ABSTRACT
Despite advances in modern human and veterinary medicine, gastrointestinal (GI) parasitic infections remain a significant health issue worldwide, mainly in developing countries. Increasing evidence of the multi-drug resistance of these parasites and the side effects of currently available synthetic drugs have led to increased research on alternative medicines to treat parasitic infections. The exploration of potential botanical antiparasitics, which are inexpensive and abundant, may be a promising alternative in this context. This study summarizes the in vitro/in vivo antiparasitic efficacy of different medicinal plants and their components against GI parasites. Published literature from 1990–2020 was retrieved from Google Scholar, Web of Science, PubMed and Scopus. A total of 68 plant species belonging to 32 families have been evaluated as antiparasitic agents against GI parasites worldwide. The majority of studies (70%) were conducted in vitro. Most plants were from the Fabaceae family (53%, n = 18). Methanol (37%, n = 35) was the most used solvent. Leaf (22%, n = 16) was the most used plant part, followed by seed and rhizome (each 12%, n = 9). These studies suggest that herbal medicines hold a great scope for new drug discoveries against parasitic diseases and that the derivatives of these plants are useful structures for drug synthesis and bioactivity optimization.
1. Introduction
Gastrointestinal (GI) parasitic diseases have become a significant health problem affecting billions of humans and livestock worldwide, particularly in developing countries. An estimated 3.5 billion people are affected globally, while 450 million are symptomatic, and more than 200,000 annual deaths are reported [Citation1]. Diseases caused by GI parasites in animals also create severe disease burdens and significant economic losses associated with food production in many regions of the globe [Citation2,Citation3]. For example, the estimated annual cost of GI parasitic diseases in the Australian sheep industry is AUD 436 million [Citation4], and in the European ruminant livestock industry is more than €1.8 billion [Citation5].
The most important GI parasites in humans consist of both helminths and protozoans. The main helminth infections are those transmitted through contaminated soil (soil-transmitted helminths or geohelminths). They include Ascaris lumbricoides (commonly called roundworm), Trichuris trichiura (whipworm), Ancylostoma duodenale (old world hookworm), and Necator americanus (new world hookworm) [Citation6]. Approximately 1.5 billion people (24% of the world’s population) are infected with at least one of these helminths during their life span; they are among the most common infectious disease agents reported in humans [Citation7]. The estimated number of A. lumbricoides (roundworm) infections exceeds a billion, while T. trichiura and N. americanus account for 795 million and 740 million infections, respectively [Citation8].
Protozoan parasites infect about one-third of the human population worldwide [Citation9]. Giardia duodenalis (syn. Giardia lamblia, Giardia intestinalis), Entamoeba histolytica, and Cryptosporidium spp. are the most common GI protozoan parasites reported in humans [Citation10]. Given the high prevalence in people mainly in resource-poor areas, both G. duodenalis and Cryptosporidium spp. were included in the WHO Neglected Diseases in 2004 [Citation11,Citation12]. Further, the rising incidence of giardiasis in developed nations has led to its designation as a reemerging infectious disease [Citation13]. As shown in epidemiological studies, the prevalence of G. duodenalis varies from 2–5% in developed countries to 20–30% in developing countries [Citation14,Citation15]. The World Health Organisation annually notifies approximately 50 million cases of E. histolytica with invasive diseases such as amoebic liver abscesses [Citation16]. Other accounts claim that E. histolytica causes 55,000 deaths annually, making it the second most common cause of death from infectious parasitic diseases [Citation17]. Of the 46 Cryptosporidium spp. characterized, C. hominis and C. parvum are the most frequently detected species in humans and are responsible for approximately a million deaths yearly [Citation18]. The approximate overall Cryptosporidium prevalence in HIV/AIDS patients is 8.69% [Citation19].
Each of these enteric parasites has a direct life cycle. Human-to-human transmission mainly occurs through the fecal-oral route, during which cysts/oocysts or eggs are shed in human feces. Lack of environmental sanitation, contaminated food and water, poor public and personal hygiene standards, and poor socio-economic and demographic conditions promote the spread of such parasites [Citation20], [Citation21].
Gastrointestinal parasites can severely affect the health and nutritional status of the host and cause a range of adverse health problems [Citation22,Citation23]. For instance, soil-transmitted helminths cause a broad spectrum of illnesses, including maldigestion and malabsorption, impaired growth, and anemia [Citation24,Citation25]. Anemia and micronutrient deficiencies, including iron, vitamins, and folate, can lead to reduced work capacity, poor cognitive function, and pregnancy disorders [Citation26,Citation27]. Tissue migration of the larval stages of roundworms and hookworms leads to acute dermatitis or eosinophilic pneumonitis. Colonic bleeding and severe dysentery-like syndrome can occur due to trichuriasis [Citation28]. In addition to these morbidities, deaths have also been reported due to acute infections with soil-transmitted helminths [Citation29].
Giardia duodenalis and E. histolytica infections usually cause diarrhea or dysentery and a complex of symptoms such as stomach ache, cramps, bloating, tenderness, nausea, and weight loss [Citation30]. Giardia duodenalis generally affects the small intestine (mainly the duodenum), leading to nutritional deficiencies and significant morbidity and mortality, especially among children, the elderly, travelers in developing countries and immunocompromised patients [Citation31,Citation32]. Entamoeba histolytica infects the large intestine, and amoebic abscesses from the large intestine can spread to the liver, pleura, pericardium, and brain [Citation33].
Infection with Cryptosporidium spp. is usually self-limiting in immunocompetent people; however, it can be devastating to immunocompromised individuals. In HIV-positive patients, symptoms may include chronic or protracted diarrhea that can become life-threatening. These patients may develop extraintestinal manifestations, spreading to other sites, including the gall bladder, biliary tract, pancreas, and pulmonary system [Citation34,Citation35]. In developing countries, malnutrition in children can lead to significantly higher rates of infection [Citation36], and a single episode of C. parvum infection during the first two years of life can result in growth deficits [Citation36,Citation37].
Gastrointestinal helminths are a significant cause of mortality and morbidity of ruminants and are responsible for high costs and production losses throughout the world [Citation38]. For example, the annual cost of GI nematode infections in Europe is estimated to be € 686 million [Citation5]. One of the most important GI helminth parasites of livestock is Haemonchus contortus, a highly virulent parasite, primarily of small ruminants in tropical, subtropical, and warm temperate regions [Citation39,Citation40]. It is a frequent cause of mortalities in sheep, goats, and other ruminants due to its blood-feeding behavior, which can result in ascites, weight loss, anemia, and death [Citation3,Citation41,Citation42]. The estimated annual global economic loss caused by H. contortus to the livestock industry is $30–300 million [Citation3,Citation43] and makes H. contortus one of the most economically important parasitic nematodes in its main endemic zones [Citation38,Citation44,Citation45].
Protozoan parasites are also of concern to the livestock industry, with species of Cryptosporidium, Giardia and Eimeria perhaps the most important. Cryptosporidium infection affects many animal hosts worldwide, including the main livestock animals such as cattle, sheep, goats, and pigs, causing significant growth and production losses [Citation46]. Neonatal (<6 weeks old) calves are very vulnerable to cryptosporidiosis [Citation47]. Infected animals may develop clinical signs including watery diarrhea, reluctance to feed and dehydration, which in severe cases, can result in death [Citation47,Citation48]. The gut takes a few weeks to recover from the infection and regain its ability to absorb nutrients effectively [Citation48].
Giardia duodenalis infections are commonly reported in livestock worldwide [Citation49]. Young animals have higher prevalence rates than adults due to underdeveloped immune systems [Citation50]. In livestock, giardiasis can cause diarrhea, growth retardation, weight loss, reduced productivity and even death, resulting in significant production losses [Citation50,Citation51]. Subclinical infections can also lead to poor growth and productivity losses in these animals [Citation50].
Coccidiosis, caused by Eimeria spp., is an important and widespread enteric disease in livestock ruminants and poultry worldwide, with high morbidities and significant mortalities. Young animals are more vulnerable to infection, and symptoms include diarrhea, dysentery, anemia, dehydration, weakness, anorexia, and emaciation [Citation52]. The worldwide annual economic consequences of coccidiosis in cattle and bison were estimated to be $400 million in 1995 [Citation53]. The recent estimates suggest a total nominal financial cost of £99.23 million per annum, a 2.6–fold increase from the last calculation in 1995 [Citation54]. The cost of coccidiosis to the global poultry industry has been estimated to exceed US$ 3 billion per annum [Citation55].
1.1 Conventional management of gastrointestinal parasites
Chemotherapy remains the mainstay for the control of all GI parasites. However, the use of conventional antiparasitic drugs is often inhibited due to limitations in availability, side effects of treatments, or parasite resistance.
1.1.1. Limitations in availability
Controlling GI helminths is achieved by periodic deworming with one of several drug compounds. Benzimidazoles (mebendazole and albendazole) along with pyrantel pamoate and levamisole, are the most frequently used anthelmintics for soil-transmitted helminths [Citation56]. However, the global coverage of periodic deworming is still not sufficiently meeting target levels in all endemic countries. In 2020, only 42% of children requiring treatment for GI helminths had access to available anthelmintic medicines [Citation57].
Metronidazole, a 5-nitroimidazole derivative, is the gold standard for protozoan parasites such as E. histolytica and G. duodenalis. Following oral administration, metronidazole is quickly absorbed and penetrates body tissues and secretions such as saliva, breast milk, vaginal secretions, and semen [Citation58]. Therefore, it should be avoided in the first trimester of pregnancy.
There is no effective chemotherapeutic intervention for Cryptosporidium species, even though several compounds have been tested for their anti-cryptosporidial effects [Citation59]. Currently, nitazoxanide, the only drug recommended by the United States Food and Drug Administration (FDA), has some promise in immunocompetent people [Citation60]. However, it does not benefit malnourished children and immunocompromised patients with cryptosporidiosis [Citation61,Citation62]. Paromomycin and azithromycin are other alternatives that have been used. However, these drugs have not been approved for cryptosporidiosis.
Managing livestock helminthic infections is based mainly on the preventive or curative use of chemotherapeutics. Several anthelmintic groups with distinct mechanisms, such as benzimidazoles, imidazothiazoles and macrocyclic lactones, are available for blood-feeding parasites like H. contortus [Citation63].
However, no licensed therapeutics are currently available to prevent cryptosporidiosis in livestock [Citation64]. Similarly, there are no approved drugs for G. duodenalis in livestock, although anthelmintic drugs, such as albendazole and fenbendazole, have been used effectively [Citation50]. Numerous anticoccidial drugs have been developed, tested, and used to prevent or reduce ruminant production losses, but none are 100% effective.
1.1.2 Side effects of treatments
Long-term use and sometimes high-dose treatment regimens can be toxic to the host. For instance, high doses of mebendazole induce alopecia, allergic skin reactions, hepatitis, headache, vertigo, and oligospermia. Reversible bone marrow suppression with neutropenia has also been reported [Citation65].
