 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Cell geometry is tightly coupled to gene expression patterns within the tissue microenvironment. This perspective synthesizes evidence that the 3D organization of chromosomes is a critical intermediate for geometric control of genomic programs. Using a combination of experiments and modeling we outline approaches to decipher the mechano-genomic code that governs cellular homeostasis and reprogramming.
Introduction
Over the past decades great progress has been made in high-resolution genome sequencing and visualization of the chromosome arrangements and their contact maps.Citation24,46 However, missing in this picture are the mechanical constraints imposed on the nucleus and the genome by cell and tissue geometry.Citation38,47 While paradigm-shifting methods to reprogram cells have been successful in tissue contexts,Citation17,42 our understanding of the nuclear mechanical mechanisms controlling these processes is very limited. Although the genetic basis for reprogramming events are beginning to be understood, the in vivo transitions between different cell types requires deciphering the codes that connect the geometry of cells, the spatial organization of the genome, and its expression. These codes are central to understanding how cells perceive extracellular microenvironments and integrate these signaling inputs to differentially turn on expression programs.
Genetic information is contained in a meter-long polymer chain that has to be tightly packed into a limited nuclear space satisfying a number of physical and chemical constraints.Citation2 The folding of the DNA is facilitated by histone and non-histone proteins and accompanied by a number of posttranslational modifications. The DNA is then further condensed into interphase chromosomes.Citation16,20,32,48 The nucleus is held under a pre-stress tension by links to the cytoskeleton and the extracellular matrix resulting in a homeostatic balance of the organization of interphase chromosomes.Citation31 A number of studies using spectral karyotyping and chromosome conformation capture have revealed that chromosomes in the nucleus are non-randomly organized into approximately ellipsoidal-shaped domains and that these arrangements follow certain probabilistic trends.Citation3,28,39
In our body we have about 200 different cell types with distinct geometries; epithelial cells, for example, are columnar, while connective tissue cells are flat ellipsoidal. In addition, cell types differ in their mechanical stiffness; bone cells are highly rigid, while brain tissue cells are extremely soft.Citation10 Although Cajal in his drawings projected the role of cell geometry in tissue homeostasis already about a century ago,Citation15 we are far from understanding the tissue-specific rules governing the non-random genome arrangements and how they emerge during differentiation or reprogramming and in maintaining cell-fate memory.Citation6,26,34 The emerging hypothesis that is outlined in this perspective is that the geometry and stiffness constraints in the various tissues impose a distinct spatial organization of the chromosomes leading to lineage-specific gene expression programs.
Cells seamlessly transition through the epigenetic landscape during differentiation and trans-differentiation programs in our body. These transitions are initiated at the molecular level: cells sense the geometry and stiffness of the microenvironment and perceive a number of biochemical signals via proteins on the cell membrane.Citation18,19 These signals have to integrate with various chromatin remodeling enzymes to impose various posttranscriptional modifications and eventually regulate individual genes or groups of genes.Citation38 However, our understanding of how these different extracellular microenvironment signals are sensed at the local cellular level and subsequently lead to nuclear remodeling of the genome in the different tissues is very poor.Citation1,5,8,9,13,25
Here, we provide our perspective on how to integrate experimental and modeling efforts in order to decipher the geometric mechano-genomic code that underlies cell-type specificity in tissue microenvironments (Fig. 1). Establishing this code has important implications on our understanding of differentiation and reprogramming processes, genomic processes underlying diseases including cancer, host pathogen interactions, and the development of single-cell disease diagnostics.
Cell geometry impacts nuclear and chromatin plasticity
Recent developments in microfabrication technologies provide means of controlling cell geometry in 2D or in 3D.Citation43 This is important since cells in our body have precise shapes imposed by the tissue microenvironment. Micro-contact printing techniques enable patterning ligands on a substrate resulting in cells adhering onto these patterns. This means that the geometry of a cell can be very precisely defined and controlled. As a result, we can now start to study the role of tissue microenvironments on gene expression.
