ABSTRACT
The aim of this study was to determine the effect of mash enzymatic maceration and heating treatments before pressing on the antioxidant activities in selected berry juices. Total phenolic contents were determined by the Folin–Ciocalteau assay, antioxidant activities were determined by means of the oxygen radical absorbance capacity (ORAC) assay, and the intracellular antioxidant potentials were determined through the cellular antioxidant activity (CAA) assay. Blueberry juices exhibited the highest antioxidant activity, followed by black currant and raspberry juices. The mash enzyme treatment with Pectinex® Ultra Color enzyme preparation, improved the antioxidant activities in vitro. ORAC values correlated with CAA values (R2 = 0.91) and a slightly positive correlation was observed between Folin–Ciocalteau and CAA assays (R2 = 0.56). The findings support the observation that the mash enzymatic treatment of macerated fruits enhances their antioxidant activity. Considering the chemical assays (ORAC and total phenolic content), a combined treatment with heating to 85°C followed by mash enzymatic treatment was the most effective. Considering a biological model through the CAA assay, however, a comparable improvement in antioxidant activity resulted from the enzymatic and the combined treatments. Overall, the data showed that maceration enzymatic treatment improves the intracellular antioxidant activity in HepG2 human cells. To the best of our knowledge, this is the first report on the effect of the antioxidant potential of mash enzymatic and heating treatments assessed by means of a cellular approach.
RESUMEN
El objetivo de este estudio fue determinar el efecto de los tratamientos de maceración enzimática y térmico antes del prensado en las actividades antioxidantes de los zumos de los frutos del bosque seleccionados. Se determinó el contenido total fenólico mediante el ensayo de Folin-Ciocalteau, las actividades antioxidantes se determinaron mediante el ensayo de la capacidad de absorción de radicales de oxígeno (ORAC), además los potenciales antioxidantes intracelulares se determinaron mediante el ensayo de actividad antioxidante celular (CAA). Los zumos de arándano azul exhibieron la mayor actividad antioxidante, seguidos de los zumos de grosella negra y frambuesa. El tratamiento de maceración enzimática con preparación enzimática Pectinex® Ultra Color mejoró la actividad antioxidante in vitro. Los valores de ORAC mostraron correlación con los valores de CAA (R2=0,91) y se observó una ligera correlación positiva entre los ensayos de Folin-Ciocalteau y CAA (R2=0,56). Los resultados apoyan la observación de que el tratamiento de maceración enzimática de los frutos macerados mejoró su actividad antioxidante. Considerando los ensayos químicos (ORAC y el contenido fenólico total), el más efectivo fue el tratamiento combinado de calor a 85°C seguido por el tratamiento de maceración enzimática. Sin embargo, considerando un modelo biológico mediante el ensayo de CAA, se obtuvo una mejora comparable en la actividad antioxidante como resultado de los tratamientos enzimáticos y combinados. En general, los datos mostraron que el tratamiento de maceración enzimática mejora la actividad antioxidante intracelular en las células humanas HepG2. Según nuestros conocimientos, este es el primer estudio sobre el efecto del potencial antioxidante de los tratamientos de maceración enzimática y térmico examinados a través de un enfoque celular.
Introduction
Blueberry and black currant are considered fruits rich in phenolic compounds, ascorbic acid, and other antioxidants (Wang, Cao, & Prior, Citation1996). The dark red or blue coloration of their juices is a result of the high level of anthocyanins present (Landbo & Meyer, Citation2004). Phenolic compounds and vitamin C have been implicated in the antioxidant activity of fruits and derived beverages (Prior et al., Citation1998). Moyer, Hummer, Finn, Frei, and Wrolstad (Citation2002) demonstrated for diverse species of Vaccinium, Rubus, and Ribes that their phenolic compounds contribute to the antioxidant capacity. Nevertheless, the phenolic compounds are located primarily in the peel of most berry fruits (Meyer, Citation2002). So, even if a large proportion of them pass into the juice, the pressed residue left behind is still rich in anthocyanins and other phenolic compounds. However, their release into the juice during processing could be enhanced by processing parameters such as mash heating or mash maceration prior to juice pressing.