The prolonged use and high doses of metronidazole can lead to side effects such as a metallic taste in the mouth, dry mouth, headache, glossitis, and nausea [Citation30,Citation66]. Rare adverse effects caused by the drug include thrush, vertigo, neutropenia, seizures, and encephalopathies due to the toxic effects on the central nervous system. Metronidazole has shown mutagenic action in bacteria. Its carcinogenic activity has been evident in mice and rats at high doses over prolonged periods [Citation67,Citation68]. However, its mutagenicity has never been reported in humans [Citation69].
There are several examples of a reduction in the efficacy of drug treatments over time. Low cure (7.6%) and egg count reduction (52.1%) rates were observed in school children treated with mebendazole in Pemba Island, Zanzibar [Citation70] compared to the results before exposure to treatment, in which mebendazole had a comparatively high cure rate (22.4%) and egg reduction rate (82.4%) [Citation71]. No reduction in the number of N. americanus eggs was observed after treatment with a single dose of mebendazole (500) mg in patients in Mali [Citation72]. The failure of pyrantel (10 mg/kg) in treating human A. duodenale infections was reported in Australia [Citation73]. Treatment of T. trichiura with single oral doses of current anthelmintics is unsatisfactory. Poor efficacy of single-dose albendazole against T. trichiura was reported in school children in Jimma Town, Ethiopia [Citation74]. Reduced single-dose albendazole (400 mg) efficacy against A. lumbricoides is reported in school children in Rwanda [Citation75].
Both paromomycin and azithromycin were reported to have partial efficacy in C. parvum infections in HIV/AIDS patients [Citation76,Citation77]. Nitazoxanide, the FDA-approved drug for human use, did not show a therapeutic effect in immunocompromised patients [Citation61]. The efficacy of metronidazole is variable; with evidence, metronidazole alone is often insufficient to eliminate amoebic cysts from the colonic lumen [Citation78]. Giardia duodenalis trophozoites within cysts are less affected by nitroimidazoles, probably due to the poor penetration of the drug through the cyst wall [Citation79].
These examples of treatment failure may be due to poor patient compliance, incorrect dosage, or inappropriate administration procedures. Still, they may also indicate the development of drug resistance in the parasite population.
1.1.3 Parasite drug resistance
Drug resistance occurs through a genetic change in a parasite population in response to selection by an antiparasitic drug that impairs the treatment and control of parasitic infection [Citation80]. In contrast to human GI parasites, drug resistance is now a well-established fact in parasites of livestock. The often excessive and frequent use of the same drug compounds for controlling parasites in livestock has led to high resistance levels, threatening the sustainability of livestock industries [Citation81].
Anthelmintic resistance is an escalating problem in sheep, goats, and horses in industrial livestock systems worldwide [Citation82,Citation83]. Haemonchus contortus, for instance, has shown a remarkable ability to develop resistance to all major anthelmintic drug classes worldwide [Citation63,Citation84]. The resistance has appeared in most cases less than ten years of the introduction of each drug group. Resistance of H. concortus to benzimidazole was reported as early as 1964 in the U.S.A [Citation85]. Resistant of H. contortus to moxidectin, a broad-spectrum antiparasitic, in small ruminants was reported in Europe [Citation86]. Ivermectin and benzimidazole-resistant Haemonchus spp. were detected in the sheep flocks in Ontario, Canada [Citation87]. Moxidectin-resistant H. contortus populations were identified in sheep farms in Queensland, Australia [Citation88]. Resistance in H. contortus to the most recently introduced amino-acetonitrile derivates (monepantel) has also been reported [Citation89]. Ivermectin resistance in horse nematodes was reported in an in vivo study in Lithuania [Citation90].
Multiple anthelmintic resistance of sheep and cattle is also a significant concern [Citation91]. Multiple anthelmintic resistance of H. contortus to milbemycins and avermectins, moxidectin and doramectin, and fenbendazole has been evident in a sheep flock in Europe [Citation82]. Cattle nematodes resistant to multiple anthelmintic classes were also reported in New Zealand and Argentina [Citation92,Citation93]. Broad-spectrum anthelmintic resistance of H. contortus was evident in sheep in Northern New South Wales, Australia [Citation94].
The resistance of GI protozoa, particularly G. duodenalis, has been reported in several in vitro studies. High in vitro susceptibility of G. duodenalis isolates to albendazole was reported compared to metronidazole [Citation95]. A laboratory-induced metronidazole-resistant line of G. duodenalis was reported, which grows in low, sub-lethal concentrations of the drug [Citation96]. Subsequently, laboratory-induced metronidazole and furazolidone-resistant lines of G. duodenalis were established in concentrations of drug lethal to the parent stock [Citation97]. Giardia duodenalis cell lines isolated from refractory patients proved metronidazole and albendazole resistant in a mouse model [Citation98]. Resistance of C. parvum to the methionyl-tRNA (CpMetRS) inhibitor 2093 was reported in the neonatal dairy calf model after four days of drug exposure [Citation99].
The need for new drug compounds against GI parasites which are safe, effective, and cheap is, therefore, imperative if we are to effectively deal with the current global burdens of parasitic disease in people and livestock. Screening of naturally occurring antiparasitic substances, such as those found in plants, which are inexpensive and abundant, appears as a promising alternative in this context. Plants have been a rich source of bioactive compounds and have been used in traditional medicine for centuries.
1.2. The use of plants in traditional medical systems
Plants have been the basis of many traditional medical systems across the world, such as Indian (Ayurveda), Chinese, and Arabic (Unani) [Citation100]. The therapies of these traditional medical systems are based on the empirical findings of thousands of years, and in some medical systems, these findings have been comprehensively documented [Citation101,Citation102]. These ancient systems of medicine use a mixture of plants or extracts, which comprise hundreds of different constituents with widely differing physiochemical properties.
Ayurveda, the Science of Life, is a comprehensive medical system originating approximately 5000 years ago in India and is considered the most ancient of all medicinal traditions. Its primary emphasis is on the prevention of disease and health promotion. The focus of Ayurveda is to achieve optimal health and well-being through a holistic approach that considers each individual’s physical, emotional, mental and spiritual aspects [Citation103]. Several thousand medicinal plants are employed in Ayurveda medicine, many of which have known antiparasitic properties. Some of the most popular plants in the Ayurveda system are Allium sativum (garlic), Alstonia scholaris (blackboard tree), Artemisia annua (sweet wormwood), Azadirachta indica (neem), Centella asiatica (Gotu kola), Curcuma longa (turmeric), Justicia adhatoda (Malabar nut) and Moringa oleifera (drumstick).
Traditional Chinese Medicine (TCM) is an ancient healing system practised for about 2000 years in China. This medical system is based on two fundamental theories (Yin-yang) that govern good health and longevity and five elements (Wuxing) that together explain all natural phenomena in the universe, including human beings [Citation104]. These natural phenomena are applied in the diagnosis and treatment of diseases. Medicine is used to restore or maintain the balance between these elements and to grant vital energy [Citation105]. Common plants used in TCM include Angelica polymorpha (female ginseng), Ephedra sinica (Chinese ephedra), Ginkgo biloba (ginkgo), Paeonia lactiflora (Chinese peony), Panax ginseng (ginseng), Rheum palmatum (Chinese rhubarb), Glycyrrhiza glabra (licorice), and Zingiber officinale (ginger).
The traditional Arabic medical system is also known as Unani or Islamic medicine. The term Unani means ‘Greek’. The Unani medical system originated in Greece, and it is believed that this system is based on the experiences of the Greek physicians Hippocrates and Galen [Citation106]. The traditional Unani healers widely used plant, mineral, and animal-origin drugs and have formulated many polyherbal-mineral recipes [Citation106]. Allium cepa (onion), Carum carvi (caraway), Crocus sativus (saffron), Ferula asafoetida (asafoetida), Papaver somniferum (opium poppy), Ricinus communis (castor), Trachyspermum ammi (ajwain) are among popular Unani plants.
1.2.1. Ethnoveterinary uses of plants
It is important to highlight that plants are not only used in traditional medicine for human treatment but also used in veterinary medicine to treat a range of ailments in animals. Ethnoveterinary medicine is crucial in many rural areas with limited access to modern drugs. People living in these remote areas rely on traditional therapies to treat domestic animals. shows the traditional use of plants to treat veterinary ailments.
Table 1. Traditional uses of medicinal plants to treat veterinary ailments.
1.3 Plant-derived synthetic medicines
The traditional use of plants has led to the isolation of promising lead molecules from plant products and the synthesis of new drugs [Citation107,Citation108]. Isolation of morphine from the plant opium was reported in 1803 and was the first pure, naturally derived medicine [Citation109]. Subsequently, many well-known plant compounds have been identified and synthesized for medicinal use (). More than 50% of the currently available synthetic drugs are based on plant products [Citation110]. These successful inventions have undoubtedly revolutionized medicine. However, they have undergone a complex, time-consuming, and expensive process. The estimated time from discovering a new drug to reaching the clinic is 12 years, involving more than $1 billion investment in today’s context.
Table 2. Plants and their compounds that have led to synthetic medicines.
1.4 Plants in the antiparasitic drug discovery pipeline
Although medicinal plants have been used to prevent and treat GI parasitic infections in humans and animals for centuries, many such plants lack systematic scientific evaluation for their efficacy, mode of action and active chemicals. More recently, however, an increase in controlled experimental studies has been reported aiming to validate and quantify the antiparasitic activities of plants and plant products used in traditional medicine [Citation111–113]. In addition, advances in instrumental technology have led to the identification, fingerprinting and chemical characterization of individual compounds in plant extracts [Citation114,Citation115]. For example, separation techniques such as gas chromatography (GC), liquid chromatography (LC), high-performance liquid chromatography (HPLC) and capillary electrophoresis (CE) have been used to separate phytochemicals in crude extracts to identify each specific component [Citation116]. Spectroscopic detection technologies, such as mass spectrometry (MS), infrared spectroscopy (IR), and nuclear magnetic resonance (NMR), have also been used to analyze molecular structures [Citation117]. The combination of these techniques has led to the expansion of medicinal plant analytics. Furthermore, with the rapid development of nanomedicine, nanoparticles have attracted researchers’ attention for antiparasitic drug discovery.
This review was undertaken to synthesize the available literature on the efficacy of different plants and active compounds and their potential mode of action against common human GI parasites and parasitic models. An extensive literature search was performed using electronic databases, including Google Scholar, Web of Science, PubMed and Scopus, for published experimental studies that aimed to validate and quantify such antiparasitic plant activities between 1990 and 2020. The following keywords and MESH headings were used. Ayurved* TCM OR Plant* OR Herb* OR Medic* OR ‘Plant extracts’ OR ‘Plants Medicinal’ AND Parasit* OR Gastrointestinal Protozoa OR Helminth* AND ‘In vitro’ OR ‘In vivo’. All peer-reviewed full-text articles of in vitro and in vivo studies investigating the antiparasitic effects of a plant or plant compound on any GI parasite capable of infecting humans or appropriate model parasites were included. Plant names, families, plant parts used, solvents used for extraction, parasites targeted, in vitro and in vivo evaluation methods and outcomes were extracted and summarized.