Changes in cell geometry alter the mechanical tension that is built up in a cell. For example, in fibroblasts the cytoskeleton compresses the nucleus into a flat shape. Changing the shape of these cells, from a polarized state into an isotropic state, results in major remodeling of the nuclear morphology.Citation27 In addition, these cytoskeletal rearrangements modulate the nucleus from a highly rigid state into a plastic state, suggesting that these alterations have an important role in chromatin organization.Citation21 For example, stem cells exhibit similar level of nuclear plasticity as fibroblast cells with reduced matrix attachment. Furthermore, stem cells expressing nuclear architectural proteins, such as lamins, exhibit stiffer nuclei.Citation40 Hence changes in cell geometry provide quantitative handles to tune the stiffness of the nucleus in terminally differentiated cells.
A number of experiments have begun to reveal the coupling between cell geometry, nuclear plasticity and chromatin organization, an essential physical intermediate in genome regulation. Time-lapse imaging experiments of chromatin organization in living cells have revealed that cells with reduced matrix contact exhibit higher chromatin dynamics.44 In addition, quantitative fluorescence polarization imaging of chromatin compaction states has shown differential exposure to regulatory sites.Citation41 Furthermore, secondary RNAi screens are now beginning to identify important proteins that link the cytoskeleton through the nuclear envelope to the chromatin. Ablation of these cytoskeletal links results in modulating both, cell geometry and the histone-DNA interactions, thus altering the condensation states of the chromatin.Citation35,44
To summarize, changes in cell geometry lead to alterations in nuclear plasticity and chromatin accessibility. This suggests the existence of highly precise geometric codes that translate cell mechanical signals into precise genetic outputs.
Cell geometry impacts gene expression
To test if these differential chromatin condensation states have functional impact, it is important to analyze the link between cellular geometric signals and the whole-genome transcriptome. This can be probed by forcing cells into specific geometries and taking whole-genome microarray or RNA-seq measurements. A heat map of gene expression as cell shape or size changes suggests that the geometric and the regulatory space are tightly coupled. Interestingly, polarized cells mainly turn on the cell matrix genes, through the serum response pathway, reinforcing attachment to the matrix. In contrast, when the shape of the cell is changed into an isotropic shape, the expression profile shows mainly the cell cycle genes.Citation23 In particular, single-cell mechanical measurements have revealed that changes in the extracellular matrix have an impact on the shuttling of transcription factors between the cytoplasm and the nucleus.Citation12,22 These results suggest that the nucleus shape, which is regulated by extracellular matrix contacts, can switch the transcription program in a modular manner.
As explained in the previous paragraphs, cell geometric constraints modulate nuclear morphology. In addition, the development of chromosome capture and Hi-C techniques in recent years have resulted in insightful new data on chromosome contacts that exist within living cells.Citation14 We hypothesize the existence of a map between cell geometry and chromosome organization ensuring that target genes of cell-type specific pathways are clustered in specific spatial domains. Our goal is to establish a geometric mechano-genomic zip code that explains modular gene expression patterns. Such codes will reveal how cell geometric alterations change chromosome contact maps and transcriptional programs.
Some evidence for our hypothesis has already been found: For example, when cells are polarized and turn on cell-matrix genes, single-cell imaging of chromosome intermingling and whole transcriptome analysis have revealed functional gene clusters that are enriched with Pol-II and the transcription factors associated to these genes. In contrast, in isotropic cells cell-cycle genes are co-clustered with their respective transcription factors and Pol-II (Wang et al., unpublished). These findings suggest that the chromosome contact maps are optimized for the cell-type specific expression profiles within the cell geometric constraints.
Geometric models for genome regulation
The spatial organization of chromosomes under different geometric constraints on the cell nucleus can be viewed as a shape-packing problem. Such problems have been a popular area of research in discrete mathematics and optimization over many years.Citation29,36 Typically, shape-packing problems study how uniform objects can be packed without overlap into a larger container or into a space of infinite extent with maximum density.Citation7 Most research has concentrated on the special case of packing spheres. A related prominent problem is sphere covering, where the goal is to find an arrangement that covers the space with a set of uniform spheres with as little overlap as possible.Citation11,36
In the chromosome arrangement problem we are interested in packing approximately ellipsoidal-shaped non-uniform domains, the interphase chromosomes, into a container, the cell nucleus. The shape of the container is defined by the geometric constraints of the different tissues. The chromosomes are well-distributed in the nucleus, with little overlap between them. At the same time, a certain amount of intermingling of the chromosomes has been observed and is important in order to facilitate the co-regulation of genes.Citation4,14,20,30,33,37 This leads to an interesting model of the spatial arrangement of chromosomes obtained by solving a constrained optimization problem: we seek a minimal overlap arrangement of ellipsoids of a given size and shape into an enclosing container that allows for the co-regulated genes to come close in space. This is a highly non-convex optimization problem; Uhler et al.45 described an algorithm for finding such locally optimal ellipsoid arrangements. This algorithm, given a starting configuration, allows exploring the consequences of changing the geometric constraints on a cell as outlined below.
