ABSTRACT
Tumor-associated macrophages (TAMs) play an important role in tumor progression, suppression of antitumor immunity and dissemination. Blood monocytes infiltrate the tumor region and are primed by local microenvironmental conditions to promote tumor growth and invasion. Although many of the interacting cytokines and factors are known for the tumor-macrophage interactions, the putative contribution of circulating tumor cells (CTCs) is not known so far. These specialized cells are characterized by increased mobility, ability to degrade the extracellular matrix (ECM) and to enter the blood stream and generate secondary lesions which is a leading cause of death for the majority of tumor patients. The first establishment of two permanent CTC lines, namely BHGc7 and 10, from blood samples of advanced stage small cell lung cancer (SCLC) patients allowed us to investigate the CTC-immune cell interaction. Cocultures of peripheral blood mononuclear cells (PBMNCs) with CTCs or addition of CTC-conditioned medium (CTC-CM) in vitro resulted in monocyte-macrophage differentiation and appearance of CD14+, CD163weak and CD68+ macrophages expressing markers of TAMs. Furthermore, we screened the supernatants of CTC-primed macrophages for presence of approximately 100 cytokines and compared the expression with those induced by the local metastatic SCLC26A cell line. Macrophages recruited by SCLC26A-CM showed expression of osteopontin (OPN), monocyte chemoattractant protein-1 (MCP-1), IL-8, chitinase3-like 1 (CHI3L1), platelet factor (Pf4), IL-1ra and matrix metalloproteinase-9 (MMP-9) among other minor cytokines/chemokines. In contrast, BHGc7-CM induced marked overexpression of complement factor D (CFD)/adipsin and vitamin D-BP (VDBP), as well as increased secretion of OPN, lipocalin-2 (LCN2), CHI3L1, uPAR, MIP-1 and GDF-15/MIC-1. BHGc10, derived independently from relapsed SCLC, revealed an almost identical pattern with added expression of ENA-78/CXCL5. CMs of the non-tumor HEK293 cell line revealed no induction of macrophages, whereas incubation of PBMNCs with recombinant CHI3L1 gave positive results. Thus, the specific contributions of CTCs in SCLC affect CFD/adipsin, possibly involved in immunity/cachexia, VDBP which gives rise to group-specific component protein-derived macrophage-activating factor (GcMAF), GDF-15/MIC-1 which enhances the malignant phenotype of tumor cells and ENA-78/CXCL5 which attracts angiogenic neutrophils. In conclusion, CTCs are competent to specifically manipulate TAMs to increase invasiveness, angiogenesis, immunosuppression and possibly lipid catabolism.
Introduction
Metastatic disease is the major cause of cancer death and the SCLC variant of lung tumors is distinguished by early dissemination and poor survival rates.Citation1,2 Despite excellent initial responses to platinum-based chemotherapy, SCLC recurs within approximately one year as chemoresistant tumor, not amenable to effective further treatment.Citation3 Furthermore, this malignancy exhibits comparatively high numbers of CTCs, an underlying cause of early tumor spread.Citation4 It is increasingly clear that tumor-immune effector cell interactions enhance tumor growth and invasion as well as local immunosuppression in order to escape from antitumor immune responses and to achieve effective extravasation at prospective metastatic sites.Citation5 Thus cancers and, possibly, CTCs recruit immunosuppressive cells, particularly belonging to the myeloid-macrophage lineage, which undergo functional polarization in dependence of tumor-derived factors.Citation6 These “tumor-educated” macrophages promote invasion, intravasation as well as survival in the circulation and durable growth at secondary lesions.Citation5,7 TAMs are recruited by various cytokines and chemokines, suppress the activity of cytotoxic T-lymphocytes via programmed cell death 1 ligand 1 (PD-L1) or B7-H4 and other receptors/mediators.Citation7,8 The mechanisms by which macrophages acquire prometastatic abilities have not been fully characterized.
The processes associated with tumor spread could not be studied in detail as cells determined to disseminate the tumor, namely CTCs, are scarce in blood and could not be kept and expanded in tissue culture except for one case of a colon CTC line and several breast cancer CTC lines, established recently.Citation9,10 We were able to set up two permanent CTC lines from SCLC patients with extended disease, allowing us for the first time to investigate markers, kinases, secreted cytokines/chemokines and proteases of pure SCLC CTCs in detail in vitro.Citation11 Experiments showed that both cell lines were effective to induce monocyte-macrophage differentiation in vitro in coculture or by exposing PBMNCs to conditioned media (CM) derived from the CTC lines. In the present work, we studied the CTC-induced macrophages in respect to secreted cytokines/chemokines which are expected to be involved in promoting invasion/extravasation of CTCs in SCLC. Since we have previously found similar features of SCLC CTCs with other tumors, such as highly malignant glioblastoma, the findings reported here may potentially hold true for other malignancies as well.Citation12
Results
CTC-induced monocyte-macrophage differentiation and recruitment
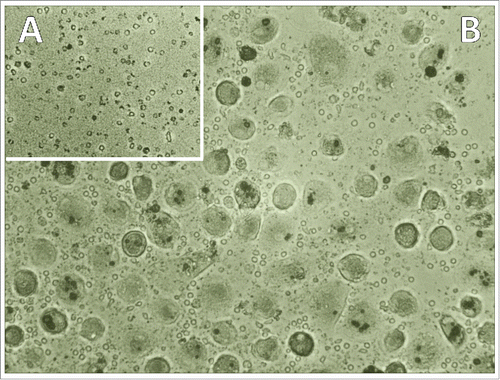
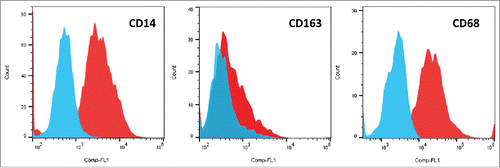
Application of CTC cell lines conditioned medium (CM) to isolated PBMCs in tissue culture medium for 10 d resulted in appearance of numerous macrophages, whereas in basic medium controls only some residual lymphocytes and cellular debris were still detectable (.). Similar experiments using CM of the local metastatic SCLC26A cell line showed analogous monocyte-macrophage induction but at a much lower frequency (data not shown). Flow cytometric analysis of the detached cells showed significant expression of CD14 as marker of monocytes/macrophages and weak expression of the CD163, a member of the B scavenger receptor cysteine-rich superfamily, as well as strong expression of CD68/macrosialin (.). Staining of cells with antibodies to immune checkpoint proteins revealed low expression of CD274 (B7-H1/PD-L1) and low or absent staining of B7-H4 (ratios of relative fluorescence for antibody/isotype control: 2.0 ± 0.49 for PD-L1 and 1.12 ± 0.9 for B7-H4 (n.s.), respectively). The two CTC cell lines, BHGc7 and BHGc10, express low amounts of CD274/PD-L1 and lack B7-H4 (data not shown).