Mash maceration treatment with enzyme preparation is a commonly applied step in berry juice processing. It is expected to increase the juice yield and improve the extraction of bioactive components as well as reduce processing time. There are many commercial enzyme preparations available, most of which are of microbial origin and generally have multicomponent enzyme activities and destroy the cell wall structure, that is, by cleaving pectins from the cell wall and degrading soluble pectins (McLellan & Padilla-Zakour, Citation2005; Zielinski et al., Citation2014).
Berry mashes which have high pectin content, for example, black currant, have a high mash viscosity after crushing and often suffer from complications during pressing to extract the juice, thus making the use of the press in industrial production inefficient (Höhn, Sun, & Nolle, Citation2005; Rodrigues, Citation2012).
Studies on black currant have demonstrated that enzymatic mash treatment enhanced the release of anthocyanins and other phenolic compounds into the juice (Bagger-Jørgensen & Meyer, Citation2004; Landbo & Meyer, Citation2004). Additionally, Borowska, Szajdek, and Czaplicki (Citation2009) demonstrated that heat treatment followed by enzymatic maceration of chokeberry mash, prior to juice pressing, is recommended for maximizing the phenolic content and the antioxidant properties.
Great interest is demonstrated by producers and consumers for the antioxidant potential exerted by polyphenol-rich beverages, therefore, manufacturing procedures that maintain or increment antioxidant potential are an attractive option especially for producers. Polyphenols are very important plant metabolites because of their ability in trapping free radicals. As antioxidants, polyphenols may contribute to antioxidative action and thus are believed to play a protective role in various degenerative diseases associated with oxidative stress.
The methods employed to determine the antioxidant capacity of polyphenol-rich preparations are mainly based on chemical reactions. These chemical assays provide important basic information on the in vitro antiradical activity, an evaluation that remains crucial in the first steps of the screening for the antioxidant capacity. Currently, the oxygen radical absorbance capacity (ORAC) test is a relevant measure used to assess the radical scavenging capacity in foods (Cao, Alessio, & Cutler, Citation1993; Ou, Hampsch-Woodill, & Prior, Citation2001; Prior, Wu, & Schaich, Citation2005) and concerns the inhibition of the peroxyl radical-induced oxidation which is initiated by thermal decomposition of azo-compounds (Glazer, Citation1990). The ORAC test measures the capacity of an antioxidant to directly quench free radicals (Prior et al., Citation1998).
However, there are questions concerning whether the results obtained with chemical methods can represent the antioxidant activity within the human body. This has led to the development of supplementary methods applying biological systems, for example, with human cells and enzymes as the test model. Thus, to further characterize the antioxidant activity of the selected berry juices in a valid biological system this study provides the first data on the intracellular antioxidant activity.
In this study the cellular antioxidant activity (CAA) assay was used, a method similar to the ORAC test that is performed within cells. Some of the advantages of using this method are that it considers additional aspects, such as cell uptake, metabolism, and distribution of the antioxidants within cells (Wolfe & Liu, Citation2007). The method measures the capacity of the antioxidants in counteracting the oxidation of a fluorescent probe by peroxyl radicals produced inside human cells. The decrease in the intracellular fluorescence when compared to control cells is directly related with the intracellular antioxidant capacity of the samples.
The aim of this study was to evaluate the effect of different mash treatments for blueberry, black currant, and raspberry mashes prior to juice pressing on juices antioxidant potentials. Recent studies quantified the antioxidant capacity of fruit juices using chemical assays. Targeted improvement of processing methods guided by a biological assay of antioxidant activity seems to be interesting with the increasing demand for antioxidant-rich beverages.
Material and methods
Plant material
Frozen black currant (Ribes nigrum), raspberry (Rubus idaeus), and blueberry (Vaccinium myrtillus) were obtained from Mainfrucht GmbH & Co. KG (Gochsheim, Germany) and stored at −20°C until use.