2. Results and Discussion
This review included 68 research articles published between 1990 and 2020. Overall, 87 plant species belonging to 34 families were screened for their antiparasitic effect against GI parasites. The majority (70%, n = 50) were in vitro studies. Most plants were from the Fabaceae family (53%, n = 18). Methanol (37%, n = 35) was the most used solvent. Leaf (22%, n = 16) was the most used plant part, followed by seed and rhizome (each 12%, n = 9).
2.1. Experimental approaches
2.1.1 In vitro and in vivo susceptibility assays
Several in vitro and in vivo screening assays for antiprotozoals and anthelmintics have been developed to evaluate the viability and motility of parasites [Citation118]. Most of the research evidence found in the literature is focused on in vitro rather than in vivo evaluation of plants against GI parasites. This is mainly due to low cost, higher throughput, quick turnover of results, and ethical approaches to reducing the use of animals [Citation119]. Moreover, in vitro studies allow screening against the different stages of parasite life cycles. For example, Hussain et al. [Citation120] tested the in vitro anthelmintic activity of the crude aqueous methanolic extract of the plants Musa paradisiaca (banana) and Trianthema portulacastrum (black pigweed) against both mature female H. contortus and their eggs.
Compared to in vitro studies, in vivo studies are more realistic; however, they are more time-consuming, costly, and challenging to reproduce. In vivo studies are designed to evaluate the safety, toxicity, and efficacy of a drug candidate in the host of interest or a model organism. However, most of the in vivo studies found for this review have focused on the efficacy of the candidate. Only a few studies have evaluated the acute toxicity of the drug candidate on the host using toxicity assays [Citation113,Citation121,Citation122].
Successful results from in vitro tests are advantageous before in vivo assays; however, compounds that have shown efficacy in vitro may or may not be effective in vivo to the same extent. For example, Thymus vulgaris (thyme) essential oil was ineffective in reducing the egg production of female H. contortus in in vivo tests. However, it was effective against the three main life stages of the parasite in vitro [Citation112]. Similarly, despite the significant in vitro activity, the ethyl acetate extract of husk fiber of Cocos nucifera (coconut) showed no activity against H. contortus in vivo [Citation122]. In contrast, some plants have shown more potency within in vivo studies than in vitro. For example, in vivo efficacy of the aqueous extract of the seeds of Coriandrum sativum (coriander) was greater than its in vitro efficacy against H. contortus [Citation123].
2.1.2 Experimental parasitic models
Some parasites of humans and livestock can be successfully cultured and studied in the laboratory. For example, advanced in vitro models have been established to cultivate protozoan parasites in the laboratory. Air-liquid interface culture derived from stem cells [Citation124], 2D cell culture platform using human esophagus squamous cell carcinoma (COLO-680N) cell line [Citation125], and 3D organoid culture using hollow fiber technology [Citation126] have been used to facilitate complete life cycle development and long-term growth of C. parvum. Giardia duodenalis trophozoites are primarily cultured axenically in a medium supplemented with bile due to the infeasibility of co-culturing parasitic trophozoites and intestinal epithelial cells. A co-culture model of trophozoites and human colorectal adenocarcinoma (Caco-2) cells has been introduced, mimicking the small intestinal epithelium [Citation127]. This model has provided a viable system for the long-term culture of G. duodenalis and helps explore host-parasite interactions [Citation127].
Similarly, the infective L3 stage of H. contortus is easy to establish in experimental conditions and can be stored at 4 °C for prolonged periods [Citation128]. Adults of H. contortus are relatively large, highly fecund and can stay viable in culture for 2–3 days [Citation128,Citation129]. The adult nematode can be used as a small animal model to screen compounds before assessing them in infected sheep. Most in vitro studies in this search have used H. contortus to screen anthelmintic drugs.
However, many important GI parasites, especially helminths, cannot be easily cultured in the laboratory and model species have been used for in vitro studies of anthelmintics.
2.1.2.1 Non-parasitic models for human and animal parasites
Free-living worms such as Pheretima posthuma and Caenorhabditis elegans are often exploited as models of parasitic nematodes of medical and veterinary importance because of their anatomical and physiological similarities [Citation130]. These worms are abundant and can be easily maintained in the laboratory. However, not being parasitic species, they lack the process essential for parasitic life cycles.
Pheretima posthuma (Phylum Annelida; Class Oligochaeta), commonly known as the Indian earthworm, is found mainly in parts of Southern Asia, where it lives in burrows made in moist soil [Citation131]. This worm is considered a model with significant experimental advantages in cellular and molecular biology experiments because of its short life cycle and ability to generate transgenic strains [Citation132].
Caenorhabditis elegans (Phylum Nematoda; Class Chromadorea) is naturally found in temperate soil environments [Citation133] and can be easily maintained in the laboratory on agar plates [Citation134]. Caenorhabditis elegans has a short life cycle and can be frozen and stored in liquid nitrogen or at −70 °C, simplifying stock-keeping. Despite its morphological simplicity, C. elegans comprises a diversity of complex organs and tissues like muscles, intestine, hypodermis, and a well-established nervous system present in more complex organisms. Because of the complexity of the nervous system, C. elegans has been used to study a large variety of behaviors and responses [Citation134,Citation135]. Due to its small genome size, C. elegans has widely been used in genetic and molecular research [Citation134]. Its genome has been comprehensively studied and mapped. A recent study revealed that the C. elegans genome possesses homologs of about two-thirds of all human disease genes [Citation136].
2.1.2.2 Veterinary parasites as models for human parasites
As the major helminthic parasites of humans are relatively host-specific and do not efficiently infect laboratory animals [Citation137], helminths of veterinary importance are sometimes used as models in experimental studies of human parasitic diseases. The rodent parasites Heligmosomoides bakeri (also known as H. polygyrus or H. polygyrus bakeri) and the ruminant parasite H. contortus are among this group.
Heligmosomoides bakeri is a nematode that has been used as a laboratory model for intestinal nematode infections for over half a century [Citation138]. This nematode is a convenient and manipulable model for hookworm infections in humans and the economically important trichostrongyloid nematodes of sheep and cattle because of similarities to these parasites in its life cycle [Citation138–140]. Unlike other rodent models, H. bakeri causes chronic infections and provides a useful paradigm for exploring the host-protective mechanisms in the intestinal mucosa [Citation140]. Heligmosomoides bakeri is also helpful in exploring the genetic basis of resistance and immunological aspects of chronic intestinal nematode infections [Citation138].
2.2. Experimental evidence of plant antiparasitic activities
2.2.1 The importance of solvents in the preparation of plant extracts
The discovery of possible plant antiparasitic activities typically begins with extractions containing the active molecule(s). Different solvents are used to separate medicinally active molecule(s) from various plant materials [Citation141]. Generally, the solvents are chosen based on the polarity of the solute of interest; a solvent with similar polarity to the solute will properly dissolve the solute [Citation142]. In the literature, a diverse range of solvents and solvent mixtures such as water (H2O), methanol (MeOH), ethanol (EtOH), hexane (HEX), dichloromethane (DCM), ethyl acetate (EtOAc), chloroform (CF), petroleum ether (PET), and tetrahydrofuran (THF) have been used to obtain crude extracts. Methanol is the most widely used solvent and is highly effective at extracting high polar phytochemicals (e.g. phenolics and alkaloids), resulting in high extraction yields [Citation143].
The bioactivities of plant extracts depend on the solubility of active molecules in the solvent. In some studies, alcohol or other organic extracts were more potent than aqueous extracts of the same concentration. For instance, Eguale et al. [Citation123] found that hydro-alcoholic extract of C. sativum seeds showed better in vitro activity against adult parasites of H. contortus than its aqueous extract. Similarly, among the hot water, cold water and ethanol extracts of Cucurbita pepo (pumpkin) seeds on model nematodes such as C. elegans and H. bakeri, ethanol extracts demonstrated the maximum diversity of active components and the most significant inhibitory effect on egg-hatching [Citation144]. In contrast, the aqueous extracts of the plant Iris kashmiriana (Kashmir iris) exhibited greater anthelmintic activity than its methanolic extracts under both in vitro and in vivo conditions against H. contortus [Citation145]. This study suggested that favorable results were due to water-soluble active ingredients in the plant extract.
In some studies, different solvents have demonstrated variations in phytochemical profiles of the same part of the plant. For example, flavonoids and tannins were the identified phytochemicals from the ethanolic extracts of the stem of Piper betle (betel betle), while only steroids were found in the aqueous extract [Citation146]. The presence of phytochemicals can also vary according to the plant part where they are deposited. Methanolic extracts of A. indica seeds were found to contain azadirachtin M and azadirachtin N [Citation147], while methanolic extracts of its flowers have shown the presence of prenylated flavonoids [Citation148].
2.2.2 Plants with potential antiparasitic activities
Several medicinal plants and isolated secondary metabolites have been screened for antiparasitic activity. A complete list of the plants screened against GI parasites in vitro and in vivo can be found in the supplementary material (Supplementary material Table S1 and S2). In most studies, authors have opted for a serial concentration of plant extracts to test against parasites. lists the in vitro and in vivo measures used to evaluate plant compounds’ efficacy against parasites. Some plants that have demonstrated antiparasitic activity in experimental studies are described below.
Table 3. In vitro and in vivo measures for the efficacy of plant compounds against parasites.
Allium sativum has been shown to possess potential antiparasitic activity against many pathogens, including G. duodenalis, E. histolytica, and H. contortus. The freeze-dried whole A. sativum extract (dissolved in sterile media) has shown inhibitory actions on the in vitro growth of G. duodenalis (IC50 = 0.3 mg/mL) [Citation149]. A clinical study performed on patients suffering from giardiasis showed a rapid reduction of clinical symptoms in the patients treated with A. sativum extract compared to metronidazole [Citation150]. A methanolic extract of A. sativum has shown moderate in vitro antiprotozoal activity against E. histolytica (IC50 = 61.8 µg/mL) [Citation151]. In another in vitro study, the same extract killed 100% of test H. contortus at six hours post-exposure [Citation152].
Zingiber officinale has also exhibited antiparasitic activity against both helminths and protozoa. Methanolic extract of its rhizome killed 100% of H. contortus at two hours post-exposure in an in vitro test [Citation152]. Furthermore, the in vitro worm paralysis rate of its methanolic extract on Ascaridia galli (86% at 100 mg/mL) was similar to that of the control drug, albendazole (87% at 7.5 mg/mL) [Citation153]. The hydro-alcoholic (70% ethanol) extract of Z. officinale led to significantly greater mortality of P. posthuma at 50 mg/mL compared to the control drug, piperazine citrate [Citation154]. The reduction of fecal G. duodenalis cyst counts and trophozoite counts was significantly more significant in the dichloromethane Z. officinale extract-treated albino rats compared to the infected nontreated group [Citation155].
