ABSTRACT
Molting is an essential developmental process for the majority of animal species on Earth. During the molting process, which is a specialized form of extracellular matrix (ECM) remodeling, the old apical ECM, or cuticle, is replaced with a new one. Many of the genes and pathways identified as important for molting in nematodes are highly conserved in vertebrates and include regulators and components of vesicular trafficking, steroid-hormone signaling, developmental timers, and hedgehog-like signaling. In this review, we discuss what is known about molting, with a focus on studies in Caenorhabditis elegans. We also describe the key structural elements of the cuticle that must be released, newly synthesized, or remodeled for proper molting to occur.
Introduction
The majority of animal species undergo molts during their life cycles. This includes all arthropods (insects, arachnids, and crustaceans), nematodes (roundworms), and other members of the Ecdysozoa superphylum.Citation1,2 Molting is the process by which animals replace their old exoskeleton, also termed the cuticle, with a new one. Depending on the species and the stage of development, molting is essential for normal growth or developmental progression, or for both processes. Molting is regulated by a combination of genetic and environmental factors and must be closely coordinated with other developmental events. Interest in the mechanisms controlling molting is due in part to the significant medical and economic consequences caused by parasitic Ecdysozoa species. As such, agents that interfere with molting, such as drugs or pesticides, have been a focus of pharmacological research.Citation3-6 In addition, the analysis of molting provides a good opportunity for understanding a variety of conserved developmental processes. For example, molting can reveal details about ECM remodeling, developmental timing, steroid hormone functions, signal transduction pathways, intracellular trafficking, and other fundamental cellular mechanisms that are evolutionarily conserved. Moreover, molting can be studied in well-developed and streamlined genetic systems such as the fruit fly, Drosophila melanogaster, and the roundworm, Caenorhabditis elegans.
Functions of molting
Although molting is required for development in a wide range of species, its primary functions are likely to vary across different groups of animals. In arthropods, which contain a rigid chitin-based cuticle, molting is essential for body size expansion. In the case of most insects, molting occurs prior to adulthood, whereas crustaceans can undergo periodic molts, coupled with continued growth, as adults. In contrast to arthropods, the nematode body is covered with a collagen-based cuticle that allows growth and expansion between molting periods. In fact, some authors reject the premise that molting is required for growth in nematodes, especially in parasitic species.Citation7,8 This is supported by the observation that infective L2 larvae of plant parasites from the nematode genus Meloidogyne can grow substantially before reaching the L3 molt.Citation9,10 Moreover, the L3 and L4 stages in Meloidogyne occur without intervening feeding or growth. In such cases, molting may be important for the development of anatomical structures in the mouth region, which are required for normal feeding and growth at the adult stage.
Another example in which molting is not essential for nematode growth was observed in the parasite species Ascaris lumbricoides. The adult animal increases in length from 1–2 cm after the final molt to 25–35 cm after living inside the host's intestine for several months.Citation11,12 Furthermore, the cuticle becomes thicker as the animal grows during its adult life, suggesting that, in at least some nematode species, cuticle components are actively synthesized after the final molt.Citation12 Based on these observations in parasitic species, a proposed function of nematode molting is to change the surface composition and structure of the organism to best match the different environments that it inhabits at different life stages.Citation7 Interestingly, all members of the Nematode phylum undergo four larval molts, regardless of whether or not they are parasitic or free-living.
In the case of many free-living nematodes, such as C. elegans, their ecological niche is unlikely to change dramatically during the course of their life cycle. Given this, environmental adaptation may not be a driving force behind molting or changes in cuticle composition in many free-living nematode species. Rather, molting may serve as a means for body-size expansion and may trigger periods of rapid growth. Consistent with this, C. elegans growth rates, based on volume, are linear between molting cycles but are exponential during the periods surrounding molts.Citation13 Thus, slow linear growth during each larval stage may be permitted by the elastic properties of the cuticle, whereas rapid exponential growth may be enabled by the process of molting. Consistent with this model, the new cuticle is initially highly convoluted after each molt in C. elegans, suggesting that it has a larger surface area than the previous cuticle and may therefore accommodate a rapid increase in size at the end of each molt.Citation14,15 This accordion-like structure of the nascent cuticle is facilitated by the presence of regularly spaced circumferential actin bundles, which reside at the apical epidermal surface during molting periods. These actin bundles may serve as attachment points for the new cuticle during synthesis, thereby allowing for the addition of excess cuticle material at the folds in between the bundles.Citation14
Although molting may serve primarily to promote the rapid growth of free-living nematodes, it is notable that the structural composition of the cuticle does vary somewhat between larval stages, with distinct types of collagens being expressed at specific life stages.Citation15,16 These different cuticle compositions could serve several functions. Cuticle composition may affect expansion rates or the formation of surface structures that are specific to developmental stages. For example, L1 larvae and adult-stage C. elegans, along with environmental stress–induced dauer larvae, contain cuticle striations termed alae, which have been suggested to aid in locomotion. Nevertheless, with the exception of dauer larvae, the cuticle is fairly similar in composition among the four larval stages and in adults, consistent with their sharing the same environment. As would be expected, a number of genes encoding both structural components of the cuticle and enzymes that modify cuticular proteins have been identified in screens for molting-defective mutants. These components, as well as the gross structure of the cuticle, are discussed in more detail below ().
Table 1. C. elegans genes implicated in molting that are discussed in this review.
Structure and composition of the C. Elegans cuticle
The C. elegans cuticle is a complex apical ECM containing proteins, lipids, and sugars. It serves several important functions including protection from the environment and pathogens. In addition, through its attachment to muscle cells, the cuticle functions as an exoskeleton to facilitate locomotion. As worms progress through their life cycle, the cuticle becomes somewhat thicker and more complex in its organization. As shown in , the adult cuticle is organized into five layers and is ∼0.5 µm thick.Citation15,17 From the outside in, these layers are referred to as the surface coat; the epicuticle; and the cortical, medial, and basal zones. In contrast to the adult cuticle, larval cuticles lack a medial zone, and the appearance of the basal zone differs between life-cycle stages. Furthermore, dauer larvae contain expanded basal and epicuticle layers, which may aid in their survival under stressful conditions.Citation15
Figure 1. Structural organization of the cuticle in adult C. elegans. Indicated are the five layers of the cuticle together with the apical part of epidermis (right side), and composition of cuticle layers (left side). The upper enlarged region shows the surface coat (glycocalyx), whereas the lower enlarged region indicates the presence of hemidesmosome-like structures (HDLSs) at the interface between the cuticle and epidermis. © WormAtlas. Adapted by permission of WormAtlas. Permission to reuse must be obtained from the rightsholder.Citation15
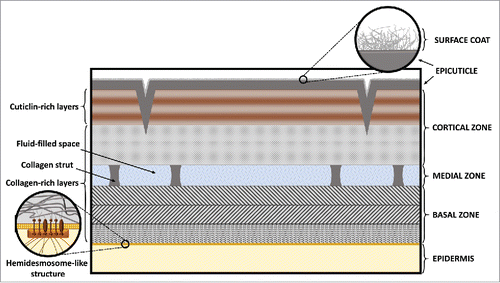
The surface coat, also termed the glycocalyx, is rich in carbohydrates and mucins. Unlike other cuticle layers, which are produced by epidermal cells, the glycocalyx is likely derived from the secretions of excretory gland cells that maintain connections to the animal's surface. Glycocalyx surface layers are found in many organisms ranging from bacteria to vertebrates and serve several important functions, including roles in development and immunity. Relatively little is known about the carbohydrate components, complex structures, or enzymatic activities required for the formation of the C. elegans glycocalyx. Recently, it was shown that cycles of rhamnose biosynthesis are in phase with C. elegans molting cycles, suggesting that rhamnose may be incorporated into the new cuticle.Citation18 Consistent with this, rhamnose is the most abundant sugar in the glycocalyx of certain parasitic amoebae.Citation19 Sugars such as rhamnose may be linked to lipid or protein cuticular components by members of the srf, bus, and bah gene families. In support of this, srf, bus, and bah mutants show changes in anti-carbohydrate antibody labeling and lectin binding at the cuticle surface.Citation20-23 Interestingly, mutations in srf, bus, and bah genes change the susceptibility to pathogens, indicating that the glycocalyx is important for protection against pathogen invasion.Citation22-25
Lying directly beneath the glycocalyx is the relatively thin layer of the epicuticle. The epicuticle is composed primarily of lipids and glycolipids.Citation15 This layer is most pronounced in dauer larvae and may enable their survival in unfavorable conditions. Because of its lipid content, the epicuticle is thought to function as a surface hydrophobic barrier. In addition, the epicuticle is likely to serve as a substrate for glycosylation pathways in the formation of the glycocalyx.