Secreted cytokines by SCLC26A cell line- and BHGc7 CTC-CM-induced macrophages
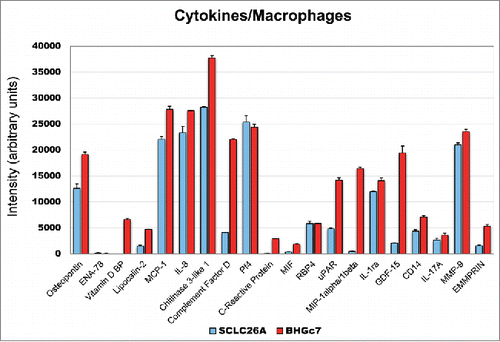
Preincubation of PBMNCs with SCLC26A-CM resulted in differentiation of monocytes to macrophages which secreted OPN, MCP-1, IL-8, CHI3L1, Pf4, IL-1ra and MMP-9, with minor amounts of LPC2, CFD, RBP4, uPAR, GDF-15, soluble CD14, IL-17A and EMMPRIN/CD147 (). In contrast, preincubation of PBMNCs with BHGc7-CM induced newly secretion of vitamin D BP (VDBP), C-reactive protein (CRP), MIP-1 and caused marked overexpression of OPN, LPC2, CFD, uPAR and GDF-15 concomitant with elevated expression of MCP-1, IL-8, CHI3L1, IL-1ra, CD14, MMP-9 and EMMPRIN/CD147.
Figure 3. Expression of cytokines/chemokines of SCLC26A and BHGc7-primed macrophages. The figure shows the significantly expressed cytokines/chemokines expressed by macrophages induced by preincubation with CM of SCLC26A and BHBc7 CTC line, respectively (mean ± SD). All differences are statistically significant, except for PF4, RBP4 and IL-17A.
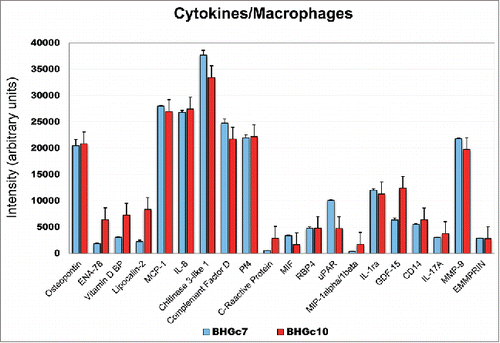
Secreted cytokines by BHGc10- and BHGc7 CTC-induced macrophages
Cytokines/chemokines secreted by CTC-induced macrophages were compared for two different donors of PBMNCs following preincubation with tissue culture supernatants of BHGc7 and BHGc10, respectively (). This comparison using different preparations of PBMNCs from experiments described above and the BHGc10 CTC line CM yielded an almost identical pattern of the cytokines/chemokines secreted by the CTC-induced macrophages, except for higher quantities of ENA-78, VDBP, LPC2 and GDF-15. Comparison of BHGc7 and BHGc10 employing the same PBMNCs revealed increased secretion of ENA-78, VDBP, CDF and GDF-15 by BHGc10-primed macrophages (data not shown).
Figure 4. Expression of cytokines/chemokines of SCLC26A and BHGc7-primed macrophages. The figure shows the significantly expressed cytokines/chemokines expressed by macrophages induced by preincubation with CM of BHGc7 and BHBc10 CTC lines, respectively (mean ± SD). All differences are statistically non-significant, except for ENA-78, VDBP, LPC2, CRP, uPAR, MIP-1 and GDF-15.
Induction of monocyte-macrophage differentiation by chitinase-3-like-1 (CHI3L1)
In controls, PBMNCs from two healthy donors were incubated with CM of human embryonic kidney 293 (HEK293) cells for up to 10 d. At this time point cultures contained isolated residual monocytes and showed no signs of monocyte-macrophage differentiation, as observed in case of the SCLC CTC lines (). In contrast, exposure of the same PBMNC preparations to 2 ng/mL recombinant CHI3L1 revealed development of macrophages within 7–10 d of initiation of this experiment ().
Figure 5. Exposure of PBMNCs to control HEK293-CM and recombinant CHI3L1. Incubation of normal PBMNCs with HEK-293 CM resulted in attachment of residual monocytes (A) in contrast to exposure with 2 ng/mL recombinant CHI3l1 which resulted in appearance of differentiated macrophages (B).