We model the chromosomes by ellipsoids ϵi, i = 1, …, n, of a given size and shape. To allow some plasticity, we only put restrictions on the minimal and maximal axes lengths and the sum of the axes. The nucleus, i.e. the enclosing container, is denoted by Ω and is assumed to be a convex body represented by quadratic or linear constraints for computational reasons. Since there is no closed-form formula for the overlap between a pair of ellipsoids, we approximate its volume by the largest inscribed ellipsoid. We denote the pairwise overlap between the ellipsoids ϵi and ϵj by ηij. This overlap can be readily computed by interior point methods. In,Citation45 we described an algorithm for finding locally optimal configurations of ellipsoids solving the following optimization problem: where the variables are the pairwise overlap vector η and the centers and orientations of the ellipsoids ϵi for i = 1, …, n, and f(η) is for example a norm of the pairwise overlaps.
In order to find configurations that reflect a cell-type specific gene expression pattern, we differentially penalize the pairwise overlap ηij between the inscribed ellipsoids by their activity distance Aij. This activity distance can be computed from the activity of each chromosome, denoted by bi, a vector containing one entry for each annotated transcription factor k = 1,…,m. This entry equals the sum of the expression levels of all genes on the chromosome to which this particular transcription factor binds. Then the activity distance Aij between 2 chromosomes is defined as a normalized activity distance between chromosomes linking transcription factors with gene expression, namely y solving the following optimization problem we obtain chromosome packing configurations that reflect a cell-type specific gene expression pattern:
The solutions to this optimization problem are configurations that link the nuclear geometry with gene expression. Such configurations for any given cell type can be used to analyze and predict the consequences of altering cell geometry, nuclear plasticity, or gene expression. Changes in cell geometry or nuclear plasticity are modeled by varying Ω, while changes in the expression profile are modeled by varying the activity distances Aij. This model will enable us to establish the geometric mechano-genomic codes that govern transitions between different cell types in a functional context.
Conclusions and future perspectives
The perspectives we have discussed will result in a holistic picture encompassing cell geometry, nuclear mechanics and transcription programs. This will push forward our understanding of how genome programs are optimized within a functional context in a tissue-specific microenvironment and enable us to establish the modular codes that drive cellular reprogramming. We envision a map that links cell geometry with the relative position of chromosomes and their intermingling, optimized for the transcription network topology of a given cell type (Fig. 1).
In order to establish such a geometric mechano-genomic map, various experimental and modeling advances are required. Proposing such a zip code and testing its validity requires new chromosome packing models that go beyond the typically used polymer physics models. To realize this, we need to advance and develop new algorithms for finding optimal shape packings under various constraints. On the experimental side, we need innovation in super-resolution imaging technologies together with single-cell chromosome capture techniques and more precise sequencing methods. Furthermore, it will be important to establish a well-annotated transcription factor database and develop novel computational methods to infer cell-type specific gene regulatory networks.
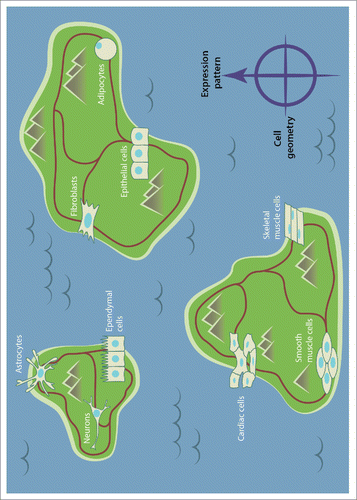
Establishing geometric mechano-genomic codes will have a major impact on our ability to remodel cells and switch their expression programs in a precise and modular manner (Fig. 2). Importantly, this will explain the role of mechanical constraints for maintaining genomic integrity and provide crucial leads for studying how alterations in these constraints can transform normal cells into disease cells. Similarly, these codes could provide insight into how pathogens use mechanical signals to invade their hosts and modulate their genomic programs. Most importantly, such studies will provide new approaches to develop biomarkers for single-cell diagnostics based on nuclear geometric cues. Collectively, understanding the mechano-genomic phase space may ultimately provide avenues for developing therapeutic interventions in personalized medicine by tuning cell geometric parameters.