In contrast to the glycocalyx and epicuticle, the cortical, medial, and basal zones are composed largely of proteins. Differences among these three layers are indicated by differences in their amino acid composition, their susceptibility to different proteases, and their solubility in sulfhydryl reducing agents.Citation17 Because the outermost portion of the cortical zone is not susceptible to digestion by collagenases, this region is thought to be composed mainly of non-collagen proteinsCitation17 and is potentially rich in cuticlins, which are an integral part of the external layers of the Ascaris lumbricoides and Ascaris suum cuticles.Citation26,27 Characterized cuticlins in C. elegans include CUT-4, which is required only at the adult stage and is important for cuticle assembly.Citation28 Another cuticlin, CUT-2, is a component of cuticles at all four larval stages. mRNA levels for cut-2 exhibit an oscillatory pattern, peaking before each molt. In addition, cut-2 mRNA is also present in adults, suggesting that new CUT-2 may be incorporated into the cuticle of aging adults.Citation29 Other cuticlins are specifically involved in the formation of alae at the L1 (CUT-3 and CUT-5) and dauer (CUT-1 and CUT-5) stages, or are important for dauer body shape (CUT-6).Citation28,30,31
The non-collagen components of the cortical layer, most notably cuticlins, are thought to be cross-linked via non-reducible covalent bonds.Citation17 This is supported by the finding that CUT-2 represents a good substrate for in vitro cross-linking reactions, leading to the formation of insoluble complexes of high molecular weight.Citation29 Other non-collagen-based components of cuticle, for which layer-specific localization has not been described, include a fibrillin-like protein, FBN-1, and two Drosophila NompA orthologs, NOAH-1 and NOAH-2.Citation32,33 In addition to producing molting defects, mutations in fbn-1 cause embryonic morphogenesis defects, which are due to the role of FBN-1 in the embryonic precursor to the cuticle, termed the sheath.Citation33
Analogous to mammalian skin, the cuticle in nematodes includes collagen as its main protein fraction. Multiple types of collagen are present in the inner parts of the cortical zone, in the struts of the medial zone, and in the basal cuticular zone.Citation17 As discussed below, certain collagens (e.g., COL-1 and DPY-2) are incorporated in cuticles at every postembryonic life stage, whereas others (e.g., BLI-1 and COL-19) are stage specific. Cuticle collagens are extensively modified after secretion and are cross-linked by disulfide bonds. Notably, a disulfide isomerase enzyme, PDI-2, is required for normal function of the collagen-modifying prolyl 4-hydroxylase complex,Citation34 which is encoded by phy-1 and phy-2.Citation35 Consistent with their proposed function in cuticle biosynthesis, phy-1, phy-2, and pdi-2 mRNA levels oscillate during larval development, reaching a maximum several hours before molting, and a similar expression pattern is observed for some collagens.Citation35
Another collagen-modifying enzymatic complex is proposed to be formed by BLI-3, a hydrogen peroxide–generating NADPH dual oxidase, and MLT-7, a peroxidase. Together, these activities are thought to be essential for the normal crosslinking of cuticular collagens.Citation36,37 BLI-3 catalyzes the crosslinking of free tyrosine ethyl esters in vitro and the formation of dityrosines and trityrosine in vivo.Citation36 Downregulation of bli-3 or mlt-7 leads to molting defects and to changes in the in vivo expression patterns of the cuticle collagens DPY-13 and COL-12.Citation37 Furthermore, bli-3(RNAi) causes extensive structural abnormalities in the medial zone, including blister formation caused by excessive liquid accumulation and collagen-strut breakage.Citation36 Collagen organization is also changed following depletion of SURO-1, a metallocarboxyprotease,Citation38 and by loss of MLT-10, a protein with oscillatory expression pattern of unknown function.Citation39
Linkage of the cuticle, epidermal cells, and muscle cells
Collagens and most other components of the cuticle are synthesized by underlying lateral epidermal cells (seam cells) and epidermal syncytia (hyp1–hyp11), which are directly adjacent to the basal zone of the cuticle. The epidermis and cuticle are attached to each other in part through hemidesmosome-like structures (HDLSs), which are part of larger pillar-like structures termed fibrous organelles. Fibrous organelles contain both apical and basal HDLSs that are connected by intermediate filaments, which span the width of the epidermis (). At the basal surface, HDLSs form attachments that connect the epidermis to the basement membrane. Likewise, muscle cells attach to the basement membrane through structures termed dense bodies, which contain integrins and are homologous to vertebrate focal adhesions.Citation40-42 Together, these linkages provide critical connections between the various layers but must also be remodeled extensively during molts.
Figure 2. Attachments between the cuticle, epidermis, and muscles. Cellular and extracellular layers are indicated on the right side. Specific attachment structures are labeled in the figure, as well as important structural elements within the layers. © WormAtlas. Adapted by permission of WormAtlas. Permission to reuse must be obtained from the rightsholder.Citation15
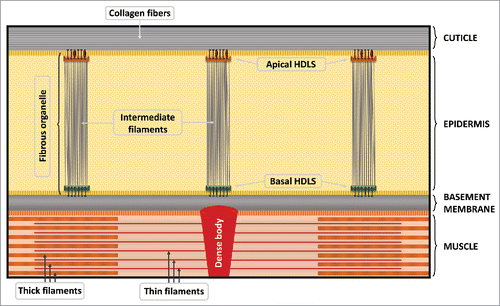
Candidate HDLS proteins include MUA-3 and MUP-4, which are related transmembrane proteins important for the establishment (MUP-4) and maintenance (MUA-3 and MUP-4) of attachments between the epidermis and the cuticle. MUA-3 may help to link the epidermal cytoskeleton to collagens in the basal zone.Citation43 Mutations in mua-3 typically cause developmental arrest during the final molt, which can be suppressed by RNAi depletion of the DPY-17 collagen.Citation44 Although both MUA-3 and MUP-4 are expressed from late embryonic stages, only MUP-4 expression is required during embryogenesis, whereas both proteins are necessary during postembryonic development. This could be because of increasing muscle activity, which may put additional stress on the interconnections between the cuticle and epidermis.Citation32,45 However, because these two proteins are believed to be expressed only at the apical epidermal membrane, other components within basal epidermal HDLSs may be necessary to connect the epidermis to the underlying basement membrane. One protein that may be specific to basal HDLSs is LET-805/myotactin. In addition, myotactin may be involved in intercellular signaling, which may guide remodeling of basement membrane attachments during molting.Citation32,46
Muscle cells are also connected to the basement ECM through dense bodies (). Expression of two dense-body proteins, PAT-3 and UNC-95, is decreased during initial stages of molting (lethargus, see below).Citation42 UNC-95 is ubiquitinated by the RING finger E3-ligase RNF-5, which may promote its degradation. Consistent with this, RNF-5 is expressed during molting, and inhibition of rnf-5 leads to molting defects.Citation42 Thus, regulated proteolysis may be a key mechanism for modulating dense bodies, as well as other structural components that are linked to the cuticle, during molting periods.Citation42 Furthermore, downregulation in the expression of LEV-11/tropomyosin in muscles or UNC-52/perlecan at the basement membrane, leads to molting defects, which are likely caused by an inability to modify muscle-epidermal attachment points.Citation32 Taken together, these findings indicate that attachments between the cuticle, epidermis, basement membrane, and muscle cells must be dynamically regulated to allow for release of the old cuticle and proper synthesis of a new one.
Internal cuticles
In addition to covering the external portions of the C. elegans body, a cuticle also lines internal epithelial surfaces that are directly connected to the body surface. Internal cuticles line the mouth (buccal cavity), pharynx (foregut), rectum, vulva, excretory duct, and excretory pore. Most of these internal cuticles appear to be simple in structure and are collagen based.Citation47 The exception is the pharyngeal cuticle, which cannot be digested by elastase or collagenase but can be degraded by bacterial pronase, suggesting that collagen is not its main protein component.Citation17 In addition, the pharyngeal cuticle contains chitin, which forms specialized anatomical elements that are important for proper food maceration.Citation48,49 Because chitin does not allow for shape changes, the pharynx can grow only during the molting period when the old cuticle is released.Citation13 Limitations in adequate food intake caused by a pharynx that is static in size and small relative to the rest of the body could be one of the triggers for molting.Citation50 Release and partial digestion of the pharyngeal cuticle may be assisted by secretions from pharyngeal gland cells, based on the observed accumulation of secretory granules in g1 gland cells during molting.Citation51-53 Furthermore, transcriptional profiling revealed that genes involved in chitin metabolic processes are upregulated during molting cycles.Citation54
The specialized structure of the pharyngeal cuticle necessitates that molting within the pharynx be partially independent of molting by the epidermis. In pharyngeal epidermal cells, molting-specific expression of abu/pqn genes, which encode prion-like glutamine and asparagine rich proteins, is necessary for normal molting and development.Citation48 Furthermore, the exoribonuclease XRN-2, which is expressed in myoepithelial pharyngeal cells, is required for cuticle formation.Citation32,55 Notably, xrn-2(RNAi) causes molting defects in the pharyngeal region as well as more general defects in cuticle shedding.Citation55 In summary, nematodes synthesize additional ECMs that cover several internal epithelia that are closely connected to the body surface. These matrices must also be shed during the molting process and, in the case of the pharynx, may have distinct properties and requirements for molting.
Molting stages and ECM remodeling
Molting can be separated into two behavioral phases: lethargus and ecdysis. Lethargus was named after the lethargic behavior exhibited by animals that have commenced molting and is most obvious during the first half of this phase. Decreased locomotion, slower pharyngeal pumping, and loss of seam cell granulation are some of the main hallmarks of lethargus.Citation51 These behavioral and morphological changes can be used to distinguish molting from non-molting animals but are labor intensive, as they require careful observations on individual animals. Simplified methods for identifying molting animals have been developed, including fluorescent reporters for genes that are expressed specifically during molts.Citation32,56 In addition, fluorescent-bead oral uptake assays have recently been described that take advantage of reduced pharyngeal pumping exhibited by molting animals.Citation56
Although lethargus is usually described as a phase of behavioral quiescence lasting from 2 to 3 hours, it encompasses alternating bouts of quiescence and activity, which last from 2 to 100 seconds.Citation57 In the first half of lethargus, quiescent periods are longer, whereas during later stages of lethargus the motile behavior becomes dominant. Studies of the quiescent phase of molting in C. elegans indicate that it could be a useful model for understanding hibernation and sleep in higher eukaryotes.Citation58 For example, the dopamine transporter encoded by dat-1 is expressed in an oscillatory manner and is upregulated during molting,Citation54,59 consistent with the role of dopamine transporters in controlling sleep in mammals.Citation60
During lethargus, animals begin to release their old cuticle, which eventually detaches from the underlying epidermis. This process, known as apolysis, starts in the head region and next occurs in the tail region and within the buccal cavity; the cuticle in the central body region is released last.Citation51 Hypomorphic mutations in the nekl–mlt gene network halt the molting process after the release of the cuticle from the head and tail regions, resulting in central body entrapment within the old cuticle (the corset phenotype).Citation61,62 In contrast, null alleles of genes within the nekl–mlt network lead to complete encasement within the old cuticle. These observations suggest that there may be somewhat different physiological or mechanistic requirements for release of the cuticle from different portions of the body.
The process of apolysis is not well understood but is thought to require the activity of proteases to degrade the old cuticle. Notably, the conserved metalloproteases NAS-36 and NAS-37,Citation63-65 as well as a cathepsin Z-like cysteine protease, CPZ-1,Citation66 are implicated in the process of old-cuticle degradation at each molt in both free-living and parasitic species.Citation65,66 Expression of nas-37 cycles in phase with the first three molts in epidermal syncytia, but it is expressed specifically in the lateral seam cells during the final molt.Citation32 Interestingly, several predicted protease inhibitors are involved in proper molting as well. For example, a cuticle blistering phenotype and molting defects are observed following depletion of BLI-5, a serine protease inhibitor.Citation32,67 Similarly, inhibition of another protease inhibitor, MLT-11, which shares a pancreatic trypsin inhibition domain with BLI-5, also causes molting defects. MLT-11 is expressed in both the main epidermal syncytia and in lateral seam cells and shows an oscillating pattern of expression, which is highest during intermolting periods.Citation32 This suggests that some protease inhibitors may suppress the activity of cuticle-degrading enzymes to prevent unwanted apolysis in periods between two molts. In addition to proteases, several other proteins with enzymatic activity are implicated in cuticle release during molting, including the selenoprotein thioredoxin reductase, TRXR-1, and GSR-1/glutathione reductase, which may facilitate removal of the old cuticle by promoting the reduction of disulfide groups in the cuticle.Citation68
Prior to complete removal of the old cuticle, however, it is essential for a new cuticle to be synthesized. Thus, during lethargus, production of the new cuticle begins while the worm is still protected by the partially detached old cuticle. Outer protein layers of the cuticle are synthesized first, whereas the basal zone components are synthesized last. As stated above, the main source of components for the new cuticle is the underlying epidermal tissue, although some superficial components are secreted from the gland cells.Citation15 It is notable that most described molting defects and molting mutants appear to affect cuticle shedding rather than cuticle synthesis. One explanation for this is that many of the components and enzymes involved in new cuticle synthesis may be required during embryogenesis for the formation of the embryonic sheath, which is essential for normal morphogenesis.Citation33,69 In addition, loss of individual structural components may have only mild effects on phenotypes, such as abnormal movement or greater susceptibility to injury or environmental toxins. Finally, it is possible that failure to synthesize a functional new cuticle could result in a failure to release the old cuticle, which could manifest as a shedding defect.
