ABSTRACT
Synbiotics combine the concepts of probiotics and prebiotics to synergistically enhance the health-associated effects of both components. Previously, we have shown that the intestinal persistence of inulin-utilizing L. plantarum Lp900 is significantly increased in rats fed an inulin-supplemented, high-calcium diet. Here we employed a competitive population dynamics approach to demonstrate that inulin and GOS can selectively enrich L. plantarum strains that utilize these substrates for growth during in vitro cultivation, but that such enrichment did not occur during intestinal transit in rats fed a GOS or inulin-supplemented diet. The intestinal persistence of all L. plantarum strains increased irrespective of their prebiotic utilization phenotype, which was dependent on the calcium level of the diet. Analysis of fecal microbiota and intestinal persistence decline rates indicated that prebiotic utilization capacity did not selectively stimulate intestinal persistence in prebiotic supplemented diets. Moreover, microbiota and organic acid profile analyses indicate that the prebiotic utilizing probiotic strains are vastly outcompeted by the endogenous prebiotic-utilizing microbiota, and that the collective enhanced persistence of all L. plantarum strains is most likely explained by their well-established tolerance to organic acids.
Introduction
Synbiotics describe a combination of probiotics and prebiotics that act in a synergistic manner to elicit health benefits greater than the separately administered pre- or pro-biotic.Citation1 Fructo-oligosaccharides (FOS), inulin and galacto-oligosaccharides (GOS) are prebiotics that are marketed on the basis of their ability to modulate the endogenous microbiota, in particular their bifidogenic effect.Citation2–7 However, dietary FOS and inulin can also stimulate potentially pathogenic enterobacteria populations in diets low in calcium.Citation6,Citation8 Yet, high calcium diets suppressed the severity of the health problems associated with challenges by pathogenic Salmonella and Escherichia coli (ETEC) in animal models and in human volunteers.Citation6,Citation9,Citation10 High calcium intake in humans could also counteract additional negative effects of dietary FOS (e.g. flatulence and increased fecal mucin excretion),Citation7,Citation11 illustrating the protective role of dietary calcium and its apparent interplay with prebiotics, the gut microbiome and gut homeostasis.
Previously, we have shown that dietary inulin significantly increases intestinal persistence of inulin-utilizing Lactiplantibacillus plantarum Lp900 when administered in a high calcium diet context.Citation12 Inulin utilization by L. plantarum Lp900 is driven by extracellular degradation of this substrate, which may compromise the competitive advantage of this capacity by “hitchhiking” of other species or strains that are equally efficient in utilizing the released breakdown products (i.e., predominantly fructose monosaccharides; Supplemental Figure SF1).Citation13 In contrast, we have previously shown that the variable utilization of GOS (i.e., DP2 versus DP3–4 (HDP) GOS) by L. plantarum strains depends on the strain-specific HDP-GOS-import capacity followed by intracellular metabolization of these substrates,Citation14 which eliminates the “hitchhiking” possibility for competing strains (Supplemental Figure SF2).
In the present study, a quantitative population dynamics approach was taken to demonstrate that dietary prebiotics (i.e., GOS and inulin) can enhance the intestinal persistence of L. plantarum strains regardless of the differential capacity of these strains to utilize the prebiotic as a substrate for growth. Moreover, previous published work on the effect of the dietary acclimatization of prebiotic and calcium-enhanced diets on microbiota composition and organic acid levels within the same cohort of rats used in the present study (one day before oral gavage),Citation15 was integrated to explore biological and physico-chemical variables in the intestinal tract that are correlated with enhanced intestinal persistence of L. plantarum strains. These findings provide new insights into the interactive effect of dietary prebiotics and probiotics strains (i.e., synbiotics).
Methods
Bacterial strains and prebiotic compounds
The seven L. plantarum strains used in the study were selected from the NIZO and Probi A/B strain collections (Supplemental Table ST1) on the basis of strain-specific growth phenotypes (i.e., GOS and inulin as growth substrate).Citation12,Citation14,Citation16 This led to the selection of L. plantarum Nizo3400, Nizo2830, Nizo2766, SD5870, Heal19, 299 v and Lp900, where the last-mentioned strain is the only strain capable of utilizing inulin,Citation16 whereas the last four strains can utilize galacto-oligosaccharides with a higher degree of polymerization (DP), designated as HDP-GOS (i.e., a DP of 3–4), while the other strains cannot.Citation14 Moreover, these strains are genetically distinguishable by their unique single nucleotide polymorphisms (SNP) present in an intergenic region (339-IR-340, Supplemental Figure SF3) that was previously employed for strain-discrimination purposes in population dynamics (20). Orafti® GOS was provided by Friesland Campina Domo (Amersfoort, the Netherlands) and had a composition of 70% galacto-oligosaccharides (DP 3–10). TURRAX®GR inulin was provided by BENEO-Orafti (Oreye, Belgium) and had a composition of 90% inulin (DP 3–60).