Juice production
Juices were produced from 2 kg berry packages. Prior to juice processing, each berry package was gently defrosted for 2 h at 23°C in a temperature-controlled cabinet and then hand-crushed with a pestle. Three different types of mash treatments were conducted, as described below:
Treatment 1 (control) [T1]: mash was heated to 50°C and then immediately filtered.
Treatment 2 [T2]: mash was heated to 50°C and incubated at 50°C with 0.35 mL/kg Pectinex® Ultra Color (Novozymes, Denmark) enzyme preparation for 60 min.
Treatment 3 [T3]: mash was heated to 85°C, held for 5 min at 85°C, and cooled to 50°C for enzyme treatment (as above).
Immediately after mash treatment, juice was extracted from the samples by pressing, using nylon filter bags and a stainless steel Para Press (Arauner Kitzingen, Germany), in which the pressure was gradually raised to 5.6 bar. Immediately after pressing, juices were filled in 110 mL bottles, pasteurized by heating to 85°C for a few seconds in a water bath and then allowed to cool at room temperature horizontally to also sterilize the lids. Juices were stored at room temperature in the dark up to 4 weeks before analysis. The juice yield was determined by weighing and is given as %. Each juice and treatment pair was undertaken twice on two successive days [-a, -b], for a total of eighteen juice samples.
Characteristic properties
Collected berry juices were examined as follows:
Ascorbic acid was determined using the Reflectoquant system RQflex 10 (Merck, Darmstadt, Germany) with the appropriate test sticks, the results are expressed as mg per liter of juice. Each measurement was done in triplicate.
Total acid measurement was done in duplicate by potentiometric titration with 1/3 mol/L NaOH (Merck, Germany) to the titration end point of pH 7.0, calculated as acidity of wine and expressed as g per liter.
The pH was measured using a pH meter EcoScan (EUTECH instruments, Netherlands).
Total soluble solids (°Brix) content was measured at 20°C using a refractometer HANNA HI 96,801 (Hanna Instruments, Vöhringen, Germany).
The viscosity was measured with a MarsIII rheometer (ThermoFisher Scientific, Karlsruhe, Germany). A concentric cylinder geometry was employed and rotational test was performed under variation of the shear rate between 0.5 and 500 s^−1. The viscosity was evaluated by Newtonian Fit of the shear rate range 1–100 s^−1 and expressed as mPa s. The tests were conducted at 20°C in triplicate.
The turbidity of juices was measured at 20°C with a turbidimeter Optek DT9011 Haze Control (optek-Danulat, Essen, Germany). A 1:4 dilution was used. Turbidity is expressed in formazin turbidity units (FTU) at 11° and 90°. Each measurement was done in triplicate.
Total phenolics
Total phenolic content was determined by the Folin–Ciocalteau method according to Singleton and Rossi (Citation1965).Total phenols are expressed as the mean of three experiments ± standard deviation and as mg of gallic acid equivalents (GAE) per liter of juice.
Oxygen radical absorbance capacity (ORAC) assay
The antioxidant capacities of berry juices were assessed in vitro by the ORAC assay (Prior et al., Citation2005) as described elsewhere (Bender, Graziano, Zimmermann, & Weidlich, Citation2014). Juice samples were centrifuged at 3000 rpm for 10 min, the supernatants were filtered through a 0.2 µm syringe filter PVDF membrane and diluted in phosphate buffer (75 mM, pH 7.4). Five serial dilutions of each sample were analyzed in duplicate wells. ORAC values are expressed as the mean of two experiments ± standard deviation and as mmol of trolox equivalents (TE) per liter of juice.
Cell culture
HepG2 cells were grown in DMEM high glucose medium supplemented with 10% heat inactivated FBS, 2 mM L-Glutamine, 50 μg/mL Penicillin, and 50 μg/mL Streptomycin. Cells were maintained at 37°C under a humidified atmosphere of 5% CO2.
