen dairy goats on performance and milk fatty acid profile. Livest Sci. 173:14–23.
- Rizzi L, Simioli M, Sardi L, Monetti PG. 2002. Carcass quality, meat chemical and fatty acid composition of lambs fed diets containing extruded soybeans and sunflower seeds. Animal Feed Sci Technol. 97:103–114.
- SAS. 2002. STAT user's guide: statistics. Version 9.1. Cary (NC): Statistical Analysis System Institute, Inc.
- Shi J, Yu J, Pohorly JE, Kakuda Y. 2003. Polyphenolics in grape seeds-biochemistry and functionality. J Med Food. 6:291–299.
- Sinclair L. 2007. Nutritional manipulation of the fatty acid composition of sheep meat: a review. J Agric Sci. 145:419–434.
- Sreelatha S, Padma P. 2009. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr. 64:303–311.
- Van Soest PV, Robertson J, Lewis B. 1991. Methods for dietary fiber, neutral detergent fiber, and non-starch polysaccharides in relation to animal nutrition. J Dairy Sci. 74:3583–3597.
- Wijendran V, Hayes K. 2004. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 24:597–615.
- Williams CD, Whitley BM, Hoyo C, Grant DJ, Iraggi JD, Newman KA, Gerber L, Taylor LA, McKeever MG, Freedland SJ. 2011. A high ratio of dietary n-6/n-3 polyunsaturated fatty acids is associated with increased risk of prostate cancer. Nutr Res. 31:1–8.
- Yu LL, Zhou KK, Parry J. 2005. Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem. 91:723–729.