Figure 1. A schematic depicting the proposed integrated approach to analyze the coupling between cell geometry and gene expression. As a first step we need to establish images of different cell types that are able to capture extracellular matrix signals, nuclear geometry, and 3d chromosome organization. Then this needs to be combined with high-throughput sequencing of the intermingled chromosomes to obtain a fine-grained picture of spatial gene neighborhoods. Gene expression analysis and mapping to the respective chromosomes will then provide insight into nanoscale functional gene clusters. In order to gain an integrated picture, we combine this single-cell analysis with geometric chromosome packing models. These models will allow us to predict the reprogramming paths between cell types. In addition, these combined experimental and modeling approaches will facilitate the development of physical biomarkers for cell-type specific alterations in disease states.
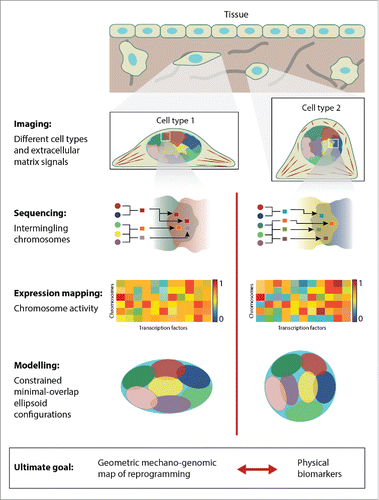
Figure 2. A schematic depicting cell types positioned within islands (representing for example different tissues) and potential reprogramming paths between these cell types. Cells can transition either within an island or between the islands by crossing local and global epigenetic barriers. We suggest the existence of a tight coupling between cell geometry and gene expression patterns. Thus, mechanical tuning of cell geometry and the induced chromosome positions could serve as an innovative approach to cross epigenetic barriers. Establishing a mechano-genomic map will provide a quantitative framework to understand how altering cellular homeostasis in local tissue microenvironments can lead to diseases such as cancer and fibrosis.
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
ACKNOWLEDGMENTS
We thank Chun Xi Wong for help with preparing the figures.
Funding
CU's research is supported by MIT start-up grants and the Austrian Science Fund (FWF) Y 903-N35. GVS is funded by the Mechanobiology Institute, Singapore, MOE-Tier3 grant Singapore and IFOM, Milan, Italy.
REFERENCES
- Augsten M. Cancer-associated fibroblasts as another polarized cell type of the tumor microenvironment. Front Oncol 2014; 4:62; PMID:24734219; http://dx.doi.org/10.3389/fonc.2014.00062
- Bickmore WA, van Steensel B. Genome architecture: domain organization of interphase chromosomes. Cell 2013; 152:1270-84; PMID:23498936; http://dx.doi.org/10.1016/j.cell.2013.02.001
- Bolzer A, Kreth G, Solovei I, Koehler D, Saracoglu K, Fauth C, Muller S, Eils R, Cremer C, Speicher MR. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol 2005; 3:e157; PMID:15839726; http://dx.doi.org/10.1371/journal.pbio.0030157
- Branco MR, Pombo A. Intermingling of chromosome territories in interphase suggests role in translocations and transcription-dependent associations. PLoS Biol 2006; 4:780; http://dx.doi.org/10.1371/journal.pbio.0040138
- Butcher DT, Alliston T, Weaver VM. A tense situation: forcing tumour progression. Nat Rev Cancer 2009; 9:108-22; PMID:19165226; http://dx.doi.org/10.1038/nrc2544
- Cavalli G, Misteli T.. Functional implications of genome topology. Nat Struct Mol Biol 2013; 20:290-9; PMID:23463314
- Conway JH, Sloane NJA. (1998). Sphere Packings, Lattices and Groups. Springer-Verlag, NY, 3rd ed.