Viability and cytotoxicity
HepG2 cell viability was determined at each experiment by trypan blue exclusion. Toxicity of the juices toward hepatocytes was assessed by measuring the cytosolic lactate dehydrogenase (LDH) activity, with the LDH cytotoxicity kit (Innoprot, Spain) and according to the manufacturer’s instructions. A dose of 10% v/v of each juice was administrated to HepG2 cells up to 24 h. Untreated cells and 1% Triton X-100 treated cells served as negative and positive controls, respectively. Free LDH concentrations from two experiments were measured spectrophotometrically at 490 nm. Nontoxic dilutions were used in the CAA assay.
Cellular antioxidant activity (CAA) assay
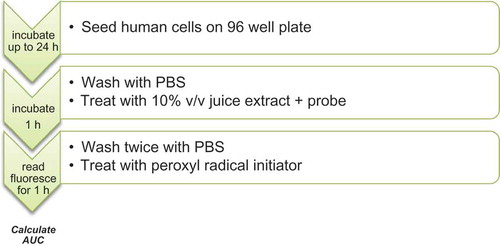
HepG2 cells (6 × 104 cells/well) were seeded in a black 96-well plate with transparent bottom (Greiner bio-one, Germany) and incubated until confluence at 37°C and 5% CO2. Intracellular oxidation was estimated by using the CAA method (Wolfe & Liu, Citation2007) with minor modifications, as reported elsewhere (Bender, Graziano, & Zimmermann, Citation2015). The simplified procedure is illustrated in . Samples were prepared via centrifugation at 3000 rpm for 10 min and the resulting supernatants were filtered through sterile 0.2 µm syringe filters with PVDF membranes. Subsequently, 200 µl of each filtrate was diluted in 800 µl of DMEM high glucose medium. Diluted samples were immediately tested in four replicate wells. The raw data were analyzed with MARS 2.0 Optima data analysis software (BMG Labtech, Germany). The integrated area under the fluorescence curve (AUC) was calculated from two experiments, for each sample, standards and controls (untreated cells), and corrected with blanks AUC. Quercetin equivalents (QE) were calculated based on the reference standard curves and CAA values were expressed as μmol of QE per liter of juice.
Statistical analysis
Standard deviations and relative standard deviation % were calculated for each test. Differences in the characteristic properties between the treatments were considered to be significant at p < 0.05 by one-way analysis of variance (ANOVA). Pearson’s test was used to assess the calibration curves linearity and correlation among different parameters. The data are represented as the mean ± standard deviation of at least two experiments.
Results and discussion
Three different methods, namely T1 (control without mash treatment), T2 (enzymatic mash treatment, and T3 (combined heating and enzymatic mash treatments) were used before pressing berry juices from blueberry (BB), black currant (BC), and raspberry (RB) fruits. shows the characteristic properties of all the berry juices. The samples -a and -b represent production replicates (semi-industrial scale) obtained on two successive days. The obtained pH between 2.75 and 3.09 showed relatively acidic but typical values for fruit juices.
Table 1. Characteristic properties performed in selected berry juices subjected to different mash treatments. Mean ± relative SD% Different letters in the same column indicate significant differences (p < 0.05); z: out of measurement range (high turbidity).
Tabla 1 . Propiedades características desarrolladas en los zumos de los frutos seleccionados sujetos a diferentes tratamientos de maceración. Promedio ± SD relativa % Las distintas letras en la misma columna indican diferencias significativas (p < 0,05); z: fuera del rango de medición (turbiedad alta).
Juices yields ranged from about 68.7% to 87.7%, except for black currant juices subjected to method T1 in which a low yield of 37.6% was obtained. For each of the three tested fruits, the lowest yield was achieved for juices subjected to method T1. In the absence of pectolytic enzymes, the soluble, not degraded pectin cause high viscosity or gelling of the mash before the juice can run off the press (Höhn et al., Citation2005). For this reason, as shown in , viscosity of the juices are also significantly higher for treatment T1 compared with T2 and T3 within each of the three tested berry juices. Thereby the lowest yield and the highest juice viscosity arose from black currant, because of its high level of pectin. This result is in accordance with previous studies (Bagger-Jørgensen & Meyer, Citation2004). ANOVA analysis showed no differences for juice viscosity between treatment T2 and T3 for all tested berries, indicating that pectin degradation is similar with or without additional heat treatment.