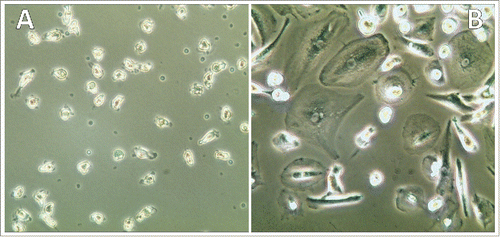
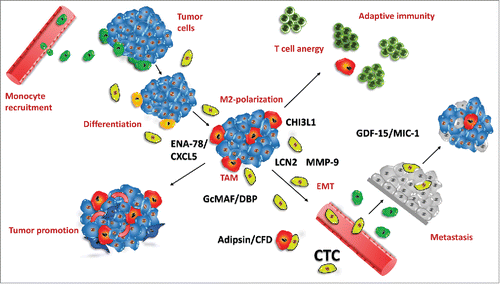
Figure 6. Tumor-associated macrophages and CTCs in tumor biology. This figure depicts the role and factors contributed by CTCs in tumor-macrophage interactions. Peripheral blood monocytes are recruited to the developing tumor (green; top, left) und undergo differentiation/polarization (yellow) under the influence of tumor-derived factors to TAMs of M2-like type (red) which results in suppression of immune responses (top, right) and enhanced neoangiogenesis/tumor progression (bottom, left). Inflammatory and tumor-derived cytokines/chemokines induce precursors of CTCs (light green) which secrete additional cytokines/chemokines, comprising ENA-78/CXCL5, LNC2, CHI3L1, CFD/adipsin, VDBP/GcMAF and MMP-9. Increased neoangiogenesis and degradation of ECM by MMP-9 and possibly other proteases induce intravasation of CTCs, most likely after EMT. At distant sites (bottom, right) CTCs extravasate capillaries and set up secondary lesions, possibly protected against immune system attack by CTC-educated macrophages. Highly effective recruitment of macrophages seems to be linked to CHI3L1 expression of CTCs as SCLC tumor cells lack expression of this pseudochitinase. CFD/adipsin may be associated with cachexia in advanced disease.
Discussion
The cellular elements of tumors can include immune cells, such as infiltrating lymphocytes, natural killer (NK) cells, macrophages, dendritic cells, eosinophils, mast cells and myeloid-derived suppressor cells which express a multitude of mediators such as cytokines, chemokines, growth factors and enzymes.Citation13 TAMs are local macrophages recruited to solid tumors which rather promote than suppress tumor progression.Citation7,14 Their infiltrate, which may be as great as half of the tumor mass, results from the so-called “cancer education” provoked by specific microenvironmental conditions.Citation15 These processes are reminiscent of a role of TAMs in tissue-repair in normal organs, employing neoangiogenesis, induction of trophic signals, tissue remodeling and immunosuppression.Citation7 Macrophages are divided into different phenotypes such as M1/M2 and macrophages with regulatory properties.Citation7 TAMs skewed toward an M2-altered functional profile play a crucial role in immune evasion within tumors. These cells are marked by the expression of CD11b, CD14, CD33 and CD68 in humans and production of lower levels of proinflammatory cytokines, such as IL-1β, TNF-α and IL-12 but higher levels of immunosuppressive mediators, such as IL-10, TGF-β and VEGF.Citation16 Accordingly, M2 macrophages with their suppressive function form about 70% of TAM populations in NSCLC and promote angiogenesis and release IL-10.Citation15 The detailed investigation of TAMs would require samples from resected tumors but only a minority of NSCLC and hardly any SCLC cases are resectable.Citation17 Bronchoalveolar lavage can partially substitute as source for the examination of cellular and humoral immune responses. Data on TAMs in SCLC are lacking and, moreover, putative contributions of CTCs at the site of their formation as well as in the vicinity of extravasation sites are not known for any kind of tumors. We have recently established the first two permanent CTC lines from different SCLC patients with advanced disease, which express typical characteristics of these tumors, and used these lines to investigate markers, secreted cytokines/chemokines, tyrosine kinases and proteases expressed (manuscripts submitted).Citation11,12 Experiments suggested marked effects of these CTC lines on PBMNCs which were studied in the present work.
Secretory phenotype of SCLC CTC-induced macrophages
The two SCLC CTC lines were found to induce monocyte-macrophage differentiation upon coculture with PBMNCs or preincubation of these blood cells with CTC-CM with high efficacy compared to CM from the local metastatic SCLC26A line. The resulting macrophages are CD14-positive and express PD-L1 and low levels of B7-H4. In detail, the phenotype of the macrophages was further characterized using antibodies to CD163, a member of the B scavenger receptor cysteine-rich superfamily and CD68/macrosialin, respectively. CD163 is a highly specific marker which is expressed primarily by M2-polarized macrophages, related to dissemination and poor prognosis.Citation18 CD68, the human homolog of macrosilin, is a pan-macrophage marker which is widely used to identify TAMs in diagnostic biopsy samples.Citation19 CD68 is a 110 kD glycoprotein, predominately expressed in cytoplasmic granules of monocytes/macrophages, dendritic cells, and granulocytes. Thus, macrophages detected in cocultures of the SCLC CTCs stain positively for CD68 and weakly for CD163, typical markers of TAMs in cancer infiltration by immune cells. It is known that MCP-1/CCL2 recruits cognate receptor-positive CCR2+ blood monocytes to tumors where they undergo a specific maturation pathway to TAMs by distinct tumor type-specific microenvironmental factors.Citation7,20 Since TAMs release a large amount of inflammatory mediators to create an corresponding environment which promotes tumor growth we screened the CTC-induced macrophage supernatants for presence of over 100 cytokines/chemokines.