Animal experiment and study design
To selectively recover the mixture of L. plantarum strains from fecal samples, the selected strains were individually pre-cultured in 2-fold diluted MRS without the addition of any carbon source (½ MRS-C) supplemented with 0.5% (w/v) glucose, plated (100 μL) on MRS (Merck, Darmstadt, Germany) agar containing rifampicin (100 μg/mL) and incubated at 37°C for 2 days. Single colony isolates of these rifampicin-resistant derivatives were designated Lp900-R, 299 v-R, Heal19-R, SD5870-R, Nizo3400-R, Nizo2830-R and Nizo2766-R. All rifampicin-resistant derivatives displayed unimpaired growth on regular MRS, and fecal pellets of the rats prior to gavage were plated on MRS supplemented with rifampicin to ensure no selection of the endogenous microbiota. These strains were cultured on overnight in ½ MRS-C supplemented with 100 μg/mL rifampicin and 0.5% glucose (w/v), harvested by centrifugation and resuspended in phosphate buffered saline solution (PBS) at a standardized density which was used to compose the strain mixtures used in the in vitro co-culture experiments as well as the intragastric gavages in the animal experiments, designated L. plantarum-R (Supplemental Table ST2). For the animal trial, specific pathogen-free male Wistar rats (WU, Harlan, Horst, The Netherlands), 8 weeks old, with a mean body weight of 348 g, were housed individually in standard rodent cages. All animals were kept in a temperature (22–24 °C) and humidity (50–60%) controlled environment in a 12-hour light–dark cycle. Rats received food and water ad libitum, were weighed twice a week and food intake was monitored daily.
L. plantarum intestinal persistence was investigated in Wistar rats (n = 8 per diet group) that were fed a powdered AIN-93-derived dietCitation17 containing a relatively high fat content (200 g fat/kg) to mimic the composition of a human Western-diet (Supplemental Figure SF4). The diets (Supplemental Table ST3) were supplemented with either 40 g/kg cellulose (Arbocel type B899 JRS, Zutphen, The Netherlands) or 40 g/kg Orafti® GR Inulin or 40 g/kg Vivinal GOS and contained either 30 or 100 mmol/kg (4.08 or 13.60 g/kg) CaHPO4 (Acros organics, Thermo Scientific, Waltham, Massachusetts, USA), designated as low calcium (Lca), low calcium with GOS (LcaGOS), high calcium (Hca), high calcium with GOS (HcaGOS) and high calcium with inulin (HcaInu). A low-calcium diet supplemented with inulin was omitted from this study because earlier work has shown that a significant increase of intestinal persistence of L. plantarum Lp900 was only observed in inulin supplemented diets with a high-calcium level.Citation12 Animals were acclimatized to the housing conditions and their group-specific diet for 14 days. Subsequently, each rat was intragastrically gavaged with a dose of 1 mL PBS containing approximately 6 × 109 CFU of the L. plantarum-R mixture (Supplemental Table ST2). The intestinal persistence of the collective mixture as well as the relative abundance of the different strains within the mixture of L. plantarum strains was followed during the subsequent 9 days (see below).
Microbial analysis of rat faecal samples
Fecal pellets were collected at baseline prior to L. plantarum-R administration and every morning post-gavage for 9 days. Fecal pellets were weighed and homogenized after the addition of 1 mL of PBS using a T10 basic ULTRA-Orafti® (IKA-Werke, Staufen im Breisgau, Germany). Fecal slurries were aliquoted (aliquots of 250 μL), one of which was immediately used for enumeration of colony forming units (CFU) of the administered rifampicin resistant L. plantarum derivative strain mixture (collective analysis). The remaining aliquots were stored at −80°C for later analyzes (see below). For the enumeration of L. plantarum-R, fecal slurries were centrifuged (3,000 × g, 5 sec, room temperature) to remove fecal debris and serially diluted (5-fold dilution steps) in PBS using 300 μl 96-Microtiter Well plates (Corning, New York, USA). Dilutions were plated on MRS agar supplemented with 50 μg/mL rifampicin followed by incubation at 37°C for two days. The lower detection limit was estimated to be approximately 2 × 103 CFU/g fecal material (wet weight). Quantization of the L. plantarum-R population in fecal samples was expressed as CFU recovery per gram fecal dry weight.
