ABSTRACT
The major challenge in the generation of bispecific IgG antibodies is enforcement of the correct heavy and light chain association. The correct association of generic light chains can be enabled using immunoglobulin domain crossover, known as CrossMAb technology, which can be combined with approaches enabling correct heavy chain association such as knobs-into-holes (KiH) technology or electrostatic steering. Since its development, this technology has proven to be very versatile, allowing the generation of various bispecific antibody formats, not only heterodimeric/asymmetric bivalent 1+1 CrossMAbs, but also tri- (2+1), tetravalent (2+2) bispecific and multispecific antibodies. Numerous CrossMAbs have been evaluated in preclinical studies, and, so far, 4 different tailor-made bispecific antibodies based on the CrossMAb technology have entered clinical studies. Here, we review the properties and activities of bispecific CrossMAbs and give an overview of the variety of CrossMAb-enabled antibody formats that differ from heterodimeric 1+1 bispecific IgG antibodies.
Introduction to CrossMAb technology
Historically, the fundamental issue in the generation of bispecific heterodimeric/asymmetric IgG antibodies has been the random association of heavy and light chains.Citation1,2 While the correct heavy chain heterodimerization was enabled early on using the knobs-into-holes (KiH) approach,Citation3 the correct association of generic light chains has remained a problem for decades.Citation2 Since 2011, when we described the CrossMAb technology as a method to enforce correct light chain association in bispecific heterodimeric IgG antibodies,Citation4 this technology has proven to be one of the most versatile antibody engineering technologies, allowing the generation of various bispecific antibody formats, including bi- (1+1), tri- (2+1) and tetra-(2+2) valent bispecific antibodies, as well as non-Fc tandem antigen-binding fragment (Fab)-based antibodies. These formats may be derived from any existing antibody pair using domain crossover, without the need for the identification of common light chains, post-translational processing/in vitro chemical assembly or the introduction of a set of mutations enforcing correct light chain association. The technology has also successfully and independently been validated by a number of academic investigators, as described below. Four different tailor-made bispecific antibodies based on the CrossMAb technology are currently in active Phase 1/2 clinical trials. These CrossMAbs can be produced using the well-established IgG production workstream based on one single standard Chinese hamster ovary cell line and typical upstream and downstream processing. The product is comparable in scale, yield, glycosylation, stability Citation5 and quality to conventional IgG antibodies.
In this review, we briefly outline the basic concept, describe the properties and activities of bispecific CrossMAbs developed by Roche and others, and give an overview of CrossMAb-enabled antibody formats that are different from heterodimeric 1+1 bispecific IgG antibodies. Since the description of the CrossMAb technology, alternative technologies have been further developed that allow the generation of bispecific IgG-based antibodies from any antibody pair, and address different aspects of antibody engineering enabled by CrossMAb technology. These include DVD-IgG Citation6-9 and CODV-Ig Citation10 technologies, common light chain approaches,Citation11-15 assembly of bispecific antibodies, e.g., Duobodies,Citation16-19 dual-action Fabs (DAFs) Citation20 or Dutafabs,Citation21 the introduction of guiding disulfide bridges in Duetmab Citation22 or the introduction of different guiding mutations in re-designed Fab moieties to enforce correct light chain association. The latter comes conceptually closest to the CrossMAb concept.Citation23-25 Several of these approaches are being applied in clinical stage bispecific antibodies as well; they are, however, not within the scope of this review, and we refer the reader to the original publications and to recent reviews on the topic of bispecific antibodies.Citation2,26,27
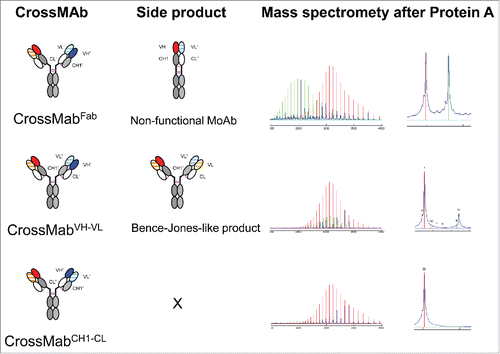
The CrossMAb technology is based on the crossover of the antibody domain within one Fab-arm of a bispecific IgG antibody in order to enable correct chain association,Citation2,4,28 whereas the correct association of heavy chains can be enforced by the KiH,Citation3 electrostatic steering Citation14,29 or alternative technologies.Citation30,31 As shown in , the complete Fab domain can be exchanged in the CrossMAbFab, or either only the variable domains in the CrossMAbVH-VL or the constant domain of the Fab arm in the CrossMAbCH1-CL. In the case of the CrossMAbCH1-CL, no theoretical side products are formed, whereas in the case of the CrossMAbFab design a non-functional monovalent antibody (MoAb/MonoMAb) is formed by the VH-CH1 and VL-CL domains, as well as a non-functional Fab from the 2 different parental antibodies. In the case of the CrossMAbVH-VL design, an antibody that carries an associated VL-CL chain in the crossed Fab-arm (VL-CH1), as known from Bence-Jones proteins, can occur as a side product. The introduction of repulsive charges into the constant CH1 and CL domains of the Fab can be done to avoid the formation of the Bence-Jones-like side product (unpublished data). In the following, we will describe different applications of the CrossMAb technology to design multispecific and multivalent antibodies of various formats.
Figure 1. Schematic overview about the 3 major CrossMAb formats: CrossMAbFab, CrossMAbVH-VL and CrossMAbCH1-CL and predicted side products that can be formed. Mass spectrometry following transient expression and purification via protein A confirmed the presence of the predicted antibody species. In the case of CrossMAbFab, a non-functional monovalent heavy chain dimer is formed; in the case CrossMAbVH-VL, an antibody with a VL-CL Bence-Jones-like associated chain can occur. KiH technology or alternative heavy chain heterodimerization technologies applied where appropriate and indicated by colors only. Constant heavy chain domains are colored in gray, constant light chain domains in white, variable heavy chains are colored uniformly, light chain domains are colored with a line pattern.