The present work indicates that SCLC26A-induced macrophages express higher levels of OPN, MCP-1, IL-8, CHI3L1, CFD, Pf4, RBP4, IL-1ra and MMP-9. OPN expressed by macrophages has been implicated in cytokine expression, phagocytosis and migration.Citation21 Furthermore, OPN is an independent predictor of tumor recurrence and survival in patients with NSCLC and promotes tumorigenicity and clonogenicity of colorectal CSCs.Citation22,23 OPN positivity was around 10% in SCLC and 70% in NSCLC and chemoresistance in NSCLC seems to correlate with higher expression this protein.Citation24,25 MCP-1 expression has been observed in both infiltrating macrophages and tumor cells as significant indicator of early relapse.Citation26 Furthermore, cocultures of macrophages with lung cancer cell lines revealed upregulation of MCP-1/CCR2 in both cell types.Citation27 Of the interleukins found, TAM-derived IL-8 was reported to induce EMT of hepatocellular carcinoma cells via activation of the JAK2/STAT3/Snail pathway.Citation28 The pseudochitinase CHI3L1/YKL-40 was found in cancers and chronic inflammatory diseases where it was strongly expressed by malignant cells and infiltrating macrophages.Citation29,30 CHI3L3 was described as typical marker of M2 macrophages in mice.Citation31 According to our results, CHI3L1 pseudochitinase is the corresponding counterpart in humans where it constitutes an important regulator of inflammation, angiogenesis and M2 macrophage differentiation in addition to its expression by SCLC CTCs.Citation12,32 Specifically, M2b macrophages are activated by immune complexes, toll-like receptor-positive lymphocytes, or IL-1ra.Citation33 Pf4/CXCL4 has been demonstrated to prevent monocyte apoptosis and to promote macrophage differentiation from peripheral blood monocytes.Citation34,35 Pf4/CXCL4-induced polarization of macrophages as found in atherosclerosis is distinct from the classical M1 and M2 phenotypes and was therefore designated M4.Citation35 Specific binding of Pf4/CXCL4, resulting in the downregulation of the IL-2-release, correlated with the inhibition of activated T cells.Citation36 Analysis of malignant pleural effusions demonstrated elevated levels of proangiogenic factors VEGF-A, PF4/CXCL4 and MMP-8.Citation37 MMP-2 and MMP-9 secreted by M2 TAMs degrade the matrix and promote tumor cell invasion.Citation7 Retinol-binding protein 4 (RBP4) is an adipokine which appears during monocytes-macrophage differentiation and is highest in differentiated macrophages.Citation38 CTC-induced macrophages lack expression of TNFα, a mediator which is typical for M1 macrophages [data not shown].
Several macrophage-derived factors, such as ENA-78, CFD, VDBP, MIP-1 and GDF-15, are specifically overinduced by CTC-CMs but not by the SCLC26A local metastatic control SCLC line. The chemokine CXCL5, which is produced in response to inflammatory cytokines IL-1 or TNFα, is also known as epithelial-derived neutrophil-activating peptide 78 (ENA-78).Citation39 CXCL5 stimulates the chemotaxis of neutrophils possessing angiogenic properties and has been implicated in connective tissue remodeling, tumor growth, migration and invasion.Citation40,41
Besides regulation of the coagulation system, complement proteins stimulate cancer invasion through enhanced EMT, degradation of ECM by proteases such as MMP-9 and induction of chemotactic stimuli and growth factors.Citation42–44 CFD is essential for alternative pathway activation and was found to be identical to the adipokine adipsin which is expressed in monocytes/macrophages.Citation45,46 Since the CTC lines were established from patients with advanced SCLC characterized by large number of these cells in the circulation, CFD may be involved in cancer-associated weight loss. Cancer cachexia is a devastating syndrome that affects around half of all lung cancer patients but the underlying mechanisms have yet to be fully elucidated.Citation47 Cachexia comprises weight loss from skeletal muscle and body fat as well as inflammation.Citation48 Cytokines, such as TNFα (also termed cachectin), IFNγ, adipsin, and IL-1/IL-6 play a role in cachectic processes in addition to other cytokines and hormones.Citation49–51 Adipsin is a novel serine protease which modulates expression of other adipocyte-specific RNAs and is implicated in both obesity and cachexia.Citation52,53 The biological activities of the adipsin include reduction of elevated free fatty acid levels, increased fatty acid oxidation in muscle cells and weight reduction.Citation54
The functions of VDBP are still being defined, but they include the transport of vitamin D in the circulation and a role as the precursor of the group-specific component protein-derived macrophage-activating factor (GcMAF) which is derived by modification of VDBP in its carbohydrate moieties by β-galactosidase from B lymphocytes or sialidase from T lymphocytes.Citation55 MAFs are lymphokines involved in cytotoxicity of macrophages to tumors.Citation56,57 GcMAF directly inhibits proliferation, migration, and uPAR expression of prostate cancer cells.Citation58 However, the enzyme α-N-acetylgalactosaminidase which is produced by cancer cells deactivates this factor and facilitates spread of tumors.Citation59 Lipocalin-2 (LCN2) is an adipokine/cytokine implicated in obesity and inflammation. A variety of malignant tumors consistently overexpress LCN2, frequently associated with tumor size, stage and invasiveness.Citation60 LCN2 plays an important role in promoting cell migration and invasion in cooperation with MMP-9 and by inducing EMT through the ERK/SLUG axis.Citation61 Growth differentiation factor 15 (GDF-15/MIC-1) belongs to the TGF-β superfamily and regulates inflammatory and apoptotic pathways in inflammation, cancer and obesity and was associated with aberrant growth and a poor prognosis.Citation62,63 Incubation of blood monocyte-derived macrophages with CM of an esophageal squamous cell carcinomas cell line induced M2 polarization and overexpression of GDF-15 as well as IL-6 and IL-8.Citation64 Macrophage Inflammatory Proteins (MIPs) belong to the family of chemotactic cytokines and the two major forms are MIP-1α/CCL3 and MIP-1β/CCL4. They activate human granulocytes (neutrophils, eosinophils and basophils) which can lead to acute neutrophilic inflammation.Citation65
The unique functional properties of TAMs are directed by tumor-derived signals which promote each step of the metastatic cascade and thus are novel targets for therapy.Citation5,7 TAMs even change their phenotype to help extravasation, survival and subsequent growth of tumor cells at secondary sites.Citation16,66 High TAM content is generally correlated with poor prognosis.Citation67 The present data indicate that CTCs in SCLC recruit macrophages through monocyte differentiation with high efficacy.Citation6 Comparison of the density of monocytes in control HEK293-CM treated PBMNCs with recombinant CHI3L1 exposed cells indicated that most of the monocytes differentiate to macrophages. The HEK293 cell line was selected since it expresses no significant amounts of GM-CSF and G-CSF, in good correspondence with the two CTC lines (R&D cytokine array; data not shown). In contrast to HEK293 cells, the two CTC lines express CHI3L1 at concentrations of approximately 2 ng/mL (R&D CHI3L1 ELISA) and supplementation of this protein induces monocyte-macrophage differentiation in PBMNC cultures. Other cytokines found in supernatants of the two CTC cell lines but not in HEK293 and possibly involved in monocyte-macrophage differentiation comprise IL-4, IL-5, pentraxin-3 (PTX-3) and VEGF. Citation68-70