Culturing of L. plantarum strain mixtures on different growth substrates
To evaluate strain-specific competition in vitro, the L. plantarum-R mixture was used as an inoculum (1:500) in growth experiments using ½ MRS-C supplemented with either 0.5% (w/v) GOS or inulin. The cultures were grown overnight at 37°C and subcultured daily (1:500) in the same medium for 8 days. Aliquots of each daily culture were collected and stored for downstream DNA isolation and relative abundance analysis of the different L. plantarum strains (see below).
DNA extraction from faeces and bacterial cultures
DNA was extracted from the fecal aliquots (4 rats per group that most consistently displayed L. plantarum-R recovery close to diet-group average for post-gavage days 1, 3, 5 and 7 for each rat), bacterial mixture used in gavages (L. plantarum-R) and in vitro subculture aliquots (starting inoculum, and after 1, 3 and 8 days of subculturing) using the repeated bead beating methodCitation18 and the QIAamp Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany), according to the manufacturer’s instruction. DNA quality and quantity were determined using a Nanodrop DeNovix DS-11 Spectrophotometer (DeNovix Inc., Wilmington, DE, USA) and QubitⓇ 4 Fluorometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA) with the QubitTM dsDNA BR Assay Kit (Thermo Fisher Scientific, Waltham, Massachusetts, USA).
Intergenic region sequencing
To evaluate the strain-specific relative abundance of the administered L. plantarum strains, the intergenic region 339-IR-340 encompassing the strain-specific SNP alleles (Supplemental Figure SF3A)Citation19 was amplified by PCR using primers S1–2 (Supplemental Table ST1), using DNA isolated from fecal samples or from in vitro bacterial cultures (see previous sections) as a template. Amplification details, its reliability and dynamic range of strain-specific detection were assessed in a technical validation of the procedure (Supplemental Figure SF3).
Amplicon concentrations were measured by DeNovix DS-11 Spectrophotometer (DeNovix Inc., Wilmington, DE, USA), and amplicons were purified using MSB Spin PCRapace (STRATEC Molecular, Germany). Purified amplicons were PCR-extended with barcoded Illumina universal index sequencing adapters and sequenced (paired-end) using the Illumina MiSeq system (performed by BaseClear BV, Leiden, The Netherlands). FASTQ files were generated using bcl2fastq2 version 2.18, initial quality assessment was based on Illumina Chastity filtering, and subsequently, reads containing PhiX control signal were removed using an in-house filtering protocol. Reads containing (partial) adapters were clipped (to a minimum read length of 50 bp). Paired-end reads were merged using PEARCitation20 and merged reads were trimmed to encompass only the 275-basepair intergenic region (339-IR-340). Trimmed reads were aligned using MUSCLE,Citation21 followed by strain-specific unique SNP position extraction and assigned to the corresponding seven allele genotypes using a custom Python script (https://github.com/m4rku5–5/IGR-caller). More than 75% of the overall sequence reads could be assigned to one of the strains in the mixture.
Microbiota analysis and faecal organic acid quantification
16S rRNA amplification, sequencing and metataxonomic analyzes as well as fecal organic acid quantifications were described previously.Citation15 The total amount of L. plantarum ASVs on day 1 or day 3 post-gavage was followed by subtraction of the number of L. plantarum ASVs on baseline measurements to represent the relative abundance of the administered L. plantarum-R cocktail.
Availability of data and materials
All IGR and 16S sequences used in this study were deposited at the European Nucleotide Archive (ENA) with the following accession numbers: ERR5902509-ERR5902553, ERR5902555- ERR5902588 (IGR) and ERR5388223, ERR5388-227, ERR5388237, ERR5388241, ERR5388-232, ERR5388239, ERR5388250, ERR5388-256, ERR5388235, ERR5388247, ERR53882-48, ERR5388252, ERR5388240, ERR53882-43, ERR5388244, ERR5388253, ERR5388219, ERR5388226, ERR5388236, ERR5388255, ERR9728100-ERR9728139 (16S). A corresponding metadata sheet (Supplemental Table ST4) is found in the supplementary materials.
Data mining and statistical analysis
Recovery of CFU per gram fecal material (wet or dry weight is specified) over time is expressed as log10 converted means ± standard error of the mean (SEM). Significant differences between intestinal persistence in the different groups of rats (diet or mode of administration differences within dietary calcium context) were detected by a two-sided non-parametric Mann–Whitney U test using GraphPad Prism version 8.00 for Windows (GraphPad Software, San Diego, California, USA). To estimate the persistence kinetics of L. plantarum-R in individual rats, the recovered CFU numbers on consecutive days post-gavage per rat were used to model the microbial density decline rate as previously described.Citation12 Significant differences between linear decline rates in the different diet groups were detected by a two-sided Student’s t-test with α = 0.05 using GraphPad Prism version 8.00 for Windows (GraphPad Software). The inverse of the linear decline rate was taken to represent the bacterial persistence rate. Redundancy analysis (RDA) was performed in CANOCO 5, according to the user manualCitation22 with log transformed ASV response data using the group specific diets as experimental variable and succinic, lactic, acetic, propionic and butyric acid, the sum of all organic acids,Citation15 and the persistence rates as supplemental variables. Arrows in the RDA plots correspond to microbial genera that display the strongest correlation with the environmental variables. Significance of the explained variation was calculated by Holms corrected p-value with α = 0.05. Spearman correlations were performed two-tailed with a confidence interval of 95% using Prism version 8.00 for Windows.