Punica granatum (pomegranate) has demonstrated antiparasitic potential against a wide range of pathogens, including A. galli [Citation156], C. parvum [Citation157], and E. histolytica [Citation151]. The worm mortality of A. galli at 25 and 50 mg/mL of P. granatum peel ethanol extract was significantly different from the control drug, fenbendazole (at 5 mg/mL) [Citation156]. Cryptosporidium parvum infected mice treated with P. granatum peel suspension displayed improvement in all parameters tested [Citation157]. A reduction of oocyst shedding was significantly less in P. granatum treated mice compared to control (untreated) mice by day 14 post-inoculation (pi) (P < 0.5), and fecal oocyst shedding was undetectable by day 28 pi [Citation157]. A methanolic extract of P. granatum was one of the most effective in vitro on E. histolytica among other tested plants with IC50<30 µg/mL [Citation151].
The expected antiparasitic activity of A. indica was investigated by feeding fresh leaves to sheep infected with H. contortus [Citation158]. The total worm count in the control group (n = 3242) was significantly higher than in the treated group (n = 1004). However, fecal egg counts (FEC) showed no significant difference between the control and treated groups (p = 0.081). The study suggested that this result could be due to the high initial egg counts and worm burdens of the animals in the control group [Citation158].
The hydro-alcoholic (70% ethanol) leaf extracts of Anacardium occidentale (cashew tree), Psidium guajava (common guava), Chenopodium ambrosioides (Mexican tea), Stachytarpheta cayennensis (blue snakeweed), and Passiflora edulis (passion fruit) all showed inhibitory effects on the in vitro growth of G. duodenalis trophozoites [Citation159]. The exhibited in vitro giardicidal activity was moderate for A. occidentale and P. guajava (250 ≤ IC50≤500 µg/mL), high for C. ambrosioides and S. cayennensis (100 ≤ IC50≤250 µg/mL); and very high for P. edulis (IC50≤100 µg/mL) [Citation159]. A methanolic extract of C. ambrosioides had a similar in vitro giardicidal activity in a different study (IC50 = 116.10 µg/mL) [Citation151].
Methanolic extracts from Acalypha phleoides (copperleaf), Cnidoscolus tehuacanensis (bad woman), Geranium niveum (geranium), Helianthella quinquenervis (little sunflower) and Teloxys graveolens (fetid goosefoot) were also found to possess in vitro antiprotozoal activity against G. duodenalis protozoa with IC50 values ranging from 2.6 to 20.6 µg/mL [Citation160]. The same plants have shown IC50 values of 4.6 to 13.7 µg/mL against E. histolytica [Citation160].
Calzada et al. [Citation151] tested the in vitro effectiveness of several plants used in traditional Mexican medicine against G. duodenalis and E. histolytica. Methanolic crude extracts of Chiranthodendron pentadactylon (monkey’s hand tree) and Annona cherimola (custard apple) were active against E. histolytica with IC50<30 µg/mL. The study included two positive controls, i.e. metronidazole and emetine, and the potency of C. pentadactylon extract (IC50 2.5 µg/mL) was close to emetine but far less than metronidazole. Methanolic extracts of Dorstenia contrajerva (snakewort), Senna villosa (senna), and Ruta chalepensis (fringed rue) were the most active plants against G. duodenalis trophozoites with IC50<38 µg/mL. The methanolic extracts of C. nucifera, T. vulgaris, and Ocimum basilicum (basil) demonstrated moderate in vitro activity with IC50 values ranging from 44.1 to 99.8 µg/mL for G. duodenalis and from 41.7 to 96.4 µg/mL for E. histolytica [Citation151].
Crude aqueous methanolic extracts of T. portulacastrum and M. paradisiaca have shown significant dose and time-dependent anthelmintic activity in vitro and in vivo [Citation120]. Methanolic M. paradisiaca extract (LC50 = 2.13 µg/mL) was found to be more potent than T. portulacastrum extract (LC50 = 2.41 µg/mL) in the egg hatch test. Both plant extracts exhibited strong in vivo anthelmintic activity compared to the untreated control. However, the in vivo maximum fecal egg count reduction of the treated groups was not significantly different from the levamisole treated group [Citation120].
The in vitro and in vivo anthelmintic activity of the ethanolic extract of C. pepo seeds on A. galli increased with the dose and treatment time. However, its anthelmintic activity was not significantly different from fenbendazole at all concentrations [Citation156].
The in vitro anthelmintic activity of the crude extracts of aerial parts of Cissus quadrangularis (veldt grape) and leaves of Schinus molle (Peruvian pepper) against H. contortus was also dose and time-dependent, with the wormicidal activity of the plant extracts at their highest concentration (10 mg/mL) were 95% and 100%, respectively [Citation161].
2.2.3 Plant combinations for added therapeutic efficacy
In traditional medical systems, plant extracts are often used in combinations. However, the literature has limited evidence for analyzing plant extracts’ combined antiparasitic effect. A study that examined combinations of the plant extracts of Picria fel-terrae (curanja), Linariantha bicolor (true baby-stars) and Lansium domesticum (langsat) against C. elegans in vitro revealed that most combinations significantly reduced the viability and survival of adult worms showing higher nematocidal and anthelminthic activities compared to the control drugs and the individual plant extracts [Citation162]. The combination of hexanic extract of A. sativum and acetone extract of Tagetes erecta (Mexican marigold) caused higher mortality of H. contortus larvae in vitro and a higher reduction of parasite burden in H. contortus infected gerbils than the individual plant extracts [Citation163]. While limited, these studies have shown that combining different plant extracts may be more effective than a sole plant extract.
2.2.4 Plant essential oils exhibited antiparasitic activities
Plant essential oils are biomolecules with biotechnological and pharmaceutical importance, particularly anthelmintic activities [Citation164,Citation165]. These are aromatic, highly volatile, hydrophobic liquids produced by different parts of plants as secondary metabolites [Citation166].
Plant-extracted essential oils from Cymbopogon martinii (palm rose), C. schoenanthus (camel grass), Mentha piperita (mint), P. betle, Syzygium aromaticum (clove), and T. vulgaris have exhibited in vitro effects against different GI parasites ().
Table 4. In vitro screening studies of essential oils against gastrointestinal parasites.
2.2.5 Plant-derived compounds against parasites
In some cases, researchers have isolated the active biomedical compounds of plants that have antiparasitic effects (). The identified metabolites are chemically diverse, comprising different chemical families. Phytochemicals are classified as polyphenols, terpenoids, alkaloids, phytosterols, and organosulfur compounds. Alkaloids, terpenoids, and polyphenols are the major biological compounds with medicinal properties [Citation178]. Matrine, oxymatrine, steroids, polysteroids, withanolides, saponins and sophocarpine are among the alkaloids isolated from plants, while flavonoids, tannin, catechins and phenols are polyphenols, and geraniol and cedrelone are terpenoids.
Table 5. In vitro screening studies of plant-derived compounds against gastrointestinal parasites.
2.2.6 Nanoparticles as antiparasitic treatments
Nanotechnology has opened up novel dimensions in the field of drug discovery research. This technology involves the synthesis and application of materials that range between 1 to 100 nanometers in size [Citation179]. Nanoparticles (NP) are emerging novel drug carriers which, because of their substantial specific surface area and strong adhesion capacity [Citation180], may overcome shortcomings of the current applications of many antiparasitic drugs; low bioavailability, poor cellular permeability, nonspecific distribution and rapid elimination from the body [Citation181,Citation182].
A wide range of plant species has been successfully used in making NPs. Bioactive molecules present in plant extracts, such as alkaloids, phenolic compounds and terpenoids, may act both as reducing agents and stabilizing agents in the synthesis of NPs [Citation183]. Silver (Ag) and gold (Au) NPs have been a specific focus in plant-based syntheses. However, studies evaluating the efficacy of plant-based nanoparticles against intestinal parasites are scarce. Said et al. [Citation184] assessed the antiparasitic potential of curcumin extracted from C. longa and silver, chitosan and curcumin NPs against G. duodenalis in vitro. Curcumin extract had the least trophozoite reduction rate (13.1%), while curcumin NPs had 54.6% trophozoite reduction. A combination of silver, chitosan, curcumin nanoforms gave 100% reduction of trophozoites [Citation184]. Plant-synthesized NPs show great potential to explore in future research.
2.3. Mode of actions of antiparasitic plants
A detailed examination of their mechanism of action is imperative for the potential clinical application of plants or plant-derived products. Studies exploring the mode of action can be complex as they need to assess every stage of the parasite life cycle to understand the therapeutic potential. Although many studies have focused on the efficacy of medicinal plants and their phytochemistry, evaluation of the mode of action has largely been neglected.
Morphological or motility changes may be used to identify the mode of action of plant products [Citation185]. The tegument/cuticle of helminth parasites has been recognized as one of the main target sites for antiparasitic drugs [Citation186,Citation187]. Morphological changes of G. duodenalis trophozoites, including loss of flagellar movement and cell motility, detachment of organisms from the reaction vessel, cell swelling and collapse of the electrochemical membrane potential, were observed after treatment with whole A. sativum extract [Citation149]. Similarly, the essential oil of S. aromaticum induced several morphological changes, including irregular ventral and dorsal surfaces, precipitates and large vacuoles in the cytoplasm, and intracellular and nuclear clearing in G. duodenalis trophozoites [Citation170]. The in vitro use of condensed tannin reduced the survival and development of sheep nematode larvae [Citation119]. Similar findings have been observed when the GI nematode larvae are treated with condensed tannin [Citation188].
The mode of action of plant extracts toward human or animal cells is generally explained through the act of secondary metabolites. These metabolites often modulate a corresponding molecular target in cells, such as proteins, biomembranes or nucleic acids [Citation189], leading to disrupted membrane permeability, neurotoxic activity, or antioxidant activity (.).
Figure 1. Schematic representation of the mechanism of action and different pharmacological targets of plant extracts/compounds.
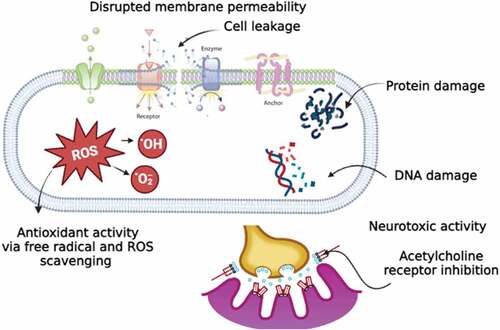
2.3.1 Disrupted membrane permeability
The suggested antiparasitic activity of A. sativum may occur because A. sativum extracts are rapidly permeable through biological membranes and enhance the membrane permeability to small molecules [Citation190]. Allium sativum also can increase intracellular nitric oxide (NO) synthase activity [Citation191]. Nitric oxide has shown cytotoxic effects on G. duodenalis, inhibiting growth and encystation of trophozoites and excystation of cysts [Citation192].