After completing synthesis of the new cuticle at the end of the lethargus phase, the partially detached old cuticle must be removed. This phase, known as ecdysis, commences with worms spinning around their long axis, a behavior believed to aid in the release of the old cuticle.Citation70 Several distinct types of motions have been observed including longitudinal contractions and expansions and forward thrusts. Foregut contractions are used to break the internal pharyngeal cuticle, so that the back portion is ingested, whereas the front part is expelled through the mouth and ultimately shed with the outer body cuticle. The old body cuticle usually breaks in the head region, after which the worm crawls out, facilitated by a series of quick body motions.Citation70
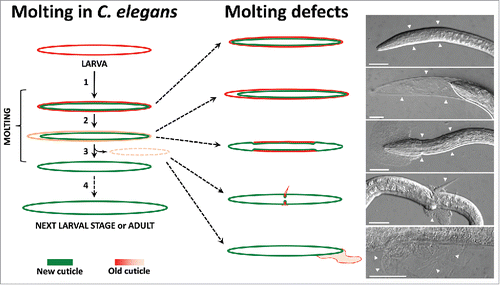
Failure to properly shed old cuticle results in a range of molting defects as shown in . The most severe phenotype is complete encasement, in which the whole body is covered with the tightly adhering old cuticle. Examples of mutants with this phenotype include null alleles in members of the NEKL–MLT kinase network. In cases where ecdysis is initiated but not completed, morphologically less severe defects can be observed. For example, after qua-1(RNAi), detachment of the old cuticle can be observed in the head region, but because the cuticle does not break, the animal remains trapped within the old cuticle. In other mutants, such as fbn-1, animals may become fully detached from the old cuticle but are unable to break through in a timely manner. Hypomorphic alleles of nekl-2, nekl-3, and mlt-4 lead to a corset phenotype, which consists of a characteristic constriction within the central portion of the body and release of the old cuticle in the head and tail regions only. Some molting-defective animals are largely successful in escaping from the old cuticle. In these cases, ecdysis fails within a small region of the body. For example, the old cuticle can form a thin constrictive ring wrapped around the body, or it can be loosely attached to the new cuticle. These less severe phenotypes can be lethal or viable, depending on the location of the old cuticle attachment, as the external constrictions can cause internal organ malfunctions or can be an obstacle for normal physiological functions, including feeding and excretion. Phenotypic differences between different genotypes may be due in some cases to the strength of individual alleles or because certain genes are required for spatially and/or temporally restricted processes during molting.
Figure 3. Schematic representation of the molting process and molting defects with representative micrograph examples. The normal physiological molting process is shown on the left. Schematic and micrograph examples of molting defects are shown on the right side, including (from top to bottom) the complete encasement phenotype of a mlt-3(fd72) mutant, the partially released cuticle in the head region of a qua-1(RNAi) larva, the corset phenotype of a nekl-3(sv3) mutant, a narrow constriction caused by the old cuticle in a nekl-3(sv3) mutant, and an old cuticle attached to the body surface after nekl-2(RNAi). White arrowheads indicate the presence of the old cuticle. Scale bars: 25µm.
Oscillations in the expression of molting genes
As molting phases are cyclical and temporally regulated, it might be expected that genes involved in molting and cuticle biogenesis would exhibit oscillatory expression patterns. To address this, Hendriks and colleagues used RNA sequencing, ribosome profiling, and RT-qPCR to analyze transcriptional and translational oscillations during C. elegans larval development.Citation71 Most protein oscillations were accomplished through changes in the level of gene transcription, with most genes showing oscillations that corresponded with molting cycles. This increase in the production of various proteins is likely to be necessary for the coordinated production of proteins that make up most of the cuticle. Accordingly, 72% of the genes encoding predicted structural components of cuticle (e.g., collagens) exhibit periodical expression patterns. Most other genes implicated in cuticle formation (27%) showed a progressive increase in their expression as animals developed.Citation71 An independent study, using microarray approaches, identified 520 genes that showed oscillations with molting cycles.Citation54 The majority of these genes encoded proteins predicted to be associated with cellular membranes, with 113 being upregulated and 68 being downregulated during molting. As expected, 19 of the identified genes that encode structural components of cuticle were expressed at higher levels during molting.Citation54
The most abundant components of the cuticle are collagens, many of which show oscillatory expression patterns. Whereas some collagens are incorporated into the cuticle at each developmental stage, others, such as BLI-1 and COL-19, are stage specific.Citation15 Oscillating collagens that are expressed at each molt, can be classified into three temporal groups—termed early (DPY-2, DPY-3, DPY-7, DPY-8, DPY-10), intermediate (COL-1, DPY-5, DPY-13, SQT-1), and late collagens (COL-12, COL-14)—with mRNA levels that peak ∼4 hours prior, 2 hours prior, and during cuticle synthesis, respectively.Citation72,73 Interestingly, mutations or depletions of specific oscillating collagens affect the structural organization of other collagens from the same temporal group, but not from other groups,Citation74 providing strong support for functional connections within temporal groups. Moreover, additive effects in the severity of cuticle-defective phenotypes are observed only in double mutants of collagens belonging to different groups,Citation74 suggesting that strong defects result from the perturbation of multiple layers of the cuticle strata.
Heterochronic genes in molting and development
Underlying both the timing of molting and the orderly progression of larval developmental events are the heterochronic genes (). Heterochronic genes, which encode a number of proteins as well as microRNAs, constitute an intrinsic developmental timer. Mutations in heterochronic genes lead to two general classes of phenotypes: precocious, in which developmental events are skipped, and retarded, in which developmental events are repeated.Citation75,76 Because the heterochronic gene network is coupled to molting cycles during larval development, both phenotypic classes of heterochronic mutants are associated with abnormal molting, such that precocious mutants undergo fewer molts than wild type, whereas retarded mutants undergo supernumerary molts. In the case of precocious mutants, such as lin-14, lin-28, lin-41, and hbl-1 loss of function, mutant animals also undergo premature termination of epidermal cell proliferation.Citation75,77-79 By contrast, retarded heterochronic mutants, such as lin-4, lin-29, and let-7 loss of function, repeat patterns of earlier larval-stage divisions and fusion events.Citation75,79,80 Interestingly, pharmacologically manipulated activity of nicotinic receptors impairs seam cell division and differentiation during the L2 larval stage, without affecting the normal L2/L3 molt. This indicates that there are two synchronized but independent timers, a developmental timer and a molting timer, and that their uncoupling can result in larval lethality.Citation81
Several studies have provided a direct link between heterochronic genes controlling developmental progression and the regulation of molting-specific factors. LIN-29, which promotes the L4-to-adult transition, is a zinc finger transcriptional regulator necessary for expression of the adult stage–specific collagen COL-19.Citation16 Furthermore, lin-29 genetically interacts with pan-1, a transmembrane protein that promotes molting and normal collagen expression.Citation82 It has also been shown that RNAi of some heterochronic genes, such as lin-14, lin-28, and lin-41, suppress supernumerary molts of hypomorphic myrf-1/pqn-47 animals. MYRF-1 is a protein linked to secretory processes in the endoplasmic reticulum of seam cells that is required for normal molting.Citation83 Another heterochronic gene, lin-42, encodes the C. elegans ortholog of PERIOD; lin-42 expression oscillates in phase with molting cycles in C. elegans and is known to control circadian rhythms in other animals.Citation84,85 In C. elegans, LIN-42 temporally controls seam cell dynamics throughout larval development and aligns seam cell developmental progression to molting cycles.Citation50,85 The paralogous let-7 and mir-84 microRNAs regulate the expression of the nuclear hormone receptors NHR-23 and NHR-25, which are the key regulators of C. elegans molting (see below).Citation86 Depletion of nhr-23 and nhr-25 leads to rescue of the let-7; mir-84 supernumerary molting phenotype, indicating that nhr-23 and nhr-25 mRNAs are likely targets for negative regulation by these microRNAs.Citation86
Hormonal control of molting
In insects, the steroid hormone ecdysone triggers molting at each developmental phase. Likewise, steroid hormone signaling is thought to regulate molting cycles in C. elegans. However, with the exception of one steroid hormone that regulates entry into the dauer stage,Citation87-90 the specific hormones that regulate normal molting cycles have not been identified. Evidence in support of a role for steroid-hormone signaling in C. elegans molting includes the finding that several nuclear hormone receptors are required for molting.Citation91,92 In addition, worms that are deprived of cholesterol undergo larval arrest, with a subset displaying defects in molting.Citation93 Notably, C. elegans cannot synthesize cholesterol de novo, and cholesterol serves as a precursor for steroid hormone biosynthesis. Below we discuss the roles of putative components of steroid signaling pathways in C. elegans molting ().