Heterodimeric/asymmetric bivalent 1+1 IgG CrossMAbs
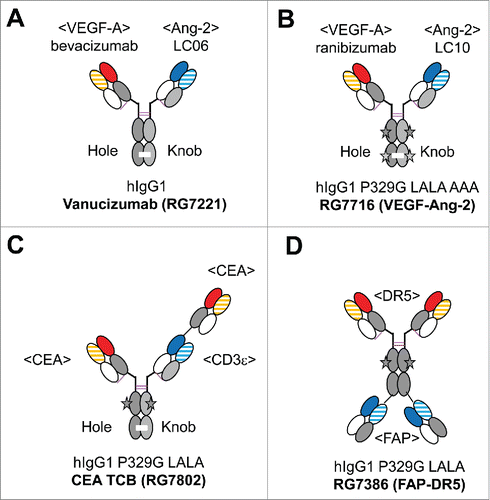
The CrossMAb technology was first described for bispecifc heterodimeric IgG antibodies recognizing vascular endothelial growth factor (VEGF)-A and angiopoietin-2 (Ang-2), [4] with the Fab carrying the crossover based on bevacizumab and the wildtype Fab based on an Ang-2 antibody termed LC06.Citation32 Based on this experience, an optimized version of the original bispecific CrossMAb was generated for clinical development. The respective bispecific anti-Ang-2/VEGF CrossMAb vanucizumab (RG7221) is of IgG1 isotype, and carries the original unmodified bevacizumab Fab on the knob arm, whereas the LC06-bearing arm is a Fab carrying a CH1-CL domain crossover.Citation33 Correct heavy chain association is enabled by use of disulfide-stabilized KiH mutations (). The potency of vanucizumab was determined using a surface plasmon resonance-based assay principle for bispecific antibodies.Citation34 Vanucizumab or a mouse specific surrogate bispecific CrossMAb showed potent antitumoral efficacy using several orthotopic/syngeneic mouse tumors, as well as patient/cell line-derived human tumor xenografts. Overall, vanucizumab or the mouse specific surrogate bispecific CrossMAb strongly inhibited angiogenesis, and resulted in an enhanced vessel maturation phenotype. Notably, and in contrast to Ang-1 inhibition, inhibition of Ang-2 did not increase the adverse effect of anti-VEGF treatment on physiologic vessels, but was able to reduce micrometastatic growth in an adjuvant setting,Citation33 in line with a study demonstrating anti-metastatic activity of Ang-2 inhibition.Citation35 In models of renal cell cancer (RCC), it significantly reduced tumor volume, vessel density, and interstitial fluid pressure compared to either monotherapy alone.Citation36
Figure 2. Overview of the clinical stage CrossMAbs: (A) Ang-2-VEGF CrossMAb vanucizumab (RG7112) for oncology, (B) VEGF-Ang-2 CrossMAb RG7716 for ophthalmology, (C) CEA TCB (RG7802) for CEA-positive solid tumors, (D) FAP-DR5 tetravalent CrossMAb RG7386. The black star depicts the P329G LALA (P329G, L235A, L234A) mutations to abolish FcγR and C1q binding, the green star Triple A (I253A, H310A, H435A) mutations to abolish FcRn binding. Constant heavy chain domains are colored in gray, constant light chain domains in white, variable heavy chains are colored uniformly, light chain domains are colored with a line pattern.
The crystal structure analysis of the isolated LC06-based anti-Ang-2 CrossFab fragment compared to the parental Fab demonstrated the structural and functional integrity of the variable domain, supporting the notion that the CrossMAb approach allows correct light chain association with unperturbed structure compared to the parental Fab structure.Citation37
Recently, Kloepper and colleagues showed that an anti-Ang-2/VEGF mouse specific surrogate bispecific CrossMAb could overcome resistance to anti-VEGF treatment in mice bearing orthotopic syngeneic or human glioblastoma models. In both models, the bispecific CrossMAb reprogrammed protumor M2 macrophages toward the antitumor M1 phenotype.Citation38
In 2012, vanucizumab became one of the first human heterodimeric bispecific IgG antibodies to enter clinical trials in cancer patients (NCT01688206).Citation39 Based on the acceptable safety profile with favorable pharmacokinetic (PK) and pharmacodynamic effects in the Phase 1 study and an extension in ovarian cancer patients,Citation40 the Phase 2 McCAVE study (NCT02141295) was initiated in 2014. In this clinical trial, vanucizumab is being evaluated in direct comparison to bevacizumab (Avastin®), both in combination with mFOLFOX-6 followed by maintenance, in patients with first line metastatic colorectal cancer. In addition, vanucizumab has been studied in a Phase 1b study in platinum-resistant ovarian cancer and is being studied in two Phase 1b studies in combination with atezolizumab or the anti-CD40 antibody RO7009789 respectively.
We and others have shown that Ang-2 plays an important role in ocular diseases characterized by angiogenesis and inflammation,Citation41-43 and that inhibition of Ang-2 alone can have an anti-angiogenic effect in the eye comparable to VEGF-A inhibition.Citation43 Based on this rationale, a tailor-made and optimized VEGF-Ang-2 CrossMAb, RG7716, was designed specifically for ophthalmology (unpublished data). RG7716 is differentiated from vanucizumab by the following features: 1) the bevacizumab Fab is replaced by ranibizumab, which exhibits higher affinity for VEGF-A in order to stronger block VEGF-A; 2) the anti-Ang-2 LC06 Fab is replaced by LC10, which is specific for Ang-2 and does not bind to Ang-1; 3) the Fc of the antibody carries P329G, L235A, L234A (P329G LALA) mutations (unpublished data) in order to abolish any undesired immune effector function of the antibody, e.g., antibody-dependent cell-meditated cytotoxicity (ADCC), phagocytosis (ADCP) and complement dependent cytotoxicity (CDC), thus reducing the potential for inflammatory events; and 4) in order to abolish FcRn recycling and reduce peripheral half-life after diffusion out of the eye into circulation, the Triple A mutations I253A, H310A, H435A have been introduced into the Fc with the goal of minimizing any potential for systemic anti-angiogenic side effects (). The introduction of these Triple A mutations also led to reduced viscosity, which is beneficial for intravitreal application through a thin needle. In preclinical models, RG7716 demonstrated potent anti-angiogenic activity superior to VEGF-A inhibition alone in a laser-induced choroidal neovascularization (CNV) model (unpublished data). In 2013, RG7716 became the first bispecific antibody to be evaluated in clinical Phase 1 studies in ophthalmology. Based on RG7716s safety and efficacy profile, the Phase 2 AVENUE study (NCT02484690) in wet age-related macular degeneration patients in which it is directly compared to ranibizumab (Lucentis®) as current standard of care was initiated in 2015. RG7716 is also being tested in the BOULEVARD Phase 2 study in patients with diabetic macular edema (CI-DME) in direct comparison to ranibizumab (NCT02699450).
Several academic investigators have used the CrossMAb technology to successfully generate bispecific antibodies. Zhang and colleagues created a bispecific heterodimeric/asymmetric CrossMAbCH1-CL using KiH technology based on an optimized variant of pertuzumab (L56TY) and trastuzumab. The resulting molecule, termed Tras-Permut CrossMAb, was shown to inhibit the progression of trastuzumab-resistant breast cancer in a mouse model.Citation44
Similarly, Zhao and colleagues used KiH technology to generate a bispecific heterodimeric/asymmetric CrossMAbCH1-CL, termed CD20-243 CrossMAb, that targeted both CD20 and HLA-DR.Citation45 The CD20-243 CrossMAb induced high levels of CDC, ADCC and anti-proliferative activity. Notably, although HLA-DR is expressed on normal and malignant cells, the CrossMAb exhibited highly anti-tumor specificity, showing efficient eradication of hematological malignancies both in vitro and in vivo.Citation45 Zhao and colleagues also constructed an analogous CrossMAbCH1-CL-based bispecific fusion protein (BiFP), termed CD20-Flex, that targeted both CD20 and Flt3.Citation46 Notably, CD20-Flex BiFP not only eliminated lymphoma, but also potentiated tumor-specific T-cell immunity, likely through the expansion and infiltration of dendritic cells into the tumor tissue.Citation46
Likewise, Asokan and colleagues generated several bispecific heterodimeric/asymmetric CrossMAbsCH1-CL using KiH technology using different broadly neutralizing antibodies (bNAbs) against the HIV-1 envelope (Env) with the aim to target different antigenically diverse HIV strains and improve coverage against the majority of these viruses.Citation47 The bispecific CrossMAbs displayed features of both antibody specificities, and, in some cases, displayed improved coverage over the individual parental antibodies. All four bispecific CrossMAbs neutralized 94% to 97% of antigenically diverse viruses in a panel of 206 HIV-1 strains. Among the bispecific antibodies tested, VRC07 × PG9-16 displayed the most favorable neutralization profile, neutralizing 97% of viruses with a median 50% inhibitory concentration (IC50) of 0.055 μg/ml. VRC07 × PG9-16 demonstrated in vivo PK parameters comparable to those of the parental bNAbs in rhesus macaques, and may qualify as a promising candidate for the prevention and treatment of HIV-1 infection.Citation47
Huang, Ho and colleagues used asymmetric CrossMAbsCH1-CL with KiH technology to generate the most potent and broad HIV neutralizing antibodies to date. One bispecific antibody, 10E8V2.0/iMab, neutralized >100 HIV-1 pseudotyped viruses and potently neutralized 99% of viruses in a second panel of 200 HIV-1 isolates belonging to clade C, the dominant subtype accounting for ∼50% of new infections worldwide. 10E8V2.0/iMab also reduced virus load substantially in HIV-1-infected humanized mice, and provided complete protection when administered prior to virus challenge. These bispecific antibodies hold promise as prophylactic or therapeutic agents for therapy of HIV-1.Citation48 In independent work, Bournazos, Nussenzweig Ravetch and colleagues created IgG3 hinge engineered bispecific anti-Env neutralizing antibodies (biNAbs) using a CH1-CL crossover based on broadly neutralizing antibodies (bNAbs) in order to improve neutralization breadth/potency. In these biNAbs the engineered hinge domain of IgG3 enabled Fab domain flexibility required for simultaneous binding to the Env trimer while retaining the functional properties of the Fc. These bispecific antibodies exhibited synergistically enhanced viral neutralization and superior control of viremiaCitation49.