Compounds in essential oils like terpenoids (e.g. thymol and geraniol) or flavonoids may also interfere with membrane permeability by disturbing cellular balance, pH, and inorganic ion balance [Citation193,Citation194]. Likewise, it has been reported that the essential oil geraniol found in O. basilicum, C. sativum and T. vulgaris could damage cell membranes, membrane-bound protein activity, and intracellular signaling pathways [Citation195]. Eugenol-rich essential oil of Ocimum gratissimum (clove basil) has been found to induce swelling of parasite mitochondrial cell membranes with a significant increase in the number of folds in its inner membrane [Citation196].
2.3.2. Neurotoxic activity
Alkaloid compounds in almost all plant families are known neurotoxic agents and act as agonists or antagonists at neuroreceptors and ion channels [Citation197,Citation198]. Alkaloids can inhibit the acetylcholine receptors (AChR) of multicellular parasites leading to muscular paralysis [Citation198]. As a result, parasitic worms attached to the intestinal walls cannot stick to the walls and can be easily removed from the gut [Citation198].
2.3.3 Antioxidant activity
It has been suggested that the nematocidal activity of phenolic compounds such as thymol is possibly derived from their inherent antioxidant properties [Citation199]. Phenolic compounds are classified as primary antioxidants, and they could form a phenoxy radical upon donating a hydrogen atom that results in antioxidant properties through the radical scavenging mechanism [Citation200]. It has been recently demonstrated that the action of thymol against H. contortus is equivalent to that of the anthelmintic drug class macrocyclic lactones [Citation201]. These drugs cause cell hyperpolarisation by enhancing chloride ion reflux, leading to the inhibition of parasite development and larval and adult motility [Citation202,Citation203].
2.4. Limitations of the use of plant-based medicines
Plant-based medicines have promising potential in the prevention and treatment of GI parasitic diseases. However, their application may be limited by bioavailability. The major phytochemicals, including glycosides, tannins and flavonoids, have poor water and lipid solubility, which limits their ability to cross biological membranes, resulting in poor absorption [Citation204]. Additionally, the pharmacokinetics of these compounds can be further modified by the highly acidic gastric pH [Citation205]. In addition, plants are subjected to different procedures such as extraction, distillation, purification, concentration or fermentation to obtain bioactive compounds. During these processes, active components are exposed to oxidation and hydrolysis, raising concerns about their stability [Citation206]. Furthermore, plant products are often prone to deterioration, particularly during storage, leading to the loss of active components and the production of metabolites with no activity [Citation207].
With the increasing global use of plant-based medicines, concerns related to their safety are also increasingly identified. Even though plants have promising potential and are extensively used, many of them remain unproven for their safety or toxicity. This leads to limited knowledge of their potential adverse effects and difficulties in the identification of the safe and most effective therapies [Citation208].
3. Conclusion and future perspectives
This review focused on studies that have evaluated plants and plant derivatives as antiparasitic agents in searching for novel drugs and lead compounds. Plants or their isolates have been tested against almost all common GI parasites, although only a few studies are available on Cryptosporidium species. Overall, the results of these experimental studies present valuable information on bioassays which can help design future studies in terms of methods, doses, and experimental models. This review found plants and plant-derived compounds with significant in vitro and in vivo effects on GI protozoan and helminth parasites. Some plant extracts have shown similar effects to broad-spectrum antiparasitic drugs. The traditional use of plants provides crucial evidence for identifying and developing synergistic drugs; however, this aspect needs to be explored further.
The studies reviewed here encourage further investigation of plants or plant derivatives as potential origins for novel therapies for GI parasitic infections. Most importantly, searching for natural alternatives is critical for parasites like Cryptosporidium spp., which do not have effective chemotherapeutics. However, these studies also point to several areas requiring further information. Most of the studies in the literature invite future research to determine the optimal dose to maximize the effectiveness of the examined plants. Conversion of the in vitro research results into in vivo trials is also essential. Moreover, clinical trials on successful animal experiments are required using the new compounds alone or with established antiparasitic drugs to prove their efficacy and safety. Future research should also focus on exploring the combined effect of plant extracts against parasites. More in-depth studies are required to evaluate the molecular mechanisms of these plant extracts and their bioactive components.
Plant products inspire synthesizing analogues with enhanced pharmacological properties, leading to new drug candidates in the development pipeline. However, many plants with claimed antiparasitic properties have not been reproduced under experimental conditions. Many such unexamined plants may be a source of valuable pharmacologically active substances against parasites and invite future research.
Acknowledgements
The authors acknowledge the financial support from the Murdoch University, Research Training Program (RTP) Scholarship.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- WHO. (2016). Essential nutrition actions: improving maternal, newborn, infant and young child health and nutrition. Retrieved from https://apps.who.int/iris/bitstream/handle/10665/326261/9789241515856-eng.pdf?ua=1
- Mehlhorn H. Encyclopedia of parasitology: volume 1, AM. 3Edn ed. Heidelberg, Germany: Springer Science & Business Media; 2008.
- Roeber F, Jex AR, Gasser RB. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance - an Australian perspective. Parasites & Vectors. 2013a;6(1):153.
- Lane J, Jubb T, Shephard R, et al. Priority list of endemic diseases for the red meat industries. ISBN: 9781741918946: North Sydney, NSW: Meat & Livestock Australia Limited, 2015.
- Charlier J, Rinaldi L, Musella V, et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev Vet Med. 2020;182:105103.
- Mascarini-Serra L. Prevention of soil-transmitted helminth infection. J Glob Infect Dis. 2011;3(2):175–182.
- WHO. (2020). Soil-transmitted helminth infections fact sheet. Retrieved from https://www.who.int/en/news-room/fact-sheets/detail/soil-transmitted-helminth-infections
- De Silva NR, Brooker S, Hotez PJ, et al. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19(12):547–551.
- Ouellette M. Biochemical and molecular mechanisms of drug resistance in parasites. Trop Med Int Health. 2001;6(11):874–882.
- Haque R. Human intestinal parasites. J Health Popul Nutr. 2007;25(4): 387–391. PMID: 18402180.
- Savioli L, Smith H, Thompson A. Giardia and cryptosporidium join the ‘neglected diseases initiative’. Trends Parasitol. 2006;22(5):203–208.
- WHO. (2015). Investing to overcome the global impact of neglected tropical diseases Retrieved from http://apps.who.int/iris/bitstream/handle/10665/152781/9789241564861_eng.pdf;jsessionid=84FC2D4652FC248959D9A5C3CB17086A?sequence=1
- Thompson RCA. Giardiasis as a re-emerging infectious disease and its zoonotic potential. Int J Parasitol. 2000;30(12):1259–1267.
- Lauritz AJ, Jerry WM, David DD, et al. Prevalence of multi-gastrointestinal infections with helminth, protozoan and campylobacter spp. in Guatemalan children. J Infect Developing Countries. 2009;3(03). DOI:10.3855/jidc.41
- Roxström-Lindquist K, Palm D, Reiner D, et al. Giardia immunity–an update. Trends Parasitol. 2006;22(1):26–31.
- Cui Z, Li J, Chen Y, et al. Molecular epidemiology, evolution, and phylogeny of Entamoeba spp. Infect Genet Evol. 2019;75:104018.
- Lozano R, Naghavi M, Foreman K, et al., Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study. Lancet. 2012;380(9859):2095–2128.
- Villanueva MT. Infectious diseases: decrypting cryptosporidium. Nat Rev Drug Discov. 2017;16(8):527.
- Wang R-J, Li J-Q, Chen Y-C, et al. Widespread occurrence of cryptosporidium infections in patients with HIV/AIDS: epidemiology, clinical feature, diagnosis, and therapy. Acta tropica. 2018;187:257–263.
- Xiao L, Alderisio K, Limor J, et al., Identification of species and sources of Cryptosporidium oocysts in storm waters with a small-subunit rRNA-based diagnostic and genotyping tool. Appl environ microbiol. 2000;66(12):5492–5498.
- Adam E, Yoder J, Gould L, et al., Giardiasis outbreaks in the United States, 1971–2011. Epidemiology & Infection. 2016;144(13):2790–2801.
- Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121(S1):S23–38.
- WHO. Soil-transmitted helminthiases: eliminating as public health problem soil-transmitted helminthiases in children: progress report 2001-2010 and strategic plan 2011-2020. In: In. Geneva: World Health Organization; 2012;1.
- Nallam NR, Paul I, Gnanamani G. Anemia and hypoalbuminia as an adjunct to soil-transmitted helminthiasis among slum school children in Visakhapatnam, South India. Asia Pac J Clin Nutr. 1998;7(2):164–169.
- Stephenson LS, Latham MC, Adams EJ, et al. Physical fitness, growth and appetite of Kenyan school boys with hookworm, Trichuris trichiura and ascaris lumbricoides infections are improved four months after a single dose of albendazole. J Nutr. 1993;123(6):1036–1046.
- Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71(5):1280S–1284S.
- Haas JD, Brownlie TT. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131(2): 676S–688S. discussion 688S-690S.
- Cappello M. Global health Impact of soil-transmitted nematodes. Pediatr Infect Dis J. 2004;23(7):663–664.
- Savioli L, Albonico M. Soil-transmitted helminthiasis. Nature Rev Microbiol. 2004;2(8):618–619.
- Kapoor K, Chandra M, Nag D, et al. Evaluation of metronidazole toxicity: a prospective study. Int J Clin Pharmacol. 1999;19(3):83–88.
- Faubert G. Immune response to Giardia duodenalis. Clinical Microbiology Reviews. 2000;13(1):35–54.
- Huang DB, White AC. An updated review oncryptosporidium and Giardia. Gastrointest Endosc Clin N Am. 2006;35(2):291–314.
- Tanyuksel M, Petri WA Jr. Laboratory diagnosis of amebiasis. Clinical Microbiology Reviews. 2003;16(4):713–729.
- Clavel A, Arnal AC, Sánchez EC, et al. Respiratory cryptosporidiosis: case series and review of the literature. Infection. 1996;24(5):341–346.
- López-Vélez R, Tarazona R, Garcia Camacho A, et al. Intestinal and extraintestinal cryptosporidiosis in AIDS patients. Eur J Clin Microbiol& Infect Di. 1995;14(8):677–681.
- Guerrant DI, Moore SR, Lima AA, et al. Association of early childhood diarrhoea and cryptosporidiosis with impaired physical fitness and cognitive function four-seven years later in a poor urban community in northeast Brazil. Am J Trop Med Hyg. 1999;61(5):707–713.
- Checkley W, Epstein LD, Gilman RH, et al. Effects of cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol. 1998;148(5):497–506.
- Perry BD. Investing in animal health research to alleviate poverty. ILRI. Kenya: (aka ILCA and ILRAD). 2002;17.
- Qamar MF, Maqbool A, Khan MS, et al. Epidemiology of haemonchosis in sheep and goats under different managemental conditions. Vet World. 2009;2(11):413–417.