Several genes encoding nuclear hormone receptors have been implicated in C. elegans molting, including nhr-23, nhr-25, and nhr-67.Citation91,92 Additionally, three other NHRs, nhr-41, nhr-85, and daf-12, control entry into the dauer stage, which is achieved through an alternative molt following the L2 stage.Citation91,92 nhr-23 and nhr-25 mRNA levels oscillate with the molting cycles according to RT-PCR measurements.Citation91 nhr-23 is an ortholog of Hr3 in Drosophila melanogaster,Citation50,94 which functions in fly molting.Citation95 Overall levels of nhr-23 mRNA are higher during intermolts,Citation91,96 suggesting that this receptor is activated before each molt and that NHR-23 initiates a molting cascade. Accordingly, NHR-23 is an important regulator in the expression of several collagens as well as hedgehog-related proteins, which are also important for normal molting.Citation97 However, it is not known whether the levels of each of the six annotated nhr-23 isoforms oscillate with the molting cycles.Citation50
NHR-25 expression oscillates in a different manner than NHR-23. Full-length nhr-25 mRNA reaches its highest expression levels during the first three molting periods and also shows a slight increase during the final molt to adulthood.Citation91 A truncated natural isoform of nhr-25, which lacks the DNA-binding domain of the full-length form, has the opposite expression profile, based on RT-PCR, and exhibits its highest levels between molts.Citation91 nhr-25 is orthologous to ftz-f1 in D. melanogaster, which is downstream from Hr3 in the fruit fly molting cascade.Citation95,98 However, nhr-25 transcriptional and translational levels are not affected by nhr-23(RNAi), suggesting that this activation cascade is not conserved in C. elegans or that NHR-25 expression is redundantly regulated by some other factor.Citation96
As might be anticipated by their differences in expression patterns, the penetrance and timing of molting defects following depletion of nhr-23 and nhr-25 are distinct. Although nhr-23(RNAi) leads to molting defects at each stage, lethal effects are rarely observed at the first molt.Citation96 In contrast, downregulation of nhr-25 leads to either embryonic arrest, due to elongation failure, or to a high frequency of arrest during the first molt.Citation99 In addition, both NHR-23 and NHR-25 function in several additional processes. For example, downregulation of either nhr-23 or nhr-25 leads to seam-cell fusion defects along with a decrease in the expression of an ACN-1::GFP reporter in epidermal seam cells.Citation100 ACN-1 is a member of the angiotensin-converting enzyme family that lacks a metalloprotease active site, and it functions in epidermal cells to promote normal seam cell fusions and molting.Citation100 Loss of nhr-23 also causes a dumpy phenotype (shorter than wild type, but with the same body diameterCitation101), which might be caused by an observed decrease in the mRNA level of dpy-7, which encodes a cuticular collagen.Citation96 Furthermore, RNAi studies indicate that in addition to acting in the epidermis, NHR-25 may control germline-specific functions.Citation96
Nuclear hormone receptors are typically activated by steroid hormones that are synthesized from cholesterol or related 4-desmethylsterols.Citation102-105 As mentioned above, however, nematodes are unable to synthesize sterols de novo fromCitation14C-labeled acetate or mevalonate,Citation103,106,107 and are unable to develop normally if the growth medium is supplemented with only early sterol precursors, including acetic acid, d,l-mevalonic acid lactone, and farnesol.Citation108 In contrast, the addition of latter sterol metabolites, including squalene, lanosterol, and cholesterol, supports nematode growth.Citation108,109 In higher eukaryotes, squalene is synthesized from farnesol, which implies that nematodes are defective in one or more biosynthetic steps leading to the production of squalene.
Although sterols comprise a very small fraction of the total lipids isolated from nematodes,Citation104,110 they are essential for the normal development and reproduction of C. elegans, Caenorhabditis briggsae, Turbatrix aceti, Panagrellus redivivus, and other nematode species.Citation93,102,103,111 Their low levels, however, suggest that sterols do not play an important structural role in nematode cell membranes but instead may be more important in processes such as steroid hormone signaling. It should be noted that the identification of steroid hormones in C. elegans has generally eluded researchers, and the only steroid ligand that has been characterized to date, dafachronic acid, specifically controls entry into the dauer stage. Dafachronic acid is produced primarily during the L2 stage and binds to DAF-12, a classical nuclear hormone receptor.Citation87-90 In the ligand-bound state, DAF-12 promotes continuation of the heterochronic circuit allowing development to progress to the L3 stage, during which normal development ensues.Citation89,112 In unfavorable environmental conditions, including those that lead to starvation and overcrowding, dafachronic acid is not produced and ligand-free DAF-12 integrates signals from several dauer-triggering pathways, including insulin, serotonin, cGMP, and TGF-β, to arrest developmental progression and promote the formation of dauer larvae.Citation112,113 Notably, the predicted 3′ untranslated region of daf-12 contains complementary sequence to the let-7 microRNA, which implies that DAF-12 expression could be regulated by let-7.Citation114
The cytochrome P450–encoding gene daf-9 acts genetically upstream of daf-12 and may be important for the biosynthesis of dafachronic acid.Citation115 DAF-9 and its potential upstream activator, SDF-9, a putative protein tyrosine phosphatase, are both expressed in postembryonic neuroendocrine XXX cells, which may be the source of dafachronic acid.Citation15,116 This is supported by the finding that ablation of XXX cells, as well as mutations in both daf-9 and sdf-9, leads to constitutive dauer formation under conditions normally conducive for reproductive growth.Citation116 Taken together, evidence supports an important role for steroid hormone signaling in the control of molting and life cycle progression in C. elegans, including the involvement of genes that are orthologous to well-known ecdysone-triggered molting cascades in D. melanogaster. However, these pathways have diverged considerably, and many gaps remain in our understanding of steroid-hormone signaling pathways in nematodes.
Cell trafficking and molting
Intracellular trafficking within the epidermis, including the coordinated processes of exocytosis and endocytosis, are considered to be critical for molting (). In the case of exocytosis, the secretion of ECM proteins and other components of the cuticle by the epidermis is essential for synthesis of the new cuticle. Consistent with this, inhibition of sec-23, which encodes a component of the COPII complex involved in transport from the endoplasmic reticulum to the Golgi, reduces protein export and leads to defects in molting.Citation117 Conversely, endocytosis may facilitate the recycling of old cuticle components and is important for the internalization of sterols from the environment. In support of this, inhibition of CHC-1, a component of clathrin-coated pits; DAB-1, an adaptor protein; and HGRS-1, an ortholog of yeast Vps27, all lead to molting defects.Citation118-120 These core endocytic factors are expressed in the epidermis, and CHC-1 localizes to apical puncta within the hyp7 syncytium.Citation62
More recently, two NIMA family kinases, NEKL-2/NEK8 and NEKL-3/NEK6/7, together with three associated ankyrin domain–rich proteins (MLT-2/ANKS6, MLT-3/ANKS3, and MLT-4/INVS), were shown to function specifically within epidermal syncytia to control each molting cycle in C. elegans.Citation61,62 Both the NEKLs and MLTs are expressed in overlapping subsets of epidermal puncta and form at least two distinct complexes consisting of NEKL-2–MLT-2–MLT-4 and NEKL-3–MLT-3. Downregulation of components within the NEKL–MLT network causes changes in the expression pattern of several endosomal markers, including CHC-1, implicating these proteins in vesicular trafficking and possible sterol uptake (discussed below).Citation61,62
Sterol uptake by the epidermis is thought to depend primarily on LRP-1, a conserved low-density lipoprotein receptor related to human megalin.Citation93 LRP-1 is expressed in an apical punctate pattern in the main epidermal syncytium, hyp7. Interestingly, mosaic analysis did not reveal a requirement for LRP-1 in the intestine,Citation93 suggesting that the epidermis, and not the intestine, is critical for the absorption of sterols by LRP-1. Depletion of LRP-1 leads to molting defects, which can occur at any of the four larval molts, and similar effects are observed after depletion of the LRP-1 endocytic adaptor protein DAB-1.Citation93,118,120,121 Furthermore, other endocytosis-related genes, including chc-1, epn-1, nekl-2, aps-2, dpy-23, abp-1, and dyn-1, are important for normal LRP-1 internalization.Citation61,118
Evidence also suggests that the intestine may play a role in sterol uptake. For example, the intestinal steroid dehydrogenase LET-767 is important for molting and other aspects of C. elegans development.Citation122 In addition, the analysis of fluorescent dehydroergosterol distribution identified the intestine and several other tissues, but not the epidermis, as the primary accumulation point for this cholesterol analog.Citation123 Similarly, intestinal adsorption of 3β-hydroxy sterols has been demonstrated by filipin staining.Citation124 However, several aspects of the C. elegans lifestyle might suggest that epidermal steroid uptake could be more important than oral uptake. For example, C. elegans eat bacteria, which do not generally contain sterols, as very few bacterial species are able to produce these organic components.Citation125 The epidermal intake of sterols might explain why C. elegans in the wild inhabit sterol-rich environments, such as the decomposing tissues of other organisms.Citation126 Furthermore, the parasitic nematode Ascaris suum primarily absorbs sterols through its body surface, as radiolabeled cholesterol intake is not impaired after occlusion of the digestive tract.Citation127
Several other proteins required for the endocytosis of sterols have been implicated in dauer development. These include ncr-1/npc-1 and ncr-2/npc-2, which encode orthologs of mammalian proteins involved in the transport of sterols.Citation128 Mutations in mammalian NPC1 lead to neurodegeneration as a result of lysosomal storage defects.Citation129,130,131 ncr-1; ncr-2 double mutants exhibit a constitutive-dauer phenotype, which can be suppressed by increasing the concentration of cholesterol in the growth medium.Citation132 Furthermore, mutations in ncr-1 cause hypersensitivity to sterol depletion, leading to larval arrest, although the phenotype of these arrested larvae was not well characterized.Citation128 Genetic analysis indicates that ncr-1 and ncr-2 function upstream of daf-9 and daf-12.Citation132 ncr-1 is expressed in multiple tissue types, including the intestine and epidermis, whereas ncr-2 is expressed together with daf-9 in XXX cells, which are thought to secrete dafachronic acid.Citation123,124,132 A role for ncr-1 and ncr-2 during normal molting cycles has not yet been demonstrated.
Hedgehog-like signaling pathways in molting
Although signaling pathways have been implicated in C. elegans molting, they have not been well characterized. Most notable are several genes with sequence similarity to components of the hedgehog–patched signaling pathway, suggesting that hedgehog-type signaling might be important for molting ().Citation32,133,134 The hedgehog signaling pathway in C. elegans has, however, diverged from that of the well-characterized hedgehog pathways in fruit flies and vertebrates.Citation133 Whereas C. elegans has three putative patched orthologs (ptc-1, ptc-2, and ptc-3), 24 patched-related genes, and two dispatched orthologs (che-14 and ptd-2), there is no obvious homolog of the hedgehog ligand or the downstream effector smoothened in this nematode species.Citation133,135 Computational analysis has, however, revealed 61 and 49 hedgehog-related open reading frames in the C. elegans and C. briggsae genomes, respectively,Citation136 suggesting that these may encode ligands in a hedgehog-like signaling pathway in nematodes.
Relevant to this discussion, patched orthologs, along with several patched-related genes, are important for proper molting, as RNAi of these genes causes molting defects and developmental arrest.Citation133 Notably, patched proteins in worms and other species contain sterol-sensing domains,Citation135 as do several other components of the hedgehog signaling pathway. Furthermore, in mammalian neuroepithelial cells, ligand binding to patched is assisted by LRP2/megalin,Citation137 a homolog of C. elegans LRP-1.Citation93 LRP2 also controls internalization and trafficking of both hedgehog and patched during forebrain development in mice,Citation137 suggesting a potential similar function for LRP-1 in C. elegans.
Of the two dispatched orthologs in C. elegans, che-14/ptd-1 has been implicated in molting.Citation138,139 CHE-14 is expressed in epidermal tissues that are covered by cuticle and is associated with the apical membrane. CHE-14 has a sterol-sensing domain, which might serve in the recognition of a sterol-modified (active) hedgehog-like ligand. Mutations in che-14 cause apical accumulation of vesicles and amorphous material, suggesting a role in epidermal exocytosis.Citation139 Notably, dispatched in D. melanogaster has a role in the secretion of hedgehog molecules from signaling cells,Citation138 suggesting that CHE-14 could be required for the secretion of hedgehog-like molecules in C. elegans.