In agreement with research by Landbo and Meyer (Citation2004), who showed that enzymatic mash treatment improved the juice clarity of black currant under specific conditions, we also found the highest turbidity (90°) values for all tested berry juices, which undergone treatment T1 (). Thereby most of the T1 samples were too turbid and out of measurement range. In case of blueberry and raspberry juices, the additional heat treatment lowers the turbidity (90°) compared with treatment T2 further, whereby no statistical difference (p < 0.05) can be seen for black currant juices between T2 and T3. Extract values ranged from 11.9 to 12.8 °Brix for blueberry, from 10.2 to 12.0 °Brix for raspberry, and the highest values represented black currant from 16.4 to 17.9 °Brix. In addition, the following tendency can be observed regarding the extract values of the treatment: T1<T2<T3. This indicates that more soluble solids are released into the juice through both the enzyme treatment and the additional heat treatment. Black currant generally showed the significant highest value of total acids, which is a measure of the free existing acids in the juice, followed by raspberry and blueberry with the lowest level (). Thereby treatment T3 leads to a higher amount compared with T1 (p < 0.05). For treatment T2 it depends on the fruit if an increase of total acids is achieved compared with T1 (as for blueberry and also for black currant but with significant lower amount compared with T3) or not (for raspberry).
According to , the amount of ascorbic acid differs significantly for each type of berry. Black currant juice had the highest amount of ascorbic acid, followed by blueberry and raspberry juices (p < 0.05.). An increase (p < 0.05) can be observed through the treatments T1<T3 for blueberry and black currant juices, indicating a positive effect of the combined enzyme and heat treatments on ascorbic acid content. For blueberry the higher significance level is also achieved through the enzyme treatment itself (T2), whereby for black currant the difference between T1 and T2 is not significant (p < 0.05). ANOVA analysis showed no differences within the tested raspberry juices.
The antioxidant activities of the berry juices were examined via chemical and biological assays, the effect of the heat and enzyme treatments on the antioxidant capacity was evaluated.
According to the Folin–Ciocalteau method, the highest content of phenolic compounds was found in black currant juices subjected to the mash treatment ((a)), being 6026.26 ± 123.5 and 5066.74 ± 65.5 mg GAE /L, respectively, for treatments T3 and T2, as a mean of the two production days. Treatment T1 represented the lowest phenolic content in black currant juices (4870.51 ± 142.1 mg GAE/L).
Figure 2. Antioxidant potential of selected berry juices as evaluated by (a) Total phenolics, (b) ORAC assay, and (c) CAA assay.All data are expressed as mean ± SD of two experiments.BB: blackberry; BC: black currant; RB: raspberry; T1: without mash enzyme treatment, T2: mash enzyme treatment, T3: combined heat and enzyme treatment. -a and -b correspond to different production days.
Figura 2 . Potencial antioxidante de los zumos de los frutos seleccionados evaluados mediante (a) Total de fenoles, (b) Ensayo de ORAC y (c) Ensayo de CAA.Todos los datos se expresan como promedio ± SD de dos experimentos.BB: mora; BC: grosella negra; RB: frambuesa; T1: sin tratamiento de maceración enzimática, T2: tratamiento de maceración enzimática, T3: tratamiento combinado de calor y enzimático. -a y -b corresponden a diferentes días de producción.
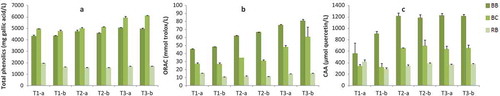
Blueberry juices ranked second in terms of phenolic content, resulting in 4343.41 ± 23.1, 4681.75 ± 111.1, and 5026.19 ± 59.7 mg GAE/L, respectively, for treatments T1, T2, and T3.