- Sissay MM, Uggla A, Waller PJ. Prevalence and seasonal incidence of nematode parasites and fluke infections of sheep and goats in eastern Ethiopia. Trop Anim Health Prod. 2007;39(7):521–531.
- Abakar A, Osman A. Haematological and biochemical changes following concurrent infections with coccidia and haemonchus contortus in desert lambs. J Anim Vet Adv. 2004;3(10): 643–647. ISSN: 1680-5593.
- Kelkele FA, Tolossa YH, Kassa GM. Experimental infection of Ethiopian highland sheep by different infective doses of haemonchus contortus (L3): haematological and parasitological parameters, serum protein concentrations and clinical responses. Ethiop Vet J. 2012;16(1):41–57.
- Emery DL, Hunt PW, Le Jambre LF. Haemonchus contortus: the then and now, and where to from here? Int J Parasitol. 2016;46(12):755–769.
- Getachew T, Dorchies P, Jacquiet P. Trends and challenges in the effective and sustainable control of haemonchus contortus infection in sheep. review. Parasite. 2007;14(1):3–14.
- McLeod R. Economic impact of worm infections in small ruminants in South East Asia, India and Australia. Worm Control for Small Ruminants in Tropical Asia.2004;23:4 .
- Robertson LJ, Björkman C, Axén C, et al. Cryptosporidiosis in farmed animals. In: Simone M. Cacciò, Giovanni Widmer, editors. Cryptosporidium: parasite and disease. Vienna: Springer; 2014. pp. 149–235.
- Thomson S, Hamilton CA, Hope JC, et al. Bovine cryptosporidiosis: impact, host-parasite interaction and control strategies. Vet Res. 2017;48(1):42.
- Klein P, Kleinová T, Volek Z, et al. Effect of Cryptosporidium parvum infection on the absorptive capacity and paracellular permeability of the small intestine in neonatal calves. Vet Parasitol. 2008;152(1–2):53–59.
- Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clinical Microbiology Reviews. 2011;24(1):110–140.
- Santin M. Cryptosporidium and giardia in ruminants. Vet Clin North Am Large Anim Pract. 2020;36(1):223–238.
- Cacciò SM, Lalle M, Svärd SG. Host specificity in the Giardia duodenalis species complex. Infect Genet Evol. 2018;66:335–345.
- Daugschies A, Najdrowski M. Eimeriosis in cattle: current understanding. J Vet Med B Infect Dis Vet Public Health. 2005;52(10):417–427.
- Williams R. A compartmentalised model for the estimation of the cost of coccidiosis to the world’s chicken production industry. Int J Parasitol. 1999;29(8):1209–1229.
- Blake DP, Knox J, Dehaeck B, et al. Re-calculating the cost of coccidiosis in chickens. Vet Res. 2020;51(1):115.
- Shirley MW, Ivens A, Gruber A, et al. The eimeriagenome projects: a sequence of events. Trends Parasitol. 2004;20(5):199–201.
- WHO. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002a;912:i-vi, 1–57. back cover.
- WHO. (2022). Soil-transmitted helminth infections fact sheet. Retrieved from https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections#
- Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clinical Microbiology Reviews. 2001a;14(1):150–164.
- Chavez MA, White AC. Novel treatment strategies and drugs in development for cryptosporidiosis. Exp Rev Anti-Infective Ther. 2018;16(8):655–661.
- Smith HV, Corcoran GD. New drugs and treatment for cryptosporidiosis. Curr Opin Infect Dis. 2004;17(6):557–564.
- Amadi B, Mwiya M, Sianongo S, et al. High dose prolonged treatment with nitazoxanide is not effective for cryptosporidiosis in HIV positive Zambian children: a randomised controlled trial. BMC Infect Dis. 2009;9(1):195.
- Sparks H, Nair G, Castellanos-Gonzalez A, et al. Treatment of cryptosporidium: what we know, gaps, and the way forward. Curr Trop Med Rep. 2015;2(3):181–187.
- Kotze AC, Prichard RK. Anthelmintic resistance in haemonchus contortus: history, mechanisms and diagnosis. Adv Parasitol. 2016;93:397–428.
- Meganck V, Hoflack G, Opsomer G. Advances in prevention and therapy of neonatal dairy calf diarrhoea: a systematical review with emphasis on colostrum management and fluid therapy. Acta Vet Scand. 2014;56(1):75.
- Kuhlmann FM, Fleckenstein JM. 157 - Antiparasitic Agents. In: Cohen J, Powderly WG, and Opal SM, editors. Infectious diseases (fourth edition). Amsterdam, Netherlands: Elsevier; 2017. pp. 1345–1372.e1342.
- Aguirre-Cruz ML, Valadez-Salazar A, Muñoz O. In vitro sensitivity of Entamoeba histolytica to metronidazole. Arch Invest Med (Mex). 1990;21 Suppl 1:23–26. PMID: 2136490.
- Falagas ME, Walker AM, Jick H, et al. Late incidence of cancer after metronidazole use: a matched metronidazole user/nonuser study. Clinl Infect Dis. 1998;26(2):384–388.
- Voogd CE. On the mutagenicity of nitroimidazoles. Mutat Res. 1981;86(3):243–277.
- Rosenkranz HS, Speck WT. Mutagenicity of metronidazole: activation by mammalian liver microsomes. Biochem Biophys Res Commun. 1975;66(2):520–525.
- Albonico M, Bickle Q, Ramsan M, et al. Efficacy of mebendazole and levamisole alone or in combination against intestinal nematode infections after repeated targeted mebendazole treatment in Zanzibar. Bullet World Health Organ. 2003;81(5): 343–352. PMID: 12856052.
- Albonico M, Smith PG, Hall A, et al. A randomized controlled trial comparing mebendazole and albendazole against Ascaris, Trichuris and hookworm infections. Trans R Soc Trop Med. 1994;88(5):585–589.
- De Clercq D, Sacko M, Behnke J, et al. Failure of mebendazole in treatment of human hookworm infections in the Southern Region of Mali. Am J Trop Med Hyg. 1997;57(1):25–30.
- Reynoldson JA, Behnke JM, Pallant LJ, et al. Failure of pyrantel in treatment of human hookworm infections (Ancylostoma duodenale) in the Kimberley region of north west Australia. Acta tropica. 1997;68(3):301–312.
- Belew S, Getachew M, Suleman S, et al. Assessment of efficacy and quality of two albendazole brands commonly used against soil-transmitted helminth infections in school children in Jimma Town, Ethiopia. PLoS negl trop dis. 2015;9(9):e0004057.
- Krücken J, Fraundorfer K, Mugisha J, et al. Reduced efficacy of albendazole against ascaris lumbricoides in Rwandan schoolchildren. Int J Parasitol: Drugs Drug Resist. 2017;7(3):262–271.
- Kadappu KK, Nagaraja MV, Rao PV, et al. Azithromycin as treatment for cryptosporidiosis in human immunodeficiency virus disease. J Postgrad Med. 2002;48(3):179–181.
- White AC Jr., Chappell CL, Hayat CS, et al. Paromomycin for cryptosporidiosis in AIDS: a prospective, double-blind trial. J Infect Dis. 1994;170(2):419–424.
- Haque Huston CD, Hughes M, Houpt E, et al. Amebiasis. N Engl J Med. 2003;348(16):1565–1573.
- Thompson RC, Reynoldson JA, Mendis AH. Giardia and giardiasis. Adv Parasitol. 1993;32:71–160.
- Bloland PB, World Health Organization. (2001). Drug resistance in malaria. (No WHO/CDS/CSR/DRS/2001.4). World Health Organization.
- Roeber F, Jex AR, Gasser RB. Advances in the diagnosis of key gastrointestinal nematode infections of livestock, with an emphasis on small ruminants. Biotechnol Adv. 2013b;31(8):1135–1152.
- Van den Brom R, Moll L, Borgsteede FH, et al. Multiple anthelmintic resistance ofhaemonchus contortus, including a case of moxidectin resistance, in a Dutch sheep flock. Vet Rec. 2013;173(22):552.
- Wirtherle N, Schnieder T, von Samson-Himmelstjerna G. Prevalence of benzimidazole resistance on horse farms in Germany. Vet Rec. 2004;154(2):39–41.
- Playford M, Smith A, Love S, et al. Prevalence and severity of anthelmintic resistance in ovine gastrointestinal nematodes in Australia (2009–2012). Aust Vet J. 2014;92(12):464–471.
- Drudge JH, Szanto J, Wyant ZN, et al. Field studies on parasite control in sheep: comparison of thiabendazole, ruelene, and phenothiazine. Am J Vet Res. 1964;25:1512–1518.
- Scheuerle MC, Mahling M, Pfister K. Anthelminthika Resistenz von Haemonchus contortus bei kleinen Wiederkäuern in der Schweiz und in Süddeutschland. Wien Klin Wochenschr. 2009;121(S3): 46–49. Suppl.
- Falzon LC, Menzies PI, Shakya KP, et al. Anthelmintic resistance in sheep flocks in Ontario, Canada. Vet Parasitol. 2013;193(1–3):150–162.
- Lyndal-Murphy M, Ehrlich WK, Mayer DG. Anthelmintic resistance in ovine gastrointestinal nematodes in inland Southern Queensland. Aust Vet J. 2014;92(11):415–420.
- Mederos AE, Ramos Z, Banchero GE. First report of monepantel haemonchus contortus resistance on sheep farms inuruguay. Parasites Vectors. 2014;7(1). DOI:10.1186/s13071-014-0598-z
- Dauparaitė E, Kupčinskas T, von Samson-Himmelstjerna G, et al. Anthelmintic resistance of horse strongyle nematodes to ivermectin and pyrantel in Lithuania. Acta Vet Scand. 2021;63(1):5.
- Sargison N, Scott P, Jackson F. Multiple anthelmintic resistance in sheep [3]. Vet Rec. 2001;149(25):778–779.
- Loveridge B, McArthur M, McKenna PB, et al. Probable multigeneric resistance to macrocyclic lactone anthelmintics in cattle in New Zealand. N Z Vet J. 2003;51(3):139–141.
- Mejía ME, Fernández Igartúa BM, Schmidt EE, et al. Multispecies and multiple anthelmintic resistance on cattle nematodes in a farm in Argentina: the beginning of high resistance? Vet Res. 2003;34(4):461–467.
- Lamb J, Elliott T, Chambers M, et al. Broad spectrum anthelmintic resistance ofhaemonchus contortus in Northern NSW of Australia. Vet Parasitol. 2017;241:48–51.
- Upcroft JA, Upcroft P. Drug susceptibility testing of anaerobic protozoa. Antimicrob Agents Chemother. 2001b;45(6):1810–1814.
- Boreham PF, Phillips RE, Shepherd RW. Altered uptake of metronidazole in vitro by stocks of giardia intestinalis with different drug sensitivities. Trans R Soc Trop Med. 1988;82(1):104–106.