Members of the hedgehog-related group of proteins typically contain a C-terminal autoproteolytic hint/hog domain and one of several types of N-terminal domains, which function in signaling and distinguish the four hedgehog subfamilies: warthog, groundhog, ground-like, and quahog.Citation133 Self-cleavage of the C-terminal domain leads to the activation of hedgehog, which subsequently binds a sterol molecule. Although many of the C. elegans hedgehog-related proteins are missing a hint/hog domain, they contain one of the conserved N-terminal domains.Citation136 Notably, truncated hedgehog orthologs containing only the N-terminal domain are active but cannot be sterol-modified, leading to a change in their hydrophobicity and regulation.Citation138,140,141 Thus, some C. elegans hedgehog-related proteins may be regulated in a different manner than their counterparts in flies and vertebrates.
C. elegans hedgehog-related genes are expressed in the epidermis and other epithelial cells and are secreted into extracellular matrices, including the internal and external cuticles.Citation136 One of the C. elegans hedgehog-related genes, qua-1, is conserved in free-living and pathogenic nematodes, as well as in higher eukaryotes.Citation134 QUA-1 is cyclically expressed in the main epidermal syncytia at each molt and is secreted into the cuticle. Loss-of-function mutations of qua-1 lead to strong molting defects and developmental arrest.Citation134 Another C. elegans hedgehog-related gene implicated in molting is wrt-5, a member of the warthog family of ligands.Citation142 Prior to and during each molt, WRT-5 is secreted by pharyngeal cells, along with other cell types, and is excreted together with the old cuticle.Citation142
Taken together, orthologs of several components of the hedgehog signaling pathway have been implicated in molting in C. elegans. A number of these proteins may be modified by sterols, which are important for molting, and could interact with the sterol-binding receptor LRP-1. These observations provide an alternative, and non-hormone-related, explanation for the role of epidermal sterol absorption in nematode molting, although it is likely that both hedgehog and nuclear hormone receptor signaling pathways have important roles in this process.
Conclusions
Molting is a complex developmental process that is carried out in all nematodes, as well as in many other invertebrate species. Molting allows for the replacement of the apical ECM, known as the cuticle, thereby enabling growth or developmental specialization. As described above, a large and diverse collection of genes have been implicated in molting control in C. elegans (). However, a clear and integrated description of the molting cascade is still missing within nematode species. Further studies will undoubtedly lead to a better understanding of this important developmental process.
Beyond an interest in understanding molting as a developmental process, molting studies may have other significant and practical applications. Notably, molting is a potential focus for antihelminthic drug development, both for human and veterinary medicine.Citation143-145 Diseases caused by nematodes are still widely distributed in the human population and affect hundreds of millions of people, according to the World Health Organization.Citation146 Nematode infections can lead to a number of long-term consequences, including death, and can further complicate other diseases. Many of these diseases are zoonoses, meaning that they are naturally transmissible from vertebrate animals to humans. Correspondingly, nematode infections of animals, as well as plants, have significant economic consequences.Citation147,148 As such, agricultural industries use extensive prophylactic measures to decrease the prevalence of parasitic nematodoses, which cause material damage and financial loss.
Although molting in nematodes might seem to constitute a specialized biological process within invertebrates, understanding the molecular basis for ECM remodeling and other fundamental events associated with molting has broad relevance for biology and our basic knowledge of disease states. Furthermore, because the C. elegans cuticle is largely collagen based, it is potentially useful for understanding dermal physiology and wound healing in higher eukaryotes.Citation149 In addition, primary tumor invasion through the ECM is an important process during tumor metastasis and shares many conserved features with ECM remodeling.Citation150-152 Notably, many genes implicated in C. elegans molting have orthologs in higher eukaryotes, indicating that this biological system represents a powerful tool for exploring the functions and molecular mechanisms underlying a wide range of conserved cellular and developmental processes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Amy Fluet for editing this manuscript. We apologize in advance for any omissions resulting from a lapse in our awareness or because of reference limitations imposed by the journal. We thank WormAtlas for allowing us to adopt the images depicted in Figures 1 and 2.
Funding
This work was supported by NIH grant GM066868 and the Wyoming INBRE P20 GM103432.
References
- Aguinaldo AMA, Turbeville JM, Linford LS, Rivera MC, Garey JR, Raff RA, Lake JA. Evidence for a clade of nematodes, arthropods and other moulting animals. Nature 1997; 387:489-93; PMID:9168109; https://doi.org/10.1038/387489a0
- Telford MJ, Bourlat SJ, Economou A, Papillon D, Rota-Stabelli O. The evolution of the Ecdysozoa. Philos Trans R Soc Lond B Biol Sci 2008; 363:1529-37; PMID:18192181; https://doi.org/10.1098/rstb.2007.2243
- Bakhetia M, Charlton W, Atkinson HJ, McPherson MJ. RNA interference of dual oxidase in the plant nematode Meloidogyne incognita. Mol Plant Microbe Interact 2005; 18:1099-106; PMID:16255249; https://doi.org/10.1094/MPMI-18-1099
- Charlton WL, Harel HY, Bakhetia M, Hibbard JK, Atkinson HJ, McPherson MJ. Additive effects of plant expressed double-stranded RNAs on root-knot nematode development. Int J Parasitol 2010; 40:855-64; PMID:20100489; https://doi.org/10.1016/j.ijpara.2010.01.003
- Page AP, Stepek G, Winter AD, Pertab D. Enzymology of the nematode cuticle: A potential drug target? Int J Parasitol Drugs Drug Resist 2014; 4:133-41; PMID:25057463; https://doi.org/10.1016/j.ijpddr.2014.05.003
- Kumar S, Chaudhary K, Foster JM, Novelli JF, Zhang Y, Wang S, Spiro D, Ghedin E, Carlow CK. Mining predicted essential genes of Brugia malayi for nematode drug targets. PLoS One 2007; 2:e1189; PMID:18000556; https://doi.org/10.1371/journal.pone.0001189
- Riddle DL, Blumenthal T, Meyer BM, Priess JR. C. ELEGANS II. Cold Spring Harbor Press, 1997.
- Lambert K, Bekal S. Introduction to Plant-Parasitic Nematodes. The Plant Health Instructor 2002; https://doi.org/10.1094/PHI-I-2002-1218-01
- Bird AF. The Attractiveness of Roots To the Plant Parasitic Nematodes Meloidogyne Javanica and M. Hapla. Nematologica 1959; 4:322-35; https://doi.org/10.1163/187529259X00534 10.1163/187529259X00345
- Bird AF, Bird J. The Structure of Nematodes, Second edition. Academic Press, INC., 1991
- Roberts FHS. The large roundworm of pigs, Ascaris lumbricoides L., 1758 : its life history in Queensland, economic importance and control. Brisbane: Dept. of Agriculture and Stock 1934
- Watson BD. The fine structure of the body-wall and the growth of the cuticle in the adult nematode Ascaris lumbricoides. J Cell Sci 1965; s3–106:83-91
- Knight CG, Patel MN, Azevedo RB, Leroi AM. A novel mode of ecdysozoan growth in Caenorhabditis elegans. Evol Dev 2002; 4:16-27; PMID:11871396; https://doi.org/10.1046/j.1525-142x.2002.01058.x
- Costa M, Draper BW, Priess JR. The role of actin filaments in patterning the Caenorhabditis elegans cuticle. Dev Biol 1997; 184:373-84; PMID:9133443; https://doi.org/10.1006/dbio.1997.8530
- Hall DH, Altun ZF. C. elegans Atlas. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press, 2008
- Rougvie AE, Ambros V. The heterochronic gene lin-29 encodes a zinc finger protein that controls a terminal differentiation event in Caenorhabditis elegans. Development 1995; 121:2491-500; PMID:7671813
- Cox GN, Kusch M, Edgar RS. Cuticle of Caenorhabditis elegans: its isolation and partial characterization. J Cell Biol 1981; 90:7-17; PMID:7251677; https://doi.org/10.1083/jcb.90.1.7
- Feng L, Shou Q, Butcher RA. Identification of a dTDP-rhamnose biosynthetic pathway that oscillates with the molting cycle in Caenorhabditis elegans. Biochem J 2016; 473:1507-21; PMID:27009306; https://doi.org/10.1042/BCJ20160142
- Villavedra M, To J, Lemke S, Birch D, Crosbie P, Adams M, Broady K, Nowak B, Raison RL, Wallach M. Characterisation of an immunodominant, high molecular weight glycoprotein on the surface of infectious Neoparamoeba spp., causative agent of amoebic gill disease (AGD) in Atlantic salmon. Fish Shellfish Immunol 2010; 29:946-55; PMID:20708082; https://doi.org/10.1016/j.fsi.2010.07.036
- Politz SM, Philipp M, Estevez M, O'Brien PJ, Chin KJ. Genes that can be mutated to unmask hidden antigenic determinants in the cuticle of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A 1990; 87:2901-5; PMID:1691498; https://doi.org/10.1073/pnas.87.8.2901
- Link CD, Silverman MA, Breen M, Watt KE, Dames SA. Characterization of Caenorhabditis elegans lectin-binding mutants. Genetics 1992; 131:867-81; PMID:1516818
- Darby C, Chakraborti A, Politz SM, Daniels CC, Tan L, Drace K. Caenorhabditis elegans mutants resistant to attachment of Yersinia biofilms. Genetics 2007; 176:221-30; PMID:17339204; https://doi.org/10.1534/genetics.106.067496