Raspberry juices represented the lowest phenolic content, accounting for 1795.91 ± 232.1, 1556.88 ± 7.0, and 1695.44 ± 9.7 mg GAE/L, respectively, for treatments T1, T2, and T3. This result is in accordance with research by Versari and colleagues (Citation1997), in which a weaker effect of enzymatic maceration on the anthocyanin content of raspberry juices was reported. A possible explanation could be that different fruits have different pectin composition and structures (Höhn et al., Citation2005), which affects the effectiveness of the enzyme preparation to cleave this polysaccharide from the cell wall.
To screen the ability of the berry juices and to scavenge free radicals the hydrophilic ORAC assay was used. The ORAC assay is a standardized method for kinetically determining the antioxidant capacity of foods (Prior et al., Citation2005), the method involves the inhibition of the peroxyl radical-induced oxidation which is initiated by thermal decomposition of azo-compounds (Glazer, Citation1990); it is a high-specific method that measures the capacity of an antioxidant to directly quench free radicals (Cao & Prior, Citation1998). On the basis of this principle the antioxidant activity of berry juices was evaluated. As illustrated in (b) all berries juices have a remarkable capacity in scavenging peroxyl radicals. Blueberry juices showed the strongest peroxyl radical scavenging activity in every treatment performed, ranging from 47.17 ± 2.1 to 78.49 ± 4.0 mg TE/L. Black currant juices showed intermediate ORAC values, ranging from 27.09 ± 0.2 to 54.74 ± 9.1 mg TE/L, depending on the treatment performed. Raspberry juices showed the lowest ORAC values ranging from 13.40 ± 3.1 to 14.87 ± 0.5 mg TE/L. In all juices, it was found that the mash treatments (T2 and T3) increased the antioxidant capacity compared with the control treatment (T1). Moreover, the combined treatment T3 was shown to improve the antioxidant capacity even further than the mash enzymatic treatment T2.
The antioxidant capacity of berry juices subjected to different treatment processes was also evaluated by means of the CAA assay. This method measures the capacity of the juice to counteract the oxidation of a fluorescent probe by peroxyl radicals produced inside human cells. The decrease in cellular fluorescence compared to control cells is proportional to the intracellular antioxidant capacity of the samples ().
Figure 3. CAA analysis for blueberry juices subjected to different preparation procedures. Kinetic curves represent a single experiment (mean, n = 4) and denote the peroxyl radical oxidation inhibition of a fluorescent probe overtime. Blue line: control preparation; red line: mash enzyme treatment; green line: combined mash treatment.
Figura 3. Análisis de CAA de los zumos de arándano azul sujetos a diferentes procedimientos de preparación. Las curvas cinéticas representan un único experimento (promedio, n = 4) e indican la inhibición oxidativa del radical de peroxil de una sonda fluorescente con el paso del tiempo. Línea azul: preparación control; Línea roja: tratamiento de maceración enzimática; Línea verde: tratamiento de maceración combinado.
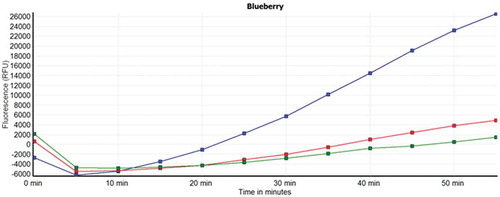
On the basis of this principle, the intracellular antioxidant potential was quantified for the selected berry juices. As illustrated in (c), all berry juices are effectively absorbed within human cells and display an intracellular antioxidant potential. The CAA values, reported as μmol QE/L, ranged from 734.06 ± 243.6 to 1222.62 ± 6.4 for blueberry juices, followed by black currant juices (333.06 ± 8.7–680.28 ± 28.9), and being much lower in raspberry juices (354.19 ± 9.8 –372.18 ± 9.6). Mash enzyme treatments, both T2 and T3, improved the intracellular antioxidant capacity to a comparable extent.