Finally, in a novel application of bispecific antibodies, Tung and colleagues used CrossMAb technology to generate bispecific antibodies against PEG and HER2 or CD19 for the specific delivery of PEGylated compounds, including PEGylated proteins, liposomes, and nanoparticles, to HER2+/CD19+ tumor cells.Citation50
In general, in our hands as well as those of external investigators (see below), the generic CrossMAb approach works very well. In exceptional cases, however, the yield or purity of the bispecific CrossMAb antibody may not fulfill all requirements and require further optimization. For example, adjusting the expression rate of the 4 different antibody chains of the CrossMabs can minimize the formation of hole-hole- or knob-knob-containing antibody species or ½ (one HC and one LC) or ¾ (lacking one LC) antibodies (unpublished data).
The CrossMAb technology can also be applied to generate the respective mouse surrogate bispecific antibodies based on murine isotypes, e.g., muIgG1, using charged residues for heterodimerization (K392D, K409D and E356K, D399K) or muIgG2a using homologous KiH mutations.Citation38
Heterodimeric/asymmetric trivalent 2+1 IgG CrossMAbs
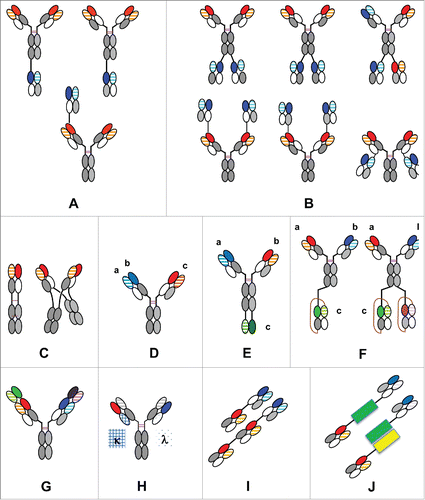
By fusing a Fab to the N-terminus of the VH domain of a CrossMAbCH-CL, a novel trivalent 2+1 IgG antibody can be generated (). Further trivalent formats can be generated by fusing the Fab to the N-terminus of the VL domain, the C-terminus of the light chain or the C-terminus of the Fc domain. Alternatively, a CrossFab can be used to fuse to the N- or C-termini of a conventional IgG antibody to generate trivalent bispecific antibodies via a flexible (G4S)2-linker. Such antibodies can be advantageous, for example, in cases where one wants to bind with avidity to an antigen A, but only in a monovalent fashion to a second antigen.
Figure 3. The CrossMAb zoo: Schematic overview about different mono-, bi- and multispecific antibody formats enabled by CrossMAb technology: (A) Heterodimeric/asymmetric trivalent 2+1 IgG CrossMAbs; (B) Symmetric tetravalent 2+2 IgG CrossMAbs; (C) MoAb (MonoMAb) and MoAb-Dimer (DuoMAb; (D) Trispecific Pan-HER family DAF-CrossMAb antibody; (E) Trispecific CrossMAb-VH-VL; (F) Tri-, tetraspecific CrossMAb-scFAb fusions; (G) DVD-CrossMAb; (H) Heterodimeric/asymmetric Kappa-Lambda-CrossMAb; (I) Fc-free Tandem Fab-CrossFab antibody. (J) Fc-free bispecific Fab fusion proteins using alternative fusion partners (green, yellow) based on Fab crossover. KiH technology or alternative heavy chain heterodimerization technologies applied where appropriate and indicated by colors only. The drawings given represent only examples, since in many cases crossed and uncrossed Fabs can be assembled in various ways. Constant heavy chain domains are colored in gray, constant light chain domains in white, variable heavy chains are colored uniformly, light chain domains are colored with a line pattern.
In the case of T cell bispecific antibodies (TCBs), the goal is to bind only monovalently to the CD3ϵ chain of the T-cell receptor (TCR), so that the TCR is only cross-linked and activated upon concomitant binding of the 2 tumor antigen binding domains of the antibody to its target on the tumor cell, resulting in crosslinking, T cell activation, and subsequent strictly tumor antigen-dependent T cell killing of the targeted cell. In 2014, CEA TCB (RG7802), a 2+1 TCB recognizing carcinoembryonic antigen (CEA), became the first T cell bispecific antibody to enter clinical Phase 1 study (NCT02324257) where such a 2+1 head-to-tail design principle was implemented using the CrossMAb technology, and it is one of the first IgG-based T cell bispecific antibodies in clinical trials ().Citation51,52 In this case, a CH1-CL crossover was introduced into the anti-CD3ϵ CrossFab to enforce correct chain pairing. Notably, the CD3ϵ binding region was introduced in an “inside” position because it allows fusion of a Fab to the N-terminus of its VH domain without loss of activity (unpublished data). Furthermore, CEA TCB has been engineered using the P329G LALA mutation to exclude any FcγR co-activation upon TCR or target cell binding.Citation51,52 CEA TCB binds simultaneously to CEA-expressing tumor and T cells, resulting in T-cell activation, secretion of cytotoxic granules and tumor cell lysis. As a consequence of bivalent CEA binding, CEA TCB activity correlates with CEA expression, which allows cells with high versus low CEA expression to be distinguished. In xenograft models, CEA TCB mediates anti-tumoral efficacy, and leads to a strong increase in frequency of tumor-infiltrating T cells. Recently, Vu and colleagues described a novel B cell maturation antigen (BCMA) specific 2+1 TCB based on this platform.Citation53,54
Symmetric tetravalent 2+2 IgG CrossMAbs
As we have described, symmetric tetravalent bispecific 2+2 antibodies can be generated by fusing either a CrossFab fragment to the C-terminus of the Fc domain of an IgG antibody. Analogous tetravalent formats can be generated by fusing the Fab to the N-terminus of the VH or VL domain or to the C-terminus of the VL domain via flexible (G4S) x-linkers. Alternatively, a Fab can be fused to the N- and C-termini of a CrossMAbCH-CL (). These antibodies are made using one heavy chain and 2 light chains.Citation55 By introduction of KiH mutations into the Fc of such tetravalent antibodies, it is also possible to generate an asymmetric tetravalent 2+2 IgG CrossMAb antibody based on 2 heavy chains and 2 light chains that carries its 2 different specificities on its 2 opposed sides (, top right).Citation56 In addition, 2+1 and 2+2 IgG CrossMAbs can also be designed using flexible connectors in the heavy and light chains.