Recruitment of macrophages may be less important during intravasation where the nearby tumor supports proinvasive factors but of significant advantage at the site of extravasation to enhance degradation of tissue components and to provide protection from immune defense.Citation71 CHI3L1 was reported to promote macrophage recruitment and angiogenesis in colorectal cancer.Citation72 High expression of CHI3L1 may be involved in monocyte-macrophage differentiation and polarization in protumor effectors of M2/M4-like effectors.Citation73 The role of VDBP is not clear as its derivative GcMAF should possess antitumor effects. MIP-1 and ENA-78/CCL5 seem to attract proinflammatory cells and overexpression of the adipokines LNC2 and CFD may be involved in cachexia in advanced metastatic disease in presence of a large number of CTCs. In conclusion, in SCLC CTCs seem to recruit and “educate” a specific type of macrophages operative in invasion, immune protection, establishment of a favorable extravasation site and possibly cachexia. Thus, the well-established role of CTCs has to be extended to specific effects on monocyte-macrophage differentiation and specific priming, possibly overlapping with the cancer stem cell characteristics.Citation74-76
Materials and methods
Cell lines and culture conditions
SCLC26A was established in our laboratory from pleural effusion of a SCLC patient before treatment and the two CTC cell lines, BHGc7 and BHGc10, were grown from peripheral blood samples of two refractory SCLC patients. Cell lines were cultured in RPMI-1640 (Sigma-Aldrich, St.Louis, MO, USA) medium supplemented with 10% fetal bovine serum (Seromed, Berlin, Germany) and antibiotics (Sigma-Aldrich, penicillin-streptomycin-neomycin solution). All cell lines grow in suspension or loosely attached and were regularly subcultivated by partial replacement of medium.
Induction of monocyte-macrophage differentiation
Normal PBMCs from four healthy volunteers were prepared using Ficoll-Paque densitiy gradient centrifugation and distributed to 75 cm2 tissue culture flasks (TPP, Trasidingen, Switzerland). Media were supplemented with 20% CM from SCLC26A or BHGc7/10 CTC cell lines, respectively. Flasks were incubated for 10 d under tissue culture conditions, then medium was aspirated, the cells washed and covered with 10 mL fresh medium. After further incubation for 3 d media were harvested and immediately used for screening of cytokines.
Flow cytometry
For analysis of cell surface markers, macrophages were detached using exposure to calcium- and magnesium-free phosphate buffered saline (Ca−/Mg− PBS; Life Technologies, Paisley, UK) and cell scrapers (TPP). Antibodies used were directed to CD14 (clone 63D3), CD163 (GHI/61), CD68 (Y1/82A), B7-H1 (29E.2A3) and B7-H4 (MIH43), respectively (Biolegend, San Diego, CA, USA). Anti-mouse-FITC labeled was used for indirect immunofluorescence (Sigma-Aldrich) and isotype controls employed from our collection of hybridomas. For cytoplasmatic staining of CD68, cells were fixed in 4% paraformaldehyde.
Western blot cytokine screening array
For assessment of the cytokines/chemokines expressed, cell culture supernatants were processed using the Human Proteome Profiler Cytokine XL Kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). In brief, this Western blot array comprises reagents to detect 102 cytokines (http://www.rndsystems.com/Products/ARY022, accessed 5/04/2015). Experiments were done in duplicate and the different arrays contain several control spots to calibrate for protein content of the samples applied. CM of the respective cell lines (500 µL) were used for performing the assay and the spots detected by chemoluminescence were analyzed using Gelanalyzer,Citation77 ImageJ and Origin 9.0 software (OriginLab, Northampton, MA, USA).
Statistics
Results were evaluated using unpaired t tests using Origin 9.0 software. p < 0.05 was regarded as statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed
Acknowledgments
The authors would like to thank the Molecular Oncology Unit of the Department of Gynecology, Medical University of Vienna for their logistics support.
References
- Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015; 121:664–72; PMID:25336398; http://dx.doi.org/10.1002/cncr.29098
- Pillai RN, Owonikoko TK. Small cell lung cancer: therapies and targets. Semin Oncol 2014; 41:133–42; PMID:24565587; http://dx.doi.org/10.1053/j.seminoncol.2013.12.015
- Kalemkerian GP. Advances in pharmacotherapy of small cell lung cancer. Expert Opin Pharmacother 2014; 15:2385–96; PMID:25255939; http://dx.doi.org/10.1517/14656566.2014.957180
- Yu N, Zhou J, Cui F, Tang X. Circulating tumor cells in lung cancer: detection methods and clinical applications. Lung. 2015; 193:157–71; PMID:25690734; http://dx.doi.org/10.1007/s00408-015-9697-7
- Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol 2015; 15:73–86; PMID:25614318; http://dx.doi.org/10.1038/nri3789
- Jiang WG, Sanders AJ, Katoh M, Ungefroren H, Gieseler F, Prince M, Thompson SK, Zollo M, Spano D, Dhawan P et al. Tissue invasion and metastasis: Molecular, biological and clinical perspectives. Semin Cancer Biol. 2015 Dec; 35:S244-75; PMID:25865774; http://dx.doi.org/10.1016/j.semcancer.2015.03.008
- Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol 2015; 36:229–239; PMID:25770924; http://dx.doi.org/10.1016/j.it.2015.02.004
- Butt AQ, Mills KH. Immunosuppressive networks and checkpoints controlling antitumor immunity and their blockade in the development of cancer immunotherapeutics and vaccines. Oncogene 2014; 33:4623–31; PMID:24141774; http://dx.doi.org/10.1038/onc.2013.432
- Cayrefourcq L, Mazard T, Joosse S, Solassol J, Ramos J, Assenat E, Schumacher U, Costes V, Maudelonde T, Pantel K, Alix-Panabières C. Establishment and characterization of a cell line from human circulating colon cancer cells. Cancer Res 2015; 75:892–901; PMID:25592149; http://dx.doi.org/10.1158/0008-5472.CAN-14-2613
- Yu M, Bardia A, Aceto N et al. Cancer therapy. Ex vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. Science 2014; 345:216–20; PMID:25013076; http://dx.doi.org/10.1126/science.1253533
- Hamilton G, Burghuber O, Zeillinger R. Circulating Tumor Cells in Small Cell Lung Cancer: Ex Vivo Expansion. Lung. 2015; 193:451–2; PMID:25821178; http://dx.doi.org/10.1007/s00408-015-9725-7
- Hamilton G, Rath B, Burghuber O. Chitinase-3-like-1/YKL-40 as marker of circulating cancer cells. Transl Lung Cancer Res 2015; 4:287–91; PMID:26207216; http://dx.doi.org/10.3978/j.issn.2218-6751.2015.04.04.