In sample RB-a T1 (raspberry juice not subjected to mash treatment) the total phenolic content, ORAC and CAA all ranked very high relative to the duplicate juice sample (RB-b, T1). One possible explanation of this result is the treatment procedure itself, which lead to a higher yield and a lower juice viscosity for sample RB-a in comparison with sample RB-b (), that is pressing more juice out of the mash and also with this juice more valuable ingredients. Former trials of raspberry juices without mash treatment showed viscosities and yields comparable to RB-b (data not shown).
Under the given conditions, comparison of the ORAC and CAA assays displayed a positive correlation, as seen in the regression analysis (R2 = 0.91). Total phenolic contents and the CAA assay correlated only weakly (R2 = 0.56).
Conclusion
The effect on the antioxidant potential of raspberry, black currant, and blueberry juices subjected to different mash treatments was studied. Juice yields ranged from 68.7% to 87.7%, being much lower in black currant control juices (37.6%) without mash treatment.
In terms of total phenolic content, black currant was the most abundant, followed by blueberry and finally raspberry juices, which had a much lower content. Mash treatments, enzymatic and combined, resulted in a higher phenolic content recorded in the blueberry and black currant samples (T3>T2) compared with control treatment (T1); while for the raspberry samples the mash enzymatic treatment seemed not to have an effect on the total content of phenolic acids when assessed by Folin–Ciocalteau.
Blueberry juices showed the highest ORAC values followed by black currant and raspberry juices. In all juices, the mash treatments increased the antioxidant capacity compared with the control treatment. Moreover, the combined treatment was shown to improve the antioxidant capacity relative to the mash enzymatic treatment alone. Under these experimental conditions, the ORAC values positively correlated with the total phenolic contents of berry juices (R2 = 0.76).
The CAA assay was performed with the HepG2 cellular line, to further investigate the antioxidant effect of berry juices subjected to different procedures with respect to their biological antioxidative capability. In this cellular assay, all the berry juices exhibited inhibition of peroxyl-radical induced fluorescence. This indicates that the berry juices are effectively absorbed into human cells, even if at different rates. It was found that blueberry ranked first in the CAA assay, followed by black currant and then raspberry, with the exception of one raspberry juice not subjected to mash treatment (RB-a, T1). In all juices, the mash enzyme treatments improved the intracellular antioxidant activity with respect to the control treatment and in a comparable extent. Under our experimental conditions, the CAA values were correlated with ORAC values (R2 = 0.91) and weakly correlated with total phenolics (R2 = 0.56). Both the ORAC and CAA assays showed that the antioxidant capacity of blueberry juices was higher than that of black currant, while that of the raspberry juice was much lower. Overall, our findings indicate that the mash enzymatic and heating treatments of berry juices have a positive effect on the improvement of the phenolic yield and the antioxidant capacity assessed by chemical assays as well as by means of a biological assay. The results obtained so far may contribute to the improvement of technological processes for the production of antioxidant-rich juices.
Acknowledgments
The authors wish to thank M.Sc. Kenneth Walsh for the language editing, and Mrs. Manuela Melcher for the assistance with Folin–Ciocalteau analysis. Donation of enzyme preparation by Novozymes (Denmark) is also gratefully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bagger-Jørgensen, R., & Meyer, A.S. (2004). Effects of different enzymatic pre-press maceration treatments on the release of phenols into blackcurrant juice. European Food Research and Technology, 219, 620–629. doi:10.1007/s00217-004-1006-2
- Bender, C., Graziano, S., & Zimmermann, B.F. (2015). Study of Stevia rebaudiana Bertoni antioxidant activities and cellular properties. International Journal of Food Sciences and Nutrition, 66, 553–558. doi:10.3109/09637486.2015.1038223
- Bender, C., Graziano, S., Zimmermann, B.F., & Weidlich, H.W. (2014). Antioxidant potential of aqueous plant extracts assessed by the cellular antioxidant assay. American Journal of Biology and Life Sciences, 2, 72–79.