- de Laat W, Duboule D. Topology of mammalian developmental enhancers and their regulatory landscapes. Nature 2013; 502:499-506; PMID:24153303; http://dx.doi.org/10.1038/nature12753
- Desprat N, Supatto W, Pouille P-A, Beaurepaire E, Farge E.. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell 2008; 15:470-7; PMID:18804441; http://dx.doi.org/10.1016/j.devcel.2008.07.009
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science 2005; 310:1139-43; PMID:16293750; http://dx.doi.org/10.1126/science.1116995
- Donev A, Cisse I, Sachs D, Variano EA, Stillinger FH, Connelly R, Torquato S, Chaikin PM. Improving the density of jammed disordered packings using ellipsoids. Science 2004; 303:990-3; PMID:14963324; http://dx.doi.org/10.1126/science.1093010
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S. Role of YAP/TAZ in mechanotransduction. Nature 2011; 474:179-83; PMID:21654799; http://dx.doi.org/10.1038/nature10137
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126:677-89; PMID:16923388; http://dx.doi.org/10.1016/j.cell.2006.06.044
- Fanucchi S, Shibayama Y, Burd S, Weinberg MS, Mhlanga MM. Chromosomal contact permits transcription between coregulated genes. Cell 2013; 155:606-20; PMID:24243018; http://dx.doi.org/10.1016/j.cell.2013.09.051
- Garcia-Lopez P, Garcia-Marin V, Freire M. The histological slides and drawings of Cajal. Front Neuroanat 2010; 4:1-16; PMID:20161990
- Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell 2007; 128:635-8; PMID:17320500; http://dx.doi.org/10.1016/j.cell.2007.02.006
- Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science 2008; 322:1811-5; PMID:19095934; http://dx.doi.org/10.1126/science.1160810
- Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Bio 2014; 15, 802-12; http://dx.doi.org/10.1038/nrm3896
- Iskratsch T, Wolfenson H, Sheetz MP. Appreciating force and shape [mdash] the rise of mechanotransduction in cell biology. Nat Rev Mol Cell Bio 2014; 15:825-33; PMID:25355507; http://dx.doi.org/10.1038/nrm3903
- Iyer KV, Maharana S, Gupta S, Libchaber A, Tlusty T, Shivashankar GV. Modeling and experimental methods to probe the link between global transcription and spatial organization of chromosomes. PLoS One 2012; 7:e46628; PMID:23049710; http://dx.doi.org/10.1371/journal.pone.0046628
- Makhija E, Jokhun DS, Shivashankar GV. Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc Natl Acad Sci USA 2016; 113(1):E32-40; PMID:26699462; http://dx.doi.org/10.1073/pnas.1513189113
- Iyer KV, Pulford S, Mogilner A, Shivashankar GV. Mechanical activation of cells reveals distinct timescales in chromatin remodeling and MKL nuclear transport Biophys J 2012; 103:1416-28; PMID:23062334; http://dx.doi.org/10.1016/j.bpj.2012.08.041
- Jain N, Iyer KV, Kumar A, Shivashankar GV. Cell geometric constraints induce modular gene-expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc Natl Acad Sci USA 2013; 110:11349-54; PMID:23798429; http://dx.doi.org/10.1073/pnas.1300801110
- Jin F, Li Y, Dixon JR, Selvaraj S, Ye Z, Lee AY, Yen C-A, Schmitt AD, Espinoza CA, Ren B. A high-resolution map of the three-dimensional chromatin interactome in human cells. Nature 2013; 503:290-4; PMID:24141950
- Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci 2010; 107:4872-7; PMID:20194780; http://dx.doi.org/10.1073/pnas.0903269107
- Lanctot C, Cheutin T, Cremer M, Cavalli G, Cremer T. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet 2007; 8:104-15; PMID:17230197; http://dx.doi.org/10.1038/nrg2041
- Li Q, Kumar A, Makhija E, Shivashankar GV. The regulation of dynamic mechanical coupling between actin cytoskeleton and nucleus by matrix geometry. Biomaterials 2014; 35:961-9; PMID:24183171; http://dx.doi.org/10.1016/j.biomaterials.2013.10.037
- Lieberman-Aiden E, van Berkum NL, Williams L, Imakaev M, Ragoczy T, Telling A, Amit I, Lajoie BR, Sabo PJ, Dorschner MO, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 2009; 326:289-93; PMID:19815776; http://dx.doi.org/10.1126/science.1181369
- Lodi A, Martello S, Monaci M. Two-dimensional packing problems: A survey. European J Oper Res 2003; 141:241-52; http://dx.doi.org/10.1016/S0377-2217(02)00123-6
- Maharana S, Iyer KV, Jain N, Nagarajan M, Wang Y, Shivashankar GV. Chromosome intermingling: The physical basis of chromosome organization in differentiated cells. Nucleic Acids Res 2016; (in press).