- Kerkar SP, Restifo NP. Cellular constituents of immune escape within the tumor microenvironment. Cancer Res 2012; 72:3125–30; PMID:22721837; http://dx.doi.org/10.1158/0008-5472.CAN-11-4094
- Lievense LA, Bezemer K, Aerts JG, Hegmans JP. Tumor-associated macrophages in thoracic malignancies. Lung Cancer 2013; 80:256–62; PMID:23489559; http://dx.doi.org/10.1016/j.lungcan.2013.02.017
- Domagala-Kulawik J, Osinska I, Hoser G. Mechanisms of immune response regulation in lung cancer. Transl Lung Cancer Res 2014; 3:15–22; PMID:25806277; http://dx.doi.org/10.3978/j.issn.2218-6751.2013.11.03.
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010; 141:39–51; PMID:20371344; http://dx.doi.org/10.1016/j.cell.2010.03.014
- Domagała-Kulawik J, Guzman J, Costabel U. Immune cells in bronchoalveolar lavage in peripheral lung cancer–analysis of 140 cases. Respiration 2003; 70:43–8; PMID:12584390; http://dx.doi.org/10.1159/000068414
- Tang X. Tumor-associated macrophages as potential diagnostic and prognostic biomarkers in breast cancer. Cancer Lett 2013; 332:3–10; PMID:23348699; http://dx.doi.org/10.1016/j.canlet.2013.01.024
- Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 2012; 12:306; PMID:22824040; http://dx.doi.org/10.1186/1471-2407-12-306
- Franklin RA, Li MO. The ontogeny of tumor-associated macrophages: a new understanding of cancer-elicited inflammation. Oncoimmunology 2014; 3:e955346; PMID:25941613; http://dx.doi.org/10.4161/21624011.2014.955346
- Rittling SR. Osteopontin in macrophage function. Expert Rev Mol Med 2011; 13:e15; PMID:21545755; http://dx.doi.org/10.1017/S1462399411001839
- Li Y, Sun BS, Pei B, Li CG, Zhang ZF, Yin YS, Wang CL. Osteopontin-expressing macrophages in non-small cell lung cancer predict survival. Ann Thorac Surg 2015; 99:1140–8; PMID:25725928; http://dx.doi.org/10.1016/j.athoracsur.2014.11.054
- Rao G, Du L, Chen Q. Osteopontin, a possible modulator of cancer stem cells and their malignant niche. Oncoimmunology 2013; 2:e24169; PMID:23762797; http://dx.doi.org/10.4161/onci.24169
- Guldur ME, Kibar Y, Deniz H, Bakir K. Comparison of osteopontin, beta-catenin and hnRNP B1 expression in lung carcinomas. Pathol Oncol Res 2010; 16:55–9; PMID:19609729; http://dx.doi.org/10.1007/s12253-009-9187-4
- Zhang J, Takahashi K, Takahashi F, Shimizu K, Ohshita F, Kameda Y, Maeda K, Nishio K, Fukuchi Y. Differential osteopontin expression in lung cancer. Cancer Lett 2001; 171:215–22; PMID:11520606; http://dx.doi.org/10.1016/S0304-3835(01)00607-3
- Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clin Cancer Res 2000; 6:3282–9; PMID:10955814
- Schmall A, Al-Tamari HM, Herold S, Kampschulte M, Weigert A, Wietelmann A, Vipotnik N, Grimminger F, Seeger W, Pullamsetti SS, Savai R. Macrophage and cancer cell cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving lung cancer. Am J Respir Crit Care Med 2015; 191:437–47; PMID:25536148; http://dx.doi.org/10.1164/rccm.201406-1137OC
- Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ, Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, Fan J. Macrophage-secreted IL-8 induces epithelial-mesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway. Int J Oncol 2015; 46:587–96; PMID:25405790; http://dx.doi.org/10.3892/ijo.2014.2761.