- Gravato-Nobre MJ, Nicholas HR, Nijland R, O'Rourke D, Whittington DE, Yook KJ, Hodgkin J. Multiple genes affect sensitivity of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics 2005; 171:1033-45; PMID:16079230; https://doi.org/10.1534/genetics.105.045716
- Drace K, McLaughlin S, Darby C. Caenorhabditis elegans BAH-1 is a DUF23 protein expressed in seam cells and required for microbial biofilm binding to the cuticle. PLoS One 2009; 4:e6741; PMID:19707590; https://doi.org/10.1371/journal.pone.0006741
- Partridge FA, Tearle AW, Gravato-Nobre MJ, Schafer WR, Hodgkin J. The C. elegans glycosyltransferase BUS-8 has two distinct and essential roles in epidermal morphogenesis. Dev Biol 2008; 317:549-59; PMID:18395708; https://doi.org/10.1016/j.ydbio.2008.02.060
- Fujimoto D, Kanaya S. Cuticlin: a noncollagen structural protein from Ascaris cuticle. Arch Biochem Biophys 1973; 157:1-6; PMID:4352055; https://doi.org/10.1016/0003-9861(73)90382-2
- Betschart B, Marti S, Glaser M. Antibodies against the cuticlin of Ascaris suum cross-react with epicuticular structures of filarial parasites. Acta Trop 1990; 47:331-8; PMID:1978533; https://doi.org/10.1016/0001-706X(90)90034-W
- Sapio MR, Hilliard MA, Cermola M, Favre R, Bazzicalupo P. The Zona Pellucida domain containing proteins, CUT-1, CUT-3 and CUT-5, play essential roles in the development of the larval alae in Caenorhabditis elegans. Dev Biol 2005; 282:231-45; PMID:15936343; https://doi.org/10.1016/j.ydbio.2005.03.011
- Lassandro F, Sebastiano M, Zei F, Bazzicalupo P. The role of dityrosine formation in the crosslinking of CUT-2, the product of a second cuticlin gene of Caenorhabditis elegans. Mol Biochem Parasitol 1994; 65:147-59; PMID:7935621; https://doi.org/10.1016/0166-6851(94)90123-6
- Sebastiano M, Lassandro F, Bazzicalupo P. cut-1 a Caenorhabditis elegans gene coding for a dauer-specific noncollagenous component of the cuticle. Dev Biol 1991; 146:519-30; PMID:1864469; https://doi.org/10.1016/0012-1606(91)90253-Y
- Muriel JM, Brannan M, Taylor K, Johnstone IL, Lithgow GJ, Tuckwell D. M142.2 (cut-6), a novel Caenorhabditis elegans matrix gene important for dauer body shape. Dev Biol 2003; 260:339-51; PMID:12921736; https://doi.org/10.1016/S0012-1606(03)00237-9
- Frand AR, Russel S, Ruvkun G. Functional genomic analysis of C. elegans molting. PLoS Biol 2005; 3:e312; PMID:16122351; https://doi.org/10.1371/journal.pbio.0030312
- Kelley M, Yochem J, Krieg M, Calixto A, Heiman MG, Kuzmanov A, Meli V, Chalfie M, Goodman MB, Shaham S, et al. FBN-1, a fibrillin-related protein, is required for resistance of the epidermis to mechanical deformation during C. elegans embryogenesis. Elife 2015; 4; https://doi.org/10.7554/eLife.06565
- Winter AD, McCormack G, Page AP. Protein disulfide isomerase activity is essential for viability and extracellular matrix formation in the nematode Caenorhabditis elegans. Dev Biol 2007; 308:449-61; PMID:17586485; https://doi.org/10.1016/j.ydbio.2007.05.041
- Winter AD, Page AP. Prolyl 4-hydroxylase is an essential procollagen-modifying enzyme required for exoskeleton formation and the maintenance of body shape in the nematode Caenorhabditis elegans. Mol Cell Biol 2000; 20:4084-93; PMID:10805750; https://doi.org/10.1128/MCB.20.11.4084-4093.2000
- Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, Edens HA, Tang X, Sullards C, Flaherty DB, et al. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol 2001; 154:879-91; PMID:11514595; https://doi.org/10.1083/jcb.200103132
- Thein MC, Winter AD, Stepek G, McCormack G, Stapleton G, Johnstone IL, Page AP. Combined extracellular matrix cross-linking activity of the peroxidase MLT-7 and the dual oxidase BLI-3 is critical for post-embryonic viability in Caenorhabditis elegans. J Biol Chem 2009; 284:17549-63; PMID:19406744; https://doi.org/10.1074/jbc.M900831200
- Kim TH, Kim YJ, Cho JW, Shim J. A novel zinc-carboxypeptidase SURO-1 regulates cuticle formation and body morphogenesis in Caenorhabditis elegans. FEBS Lett 2011; 585:121-7; PMID:21094156; https://doi.org/10.1016/j.febslet.2010.11.020
- Meli VS, Osuna B, Ruvkun G, Frand AR. MLT-10 defines a family of DUF644 and proline-rich repeat proteins involved in the molting cycle of Caenorhabditis elegans. Mol Biol Cell 2010; 21:1648-61; PMID:20335506; https://doi.org/10.1091/mbc.E08-07-0708
- Lecroisey C, Segalat L, Gieseler K. The C. elegans dense body: anchoring and signaling structure of the muscle. J Muscle Res Cell Motil 2007; 28:79-87; PMID:17492481; https://doi.org/10.1007/s10974-007-9104-y
- Cox EA, Hardin J. Sticky worms: adhesion complexes in C. elegans. J Cell Sci 2004; 117:1885-97; PMID:15090594; https://doi.org/10.1242/jcs.01176
- Zaidel-Bar R, Miller S, Kaminsky R, Broday L. Molting-specific downregulation of C. elegans body-wall muscle attachment sites: the role of RNF-5 E3 ligase. Biochem Biophys Res Commun 2010; 395:509-14; PMID:20385102; https://doi.org/10.1016/j.bbrc.2010.04.049
- Bercher M, Wahl J, Vogel BE, Lu C, Hedgecock EM, Hall DH, Plenefisch JD. mua-3, a gene required for mechanical tissue integrity in Caenorhabditis elegans, encodes a novel transmembrane protein of epithelial attachment complexes. J Cell Biol 2001; 154:415-26; PMID:11470828; https://doi.org/10.1083/jcb.200103035
- Fotopoulos P, Kim J, Hyun M, Qamari W, Lee I, You YJ. DPY-17 and MUA-3 Interact for Connective Tissue-Like Tissue Integrity in Caenorhabditis elegans: A Model for Marfan Syndrome. G3 (Bethesda) 2015; 5:1371-8; PMID:25917920; https://doi.org/10.1534/g3.115.018465
- Hong L, Elbl T, Ward J, Franzini-Armstrong C, Rybicka KK, Gatewood BK, Baillie DL, Bucher EA. MUP-4 is a novel transmembrane protein with functions in epithelial cell adhesion in Caenorhabditis elegans. J Cell Biol 2001; 154:403-14; PMID:11470827; https://doi.org/10.1083/jcb.200007075
- Hresko MC, Schriefer LA, Shrimankar P, Waterston RH. Myotactin, a novel hypodermal protein involved in muscle-cell adhesion in Caenorhabditis elegans. J Cell Biol 1999; 146:659-72; PMID:10444073; https://doi.org/10.1083/jcb.146.3.659
- Gill HK, Cohen JD, Ayala-Figueroa J, Forman-Rubinsky R, Poggioli C, Bickard K, Parry JM, Pu P, Hall DH, Sundaram MV. Integrity of Narrow Epithelial Tubes in the C. elegans excretory system requires a Transient luminal matrix. PLoS Genet 2016; 12:e1006205; PMID:27482894; https://doi.org/10.1371/journal.pgen.1006205
- George-Raizen JB, Shockley KR, Trojanowski NF, Lamb AL, Raizen DM. Dynamically-expressed prion-like proteins form a cuticle in the pharynx of Caenorhabditis elegans. Biol Open 2014; 3:1139-49; PMID:25361578; https://doi.org/10.1242/bio.20147500
- Veronico P, Gray LJ, Jones JT, Bazzicalupo P, Arbucci S, Cortese MR, Di Vito M, De Giorgi C. Nematode chitin synthases: gene structure, expression and function in Caenorhabditis elegans and the plant parasitic nematode Meloidogyne artiellia. Mol Genet Genomics 2001; 266:28-34; PMID:11589574; https://doi.org/10.1007/s004380100513
- Monsalve GC, Frand AR. Toward a unified model of developmental timing: A “molting” approach. Worm 2012; 1:221-30; PMID:24058853; https://doi.org/10.4161/worm.20874
- Singh RN, Sulston JE. Some observations on the moulting of Caenorhabditis elegans. Nematologica 1978; 24:63-71; https://doi.org/10.1163/187529278X00074
- Pilon M. Developmental genetics of the Caenorhabditis elegans pharynx. Wiley Interdiscip Rev Dev Biol 2014; 3:263-80; PMID:25262818; https://doi.org/10.1002/wdev.139
- Hall DH, Hedgecock EM. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 1991; 65:837-47; PMID:1710172; https://doi.org/10.1016/0092-8674(91)90391-B
- Turek M, Bringmann H. Gene expression changes of Caenorhabditis elegans larvae during molting and sleep-like lethargus. PLoS One 2014; 9:e113269; PMID:25409030; https://doi.org/10.1371/journal.pone.0113269
- Miki TS, Ruegger S, Gaidatzis D, Stadler MB, Grosshans H. Engineering of a conditional allele reveals multiple roles of XRN2 in Caenorhabditis elegans development and substrate specificity in microRNA turnover. Nucleic Acids Res 2014; 42:4056-67; PMID:24445807; https://doi.org/10.1093/nar/gkt1418
- Nika L, Gibson T, Konkus R, Karp X. Fluorescent beads are a versatile tool for staging Caenorhabditis elegans in different life histories. G3 (Bethesda) 2016; 6:1923-33; PMID:27172224; https://doi.org/10.1534/g3.116.030163
- Iwanir S, Tramm N, Nagy S, Wright C, Ish D, Biron D. The microarchitecture of C. elegans behavior during lethargus: homeostatic bout dynamics, a typical body posture, and regulation by a central neuron. Sleep 2013; 36:385-95; PMID:23449971; https://doi.org/10.5665/sleep.2456
- Nagy S, Raizen DM, Biron D. Measurements of behavioral quiescence in Caenorhabditis elegans. Methods 2014; 68:500-7; PMID:24642199; https://doi.org/10.1016/j.ymeth.2014.03.009
- Singh K, Ju JY, Walsh MB, DiIorio MA, Hart AC. Deep conservation of genes required for both Drosphila melanogaster and Caenorhabditis elegans sleep includes a role for dopaminergic signaling. Sleep 2014; 37:1439-51; PMID:25142568; https://doi.org/10.5665/sleep.3990
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci 2001; 21:1787-94; PMID:11222668
- Yochem J, Lazetic V, Bell L, Chen L, Fay D. C. elegans NIMA-related kinases NEKL-2 and NEKL-3 are required for the completion of molting. Dev Biol 2015; 398:255-66; PMID:25523392; https://doi.org/10.1016/j.ydbio.2014.12.008
- Lazetic V, Fay DS. Conserved Ankyrin Repeat Proteins and Their NIMA Kinase Partners Regulate Extracellular Matrix Remodeling and Intracellular Trafficking in Caenorhabditis elegans. Genetics 2017; 205:273-93; PMID:27799278; https://doi.org/10.1534/genetics.116.194464