- Mazumder A, Roopa T, Basu A, Mahadevan L, Shivashankar GV. Dynamics of chromatin decondensation reveals the structural integrity of a mechanically prestressed cell nucleus. Biophys J 2008; 95:3028-35; PMID:18556763; http://dx.doi.org/10.1529/biophysj.108.132274
- Olins DE, Olins AL. Chromatin history: our view from the bridge. Nat Rev Mol Cell Biol 2003; 4:809-14; PMID:14570061; http://dx.doi.org/10.1038/nrm1225
- Osborne CS, Chakalova L, Brown KE, Carter D, Horton A, Debrand E, Goyenechea B, Mitchell JA, Lopes S, Reik W. Active genes dynamically colocalize to shared sites of ongoing transcription. Nat Genet 2004; 36:1065-71; PMID:15361872; http://dx.doi.org/10.1038/ng1423
- Rajapakse I, Perlman MD, Scalzo D, Kooperberg C, Groudine M, Kosak ST. The emergence of lineage-specific chromosomal topologies from coordinate gene regulation. Proc Natl Acad Sci USA 2009; 106:6679-84; PMID:19276122; http://dx.doi.org/10.1073/pnas.0900986106
- Ramdas NM, Shivashankar GV. Cytoskeletal control of nuclear morphology and chromatin organization. J Mol Biol 2015; 427:695-706; PMID:25281900; http://dx.doi.org/10.1016/j.jmb.2014.09.008
- Rogers CA. Packing and Covering. Volume 54 of Cambridge Tracts in Mathematics and Mathematical Physics. Cambridge, UK: Cambridge University Press; 1964.
- Schoenfelder S, Sexton T, Chakalova L, Cope NF, Horton A, Andrews S, Kurukuti S, Mitchell JA, Umlauf D, Dimitrova DS, et al. Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat Genet 2009; 42:53-61; PMID:20010836; http://dx.doi.org/10.1038/ng.496
- Shivashankar GV. Mechanosignaling to cell nucleus and genome regulation. Annu Rev Biophys 2011; 40:361-78; PMID:21391812; http://dx.doi.org/10.1146/annurev-biophys-042910-155319
- Sun HB, Shen J, Yokota H. Size-dependent positioning of human chromosomes in interphase nuclei. Biophys J 2000; 79:184-190; PMID:10866946; http://dx.doi.org/10.1016/S0006-3495(00)76282-5
- Talwar S, Jain N, Shivashankar GV. The regulation of gene expression during onset of differentiation by nuclear mechanical heterogeneity. Biomaterials 2014; 35:2411-9; PMID:24388387; http://dx.doi.org/10.1016/j.biomaterials.2013.12.010
- Talwar S, Kumar A, Rao M, Menon G, Shivashankar GV. Correlated spatio-temporal fluctuations in chromatin compaction states characterize stem cells. Biophys J 2013; 104:553-64; PMID:23442906; http://dx.doi.org/10.1016/j.bpj.2012.12.033
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126:663-76; PMID:16904174; http://dx.doi.org/10.1016/j.cell.2006.07.024
- Théry M.. Micropatterning as a tool to decipher cell morphogenesis and functions. J Cell Sci 2010; 123:4201-13; PMID:21123618; http://dx.doi.org/10.1242/jcs.075150
- Toh KC, Ramdas N, Shivashankar GV. Actin cytoskeleton differentially alters the dynamics of lamin A/C, HP1α and H2B core histone proteins to remodel chromatin condensation state in living cells. Int. Biology 2015; (in press).
- Uhler C, Wright SJ. Packing ellipsoids with overlap. SIAM Rev 2013; 55:671-706; http://dx.doi.org/10.1137/120872309
- Van Steensel B, Dekker J. Genomics tools for unraveling chromosome architecture. Nat. Biotechnol 2010; 28:1089-95; PMID:20944601; http://dx.doi.org/10.1038/nbt.1680
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 2009; 10:75-82; PMID:19197334; http://dx.doi.org/10.1038/nrm2594
- Woodcock CL. Chromatin architecture. Curr Opin Struct Biol 2005; 16:1-8.