- Borowska, E.J., Szajdek, A., & Czaplicki, S. (2009). Effect of heat an enzyme treatment on yield, phenolic content and antioxidant capacity of juices from chokeberry mash. Italian Journal of Food Science, 21, 197–209.
- Cao, G., Alessio, H.M., & Cutler, R.G. (1993). Oxygen-radical absorbance capacity assay for antioxidants. Free Radical Biology and Medicine, 14, 303–311. doi:10.1016/0891-5849(93)90027-R
- Cao, G., & Prior, R.L. (1998). Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clinical Chemistry, 44, 1309–1315.
- Glazer, A.N. (1990). Phycoerythrin fluorescence-based assay for reactive oxygen species. Methods in Enzymology, 186, 161–168.
- Höhn, A., Sun, D., & Nolle, F. (2005). Enzymes in the fruit juice and wine industry. In D.M. Barrett (Ed.), Processing fruits - science and technology (2nd ed., pp. 97–112). Boca Raton, FL: CRC Press.
- Landbo, A.-K., & Meyer, A.S. (2004). Effects of different enzymatic maceration treatments on enhancement of anthocyanins and other phenolics in black currant juice. Innovative Food Science & Emerging Technologies, 5, 503–513. doi:10.1016/j.ifset.2004.08.003
- McLellan, M.R., & Padilla-Zakour, O.I. (2005). Juice processing. In D.M. Barrett (Ed.), Processing fruits - science and technology (2nd ed., pp. 73–96). Boca Raton, FL: CRC Press.
- Meyer, A.S. (2002). Enhanced extraction of antioxidant phenols from wine and juice press residues via enzymatic polysaccharide hydrolysis. Fruit Processing, 12, 29–33.
- Moyer, R.A., Hummer, K.E., Finn, C.E., Frei, B., & Wrolstad, R.E. (2002). Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. Journal of Agricultural and Food Chemistry, 50, 519–525. doi:10.1021/jf011062r
- Ou, B., Hampsch-Woodill, M., & Prior, R.L. (2001). Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. Journal of Agricultural and Food Chemistry, 49, 4619–4626. doi:10.1021/jf010586o
- Prior, R.L., Cao, G., Martin, A., Sofic, E., McEwen, J., O’Brien, C. … Mainland, C.M. (1998). Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of vaccinium species. Journal of Agricultural and Food Chemistry, 46, 2686–2693. doi:10.1021/jf980145d
- Prior, R.L., Wu, X., & Schaich, K. (2005). Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry, 53, 4290–4302. doi:10.1021/jf0502698
- Rodrigues, S. (2012). Enzyme maceration. In S. Rodrigues (Ed.), Advances in fruit processing technologies (pp. 235–246). Boca Raton, FL: CRC Press.
- Singleton, V.L., & Rossi, J.A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
- Versari, A., Biesenbruch, S., Barbanti, D., Farnell, P.J., & Galassi, S. (1997). Effects of pectolytic enzymes on selected phenolic compounds in strawberry and raspberry juices. Food Research International, 30, 811–817. doi:10.1016/S0963-9969(98)00050-7
- Wang, H., Cao, G., & Prior, R.L. (1996). Total antioxidant capacity of fruits. Journal of Agricultural and Food Chemistry, 44, 701–705. doi:10.1021/jf950579y
- Wolfe, K.L., & Liu, R.H. (2007). Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. Journal of Agricultural and Food Chemistry, 55, 8896–8907. doi:10.1021/jf0715166
- Zielinski, A.A.F., Alberti, A., Braga, C.M., Marques da Silva, K., Canteri, M.H.G., Mafra, L.I. … Wosiacki, G. (2014). Effect of mash maceration and ripening stage of apples on phenolic compounds and antioxidant power of cloudy juices: A study using chemometrics. LWT - Food Science and Technology, 57, 223–229. doi:10.1016/j.lwt.2014.01.029