- Townson SM, Laqua H, Upcroft P, et al. Induction of metronidazole and furazolidone resistance ingiardia. Trans R Soc Trop Med. 1992;86(5):521–522.
- Lemée V, Zaharia I, Nevez G, et al. Metronidazole and albendazole susceptibility of 11 clinical isolates of giardia duodenalis from France. J Antimicrob Chemother. 2000;46(5):819–821.
- Hasan MM, Stebbins EE, Choy RKM, et al. 2021. Spontaneous selection of cryptosporidium drug resistance in a calf model of infection. Antimicrobial agents and chemotherapyVol. 65 6pp. e00023–00021. 10.1128/AAC.00023-21
- WHO. (2002b). Traditional medicine in Asia (9290222247). Retrieved from https://apps.who.int/iris/bitstream/handle/10665/206025/B0104.pdf?sequence
- Patwardhan B. Bridging ayurveda with evidence-based scientific approaches in medicine. EPMA Journal. 2014;5(1):19–19.
- Spencer JW, Jacobs JJMD. Complementary and alternative medicine: an evidence-based approach. 2nd ed.). St ed. Louis, Mo: Mosby; 2003.
- Sharma PV. editor Caraka samhita of agnivesha (text with english translation) sutra sthana. Ch. XX, VerVols. 1-25. I. Varanasi Chaukhambha Orientalia 1981pp. 137–143
- Mei-Ling S. The contribution of traditional Chinese medicine to modern pharmacology. Trends Pharmacol Sci. 1983;4:496–500.
- Ma C. Treatment methods of traditional chinese medicines against intestinal protozoan infections. In: Mehlhorn H, Wu Z Ye B, editors. Treatment of human parasitosis in traditional Chinese medicine. Berlin Heidelberg: Springer; 2014. pp. 11–21.
- Rahman SZ. Unani medicine in India: its origin and fundamental concepts. History of Sci Phil Cult Indian Civil. 2001;4(Part 2):298–325.
- Ganesan A. The impact of natural products upon modern drug discovery. Curr Opin Chem Biol. 2008;12(3):306–317.
- Gupta R, Gabrielsen B, Ferguson SM. Nature’s medicines: traditional knowledge and intellectual property management case Studies from the National Institutes of Health (NIH), USACase Studies from the National Institutes of Health (NIH), USA. Current Drug Discovery Technologies. 2005;2(4):203–219.
- Krishnamurti C, Rao SC. The isolation of morphine by serturner. Indian J Anaesth. 2016;60(11):861–862.
- Butler MS. Natural products to drugs: natural product-derived compounds in clinical trials. Nat Prod Rep. 2005;22(2):162–195.
- Calzada F, Cervantes-Martinez JA, Yepez-Mulia L. In vitro antiprotozoal activity from the roots of geranium mexicanum and its constituents on Entamoeba histolytica and giardia lamblia. J Ethnopharmacol. 2005;98(1–2):191–193.
- Ferreira LE, Benincasa BI, Fachin AL, et al. Thymus vulgaris L. essential oil and its main component thymol: anthelmintic effects againsthaemonchus contortus from sheep. Vet Parasitol. 2016;228:70–76.
- Ndjonka D, Abladam ED, Djafsia B, et al. Anthelmintic activity of phenolic acids from the axlewood tree anogeissus leiocarpus on the filarial nematode Onchocerca ochengi and drug-resistant strains of the free-living nematode Caenorhabditis elegans. J Helminthol. 2014;88(4):481–488.
- Adegbe A, Larayetan R, Omojuwa T. Proximate analysis, physicochemical properties and chemical constituents characterization of Moringa oleifera (Moringaceae) seed oil using GC-MS analysis. Am J Chem. 2016;6(2):23–28.
- Tomou EM, Chatziathanasiadou MV, Chatzopoulou P, et al. NMR-based chemical profiling, isolation and evaluation of the cytotoxic potential of the diterpenoid siderol from cultivated Sideritis euboea heldr. Molecules. 2020;25(10):2382.
- Coskun O. Separation techniques: chromatography. North Clin Istanb. 2016;3(2):156–160.
- Zhang W, Tang Y, Shi A, et al. Recent developments in spectroscopic techniques for the detection of explosives. Materials. 2018;11(8). DOI:10.3390/ma11081364
- Murthy PK, Joseph SK, Murthy PS. Plant products in the treatment and control of filariasis and other helminth infections and assay systems for antifilarial/anthelmintic activity. Planta Med. 2011;77(6):647–661.
- Athanasiadou S, Githiori J, Kyriazakis I. Medicinal plants for helminth parasite control: facts and fiction. Animal. 2007;1(9):1392–1400.
- Hussain A, Khan MN, Iqbal Z, et al. Anthelmintic activity of trianthema portulacastrum L. and musa paradisiaca L. against gastrointestinal nematodes of sheep. Vet Parasitol. 2011;179(1–3):92–99.
- Nath P, Yadav AK. Anthelmintic activity of a standardised extract from the rhizomes of Acorus calamus Linn.(Acoraceae) against experimentally induced cestodiasis in rats. J Intercultural Ethnopharmacol. 2016;5(4):390.
- Oliveira L, Bevilaqua C, Costa C, et al. Anthelmintic activity ofcocos nucifera L. against sheep gastrointestinal nematodes. Vet Parasitol. 2009;159(1):55–59.
- Eguale T, Getachew T, Debella A, et al. In vitro and in vivo anthelmintic activity of crude extracts of Coriandrum sativum against haemonchus contortus. J Ethnopharmacol. 2007;110(3):428–433.
- Wilke G, Funkhouser-Jones LJ, Wang Y, et al. A stem-cell-derived platform enables complete cryptosporidium development in vitro and genetic tractability. Cell Host Microbe. 2019;26(1):123–134.e128.
- Miller CN, Jossé L, Brown I, et al. A cell culture platform for cryptosporidium that enables long-term cultivation and new tools for the systematic investigation of its biology. Int J Parasitol. 2018;48(3–4):197–201.
- Morada M, Lee S, Gunther-Cummins L, et al. Continuous culture of cryptosporidium parvum using hollow fiber technology. Int J Parasitol. 2016;46(1):21–29.
- Fisher BS, Estraño CE, Cole JA. Modeling long-term host cell-giardia lamblia interactions in an in vitro co-culture system. PLoS ONE. 2013;8(12):e81104.
- Geary TG. Chapter Ten - haemonchus contortus: applications in drug discovery. In: Gasser RB Samson-Himmelstjerna GV, editors Advances in parasitology. Vol. 93. 2016; pp. 429–463. DOI:10.1016/bs.apar.2016.02.013
- Lanusse CE, Alvarez LI, Lifschitz AL. Gaining insights into the pharmacology of anthelmintics using Haemonchus contortus as a model nematode. Adv Parasitol. 2016;93:465–518.
- Das SS, Dey M, Ghosh AK. Determination of anthelmintic activity of the leaf and bark extract of Tamarindus indica linn. Indian J Pharm Sci. 2011;73(1):104–107.
- Verma MK, Xavier F, Verma YK, et al. Evaluation of cytotoxic and anti-tumor activity of partially purified serine protease isolate from the Indian earthworm Pheretima posthuma. Asian Pac J Tropical Biomedicine. 2013;3(11):896–901.
- Teschendorf D, Link CD. What have worm models told us about the mechanisms of neuronal dysfunction in human neurodegenerative diseases? Mol Neurodegener. 2009;4(1):38.
- Stiernagle T. Maintenance of C. elegans. in. Minneapolis: caenorhabditis. USA: Genetics Center, University of Minnesota; 2006. pp. 51–67.
- Bürglin TR, Lobos E, Blaxter ML. Caenorhabditis elegans as a model for parasitic nematodes. Int J Parasitol. 1998;28(3):395–411.
- Cook SJ, Jarrell TA, Brittin CA, et al. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature. 2019;571(7763):63–71.
- Qin Z, Johnsen R, Yu S, et al. Genomic identification and functional characterization of essential genes in Caenorhabditis elegans. G3. 2018;8(3):981–997.
- Maizels RM, Bundy DA, Selkirk ME, et al. Immunological modulation and evasion by helminth parasites in human populations. Nature. 1993;365(6449):797–805.
- Behnke JM, Menge DM, Noyes H. Heligmosomoides bakeri: a model for exploring the biology and genetics of resistance to chronic gastrointestinal nematode infections. Parasitology. 2009;136(12):1565–1580.
- Behnke J, Harris PD. Heligmosomoides bakeri: a new name for an old worm? Trends Parasitol. 2010;26(11):524–529.
- Monroy FG, Enriquez FJ. Heligmosomoides polygyrus: a model for chronic gastrointestinal helminthiasis. Parasitology Today. 1992;8(2):49–54.
- Handa S. An overview of extraction techniques for medicinal and aromatic plants. Extract Technol for Med Aro Plants. 2008;1:21–40.
- Dai J, Mumper RJ. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15(10):7313–7352.
- Truong D-H, Nguyen DH, Ta NTA, et al. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of severinia buxifolia. J Food Qual. 2019;2019:8178294.
- Grzybek M, Kukula-Koch W, Strachecka A, et al. Evaluation of anthelmintic activity and composition of pumpkin (Cucurbita pepo L.) seed extracts—in vitro and in vivo studies. Int J Mol Sci. 2016;17(9):1456.
- Khan A, Tak H, Nazir R, et al. In vitro and in vivo anthelmintic activities of Iris kashmiriana Linn. J Saudi Soc Agri Sci. 2018;17(3):235–240.
- Adate PS, Parmesawaran S, Chauhan Y. In vitro anthelmintic activity of stem extracts of Piper betle Linn. against Pheretima posthuma. Pharmacogn J. 2012;4(29):61–65.
- Luo X, Ma Y, Wu S, et al. Two novel azadirachtin derivatives from Azadirachta indica. J Natural Prod. 1999;62(7):1022–1024.
- Nakahara K, Roy MK, Ono H, et al. Prenylated flavanones isolated from flowers of Azadirachta indica (the neem tree) as antimutagenic constituents against heterocyclic amines. J Agric Food Chemistry. 2003;51(22):6456–6460.
- Harris JC, Plummer S, Turner MP, et al. The microaerophilic flagellate giardia intestinalis: allium sativum (garlic) is an effective antigiardial. Microbiology. 2000;146(12): 3119–3127. Pt.
- Soffar SA, Mokhtar GM. Evaluation of the antiparasitic effect of aqueous garlic (Allium sativum) extract in hymenolepiasis nana and giardiasis. J Egypt Soc Parasitol. 1991;21(2):497–502.
- Calzada F, Yepez-Mulia L, Aguilar A. In vitro susceptibility of Entamoeba histolytica and giardia lamblia to plants used in Mexican traditional medicine for the treatment of gastrointestinal disorders. J Ethnopharmacol. 2006;108(3):367–370.
- Iqbal Z, Nadeem QK, Khan M, et al. In vitro anthelmintic activity of allium sativum, Zingiber officinale, curcurbita mexicana and ficus religiosa. Int J Agric Biol. 2001;3(4):454–457.