Results & discussion
In the current study we investigated the effect of dietary GOS and inulin supplementation in low and high calcium diets (Supplemental Table ST3; Supplemental Figure SF4) on strain-specific L. plantarum persistence using a competitive intestinal persistence model in Wistar rats. After a two-week acclimatization period, each rat was gavaged with a mixture of rifampicin-resistant derivatives (L. plantarum-R) with varying capacities to utilize inulin or GOS (; Supplemental Tables S1–2). Total L. plantarum abundance as well as strain-specific population dynamics were determined in fecal samples by selective enumeration of L. plantarum colony forming units (CFU), and quantitative profiling of a variable intergenic allele known in this panel of strainsCitation19 over a time period of 9 days (Supplemental Figures SF3–4).
Figure 1. Time-resolved persistence and relative abundance of intragastrically administered L. plantarum-R strains in adult male rats. (a) L. plantarum-R strain panel legend (Figure 1(a)) with growth phenotypes. A plus (+) symbol indicates that capacity of a strain to utilise >DP3 inulin or DP3–4 GOS, while a minus (-) symbol indicates the lack of this capacity (see supplemental Figures S1–2).Citation14,Citation16 Strains with a (-) phenotype fail to utilise higher DP compounds but are still capable of utilising most mono- and di-saccharides present in either prebiotic substrate. (b) Persistence of administered L. plantarum-R strain cocktail in rats on Hca (circles, open), HcaGOS (black circles, closed) and HcaInu (grey circles, closed) diets after a single gavage with approximately 6 × 109 CFU of L. plantarum-R on day 0. All CFU counts are log-transformed and expressed as mean ± SEM (n = 8 per diet group). #, the GOS-supplemented group is significantly different from the Hca control group (#: p < .05, ##: p < .01). *, the inulin-supplemented group is significantly different from the Hca control group control group (*: p < .05). (c) L. plantarum-R mixture was grown on ½ MRS-C supplemented with either 0.5% (w/v) GOS or inulin and passaged 1:500 after 24 hours of incubation for 8 days. Stacked area charts represent the relative abundance of L. plantarum strain-specific 339-IR-340 sequences amplified from isolated DNA from mixed bacterial cultures collected at different time points corresponding to estimated generations (see figure SF3 in the supplementary material). (d) Stacked area charts with relative abundance of L. plantarum strain-specific 339-IR-340 sequences amplified from isolated faecal DNA collected at different time points after L. plantarum-R gavage in rats on the different diets. Results are expressed as average of relative abundance, as fraction of the whole.
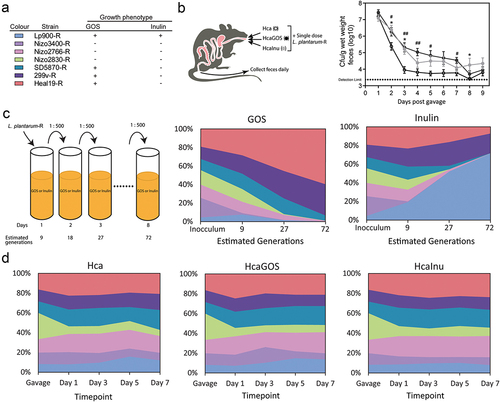
Analogous to our previous results,Citation12 inulin supplementation increased L. plantarum total persistence in high calcium diets up to 8 days post-gavage (). Similarly, increased L. plantarum persistence up to 7 days was observed for GOS supplementation in high calcium diets (), whereas L. plantarum recovery rapidly declined in rats that were fed high-calcium diets without prebiotic supplements. Notably, the extended intestinal persistence in prebiotic-containing diet was not influenced by differences in fecal dry- or wet-weight variations as a consequence of the diet and was not observed in rats that were fed supplemented low-calcium diets (Supplemental Figure SF5). The results demonstrate that the intestinal persistence of the L. plantarum-R strain mixture is influenced by the dietary regime of the rats and appears to be enhanced by prebiotic supplementation. The mechanisms by which dietary calcium modulates the intestinal microbiota have not been fully deciphered, but it has been proposed to rely on increased buffering capacity of the intestinal lumen and the consequential precipitation of cytotoxic surfactants like secondary bile acids.Citation23,Citation24 The latter compounds are known antimicrobials, with especially effective inhibitory capacities against endogenous Gram-positive bacteria like the lactobacilli.Citation25