This design principle was applied to generate RG7386, a symmetric tetravalent bispecific 2+2 CrossMAb antibody recognizing fibroblast activation protein-α (FAP) on activated tumor fibroblasts and death receptor 5 (DR5) on tumor cells ().Citation57 Activation of apoptosis in tumor cells can be triggered through DR5 hyperclustering on the cell surface; however, conventional DR5 antibodies that rely on FcγRIIb-mediated hyperclustering have failed in clinical trials.Citation58 RG7386 was designed as a bispecific antibody using a low affinity DR5 antibody to activate the extrinsic apoptotic pathway in tumor cells only by avidity-driven DR5 hyperclustering in a strictly FAP expression-dependent fashion. Due to the introduction of the P329G LALA mutation, FcγR-mediated crosslinking cannot contribute to the mechanism of action of RG7386. RG7386 potently triggers tumor cell apoptosis in vitro and in vivo in preclinical tumor models with FAP-positive stroma or FAP-expressing malignant cells in a fashion superior to conventional DR5 antibodies.Citation57 RG7386 is being tested in a clinical Phase 1 study (NCT02558140).
Similarly, a symmetric tetravalent bispecific 2+2 CrossMAb antibody targeting the inflammatory tumor necrosis factor (TNF) and interleukin (IL)-17 was generated and compared to a classical heterodimeric/asymmetric bivalent 1+1 IgG CrossMAbCH1-CL.Citation59 Blocking TNF and IL-17 showed additive/synergistic effects in promoting production of IL-6, IL-8, and granulocyte colony-stimulating factor in human fibroblast-like synoviocytes. The bispecific TNF/IL-17 CrossMAb showed superior efficacy, both in blocking cytokine and chemokine responses, in vitro. Furthermore, dual vs. single inhibition of both cytokines was more effective in inhibiting the development of inflammation and bone and cartilage destruction in arthritic mice. Importantly, in these studies the tetravalent 2+2 TNF/IL-17 CrossMAb was more potent than the bivalent 1+1 TNF/IL-17 CrossMAb due to avidity, supporting the importance of testing different antibodies formats to identify the most suitable one.Citation59
Additional applications of CrossMAb technology
Apart from the applications of the CrossMAb technology to the IgG-based bispecific antibody formats described above, the CrossMAb technology can also be applied for the engineering of other antibody formats.
A functional monovalent antibody (MoAb/MonoMAb, ) can be generated by combining 2 crossover heavy chains with either CH1-CL or the analogous VH-VL crossover (unpublished data, ref.Citation60). Surprisingly, when we expressed the corresponding 2 heavy chains without enforcing heterodimerization by KiH, in addition to the correct MoAb antibody, a dimeric MoAb (MoAb-dimer, DuoMAb) comprising 2 Fab and 2 Fc regions was formed () (unpublished data, ref. Citation61).
Fuh and colleagues have described dual-action Fabs (DAFs) that can bind 2 different antigens with the same variable region, such as HER2 and VEGF-A or EGFR/HER1 and HER3. Citation20,62 Using DAFs, tri- or tetraspecific heterodimeric/asymmetric bivalent 1+1 DAF-CrossMAbs can be generated, e.g., a trispecific pertuzumab and EGFR/HER1-HER3 DAF-based pan-HER family CrossDAF antibody (). Citation63
Trispecific antibodies may also be generated by fusing a VH domain to the C-terminus of the Fc of a heterodimeric CrossMAb, and a VL domain to the other C-terminus of the Fc as previously described Citation64,65 (trispecific CrossMAb-VH-VL, ). Alternatively, tri- or tetraspecific antibodies can be generated by fusing scFv or single chain Fab (scFab) moieties Citation66-68 to the Fc of a heterodimeric CrossMAb (). Citation69
Hu and colleagues used CrossMAb technology to generate 2 different tetraspecific, tetravalent antibodies targeting EGFR/HER1, HER2, HER3, and VEGF to therapeutically overcome crosstalk among the HER family receptors.Citation70 They generated FL518, a 4-in-one CrossMAbCH1-CL based on the EGFR/HER1-HER3 and HER2-VEGF DAF antibodies Citation20,62 and CRTB6, a tetraspecific, tetravalent DVD-CrossMAb antibody based on a bevacizumab-cetuximab CrossMAbCH1-CL extended by VH and VL domains from the HER3 antibody RG7116 Citation71,72 and trastuzumab using DVD-Ig technology ().Citation9 These tetraspecific antibodies not only inhibit signaling mediated by the receptors in vitro and in vivo, but also disrupted HER-MET crosstalk. Compared with 2-in-one antibodies and a series of bispecific antibodies in multiple tumor models, FL518 and CRTB6 were more broadly efficacious and able to inhibit the growth of anti–HER-resistant cancer cells.Citation70
Fischer and colleagues described an alternative and attractive strategy to generate natural bispecific antibodies based on a common heavy chain (CHC) without the requirement of heterodimeric Fc domains (e.g., KiH) using kappa and lambda light chains (LCs) and differential purification via kappa and lambda specific resins.Citation12 As the specificity of antibodies frequently is determined by the heavy chains (HCs), we adapted this approach using a crossover of a common light chain (CLC) combined with the use of Ckappa and Clambda domains. In the respective format, the common light chain variable domain is now part of the identical heavy chains in the heterodimeric/asymmetric Kappa-Lambda-CrossMAb, whereas the 2 different VH domains fused to Ckappa and Clambda chains, respectively, remain in the respective light chains ().Citation73
Last, but not least, the CrossMAb approach can be applied to generate bi-, tri-or multivalent Fc-free Tandem Fab-CrossFab antibodies () Citation74 similar in design and application to what has been described using site directed mutagenesis by Wu and colleagues for using a redesigned Fab interface.Citation24 Such molecules can also contain linkers between the respective light chains. Similarly, Fc-free bispecific Fab fusion proteins using various alternative fusion partners can be imagined using the CrossMab Fab approach ().
Generation of CrossMAbs
An overview of the design principle applied for the generation of bispecific CrossMAb antibodies with CH1-CL crossover is shown in . Design of the crossing point is based on the fact that the VH, Vκ and Vλ domains, as well as their constant counterparts, are structurally extremely similar. Thus, we have extracted the 4 domains VH, VL (kappa), CH1, CL (kappa) from the crystal structure of trastuzumab (PDB:1N8Z) Citation75 and VL (lambda) and CL (lambda) from the crystal structure of a lambda Fab (PDB: 7FAB Citation76). Superpositions of the 3 variable domains and the 3 constant domains were performed separately by application of a root mean square fit that included only the Cα-atoms of the β-sheets adjacent to the elbow region (displayed as spheres in the structures of , gray in the sequences) match in space. The superposition of separate domains avoids a bias for specific elbow angles. The new elbow can then be composed from the original sequences, aiming for optimal sequence homology, structural overlap, and contact between variable and constant domains, while also avoiding the generation of potential T cell epitopes.