- Shao R. YKL-40 acts as an angiogenic factor to promote tumor angiogenesis. Front Physiol 2013; 4:122; PMID:23755018; http://dx.doi.org/10.3389/fphys.2013.00122
- Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights 2007; 2:128–46; PMID:19662198
- Ishii M, Wen H, Corsa CA, Liu T, Coelho AL, Allen RM, Carson WF 4th, Cavassani KA, Li X, Lukacs NW, Hogaboam CM, Dou Y, Kunkel SL. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood 2009; 114:3244–54; PMID:19567879; http://dx.doi.org/10.1182/blood-2009-04-217620
- Lee CG, Dela Cruz CS, Herzog E, Rosenberg SM, Ahangari F, Elias JA. YKL-40, a chitinase-like protein at the intersection of inflammation and remodeling. Am J Respir Crit Care Med 2012; 185(7):692–4; PMID:22467800; http://dx.doi.org/10.1164/rccm.201202-0203ED
- Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012; 2012:948098; PMID:22778768; http://dx.doi.org/10.1155/2012/948098
- Scheuerer B, Ernst M, Dürrbaum-Landmann I, Fleischer J, Grage-Griebenow E, Brandt E, Flad HD, Petersen F. The CXC-chemokine platelet factor 4 promotes monocyte survival and induces monocyte differentiation into macrophages. Blood 2000; 95:1158–66; PMID:10666185
- Gleissner CA. Macrophage Phenotype Modulation by CXCL4 in Atherosclerosis. Front Physiol 2012; 3:1; PMID:22275902; http://dx.doi.org/10.3389/fphys.2012.00001
- Fleischer J, Grage-Griebenow E, Kasper B, Heine H, Ernst M, Brandt E, Flad HD, Petersen F. Platelet factor 4 inhibits proliferation and cytokine release of activated human T cells. J Immunol 2002; 169:770–7; PMID:12097379; http://dx.doi.org/10.4049/jimmunol.169.2.770
- Lieser EA, Croghan GA, Nevala WK, Bradshaw MJ, Markovic SN, Mansfield AS. Up-regulation of pro-angiogenic factors and establishment of tolerance in malignant pleural effusions. Lung Cancer 2013; 82:63–8; PMID:23948549; http://dx.doi.org/10.1016/j.lungcan.2013.07.007
- Broch M, Ramírez R, Auguet MT, Alcaide MJ, Aguilar C, Garcia-Espana A, Richart C. Macrophages are novel sites of expression and regulation of retinol binding protein-4 (RBP4). Physiol Res 2010; 59:299–303; PMID:19537932
- Shiels MS, Pfeiffer RM, Hildesheim A, Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE, Pinto LA, Chaturvedi AK. Circulating inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst. 2013; 105:1871–80; PMID:24249745; http://dx.doi.org/10.1093/jnci/djt309
- Arenberg DA, Keane MP, DiGiovine B, Kunkel SL, Morris SB, Xue YY, Burdick MD, Glass MC, Iannettoni MD, Strieter RM. Epithelial-neutrophil activating peptide (ENA-78) is an important angiogenic factor in non-small cell lung cancer. J Clin Invest 1998; 102:465–72; PMID:9691082; http://dx.doi.org/10.1172/JCI3145
- Xia J, Xu X, Huang P, He M, Wang X. The potential of CXCL5 as a target for liver cancer - what do we know so far? Expert Opin Ther Targets 2015; 19:141–6; PMID:25495348; http://dx.doi.org/10.1517/14728222.2014.993317
- Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Parsa AT. Cancer and the complement cascade. Mol Cancer Res 2010; 8:1453–65; PMID:20870736; http://dx.doi.org/10.1158/1541-7786.MCR-10-0225
- Sayegh ET, Bloch O, Parsa AT. Complement anaphylatoxins as immune regulators in cancer. Cancer Med 2014; 3:747–58; PMID:24711204; http://dx.doi.org/10.1002/cam4.241
- Brade V, Kreuzpaintner G. Functional active complement components secreted by guinea pig peritoneal macrophages. Immunobiology 1982; 161:315–21; PMID:7047377; http://dx.doi.org/10.1016/S0171-2985(82)80088-0
- Pio R, Corrales L, Lambris JD. The role of complement in tumor growth. Adv Exp Med Biol 2014; 772:229–62; PMID:24272362; http://dx.doi.org/10.1007/978-1-4614-5915-6_11
- White RT, Damm D, Hancock N, Rosen BS, Lowell BB, Usher P, Flier JS, Spiegelman BM. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem 1992; 267:9210–3; PMID:1374388
- Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014; 14:754–62; PMID:25291291; http://dx.doi.org/10.1038/nrc3829
- Johns N, Stephens NA, Fearon KC. Muscle wasting in cancer. Int J Biochem Cell Biol 2013; 45:2215–29; PMID:23770121; http://dx.doi.org/10.1016/j.biocel.2013.05.032
- Dalamaga M. Interplay of adipokines and myokines in cancer pathophysiology: Emerging therapeutic implications. World J Exp Med 2013; 3:26–33; PMID:24520543; http://dx.doi.org/10.5493/wjem.v3.i3.26
- Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 2009; 89:381–410; PMID:19342610; http://dx.doi.org/10.1152/physrev.00016.2008
- Reife CM. Involuntary weight loss. Med Clin North Am 1995; 79:299–313; PMID:7877392
- Min HY, Spiegelman BM. Adipsin, the adipocyte serine protease: gene structure and control of expression by tumor necrosis factor. Nucleic Acids Res. 1986 Nov 25; 14(22):8879–92; PMID:3024123
- Guerre-Millo M. Adipose tissue hormones. J Endocrinol Invest. 2002 Nov; 25(10):855–61; PMID:12508947 http://dx.doi.org/10.1007/BF03344048
- Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, White T, Spiegelman BM. Adipsin and complement factor D activity: an immune-related defect in obesity. Science 1989; 244:1483–7; PMID:2734615; http://dx.doi.org/10.1126/science.2734615
- Malik S, Fu L, Juras DJ, Karmali M, Wong BY, Gozdzik A, Cole DE. Common variants of the vitamin D binding protein gene and adverse health outcomes. Crit Rev Clin Lab Sci 2013; 50:1–22; PMID:23427793; http://dx.doi.org/10.3109/10408363.2012.750262