- Stepek G, McCormack G, Birnie AJ, Page AP. The astacin metalloprotease moulting enzyme NAS-36 is required for normal cuticle ecdysis in free-living and parasitic nematodes. Parasitology 2011; 138:237-48; PMID:20800010; https://doi.org/10.1017/S0031182010001113
- Suzuki M, Sagoh N, Iwasaki H, Inoue H, Takahashi K. Metalloproteases with EGF, CUB, and thrombospondin-1 domains function in molting of Caenorhabditis elegans. Biol Chem 2004; 385:565-8; PMID:15255192; https://doi.org/10.1515/BC.2004.069
- Davis MW, Birnie AJ, Chan AC, Page AP, Jorgensen EM. A conserved metalloprotease mediates ecdysis in Caenorhabditis elegans. Development 2004; 131:6001-8; PMID:15539494; https://doi.org/10.1242/dev.01454
- Hashmi S, Zhang J, Oksov Y, Lustigman S. The Caenorhabditis elegans cathepsin Z-like cysteine protease, Ce-CPZ-1, has a multifunctional role during the worms' development. J Biol Chem 2004; 279:6035-45; PMID:14630920; https://doi.org/10.1074/jbc.M312346200
- Page AP, McCormack G, Birnie AJ. Biosynthesis and enzymology of the Caenorhabditis elegans cuticle: identification and characterization of a novel serine protease inhibitor. Int J Parasitol 2006; 36:681-9; PMID:16500660; https://doi.org/10.1016/j.ijpara.2006.01.004
- Stenvall J, Fierro-Gonzalez JC, Swoboda P, Saamarthy K, Cheng Q, Cacho-Valadez B, Arnér ES, Persson OP, Miranda-Vizuete A, Tuck S. Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc Natl Acad Sci U S A 2011; 108:1064-9; PMID:21199936; https://doi.org/10.1073/pnas.1006328108
- Priess JR, Hirsh DI. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev Biol 1986; 117:156-73; PMID:3743895; https://doi.org/10.1016/0012-1606(86)90358-1
- Singh RN, Sulston JE. Some observations on moulting in Caenorhabditis elegans. Nematologica 1978; 24:63-71; https://doi.org/10.1163/187529278X00074
- Hendriks GJ, Gaidatzis D, Aeschimann F, Grosshans H. Extensive oscillatory gene expression during C. elegans larval development. Mol Cell 2014; 53:380-92; PMID:24440504; https://doi.org/10.1016/j.molcel.2013.12.013
- Johnstone IL, Barry JD. Temporal reiteration of a precise gene expression pattern during nematode development. EMBO J 1996; 15:3633-9; PMID:8670866
- Page AP, Johnstone IL. The cuticle. WormBook 2007:1-15; PMID:18050497; https://doi.org/10.1895/wormbook.1.138.1
- McMahon L, Muriel JM, Roberts B, Quinn M, Johnstone IL. Two sets of interacting collagens form functionally distinct substructures within a Caenorhabditis elegans extracellular matrix. Mol Biol Cell 2003; 14:1366-78; PMID:12686594; https://doi.org/10.1091/mbc.E02-08-0479
- Ambros V, Horvitz HR. Heterochronic mutants of the nematode Caenorhabditis elegans. Science 1984; 226:409-16; PMID:6494891; https://doi.org/10.1126/science.6494891
- Moss EG. Heterochronic genes and the nature of developmental time. Curr Biol 2007; 17:R425-34; PMID:17550772; https://doi.org/10.1016/j.cub.2007.03.043
- Lin SY, Johnson SM, Abraham M, Vella MC, Pasquinelli A, Gamberi C, Gottlieb E, Slack FJ. The C elegans hunchback homolog, hbl-1, controls temporal patterning and is a probable microRNA target. Dev Cell 2003; 4:639-50; PMID:12737800; https://doi.org/10.1016/S1534-5807(03)00124-2
- Slack FJ, Basson M, Liu Z, Ambros V, Horvitz HR, Ruvkun G. The lin-41 RBCC gene acts in the C. elegans heterochronic pathway between the let-7 regulatory RNA and the LIN-29 transcription factor. Mol Cell 2000; 5:659-69; PMID:10882102; https://doi.org/10.1016/S1097-2765(00)80245-2
- Vella MC, Slack FJ. C. elegans microRNAs. WormBook 2005; PMID:18050425; https://doi.org/10.1895/wormbook.1.26.1
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75:843-54; PMID:8252621; https://doi.org/10.1016/0092-8674(93)90529-Y
- Ruaud AF, Bessereau JL. Activation of nicotinic receptors uncouples a developmental timer from the molting timer in C. elegans. Development 2006; 133:2211-22; PMID:16672334; https://doi.org/10.1242/dev.02392
- Gissendanner CR, Kelley TD. The C. elegans gene pan-1 encodes novel transmembrane and cytoplasmic leucine-rich repeat proteins and promotes molting and the larva to adult transition. BMC Dev Biol 2013; 13:21; PMID:23682709; https://doi.org/10.1186/1471-213X-13-21
- Russel S, Frand AR, Ruvkun G. Regulation of the C. elegans molt by pqn-47. Dev Biol 2011; 360:297-309; PMID:21989027; https://doi.org/10.1016/j.ydbio.2011.09.025
- Edelman TL, McCulloch KA, Barr A, Frokjaer-Jensen C, Jorgensen EM, Rougvie AE. Analysis of a lin-42/period Null Allele Implicates All Three Isoforms in Regulation of Caenorhabditis elegans molting and developmental timing. G3 (Bethesda) 2016; 6:4077-86; PMID:27729432
- Monsalve GC, Van Buskirk C, Frand AR. LIN-42/PERIOD controls cyclical and developmental progression of C. elegans molts. Curr Biol 2011; 21:2033-45; PMID:22137474; https://doi.org/10.1016/j.cub.2011.10.054
- Hayes GD, Frand AR, Ruvkun G. The mir-84 and let-7 paralogous microRNA genes of Caenorhabditis elegans direct the cessation of molting via the conserved nuclear hormone receptors NHR-23 and NHR-25. Development 2006; 133:4631-41; PMID:17065234; https://doi.org/10.1242/dev.02655
- Matyash V, Entchev EV, Mende F, Wilsch-Brauninger M, Thiele C, Schmidt AW, Knölker HJ, Ward S, Kurzchalia TV. Sterol-derived hormone(s) controls entry into diapause in Caenorhabditis elegans by consecutive activation of DAF-12 and DAF-16. PLoS Biol 2004; 2:e280; PMID:15383841; https://doi.org/10.1371/journal.pbio.0020280
- Entchev EV, Kurzchalia TV. Requirement of sterols in the life cycle of the nematode Caenorhabditis elegans. Semin Cell Dev Biol 2005; 16:175-82; PMID:15797828; https://doi.org/10.1016/j.semcdb.2005.01.004
- Li TM, Chen J, Li X, Ding XJ, Wu Y, Zhao LF, Chen S, Lei X, Dong MQ. Absolute quantification of a steroid hormone that regulates development in Caenorhabditis elegans. Anal Chem 2013; 85:9281-7; PMID:24010904; https://doi.org/10.1021/ac402025c
- Motola DL, Cummins CL, Rottiers V, Sharma KK, Li T, Li Y, Suino-Powell K, Xu HE, Auchus RJ, Antebi A, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell 2006; 124:1209-23; https://doi.org/10.1016/j.cell.2006.01.037
- Gissendanner CR, Crossgrove K, Kraus KA, Maina CV, Sluder AE. Expression and function of conserved nuclear receptor genes in Caenorhabditis elegans. Dev Biol 2004; 266:399-416; PMID:14738886; https://doi.org/10.1016/j.ydbio.2003.10.014
- Antebi A. Nuclear hormone receptors in C. elegans. WormBook 2006:1-13; PMID:18050471
- Yochem J, Tuck S, Greenwald I, Han M. A gp330/megalin-related protein is required in the major epidermis of Caenorhabditis elegans for completion of molting. Development 1999; 126:597-606; PMID:9876188
- Maglich JM, Sluder A, Guan X, Shi Y, McKee DD, Carrick K, Kamdar K, Willson TM, Moore JT. Comparison of complete nuclear receptor sets from the human, Caenorhabditis elegans and Drosophila genomes. Genome Biol 2001; 2:RESEARCH0029; PMID:11532213; https://doi.org/10.1186/gb-2001-2-8-research0029
- Lam G, Hall BL, Bender M, Thummel CS. DHR3 is required for the prepupal-pupal transition and differentiation of adult structures during Drosophila metamorphosis. Dev Biol 1999; 212:204-16; PMID:10419696; https://doi.org/10.1006/dbio.1999.9343
- Kostrouchova M, Krause M, Kostrouch Z, Rall JE. Nuclear hormone receptor CHR3 is a critical regulator of all four larval molts of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A 2001; 98:7360-5; PMID:11416209; https://doi.org/10.1073/pnas.131171898
- Kouns NA, Nakielna J, Behensky F, Krause MW, Kostrouch Z, Kostrouchova M. NHR-23 dependent collagen and hedgehog-related genes required for molting. Biochem Biophys Res Commun 2011; 413:515-20; PMID:21910973; https://doi.org/10.1016/j.bbrc.2011.08.124
- Asahina M, Ishihara T, Jindra M, Kohara Y, Katsura I, Hirose S. The conserved nuclear receptor Ftz-F1 is required for embryogenesis, moulting and reproduction in Caenorhabditis elegans. Genes Cells 2000; 5:711-23; PMID:10971653; https://doi.org/10.1046/j.1365-2443.2000.00361.x
- Gissendanner CR, Sluder AE. nhr-25, the Caenorhabditis elegans ortholog of ftz-f1, is required for epidermal and somatic gonad development. Dev Biol 2000; 221:259-72; PMID:10772806; https://doi.org/10.1006/dbio.2000.9679
- Brooks DR, Appleford PJ, Murray L, Isaac RE. An essential role in molting and morphogenesis of Caenorhabditis elegans for ACN-1, a novel member of the angiotensin-converting enzyme family that lacks a metallopeptidase active site. J Biol Chem 2003; 278:52340-6; PMID:14559923; https://doi.org/10.1074/jbc.M308858200
- Brenner S. The genetics of Caenorhabditis elegans. Genetics 1974; 77:71-94; PMID:4366476
- Hieb WF, Rothstein M. Sterol requirement for reproduction of a free-living nematode. Science 1968; 160:778-80; PMID:4869093; https://doi.org/10.1126/science.160.3829.778
- Cole RJ, Dutky SR. A Sterol Requirement in Turbatrix aceti and Panagrellus redivivus. J Nematol 1969; 1:72-5; PMID:19325657
- Chitwood DJ. Biochemistry and function of nematode steroids. Crit Rev Biochem Mol Biol 1999; 34:273-84; PMID:10517647; https://doi.org/10.1080/10409239991209309
- Ritter KS. Steinernema feltiae (=Neoaplectana carpocapsae): effect of sterols and hypolipidemic agents on development. Exp Parasitol 1988; 67:257-67; PMID:3191959; https://doi.org/10.1016/0014-4894(88)90073-2
- Barrett J, Cain GD, Fairbairn D. Sterols in Ascaris lumbricoides (Nematoda), Macracanthorhynchus hirudinaceus and Moniliformis dubius (Acanthocephala), and Echinostoma revolutum (Trematoda). J Parasitol 1970; 56:1004-8; PMID:5504522; https://doi.org/10.2307/3277525