- Bazh EK, El-Bahy NM. In vitro and in vivo screening of anthelmintic activity of ginger and curcumin on ascaridia galli. Parasitol Res. 2013;112(11):3679–3686.
- Singh R, Mehta A, Mehta P, et al. Anthelmintic activity of rhizome extracts of curcuma longa and Zingiber officinale (Zingiberaceae). Int J Pharm Pharm Sci. 2011;3(2):236–237.
- Mahmoud A, Attia R, Said S, et al. Ginger and cinnamon: can this household remedy treat giardiasis? Parasitological and histopathological studies. Iran j parasitol. 2014;9(4):530–540.
- Abdel Aziz AR, AbouLaila MR, Aziz M, et al. In vitro and in vivo anthelmintic activity of pumpkin seeds and pomegranate peels extracts against Ascaridia galli. Beni-Suef Univ J Basic Appl Sci. 2018;7(2):231–234.
- Al-Mathal EM, Alsalem AM. Pomegranate (Punica granatum) peel is effective in a murine model of experimental cryptosporidium parvum. Exp Parasitol. 2012;131(3):350–357.
- Chandrawathani P, Chang KW, Nurulaini R, et al. Daily feeding of fresh neem leaves (Azadirachta indica) for worm control in sheep. Trop Biomed. 2006;23(1):23.
- Neiva VDA, Ribeiro MNS, Nascimento FRF, et al. Plant species used in giardiasis treatment: ethnopharmacology and in vitro evaluation of anti-giardia activity. Revista Brasileira de Farmacognosia. 2014;24(2):215–224.
- Calzada F, Alanis AD, Meckes M, et al. In vitro susceptibility of Entamoeba histolytica and giardia lamblia to some medicinal plants used by the people of Southern Mexico. Phytother Res. 1998;12(1):70–72.
- Zenebe S, Feyera T, Assefa S. In vitro anthelmintic activity of crude extracts of aerial parts of cissus quadrangularis l. and leaves of Schinus molle l. against haemonchus contortus. Bio Med Res Int. 2017:6 2017. 10.1155/2017/1905987
- Kumarasingha R, Palombo EA, Bhave M, et al. Enhancing a search for traditional medicinal plants with anthelmintic action by using wild type and stress reporter Caenorhabditis elegans strains as screening tools. Int J Parasitol. 2014;44(5):291–298.
- Palacio-Landin J, Mendoza-de Gives P, Salinas-Sanchez DO, et al. In vitro and in vivo nematocidal activity of allium sativum and tagetes erecta extracts against haemonchus contortus. Turk parazitolojii derg. 2015;39(4):260–264.
- Hashmi LSA, Hossain MA, Weli AM, et al. Gas chromatography-mass spectrometry analysis of different organic crude extracts from the local medicinal plant of Thymus vulgaris L. Asian Pac J Tropical Biomedicine. 2013;3(1):69–73.
- Staniek A, Bouwmeester H, Fraser PD, et al. Natural products - modifying metabolite pathways in plants. Biotechnol J. 2013;8(10):1159–1171.
- Russo R, Corasaniti MT, Bagetta G, et al. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid Based Complement Alternat Med. 2015;2015:397821.
- Katiki LM, Chagas AC, Bizzo HR, et al. Anthelmintic activity of Cymbopogon martinii, Cymbopogon schoenanthus and Mentha piperita essential oils evaluated in four different in vitro tests. Vet Parasitol. 2011;183(1–2):103–108.
- Kumaran AM, D’souza P, Agarwal A, et al. Geraniol, the putative anthelmintic principle of Cymbopogon martinii. Phytother Res. 2003;17(8):957.
- Garg SC, Jain R. Biological activity of the essential oil of piper betle L. J Essent Oil Res. 1992;4(6):601–606.
- Machado M, Dinis AM, Salgueiro L, et al. Anti-giardia activity of Syzygium aromaticum essential oil and eugenol: effects on growth, viability, adherence and ultrastructure. Exp Parasitol. 2011;127(4):732–739.
- Behnia M, Haghighi A, Komeylizadeh H, et al. Inhibitory effects of Iranian thymus vulgaris extracts on in vitro growth of Entamoeba histolytica. Korean J Parasitol. 2008;46(3):153–156.
- Katiki LM, Ferreira JFS, Gonzalez JM, et al. Anthelmintic effect of plant extracts containing condensed and hydrolyzable tannins on Caenorhabditis elegans, and their antioxidant capacity. Vet Parasitol. 2013;192(1):218–227.
- Domingues LF, Giglioti R, Feitosa KA, et al. In vitro and in vivo evaluation of the activity of pineapple (Ananas comosus) on haemonchus contortus in Santa ines sheep. Vet Parasitol. 2013;197(1–2):263–270.
- Jin Z, Ma J, Zhu G, et al., Discovery of novel anti-cryptosporidial activities from natural products by in vitro high-throughput phenotypic screening. Front Microbiol. 10: 1999. 2019; 10.3389/fmicb.2019.01999.
- Chaweeborisuit P, Suriyonplengsaeng C, Suphamungmee W, et al. Nematicidal effect of plumbagin on Caenorhabditis elegans: a model for testing a nematicidal drug. Z Naturforsch C J Biosci. 2016;71(5–6):121–131.
- Luo YP, Zhang Y, Zhang HM, et al. Anti-parasitic effects of water-soluble alkaloid fractions from ethanolic extracts of Sophora moorcroftiana seeds in Caenorhabditis elegans. Chin J Nat Med. 2018;16(9):665–673.
- Arrieta J, Reyes B, Calzada F, et al. Amoebicidal and giardicidal compounds from the leaves of Zanthoxylum liebmannianun. Fitoterapia. 2001;72(3):295–297.
- Lesney M. Nature’s pharmaceuticals natural products from plants remain at the core of modern medicinal chemistry. Todays Chemist at Work. 2004;13(7):26–33.
- Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–71.
- Wagner V, Dullaart A, Bock A-K, et al. The emerging nanomedicine landscape. Nature Biotechnol. 2006;24(10):1211–1217.
- Müller RH, Mäder K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur J Pharm Biopharm. 2000;50(1):161–177.
- Xie S, Tao Y, Pan Y, et al. …Biodegradable nanoparticles for intracellular delivery of antimicrobial agents. JControlled Release. 2014;187:101–117.
- Kumar V, Yadav SK. Plant‐mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol Int J Environ Sci Technol. 2009;84(2):151–157.
- Said D, Elsamad L, Gohar Y. Validity of silver, chitosan, and curcumin nanoparticles as anti-giardia agents. Parasitol Res. 2012;111(2):545–554.
- Monzote L, Alarcon O, Setzer NW. Antiprotozoal activity of essential oils. Agric Conspec Sc. 2012;77(4):167–169.
- Mehlhorn H, Becker B, Andrews P, et al. In vivo and in vitro experiments on the effects of praziquantel on Schistosoma mansoni. A light and electron microscopic study. Arzneimittelforschung. 1981;31(3a):544–554.
- Mottier L, Alvarez L, Ceballos L, et al. Drug transport mechanisms in helminth parasites: passive diffusion of benzimidazole anthelmintics. Exp Parasitol. 2006;113(1):49–57.
- Molan AL, Hoskin SO, Barry TN, et al. Effect of condensed tannins extracted from four forages on the viability of the larvae of deer lungworms and gastrointestinal nematodes. Vet Rec. 2000;147(2):44–48.
- Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines (Basel). 2015;2(3):251–286.
- Bogin E (1973). Studies on the effects of antibacterial compounds from garlic on biological membranes. Paper presented at the proceedings of the international congress of Biochem (9th), Vienna. p. 271.
- Das I, Khan NS, Sooranna SR. Potent activation of nitric oxide synthase by garlic: a basis for its therapeutic applications. Curr Med Res Opin. 1995;13(5):257–263.
- Eckmann L, Laurent F, Langford TD, et al. Nitric oxide production by human intestinal epithelial cells and competition for arginine as potential determinants of host defence against the lumen-dwelling pathogen Giardia lamblia. J Immunol. 2000;164(3):1478–1487.
- Carnesecchi S, Langley K, Exinger F, et al. Geraniol, a component of plant essential oils, sensitizes human colonic cancer cells to 5-fluorouracil treatment. J Pharmacol Exp Ther. 2002;301(2):625–630.
- Lambert RJ, Skandamis PN, Coote PJ, et al. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J Appl Microbiol. 2001;91(3):453–462.
- Lei J, Leser M, Enan E. Nematicidal activity of two monoterpenoids and SER-2 tyramine receptor of Caenorhabditis elegans. Biochem Pharmacol. 2010;79(7):1062–1071.
- Ueda-Nakamura T, Mendonça-Filho RR, Morgado-Díaz JA, et al. Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum. Parasitol Int. 2006;55(2):99–105.
- Wink M. Interference of alkaloids with neuroreceptors and ion channels. In: Atta-ur-Rahman, editor. Studies in natural products chemistry. Vol. 21. Amsterdam, Netherlands: Elsevier; 2000. pp. 3–122.
- Wink M, Schimmer O. Molecular modes of action of defensive secondary metabolites. Fun Biotech Plant Sec Met. 2018;39:21–161.
- Ali HM, Abo-Shady A, Sharaf Eldeen HA, et al. Structural features, kinetics and SAR study of radical scavenging and antioxidant activities of phenolic and anilinic compounds. Chem Cent J. 2013;7(1): 53-53. DOI:10.1186/1752-153X-7-53
- Kiokias S, Varzakas T, Oreopoulou V. In vitro activity of vitamins, flavonoids, and natural phenolic antioxidants against the oxidative deterioration of oil-based systems. Crit Rev Food Sci Nutr. 2008;48(1):78–93.
- Lynagh T, Cromer BA, Dufour V, et al. Comparative pharmacology of flatworm and roundworm glutamate-gated chloride channels: implications for potential anthelmintics. Int J Parasitol: Drugs Drug Resist. 2014;4(3):244–255.
- Kotze AC, Dobson RJ, Tyrrell KL, et al. High-level ivermectin resistance in a field isolate ofhaemonchus contortus associated with a low level of resistance in the larval stage: implications for resistance detection. Vet Parasitol. 2002;108(3):255–263.
- Wolstenholme AJ. Ion channels and receptor as targets for the control of parasitic nematodes. Int J Parasitol Drugs Drug Resist. 2011;1(1):2–13.
- Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747.
- Gao S, Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev Med Chem. 2010;10(6):550–567.
- Rangari VD. Pharmacognosy & Phytochemistry. Vol. 1. India: Career publications; 2009.
- Thakur L, Ghodasra U, Patel N, et al. Novel approaches for stability improvement in natural medicines. Pharmacogn Revi. 2011;5(9):48.
- World Health Organization. (2002). Traditional medicine in asia (No. Regional Publication No. 39). WHO Regional Office for South-East Asia.