- Thyer L, Ward E, Smith R, Fiore MG, Magherini S, Branca JJ, Morucci G, Gulisano M, Ruggiero M, Pacini S. A novel role for a major component of the vitamin D axis: vitamin D binding protein-derived macrophage activating factor induces human breast cancer cell apoptosis through stimulation of macrophages. Nutrients 2013; 5:2577–89; PMID:23857228; http://dx.doi.org/10.3390/nu5072577
- Thyer L, Ward E, Smith R, Branca JJ, Morucci G, Gulisano M, Noakes D, Eslinger R, Pacini S. GC protein-derived macrophage-activating factor decreases α-N-acetylgalactosaminidase levels in advanced cancer patients. Oncoimmunology 2013; 2:e25769; PMID:24179708; http://dx.doi.org/10.4161/onci.25769
- Gregory KJ, Zhao B, Bielenberg DR, Dridi S, Wu J, Jiang W, Huang B, Pirie-Shepherd S, Fannon M. Vitamin D binding protein-macrophage activating factor directly inhibits proliferation, migration, and uPAR expression of prostate cancer cells. PLoS One 2010; 5:e13428; PMID:20976141; http://dx.doi.org/10.1371/journal.pone.0013428
- Ghanei M, Shohrati M, Saburi A. The new aspects of immunotherapy in prostate cancer. Cancer Immunol Immunother. 2012; 61:2375–6; PMID:22736256; http://dx.doi.org/10.1007/s00262-012-1309-2
- Lippi G, Meschi T, Nouvenne A, Mattiuzzi C, Borghi L. Neutrophil gelatinase-associated lipocalin in cancer. Adv Clin Chem 2014; 64:179–219; PMID:24938019; http://dx.doi.org/10.1016/B978-0-12-800263-6.00004-5
- Ding G, Fang J, Tong S, Qu L, Jiang H, Ding Q, Liu J. Over-expression of lipocalin 2 promotes cell migration and invasion through activating ERK signaling to increase SLUG expression in prostate cancer. Prostate 2015 Feb 25; 75(9):957–68; PMID: 25728945; http://dx.doi.org/10.1002/pros.22978
- Corre J, Hébraud B, Bourin P. Concise review: growth differentiation factor 15 in pathology: a clinical role? Stem Cells Transl Med 2013; 2:946–52; PMID:24191265; http://dx.doi.org/10.5966/sctm.2013-0055
- Unsicker K, Spittau B, Krieglstein K. The multiple facets of the TGF-β family cytokine growth/differentiation factor-15/ macrophage inhibitory cytokine-1. Cytokine Growth Factor Rev 2013; 24:373–84; PMID:23787157; http://dx.doi.org/10.1016/j.cytogfr.2013.05.003
- Urakawa N, Utsunomiya S, Nishio M, Shigeoka M, Takase N, Arai N, Kakeji Y, Koma Y, Yokozaki H. GDF15 derived from both tumor-associated macrophages and esophageal squamous cell carcinomas contributes to tumor progression via Akt and Erk pathways. Lab Invest 2015; 95:491–503; PMID:25730371; http://dx.doi.org/10.1038/labinvest.2015.36
- Baay M, Brouwer A, Pauwels P, Peeters M, Lardon F. Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin Dev Immunol 2011; 2011:565187; PMID:22162712; http://dx.doi.org/10.1155/2011/565187
- Montuenga LM, Pio R. Tumour-associated macrophages in nonsmall cell lung cancer: the role of interleukin-10. Eur Respir J 2007; 30:608–10; PMID:17906081; http://dx.doi.org/10.1183/09031936.00091707
- Quatromoni JG, Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am J Transl Res 2012; 4:376–89; PMID:23145206
- Sousa S, Brion R, Lintunen M, Kronqvist P, Sandholm J, Mönkkönen J, Kellokumpu-Lehtinen PL, Lauttia S, Tynninen O, Joensuu H et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res 2015; 17:101; PMID:26243145; http://dx.doi.org/10.1186/s13058-015-0621-0
- Locatelli M, Ferrero S, Martinelli Boneschi F, Boiocchi L, Zavanone M, Maria Gaini S, Bello L, Valentino S, Barbati E, Nebuloni M et al. The long pentraxin PTX3 as a correlate of cancer-related inflammation and prognosis of malignancy in gliomas. J Neuroimmunol 2013; 260:99–106; PMID:23664694; http://dx.doi.org/10.1016/j.jneuroim.2013.04.009
- Honda T, Inagawa H, Yamamoto I. Differential expression of mRNA in human monocytes following interaction with human colon cancer cells. Anticancer Res. 2011; 31:2493–7; PMID:21873165
- Spary LK, Salimu J, Webber JP, Clayton A, Mason MD, Tabi Z. Tumor stroma-derived factors skew monocyte to dendritic cell differentiation toward a suppressive CD14+ PD-L1+ phenotype in prostate cancer. Oncoimmunology 2014; 3:e955331; PMID:25941611; http://dx.doi.org/10.4161/21624011.2014.955331
- Kawada M, Seno H, Kanda K, Nakanishi Y, Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E, Chiba T. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene 2012; 31:3111–23; PMID:22056877; http://dx.doi.org/10.1038/onc.2011.498
- Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24:207–12; PMID:22236695; http://dx.doi.org/10.1016/j.coi.2011.12.009
- Paterlini-Bréchot P. Circulating Tumor Cells: Who is the Killer? Cancer Microenviron 2014; 7:161–76; PMID:25527469; http://dx.doi.org/10.1007/s12307-014-0164-4
- Hou JM, Krebs M, Ward T, Sloane R, Priest L, Hughes A, Clack G, Ranson M, Blackhall F, Dive C. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol 2011; 178:989–96; PMID:21356352; http://dx.doi.org/10.1016/j.ajpath.2010.12.003
- Yamashina T, Baghdadi M, Yoneda A, Kinoshita I, Suzu S, Dosaka-Akita H, Jinushi M. Cancer stem-like cells derived from chemoresistant tumors have a unique capacity to prime tumorigenic myeloid cells. Cancer Res 2014; 74:2698–709; PMID:24638980; http://dx.doi.org/10.1158/0008-5472.CAN-13-2169
- Lazar I, Lazar I. Gel Analyzer 2010a: Freeware 1D gel electrophoresis image analysis software, 2010. http://www.gelanalyzer.com, accessed on 3rd March 2015.