Figure 4. Design of CrossMAbs with CH1-CL crossover: Top: Typical structures of VH, Vk and Vλ domains and their superposition in the sense that the Cα atoms of the β-sheets adjacent to the elbow region (displayed as spheres in the structures, gray in the sequences) match in space. Middle: the same applied to the CH1, Cκ and Cλ domains. Bottom: wildtype and designed CrossMAb sequences; β-sheets adjacent to the elbow regions are colored gray. The newly designed sequence is shown in red.
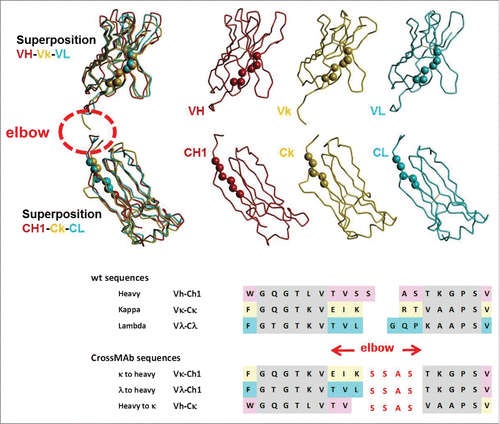
From many possibilities, the elbow sequence “SSAS” (red sequence in ) was selected in the initial CrossMAb for both the VH-Cκ- as well as the Vκ and Vλ−CH1 transitions successfully. This means that on both sides of the CrossMAb this stretch is part of a wildtype sequence (SSAS-TKGPS for Vκ-CH1 and GTLVTV-SSAS for VH-Cκ); new solvent-exposed sequences do not occur as consequences of the crossover. Since the constant domains are not involved in antigen binding, there is no need to design separate VH-Cκ and VH-Cλ sequences; thus, typically VH-Cκ is used.
As mentioned above, several elbow sequences and variations in length are imaginable, and therefore we conducted a matrix approach, testing variations in length between VH-Cκ and VL-CH1 by addition or deletion of amino acids and utilizing different amino acids in individual positions. In general, there are many elbow variations possible and functional. To identify the most suitable elbow sequences, we assessed the thermal stability of crossed Fabs, measured as aggregation onset temperature by dynamic light scattering, in dependency of an increasing temperature. To our surprise, there was no better elbow sequence included than the initially proposed one (unpublished data). and give on overview of typical elbow sequences for Cκ and Cλ containing light chains. Thermal stability and productivity can slightly differ between these elbow variants.
Table 1. Typical elbow sequences, * typically Cκ is used.
Conclusion
Based on the ongoing clinical validation of several CrossMAb-based bispecific antibodies and the successful application by independent academic researchers, the CrossMAb approach and platform has been validated as a powerful strategy to generate diverse bi- and multispecific antibodies. Additional CrossMAb-based bispecific antibodies are expected to advance into clinical trials in the future, and ultimately they may contribute to the improvement of patients' outcomes.
Disclosure of potential conflicts of interest
All authors are employees of Roche. CK, WS and JR are co-inventors on various CrossMAb technology related patent applications and issued patents.
Acknowledgment
We thank all the numerous contributors from Roche Pharmaceutical Research and Early Development (pRED) to the development of the CrossMAb technology and the team members from pRED and Roche Pharma Technical Development working on the development of specific CrossMAbs for all their support, ideas and effort over the last 10 y.
References
- Schaefer W, Volger HR, Lorenz S, Imhof-Jung S, Regula JT, Klein C, Molhoj M. Heavy and light chain pairing of bivalent quadroma and knobs-into-holes antibodies analyzed by uhr-esi-qtof mass spectrometry. MAbs 2016; 8(1):49-55; PMID:26496506; http://dx.doi.org/10.1080/19420862.2015.1111498
- Klein C, Sustmann C, Thomas M, Stubenrauch K, Croasdale R, Schanzer J, Brinkmann U, Kettenberger H, Regula JT, Schaefer W. Progress in overcoming the chain association issue in bispecific heterodimeric igg antibodies. MAbs 2012; 4(6):653-63; PMID:22925968; http://dx.doi.org/10.4161/mabs.21379
- Merchant AM, Zhu Z, Yuan JQ, Goddard A, Adams CW, Presta LG, Carter P. An efficient route to human bispecific igg. Nat Biotechnol 1998; 16(7):677-81; PMID:9661204; http://dx.doi.org/10.1038/nbt0798-677
- Schaefer W, Regula JT, Bahner M, Schanzer J, Croasdale R, Durr H, Gassner C, Georges G, Kettenberger H, Imhof-Jung S, et al. Immunoglobulin domain crossover as a generic approach for the production of bispecific igg antibodies. Proc Natl Acad Sci U S A 2011; 108(27):11187-92; PMID:21690412; http://dx.doi.org/10.1073/pnas.1019002108
- Dengl S, Wehmer M, Hesse F, Lipsmeier F, Popp O, Lang K. Aggregation and chemical modification of monoclonal antibodies under upstream processing conditions. Pharm Res 2013; 30(5):1380-99; PMID:23322133; http://dx.doi.org/10.1007/s11095-013-0977-8
- Gu J, Yang J, Chang Q, Liu Z, Ghayur T. Identification of anti-egfr and anti-erbb3 dual variable domains immunoglobulin (dvd-ig) proteins with unique activities. PLoS One 2015; 10(5):e0124135; PMID:25997020; http://dx.doi.org/10.1371/journal.pone.0124135
- Correia I, Sung J, Burton R, Jakob CG, Carragher B, Ghayur T, Radziejewski C. The structure of dual-variable-domain immunoglobulin molecules alone and bound to antigen. MAbs 2013; 5(3):364-72; PMID:23572180; http://dx.doi.org/10.4161/mabs.24258
- Jakob CG, Edalji R, Judge RA, DiGiammarino E, Li Y, Gu J, Ghayur T. Structure reveals function of the dual variable domain immunoglobulin (dvd-ig) molecule. MAbs 2013; 5(3):358-63; PMID:23549062; http://dx.doi.org/10.4161/mabs.23977
- Wu C, Ying H, Grinnell C, Bryant S, Miller R, Clabbers A, Bose S, McCarthy D, Zhu RR, Santora L, Davis-Taber R, et al. Simultaneous targeting of multiple disease mediators by a dual-variable-domain immunoglobulin. Nat Biotechnol 2007; 25(11):1290-7; PMID:17934452; http://dx.doi.org/10.1038/nbt1345
- Steinmetz A, Vallee F, Beil C, Lange C, Baurin N, Beninga J, Capdevila C, Corvey C, Dupuy A, Ferrari P, et al. Codv-ig, a universal bispecific tetravalent and multifunctional immunoglobulin format for medical applications. MAbs 2016 July; 8(5): 867—78; PMID:26984268; http://dx.doi.org/10.1080/19420862.2016.1162932