The L. plantarum-R mixture used in the gavage was also co-cultured in vitro for approximately 9, 27 and 72 generations in rich laboratory (MRS-based) media containing GOS or inulin as a sole carbon source (). Strain-specific relative abundance was greatly influenced by the prebiotic used as carbon source. The strains Heal19, 299 v and SD5870 are HDP-GOS utilizers and outcompeted all strains that cannot utilize HDP-GOS (i.e., Nizo3400, Nizo2766 and Nizo2830) but also one of the HDP-GOS utilizing strains (Lp900). Inulin containing media clearly enriched the only inulin utilizing strain Lp900 (comprising 51% and 70% of the community after 27 and 72 generations, respectively) (). Notably, besides Lp900, the strains Heal19 and 299 v also remained prominently present in the mixed culture (~30% of the community after 72 generations; ), which may reflect the effectiveness of these strains to utilize the fructose liberated by the β-fructosidase produced by strain Lp900 (i.e., “hitchhiking”). The difference in the mechanisms involved in GOS and inulin utilization in L. plantarum could significantly impact the population dynamics of prebiotic utilizing and non-utilizing strains when grown in competition. GOS is imported and intracellularly metabolized (Supplemental Figure S2) providing a competitive exclusion advantageCitation26 in environments containing GOS. Conversely, inulin is hydrolyzed extracellularly by the cell-wall anchored FosE enzyme,Citation12 followed by import of the released fructose moieties (Supplemental Figure SF1). Consequently, the fructose released is probably also available for competing strains that lack the inulin-hydrolysis capacity but can import and utilize fructose. Such “hitchhiking” possibility is further stimulated by the partial release of FosE by L. plantarum Lp900 into its culture supernatant.Citation12
Intriguingly, the robust effects of GOS and inulin to drive competition between utilizing and non-utilizing strains in vitro was not reflected in intestinal persistence in vivo (). The relative population of L. plantarum remained virtually identical in fecal samples collected over time for each dietary group, and none of the strains appeared to be selectively enriched over time. The marginal increases observed for some strains are likely caused by the consistently observed decrease in Nizo2830 and Nizo3400 populations, which could reflect the diet-independent lower survival and persistence of these strains in the intestine (Supplemental Table S1).Citation19
The present study reveals that the observed increase in L. plantarum-R intestinal persistence is independent of the capacity of a subset of the L. plantarum strain(s) to effectively utilize inulin or GOS as a substrate for growth, which does not enhance a strain’s in situ fitness in the intestinal tract compared to non-utilizing strains of the same species. Intriguingly, comparable competition experiments in GOS-fed gnotobiotic mice, using Limosilactobacillus reuteri 6475 and its isogenic derivative that is unable to utilize GOS, also failed to reveal a significant competitive advantage for GOS-utilizing strains.Citation27
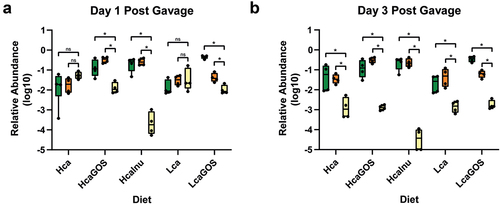
Previously, we have reported about the fecal microbiota composition and their corresponding fermentation end-product profiles (including succinic and lactic acid) in the rats used in this study, following their two-week diet-acclimatization period (i.e., prior to the L. plantarum-R gavage).Citation15 Primary outputs of this analysis were the pronounced proliferation of Bifidobacterium and Faecalibaculum in prebiotic supplemented diets in a high calcium background, together with increased organic acid levels, in particular lactic and acetic acid. Notably, L. plantarum gavages did not lead to significant changes in the intestinal microbiota composition nor fecal organic acid levels as compared to those published previously (data not shown;Citation15). Moreover, the fecal level of the main metabolic output from L. plantarum strains, i.e., lactic acid, did not change significantly between pre-gavage and 3 days post-gavage (Supplemental Figure S6). Based on this lack of change in the intestinal measurements (microbiome and acid profiles) and since GOS or inulin utilization capacity did not affect strain-specific persistence, we explored these intestinal measurements as potential persistence drivers for the L. plantarum-R population as a whole. Redundancy analysis revealed that the diets that increased L. plantarum-R persistence (i.e., the HcaInu and HcaGOS diets) were associated with increased relative abundance of Bifidobacterium and Faecalibaculum (). Various genes associated with inulin and GOS utilization are regularly found in representatives within the Bifidobacterium genus.Citation28,Citation29 Furthermore, public Faecalibaculum rodentium genomes encode secreted proteins with canonical inulin-hydrolyzing glycoside hydrolase (GH) family 32 domains, as well as lactose-PTS systems and 6-P-β-galactosidases that are resembling genes and proteins found to convey GOS utilization capacity in Lactobacillus gasseri.Citation30 These findings implicate that the increased relative abundance of these bacterial taxa in rats on prebiotic-supplemented diets depends on their capacity to utilize the substrates. Interestingly, microbiota composition analyses using fecal samples collected on days 1 and 3 