- Rothstein M. Nematode biochemistry. IX. Lack of sterol biosynthesis in free-living nematodes. Comp Biochem Physiol 1968; 27:309-17; PMID:5758374; https://doi.org/10.1016/0010-406X(68)90773-1
- Lu NC, Newton C, Stokstad ELR. The Requirement of Sterol and Various Sterol Precursors in Free-Living Nematodes Nematologica. 1977; 23:57-61
- Willett JD, Downey WL. Sterol biosynthesis in the free-living nematode Panagrellus redivivus. Biochem J 1974; 138:233-7; PMID:4822732; https://doi.org/10.1042/bj1380233
- Cole RJ, Krusberg LR. Sterol composition of the nematodes Ditylenchus triformis and Ditylenchus dipsaci, and host tissues. Exp Parasitol 1967; 21:232-9; PMID:6080012; https://doi.org/10.1016/0014-4894(67)90085-9
- Cheong MC, Na K, Kim H, Jeong SK, Joo HJ, Chitwood DJ, Paik YK. A potential biochemical mechanism underlying the influence of sterol deprivation stress on Caenorhabditis elegans longevity. J Biol Chem 2011; 286:7248-56; PMID:21186286; https://doi.org/10.1074/jbc.M110.189183
- Fielenbach N, Antebi A. C. elegans dauer formation and the molecular basis of plasticity. Genes Dev 2008; 22:2149-65; PMID:18708575; https://doi.org/10.1101/gad.1701508
- Vowels JJ, Thomas JH. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics 1992; 130:105-23; PMID:1732156
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000; 403:901-6; PMID:10706289; https://doi.org/10.1038/35002607
- Jia K, Albert PS, Riddle DL. DAF-9, a cytochrome P450 regulating C. elegans larval development and adult longevity. Development 2002; 129:221-31; PMID:11782415
- Ohkura K, Suzuki N, Ishihara T, Katsura I. SDF-9, a protein tyrosine phosphatase-like molecule, regulates the L3/dauer developmental decision through hormonal signaling in C. elegans. Development 2003; 130:3237-48
- Roberts B, Clucas C, Johnstone IL. Loss of SEC-23 in Caenorhabditis elegans causes defects in oogenesis, morphogenesis, and extracellular matrix secretion. Mol Biol Cell 2003; 14:4414-26; PMID:14551256; https://doi.org/10.1091/mbc.E03-03-0162
- Kang YL, Yochem J, Bell L, Sorensen EB, Chen L, Conner SD. Caenorhabditis elegans reveals a FxNPxY-independent low-density lipoprotein receptor internalization mechanism mediated by epsin1. Mol Biol Cell 2013; 24:308-18; PMID:23242996; https://doi.org/10.1091/mbc.E12-02-0163
- Roudier N, Lefebvre C, Legouis R. CeVPS-27 is an endosomal protein required for the molting and the endocytic trafficking of the low-density lipoprotein receptor-related protein 1 in Caenorhabditis elegans. Traffic 2005; 6:695-705; PMID:15998324; https://doi.org/10.1111/j.1600-0854.2005.00309.x
- Kamikura DM, Cooper JA. Lipoprotein receptors and a disabled family cytoplasmic adaptor protein regulate EGL-17/FGF export in C. elegans. Genes Dev 2003; 17:2798-811; PMID:14630941; https://doi.org/10.1101/gad.1136103
- Holmes A, Flett A, Coudreuse D, Korswagen HC, Pettitt J. C. elegans Disabled is required for cell-type specific endocytosis and is essential in animals lacking the AP-3 adaptor complex. J Cell Sci 2007; 120:2741-51; PMID:17636000; https://doi.org/10.1242/jcs.03474
- Kuervers LM, Jones CL, O'Neil NJ, Baillie DL. The sterol modifying enzyme LET-767 is essential for growth, reproduction and development in Caenorhabditis elegans. Mol Genet Genomics 2003; 270:121-31; PMID:12905072; https://doi.org/10.1007/s00438-003-0900-9
- Matyash V, Geier C, Henske A, Mukherjee S, Hirsh D, Thiele C, Grant B, Maxfield FR, Kurzchalia TV. Distribution and transport of cholesterol in Caenorhabditis elegans. Mol Biol Cell 2001; 12:1725-36; PMID:11408580; https://doi.org/10.1091/mbc.12.6.1725
- Merris M, Wadsworth WG, Khamrai U, Bittman R, Chitwood DJ, Lenard J. Sterol effects and sites of sterol accumulation in Caenorhabditis elegans: developmental requirement for 4alpha-methyl sterols. J Lipid Res 2003; 44:172-81; PMID:12518036; https://doi.org/10.1194/jlr.M200323-JLR200
- Wei JH, Yin X, Welander PV. Sterol Synthesis in Diverse Bacteria. Front Microbiol 2016; 7:990; PMID:27446030; https://doi.org/10.3389/fmicb.2016.00990
- Kiontke K, Sudhaus W. Ecology of Caenorhabditis species. WormBook 2006:1-14; PMID:18050464
- Fleming MW, Fetterer RH. Ascaris suum: continuous perfusion of the pseudocoelom and nutrient absorption. Exp Parasitol 1984; 57:142-8; PMID:6201385; https://doi.org/10.1016/0014-4894(84)90073-0
- Sym M, Basson M, Johnson C. A model for niemann-pick type C disease in the nematode Caenorhabditis elegans. Curr Biol 2000; 10:527-30; PMID:10801441; https://doi.org/10.1016/S0960-9822(00)00468-1
- Neufeld EB, Wastney M, Patel S, Suresh S, Cooney AM, Dwyer NK, Roff CF, Ohno K, Morris JA, Carstea ED, et al. The Niemann-Pick C1 protein resides in a vesicular compartment linked to retrograde transport of multiple lysosomal cargo. J Biol Chem 1999; 274:9627-35; PMID:10092649; https://doi.org/10.1074/jbc.274.14.9627
- Vanier MT. Niemann-Pick disease type C. Orphanet J Rare Dis 2010; 5:16; PMID:20525256; https://doi.org/10.1186/1750-1172-5-16
- Millat G, Marcais C, Tomasetto C, Chikh K, Fensom AH, Harzer K, Wenger DA, Ohno K, Vanier MT. Niemann-Pick C1 disease: correlations between NPC1 mutations, levels of NPC1 protein, and phenotypes emphasize the functional significance of the putative sterol-sensing domain and of the cysteine-rich luminal loop. Am J Hum Genet 2001; 68:1373-85; PMID:11333381; https://doi.org/10.1086/320606
- Li J, Brown G, Ailion M, Lee S, Thomas JH. NCR-1 and NCR-2, the C. elegans homologs of the human Niemann-Pick type C1 disease protein, function upstream of DAF-9 in the dauer formation pathways. Development 2004; 131:5741-52; PMID:15509773; https://doi.org/10.1242/dev.01408
- Zugasti O, Rajan J, Kuwabara PE. The function and expansion of the Patched- and Hedgehog-related homologs in C. elegans. Genome Res 2005; 15:1402-10; PMID:16204193; https://doi.org/10.1101/gr.3935405
- Hao L, Mukherjee K, Liegeois S, Baillie D, Labouesse M, Burglin TR. The hedgehog-related gene qua-1 is required for molting in Caenorhabditis elegans. Dev Dyn 2006; 235:1469-81; PMID:16502424; https://doi.org/10.1002/dvdy.20721
- Kuwabara PE, Lee MH, Schedl T, Jefferis GS. A C. elegans patched gene, ptc-1, functions in germ-line cytokinesis. Genes Dev 2000; 14:1933-44; PMID:10921907
- Hao L, Johnsen R, Lauter G, Baillie D, Burglin TR. Comprehensive analysis of gene expression patterns of hedgehog-related genes. BMC Genomics 2006; 7:280; PMID:; PMID:17076889; https://doi.org/10.1186/1471-2164-7-280
- Christ A, Christa A, Kur E, Lioubinski O, Bachmann S, Willnow TE, Hammes A. LRP2 is an auxiliary SHH receptor required to condition the forebrain ventral midline for inductive signals. Dev Cell 2012; 22:268-78; PMID:22340494; https://doi.org/10.1016/j.devcel.2011.11.023
- Burke R, Nellen D, Bellotto M, Hafen E, Senti KA, Dickson BJ, Basler K. Dispatched, a novel sterol-sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell 1999; 99:803-15; PMID:10619433; https://doi.org/10.1016/S0092-8674(00)81677-3
- Michaux G, Gansmuller A, Hindelang C, Labouesse M. CHE-14, a protein with a sterol-sensing domain, is required for apical sorting in C. elegans ectodermal epithelial cells. Curr Biol 2000; 10:1098-107; PMID:10996790; https://doi.org/10.1016/S0960-9822(00)00695-3
- Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, McMahon AP. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell 2001; 105:599-612; PMID:11389830; https://doi.org/10.1016/S0092-8674(01)00369-5
- Porter JA, Ekker SC, Park WJ, von Kessler DP, Young KE, Chen CH, Ma Y, Woods AS, Cotter RJ, Koonin EV. Hedgehog patterning activity: role of a lipophilic modification mediated by the carboxy-terminal autoprocessing domain. Cell 1996; 86:21-34; PMID:8689684; https://doi.org/10.1016/S0092-8674(00)80074-4
- Hao L, Aspock G, Burglin TR. The hedgehog-related gene wrt-5 is essential for hypodermal development in Caenorhabditis elegans. Dev Biol 2006; 290:323-36; PMID:16413526; https://doi.org/10.1016/j.ydbio.2005.11.028
- Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol 2004; 20:477-81; PMID:15363441; https://doi.org/10.1016/j.pt.2004.08.001
- Geurden T, Chartier C, Fanke J, di Regalbono AF, Traversa D, von Samson-Himmelstjerna G, Demeler J, Vanimisetti HB, Bartram DJ, Denwood MJ. Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe. Int J Parasitol Drugs Drug Resist 2015; 5:163-71; PMID:26448902; https://doi.org/10.1016/j.ijpddr.2015.08.001
- Srivastava M, Misra-Bhattacharya S. Overcoming drug resistance for macro parasites. Future Microbiology 2015; 10:1783-9; PMID:26517758; https://doi.org/10.2217/fmb.15.73
- WHO. WHO Model Prescribing Information: Drugs used in Parasitic Diseases, Second edition. 1995
- Roeber F, Jex AR, Gasser RB. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance - an Australian perspective. Parasit Vectors 2013; 6:153; PMID:23711194; https://doi.org/10.1186/1756-3305-6-153
- Nicol JM, Turner SJ, Coyne DL, den Nijs L, Hockland S, Tahna Maafi Z. Genomics and molecular genetics of plant-nematode interactions: Current Nematode Threats to World Agriculture. 2011
- Chisholm AD, Xu S. The Caenorhabditis elegans epidermis as a model skin. II: differentiation and physiological roles. Wiley Interdiscip Rev Dev Biol 2012; 1:879-902; PMID:23539358; https://doi.org/10.1002/wdev.79 10.1002/wdev.77
- Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 2011; 4:165-78; PMID:21324931; https://doi.org/10.1242/dmm.004077
- Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol 2011; 3; PMID:21917992; https://doi.org/10.1101/cshperspect.a005058
- Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012; 196:395-406; PMID:22351925; https://doi.org/10.1083/jcb.201102147