- Smith EJ, Olson K, Haber LJ, Varghese B, Duramad P, Tustian AD, Oyejide A, Kirshner JR, Canova L, Menon J, et al. A novel, native-format bispecific antibody triggering t-cell killing of b-cells is robustly active in mouse tumor models and cynomolgus monkeys. Sci Rep 2015; 5:17943; PMID:26659273; http://dx.doi.org/10.1038/srep17943
- Fischer N, Elson G, Magistrelli G, Dheilly E, Fouque N, Laurendon A, Gueneau F, Ravn U, Depoisier JF, Moine V, et al. Exploiting light chains for the scalable generation and platform purification of native human bispecific igg. Nat Commun 2015; 6:6113; PMID:25672245; http://dx.doi.org/10.1038/ncomms7113
- Sampei Z, Igawa T, Soeda T, Okuyama-Nishida Y, Moriyama C, Wakabayashi T, Tanaka E, Muto A, Kojima T, Kitazawa T, et al. Identification and multidimensional optimization of an asymmetric bispecific igg antibody mimicking the function of factor viii cofactor activity. PLoS One 2013; 8(2):e57479; PMID:23468998; http://dx.doi.org/10.1371/journal.pone.0057479
- Kitazawa T, Igawa T, Sampei Z, Muto A, Kojima T, Soeda T, Yoshihashi K, Okuyama-Nishida Y, Saito H, Tsunoda H, et al. A bispecific antibody to factors ixa and x restores factor viii hemostatic activity in a hemophilia a model. Nat Med 2012; 18(10):1570-4; PMID:23023498; http://dx.doi.org/10.1038/nm.2942
- Tustian AD, Endicott C, Adams B, Mattila J, Bak H. Development of purification processes for fully human bispecific antibodies based upon modification of protein a binding avidity. MAbs 2016; 8(4):828-38; PMID:26963837; http://dx.doi.org/10.1080/19420862.2016.1160192
- Spiess C, Merchant M, Huang A, Zheng Z, Yang NY, Peng J, Ellerman D, Shatz W, Reilly D, Yansura DG, et al. Bispecific antibodies with natural architecture produced by co-culture of bacteria expressing two distinct half-antibodies. Nat Biotechnol 2013; 31(8):753-8; PMID:23831709; http://dx.doi.org/10.1038/nbt.2621
- Labrijn AF, Meesters JI, de Goeij BE, van den Bremer ET, Neijssen J, van Kampen MD, Strumane K, Verploegen S, Kundu A, Gramer MJ, et al. Efficient generation of stable bispecific igg1 by controlled fab-arm exchange. Proc Natl Acad Sci U S A 2013; 110(13):5145-50; PMID:23479652; http://dx.doi.org/10.1073/pnas.1220145110
- Spiess C, Bevers J, 3rd, Jackman J, Chiang N, Nakamura G, Dillon M, Liu H, Molina P, Elliott JM, Shatz W, et al. Development of a human igg4 bispecific antibody for dual targeting of interleukin-4 (il-4) and interleukin-13 (il-13) cytokines. J Biol Chem 2013; 288(37):26583-93; PMID:23880771; http://dx.doi.org/10.1074/jbc.M113.480483
- Strop P, Ho WH, Boustany LM, Abdiche YN, Lindquist KC, Farias SE, Rickert M, Appah CT, Pascua E, Radcliffe T, et al. Generating bispecific human igg1 and igg2 antibodies from any antibody pair. J Mol Biol 2012; 420(3):204-19; PMID:22543237; http://dx.doi.org/10.1016/j.jmb.2012.04.020
- Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, Totpal K, Wong A, Lee CV, Stawicki S, et al. A two-in-one antibody against her3 and egfr has superior inhibitory activity compared with monospecific antibodies. Cancer Cell 2011; 20(4):472-86; PMID:22014573; http://dx.doi.org/10.1016/j.ccr.2011.09.003
- Beckmann R. Wo2012163520): Dual targeting. 2012
- Mazor Y, Oganesyan V, Yang C, Hansen A, Wang J, Liu H, Sachsenmeier K, Carlson M, Gadre DV, Borrok MJ, et al. Improving target cell specificity using a novel monovalent bispecific igg design. MAbs 2015; 7(2):377-89; PMID:25621507; http://dx.doi.org/10.1080/19420862.2015.1007816
- Liu Z, Leng EC, Gunasekaran K, Pentony M, Shen M, Howard M, Stoops J, Manchulenko K, Razinkov V, Liu H, et al. A novel antibody engineering strategy for making monovalent bispecific heterodimeric igg antibodies by electrostatic steering mechanism. J Biol Chem 2015; 290(12):7535-62; PMID:25583986; http://dx.doi.org/10.1074/jbc.M114.620260
- Wu X, Sereno AJ, Huang F, Lewis SM, Lieu RL, Weldon C, Torres C, Fine C, Batt MA, Fitchett JR, et al. Fab-based bispecific antibody formats with robust biophysical properties and biological activity. MAbs 2015; 7(3):470-82; PMID:25774965; http://dx.doi.org/10.1080/19420862.2015.1022694
- Lewis SM, Wu X, Pustilnik A, Sereno A, Huang F, Rick HL, Guntas G, Leaver-Fay A, Smith EM, Ho C, et al. Generation of bispecific igg antibodies by structure-based design of an orthogonal fab interface. Nat Biotechnol 2014; 32(2):191-8; PMID:24463572; http://dx.doi.org/10.1038/nbt.2797
- Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today 2015; 20(7):838-47; PMID:25728220; http://dx.doi.org/10.1016/j.drudis.2015.02.008
- Spiess C, Zhai Q, Carter PJ. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol Immunol 2015; 67(2 Pt A):95-106; PMID:25637431; http://dx.doi.org/10.1016/j.molimm.2015.01.003
- Grote M, Haas AK, Klein C, Schaefer W, Brinkmann U. Bispecific antibody derivatives based on full-length igg formats. Methods Mol Biol 2012; 901(247-63; PMID:22723106; http://dx.doi.org/10.1007/978-1-61779-931-0_16
- Gunasekaran K, Pentony M, Shen M, Garrett L, Forte C, Woodward A, Ng SB, Born T, Retter M, Manchulenko K, et al. Enhancing antibody fc heterodimer formation through electrostatic steering effects: Applications to bispecific molecules and monovalent igg. J Biol Chem 2010; 285(25):19637-46; PMID:20400508; http://dx.doi.org/10.1074/jbc.M110.117382
- Moore GL, Bautista C, Pong E, Nguyen DH, Jacinto J, Eivazi A, Muchhal US, Karki S, Chu SY, Lazar GA. A novel bispecific antibody format enables simultaneous bivalent and monovalent co-engagement of distinct target antigens. MAbs 2011; 3(6):546-57; PMID:22123055; http://dx.doi.org/10.4161/mabs.3.6.18123