post-gavage, showed that the strains administered through the L. plantarum-R cocktail were on average 240- and 1850-fold outnumbered by the endogenous Bifidobacterium and Faecalibaculum in the prebiotic supplemented diets on days 1 and 3, respectively (). This finding indicates that the administered L. plantarum-R are incapable to compete for prebiotic utilization with the ma-ssively outnumbering Bifidobacterium and Faecalibaculum, explaining that the possible fitness advantage provided by prebiotic utilization in specific L. plantarum strains is nullified in vivo in the intestine and does not explain the enhanced intestinal persistence observed. The latter conclusion implies that explanations for the increased L. plantarum-R persistence in prebiotic-supplemented diets have to be found elsewhere and could very well depend on diet induced physical and chemical differences in the intestinal habitat, which may very well involve a pivotal role of the endogenous microbiota. The HcaInu and HcaGOS diets were associated with increased persistence of the L. plantarum-R population and also induced increased total organic acid concentrations in fecal samples, particularly driven by increased lactic and acetic acid levels ().Citation15 Conversely, the increased levels of propionic acid observed in de Lca diets supplemented with either GOS or inulin did not appear to significantly enhance L. plantarum-R persistence. The association of increased persistence of L. plantarum-R with increased levels of fecal organic acids was also tentatively supported by a trending spearman correlation (ρ = −0.29; p = 0.066) between the overall organic acid levels and population decline rates using the data from all animals (data not shown).
Figure 2. Exploring the relation between persistence, microbiota and fecal organic acid levels. RDA with the endogenous microbiota (genera) as response variables and the explanatory variable of diet: Hca (yellow squares), Lca (yellow circles), HcaGOS (blue squares), LcaGOS (blue circles) and HcaInu (red squares) with fecal organic lactic (LA), succinic (SA), acetic (AA), propionic (PA) and butyric (BA) acid levels, total acid levels and the persistence rate (i.e., inverse of linear decline rate) as supplementary variables. Blue arrows correspond to selected genera associated with the dietary regimes.
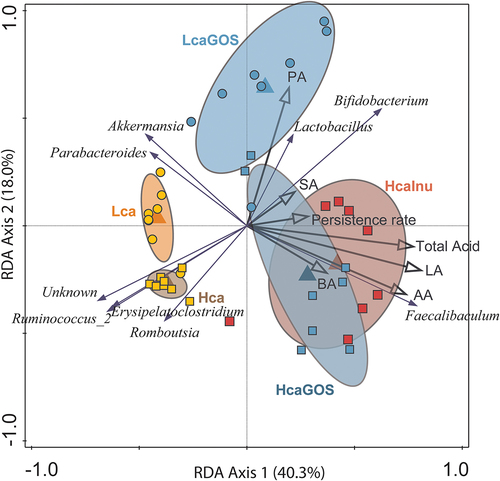
Figure 3. Relative abundance of L. plantarum-R compared to endogenous Bifidobacterium, Faecalibaculum abundance post-gavage. Relative abundance of Bifidobacterium (green), Faecalibaculum (orange), and the L. plantarum-R cocktail (yellow) after (a) one day post-gavage and (b) three days post-gavage. Asterisks (*) indicate significance after a two-sided Mann-Whitney t test. *: p < .05.
Conclusion
In conclusion, irrespective of the mechanisms involved in prebiotic utilization (i.e., import versus extracellular hydrolysis) the constant relative abundance of the L. plantarum strains in vivo in the intestine underpins that neither substrate utilization nor potential cross-feeding contribute to in situ fitness in the intestinal tract. This is likely explained by the observation that these bacteria are vastly outnumbered by members of the endogenous microbiota that effectively digest the prebiotic substrate. Consequently, the contribution of the capacity of individual L. plantarum strains to utilize the same prebiotic substrate is nullified and irrelevant for strain-specific intestinal colonization and persistence efficacy. Therefore, we propose that the substantial changes in L. plantarum intestinal persistence in rats fed a prebiotic supplemented diet is caused by the effects of prebiotics on intestinal characteristics, including the changes in microbiota composition and cognate SCFA levels in the large intestine and feces,Citation15 which is in good agreement with the observation that L. plantarum is known to tolerate relatively high levels of these organic acids. These results elucidate the principal mechanisms that underlie synergistic effects associated with a combination of pre- and pro-biotics (i.e., synbiotics).Citation1 Moreover, dietary (micro-)nutrients such as dietary calcium co-modulate the L. plantarum persistence. The dietary calcium regimes used in this study reflect the higher and lower intake levels in typical northern and southern European diets, respectively. Taken together, the non-dependence of prebiotic utilization capacity for the persistence of administered probiotics, in concert with dietary calcium backgrounds, provides critical insight in the parameters that should be taken into account when setting up probiotic or synbiotic intervention trials, to ensure that the establishment of the intestinal milieu supports probiotic delivery in such trials.