- Davis JH, Aperlo C, Li Y, Kurosawa E, Lan Y, Lo KM, Huston JS. Seedbodies: Fusion proteins based on strand-exchange engineered domain (seed) ch3 heterodimers in an fc analogue platform for asymmetric binders or immunofusions and bispecific antibodies. Protein Eng Des Sel 2010; 23(4):195-202; PMID:20299542; http://dx.doi.org/10.1093/protein/gzp094
- Thomas M, Kienast Y, Scheuer W, Bahner M, Kaluza K, Gassner C, Herting F, Brinkmann U, Seeber S, Kavlie A, et al. A novel angiopoietin-2 selective fully human antibody with potent anti-tumoral and anti-angiogenic efficacy and superior side effect profile compared to pan-angiopoietin-1/-2 inhibitors. PLoS One 2013; 8(2):e54923; PMID:23405099; http://dx.doi.org/10.1371/journal.pone.0054923
- Kienast Y, Klein C, Scheuer W, Raemsch R, Lorenzon E, Bernicke D, Herting F, Yu S, The HH, Martarello L, et al. Ang-2-vegf-a crossmab, a novel bispecific human igg1 antibody blocking vegf-a and ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin Cancer Res 2013; 19(24):6730-40; PMID:24097868; http://dx.doi.org/10.1158/1078-0432.CCR-13-0081
- Gassner C, Lipsmeier F, Metzger P, Beck H, Schnueriger A, Regula JT, Moelleken J. Development and validation of a novel spr-based assay principle for bispecific molecules. J Pharm Biomed Anal 2015; 102:144-9; PMID:25277666; http://dx.doi.org/10.1016/j.jpba.2014.09.007
- Srivastava K, Hu J, Korn C, Savant S, Teichert M, Kapel SS, Jugold M, Besemfelder E, Thomas M, Pasparakis M, et al. Postsurgical adjuvant tumor therapy by combining anti-angiopoietin-2 and metronomic chemotherapy limits metastatic growth. Cancer Cell 2014; 26(6):880-95; PMID:25490450; http://dx.doi.org/10.1016/j.ccell.2014.11.005
- Bessho H, Wong B, Huang D, Tan J, Ong CK, Iwamura M, Hart S, Dangl M, Thomas M, Teh BT. Effect of ang-2-vegf-a bispecific antibody in renal cell carcinoma. Cancer Invest 2015; 33(8):378-86; PMID:26115098; http://dx.doi.org/10.3109/07357907.2015.1047505
- Fenn S, Schiller CB, Griese JJ, Duerr H, Imhof-Jung S, Gassner C, Moelleken J, Regula JT, Schaefer W, Thomas M, et al. Crystal structure of an anti-ang2 crossfab demonstrates complete structural and functional integrity of the variable domain. PLoS One 2013; 8(4):e61953; PMID:23613981; http://dx.doi.org/10.1371/journal.pone.0061953
- Kloepper J, Riedemann L, Amoozgar Z, Seano G, Susek K, Yu V, Dalvie N, Amelung RL, Datta M, Song JW, et al. Ang-2/vegf bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci U S A 2016; 113:4476-81; PMID:27044098
- Hidalgo M, Le Tourneau C, Massard C, Boni V, Calvo E, Albanell J, Taus A, Sablin MP, Varga A, Bahleda R, et al. Results from the first-in-human (fih) phase i study of ro5520985 (rg7221), a novel bispecific human anti-ang-2/anti-vegf-a antibody, administered as an intravenous infusion to patients with advanced solid tumors. J Clin Oncol 2014; 32(15)
- Oaknin A, Floquet A, Le Tourneau C, Ray-Coquard IL, Joly F, Hidalgo M, Leary A, Krieter O, Lahr A, Rossomanno S, et al. Single agent vanucizumab (ro5520985) for platinum (pt)-resistant recurrent ovarian cancer (oc): Results from a single arm extension phase of the phase i fih study. J Clin Oncol 2015; 33(15)
- Otani A, Takagi H, Oh H, Koyama S, Matsumura M, Honda Y. Expressions of angiopoietins and tie2 in human choroidal neovascular membranes. Invest Ophthalmol Vis Sci 1999; 40(9):1912-20; PMID:10440243
- Takagi H, Koyama S, Seike H, Oh H, Otani A, Matsumura M, Honda Y. Potential role of the angiopoietin/tie2 system in ischemia-induced retinal neovascularization. Invest Ophthalmol Vis Sci 2003; 44(1):393-402; PMID:12506101; http://dx.doi.org/10.1167/iovs.02-0276
- Rennel ES, Regula JT, Harper SJ, Thomas M, Klein C, Bates DO. A human neutralizing antibody specific to ang-2 inhibits ocular angiogenesis. Microcirculation 2011; 18(7):598-607; PMID:21851472; http://dx.doi.org/10.1111/j.1549-8719.2011.00120.x
- Zhang F, Zhang J, Liu M, Zhao L, LingHu R, Feng F, Gao X, Jiao S, Hu Y, Yang J. Combating her2-overexpressing breast cancer through induction of calreticulin exposure by tras-permut crossmab. Oncoimmunology 2015; 4(3):e994391; PMID:25949918; http://dx.doi.org/10.4161/2162402X.2014.994391
- Zhao L, Xie F, Tong X, Li H, Chen Y, Qian W, Duan S, Zheng J, Zhao Z, Li B, et al. Combating non-hodgkin lymphoma by targeting both cd20 and hla-dr through cd20-243 crossmab. MAbs 2014; 6(3):740-8; PMID:24670986; http://dx.doi.org/10.4161/mabs.28613
- Zhao L, Tong Q, Qian W, Li B, Zhang D, Fu T, Duan S, Zhang X, Zhao J, Dai J, et al. Eradication of non-hodgkin lymphoma through the induction of tumor-specific t-cell immunity by cd20-flex bifp. Blood 2013; 122(26):4230-6; PMID:24178967; http://dx.doi.org/10.1182/blood-2013-04-496554
- Asokan M, Rudicell RS, Louder M, McKee K, O'Dell S, Stewart-Jones G, Wang K, Xu L, Chen X, Choe M, et al. Bispecific antibodies targeting different epitopes on the hiv-1 envelope exhibit broad and potent neutralization. J Virol 2015; 89(24):12501-12; PMID:26446600; http://dx.doi.org/10.1128/JVI.02097-15
- Huang Y, Yu J, Lanzi A, Yao X, Andrews CD, Tsai L, Gajjar M, Sun M, Seaman M, Padte NN, et al. Engineered bispecific antibodies with exquisite hiv-1-neutralizing activity. Cell 2016 June 16; 165(7):1621-1631; PMID:27315479; http://dx.doi.org/10.1016/j.cell.2016.05.024
- Bispecific Anti-HIV-1 Antibodies with Enhanced Breadth and Potency. Bournazos S, Gazumyan A, Seaman MS, Nussenzweig MC, Ravetch JV. Cell. 2016 Jun 16;165(7):1609-1620. doi:10.1016/j.cell.2016.04.050. PMID: 27315478.
- Tung HY, Su YC, Chen BM, Burnouf PA, Huang WC, Chuang KH, Yan YT, Cheng TL, Roffler SR. Selective delivery of pegylated compounds to tumor cells by anti-peg hybrid antibodies. Mol Cancer Ther 2015; 14(6):1317-26; PMID:25852063; http://dx.doi.org/10.1158/1535-7163.MCT-15-0151
- Lehmann S, Perera R, Grimm HP, Sam J, Colombetti S, Fauti T, Fahrni L, Schaller T, Freimoser-Grundschober A, Zielonka J, et al. In vivo imaging of the activity of cea tcb, a novel t-cell bispecific antibody, reveals specific tumor targeting and fast induction of t-cell mediated tumor killing. Clin Cancer Res 2016 Apr 26; PMID:27117182
- Bacac M, Fauti T, Sam J, Colombetti S, Weinzierl T, Ouaret D, Bodmer W, Lehmann S, Hofer T, Hosse RJ, et al. A novel carcinoembryonic antigen t-cell bispecific antibody (cea tcb) for the treatment of solid tumors. Clin Cancer Res 2016 Feb 9; PMID:26861458
- Vu MD, Moser S, Delon C, Latzko M, Gianotti R, Luoend R, Friang C, Murr R, Duerner LJ, Weinzierl T, et al. A new class of t-cell bispecific antibodies for the treatment of multiple myeloma, binding to b cell maturation antigen and cd3 and showing potent, specific antitumor activity in myeloma cells and long duration of action in cynomolgus monkeys. Blood 2015; 126(23)
- Seckinger A, Delgado JA, Moreno L, Neuber B, Grab A, Lipp S, Merino J, Vu MD, Strein K, Prosper F, et al. Target expression, preclinical activity and mechanism of action of em801: A novel first-in-class bcma t-cell bispecific antibody for the treatment of multiple myeloma. Blood 2015; 126(23); PMID:NOT_FOUND
- Imhof-Jung S, Klein C, Regula J, Schaefer W, Schanzer J. Wo2010145792: Bispecific antigen binding proteins. 2010
- Imhof-Jung S, Klein C, Regula J, Schaefer W, Schanzer J. Wo2010145793: Bispecific, tetravalent antigen binding proteins. 2010
- Brunker P, Wartha K, Friess T, Grau-Richards S, Waldhauer I, Ferrara Koller C, Weiser B, Majety M, Runza V, Niu H, et al. Rg7386, a novel tetravalent fap-dr5 antibody, effectively triggers fap-dependent, avidity-driven dr5 hyperclustering and tumor cell apoptosis. Mol Cancer Ther 2016; 15:946-57; PMID:27037412
- Kim JM, Ashkenazi A. Fcgamma receptors enable anticancer action of proapoptotic and immune-modulatory antibodies. J Exp Med 2013; 210(9):1647-51; PMID:23980122; http://dx.doi.org/10.1084/jem.20131625
- Fischer JA, Hueber AJ, Wilson S, Galm M, Baum W, Kitson C, Auer J, Lorenz SH, Moelleken J, Bader M, et al. Combined inhibition of tumor necrosis factor alpha and interleukin-17 as a therapeutic opportunity in rheumatoid arthritis: Development and characterization of a novel bispecific antibody. Arthritis Rheumatol 2015; 67(1):51-62; PMID:25303306; http://dx.doi.org/10.1002/art.38896
- Bossenmaier B, Kettenberger H, Klein C, Kuenkele KP, Regula J, Schaefer W, Schwaiger M, Sustmann C. Wo2012116927: Monovalent antigen binding protein. 2012
- Bossenmaier B, Kettenberger H, Klein C, Kuenkele KP, Regula J, Schaefer W, Schwaiger M, Sustmann C. Wo2012116926a1: Antigen binding proteins. 2012
- Bostrom J, Yu SF, Kan D, Appleton BA, Lee CV, Billeci K, Man W, Peale F, Ross S, Wiesmann C, et al. Variants of the antibody herceptin that interact with her2 and vegf at the antigen binding site. Science 2009; 323(5921):1610-4; PMID:19299620; http://dx.doi.org/10.1126/science.1165480
- Castoldi F, Hass A, Klein C, Schaefer W, Sustmann C. Wo2013174873a1: Multispecific antibodies. 2013
- Brinkmann U, Croasdale R, Hoffmann E, Klein C, Moessner E, Schanzer J, Umana P. Wo2010115589: Trivalent bispecific antibodies. 2010
- Metz S, Panke C, Haas AK, Schanzer J, Lau W, Croasdale R, Hoffmann E, Schneider B, Auer J, Gassner C, et al. Bispecific antibody derivatives with restricted binding functionalities that are activated by proteolytic processing. Protein Eng Des Sel 2012; 25(10):571-80; PMID:22976197; http://dx.doi.org/10.1093/protein/gzs064
- Castoldi R, Ecker V, Wiehle L, Majety M, Busl-Schuller R, Asmussen M, Nopora A, Jucknischke U, Osl F, Kobold S, et al. A novel bispecific egfr/met antibody blocks tumor-promoting phenotypic effects induced by resistance to egfr inhibition and has potent antitumor activity. Oncogene 2013; 32(50):5593-5601; PMID:23812422; http://dx.doi.org/10.1038/onc.2013.245
- Castoldi R, Jucknischke U, Pradel LP, Arnold E, Klein C, Scheiblich S, Niederfellner G, Sustmann C. Molecular characterization of novel trispecific erbb-cmet-igf1r antibodies and their antigen-binding properties. Protein Eng Des Sel 2012; 25(10):551-9; PMID:22936109; http://dx.doi.org/10.1093/protein/gzs048
- Schanzer JM, Wartha K, Croasdale R, Moser S, Kunkele KP, Ries C, Scheuer W, Duerr H, Pompiati S, Pollman J, et al. A novel glycoengineered bispecific antibody format for targeted inhibition of epidermal growth factor receptor (egfr) and insulin-like growth factor receptor type i (igf-1r) demonstrating unique molecular properties. J Biol Chem 2014; 289(27):18693-706; PMID:24841203; http://dx.doi.org/10.1074/jbc.M113.528109
- Croasdale R, Klein C, Schaefer W, Schanzer J. Wo2010136172: Tri- or tetraspecific antibodies. 2010
- Hu S, Fu W, Xu W, Yang Y, Cruz M, Berezov SD, Jorissen D, Takeda H, Zhu W. Four-in-one antibodies have superior cancer inhibitory activity against egfr, her2, her3, and vegf through disruption of her/met crosstalk. Cancer Res 2015; 75(1):159-70; PMID:25371409; http://dx.doi.org/10.1158/0008-5472.CAN-14-1670
- Mirschberger C, Schiller CB, Schraml M, Dimoudis N, Friess T, Gerdes CA, Reiff U, Lifke V, Hoelzlwimmer G, Kolm I, et al. Rg7116, a therapeutic antibody that binds the inactive her3 receptor and is optimized for immune effector activation. Cancer Res 2013; 73(16):5183-94; PMID:23780344; http://dx.doi.org/10.1158/0008-5472.CAN-13-0099
- Meneses-Lorente G, Friess T, Kolm I, Holzlwimmer G, Bader S, Meille C, Thomas M, Bossenmaier B. Preclinical pharmacokinetics, pharmacodynamics, and efficacy of rg7116: A novel humanized, glycoengineered anti-her3 antibody. Cancer Chemother Pharmacol 2015; 75(4):837-50; PMID:25702049; http://dx.doi.org/10.1007/s00280-015-2697-8
- Bruenker P, Jaeger C, Klein C, Moessner E, Schaefer W. Wo2015052230a1: Multispecific domain exchanged common variable light chain antibodies. 2015
- Bruenker P, Fauti T, Jaeger C, Klein C, Umana P. Wo2014056783a1: Fc-free antibodies comprising two fab fragments and methods of use. 2014
- Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr., Leahy DJ. Structure of the extracellular region of her2 alone and in complex with the herceptin fab. Nature 2003; 421(6924):756-60; PMID:12610629; http://dx.doi.org/10.1038/nature01392
- Saul FA, Poljak RJ. Crystal structure of human immunoglobulin fragment fab new refined at 2.0 a resolution. Proteins 1992; 14(3):363-71; PMID:1438175; http://dx.doi.org/10.1002/prot.340140305