Ethics approval and consent to participate
All performed animal experiments related to this study were approved by the Central Authority for Scientific Procedures on Animals (CCD) in the Netherlands.
Supplemental Material
Download PDF (1.6 MB)Acknowledgments
We would like to thank Kerstin Holmgren, Gunilla Önning and Niklas Larson from PROBI A/B for the fruitful discussions about the results obtained. We want to thank PROBI A/B and NIZO food research for providing the L. plantarum strains. Furthermore, we want to thank BENEO-Orafti and Friesland Campina DOMO for providing the inulin and Vivinal GOS, respectively. We would like to thank Prof Dr Heidy den Besten for her expert consultation on modeling bacterial decline rates. We are also grateful to the biotechnicians of the laboratory animal facility of the Wageningen University (Wageningen, the Netherlands) for their excellent technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/19490976.2024.2338946
Additional information
Funding
References
- Markowiak P, Ślizewska K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients. 2017;9:1021. doi:10.3390/nu9091021.
- Davis LMG, Martínez I, Walter J, Hutkins R. A dose dependent impact of prebiotic galactooligosaccharides on the intestinal microbiota of healthy adults. Int J Food Microbiol. 2010;144(2):285–10. doi:10.1016/j.ijfoodmicro.2010.10.007.
- Bouhnik Y, Raskine L, Simoneau G, Vicaut E, Neut C, Flourié B, Brouns F, Bornet FR. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: A double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr. 2004;80(6):1658–1664. doi:10.1093/ajcn/80.6.1658.
- Costalos C, Kapiki A, Apostolou M, Papathoma E. The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Hum Dev. 2008;84(1):45–49. doi:10.1016/j.earlhumdev.2007.03.001.
- Kleessen B, Schwarz S, Boehm A, Fuhrmann H, Richter A, Henle T, Krueger M. Jerusalem artichoke and chicory inulin in bakery products affect faecal microbiota of healthy volunteers. Br J Nutr. 2007;98(3):540–549. doi:10.1017/S0007114507730751.
- Ten Bruggencate SJM, Bovee-Oudenhoven IMJ, Lettink-Wissink MLG, Katan MB, Van Der Meer R. Dietary fructo-oligosaccharicles and inulin decrease resistance of rats to salmonella: Protective role of calcium. Gut. 2004;53(4):530–535. doi:10.1136/gut.2003.023499.
- Ten Bruggencate SJM, Bovee-Oudenhoven IMJ, Lettink-Wissink MLG, Katan MB, van der Meer R. Dietary fructooligosaccharides affect intestinal barrier function in healthy men. J Nutr. 2006;136(1):70–74. doi:10.1093/jn/136.1.70.
- Ten Bruggencate SJM, Bovee-Oudenhoven IMJ, Lettink-Wissink MLG, Van Der Meer R. Dietary fructo-oligosaccharides dose-dependently increase translocation of salmonella in rats. J Nut. 2003;133(7):2313–2318. doi:10.1093/jn/133.7.2313.
- Bovee-Oudenhoven IM, Wissink ML, Wouters JT, Van der Meer R. Dietary calcium phosphate stimulates intestinal lactobacilli and decreases the severity of as salmonella infection in rats. J Nutr. 1999;129(3):607–612. doi:10.1093/jn/129.3.607.
- Bovee-Oudenhoven IMJ, Lettink-Wissink MLG, Van Doesburg W, Witteman BJM, Van der Meer R. Diarrhea caused by enterotoxigenic Escherichia coli infection of humans is inhibited by dietary calcium. Gastroenterology. 2003;125(2):469–476. doi:10.1016/S0016-5085(03)00884-9.
- Scholtens PAMJ, Alles MS, Willemsen LEM, van den Braak C, Bindels JG, Boehm G, Govers MJAP. Dietary fructo-oligosaccharides in healthy adults do not negatively affect faecal cytotoxicity: a randomised, double-blind, placebo-controlled crossover trial. Br J Nutr. 2006;95(6):1143–1149. doi:10.1079/BJN20061765.
- Fuhren J, Schwalbe M, Rösch C, Nijland R, Wels M, Schols HA, Kleerebezem M. Dietary inulin increases lactiplantibacillus plantarum strain Lp900 persistence in rats depending on the dietary-calcium level. Appl Environ Microbiol. 2021;87(9):e00122–21. doi:10.1128/AEM.00122-21.
- Bachmann H, Molenaar D, Kleerebezem M, Van Hylckama Vlieg JE. High local substrate availability stabilizes a cooperative trait. Isme J. 2011;5(5):929–932. doi:10.1038/ismej.2010.179.
- Fuhren J, Schwalbe M, Peralta-Marzal L, Rösch C, Schols HA, Kleerebezem M. Phenotypic and genetic characterization of differential galacto-oligosaccharide utilization in Lactobacillus plantarum. Sci Rep. 2020;10(1):1–11. doi:10.1038/s41598-020-78721-4.
- Fuhren J, Schwalbe M, Boekhorst J, Rösch C, Schols HA, Kleerebezem M. Dietary calcium phosphate strongly impacts gut microbiome changes elicited by inulin and galacto-oligosaccharides consumption. Microbiome. 2021;9(1):218. doi:10.1186/s40168-021-01148-0.
- Fuhren J, Rösch C, ten Napel M, Schols HA, Kleerebezem M, McBain AJ. Synbiotic matchmaking in Lactobacillus plantarum: substrate screening and gene-trait matching to characterize strain-specific carbohydrate utilization. Appl Environ Microbiol. 2020;86(18):e01081–20. doi:10.1128/AEM.01081-20.
- Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123(11):1939–1951. doi:10.1093/jn/123.11.1939.
- Yu Z, Morrison M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques. 2004;36(5):808–812. doi:10.2144/04365ST04.
- van Bokhorst-van de Veen H, van Swam I, Wels M, Bron PA, Kleerebezem M, Driks A. Congruent strain specific intestinal persistence of Lactobacillus plantarum in an intestine-mimicking in vitro system and in human volunteers. PLoS One. 2012;7(9):e44588. doi:10.1371/journal.pone.0044588.
- Zhang J, Kobert K, Flouri T, Stamatakis A. PEAR: a fast and accurate illumina paired-end reAd mergeR. Bioinformatics. 2014;30(5):614–620. doi:10.1093/bioinformatics/btt593.
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi:10.1093/nar/gkh340.
- Ter Braak CJF, Smilauer P. CANOCO reference manual and canodraw for windows user’s guide: software for canonical community ordination (version 4.5). 2002. www.canoco.com,33,Biometris(WUMAT).
- Govers MJAP, Van Der Meer R. Effects of dietary calcium and phosphate on the intestinal interactions between calcium, phosphate, fatty acids, and bile acids. Gut. 1993;34(3):365–370. doi:10.1136/gut.34.3.365.
- Govers MJAP, Termont DSML, Lapre JA, Kleibeuker JH, Vonk RJ, van der Meer R. Calcium in milk products precipitates intestinal fatty acids and secondary bile acids and thus inhibits colonic cytotoxicity in humans. Cancer Res. 1996;56:3270–3275.
- Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev. 2005;29(4):625–651. doi:10.1016/j.femsre.2004.09.003.
- Hardin G. The competitive exclusion principle. Science (1979). 1960;131(3409):1292–1297. doi:10.1126/science.131.3409.1292.
- Rattanapprasert M, van Pijkeren JP, Ramer-Tait AE, Quintero M, Kok CR, Walter J, Hutkins RW. Genes involved in galactooligosaccharide metabolism in Lactobacillus reuteri and their ecological role in the gastrointestinal tract. Appl Environ Microbiol. 2019;85(22):1–15. doi:10.1128/AEM.01788-19.
- Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S, Matteuzzi D. Fermentation of fructooligosaccharides and inulin by bifidobacteria: A comparative study of pure and fecal cultures. Appl Environ Microbiol. 2005;71(10):6150–6158. doi:10.1128/AEM.71.10.6150-6158.2005.
- Böger M, Van Leeuwen SS, Lammerts Van Bueren A, Dijkhuizen L. Structural identity of galactooligosaccharide molecules selectively utilized by single cultures of probiotic bacterial strains. J Agric Food Chem. 2019;67(50):13969–13977. doi:10.1021/acs.jafc.9b05968.
- Honda H, Nagaoka S, Kawai Y, Kemperman R, Kok J, Yamazaki Y, Tateno Y, Kitazawa H, Saito T. Purification and characterization of two phospho-β-galactosidases, LacG1 and LacG2, from Lactobacillus gasseri ATCC33323 T. J Gen Appl Microbiol. 2012;58(1):11–17. doi:10.2323/jgam.58.11.

