Abstract
Crimean-Congo Hemorrhagic Fever (CCHF) is a severe tick-borne disease, endemic in many countries in Africa, the Middle East, Eastern Europe and Asia. Between 15–70% of reported cases are fatal with no approved vaccine available. In the present study, the attenuated poxvirus vector, Modified Vaccinia virus Ankara, was used to develop a recombinant candidate vaccine expressing the CCHF virus nucleoprotein. Cellular and humoral immunogenicity was confirmed in 2 mouse strains, including type I interferon receptor knockout mice, which are susceptible to CCHF disease. Despite the immune responses generated post-immunisation, the vaccine failed to protect animals from lethal disease in a challenge model.
Introduction
Crimean-Congo hemorrhagic fever (CCHF) virus causes a severe and frequently fatal hemorrhagic disease in people, with a mortality rate of approximately 30%.Citation1 CCHF virus has the most extensive geographical distribution of the medically important tickborne viral diseases.Citation2 It is the second most widespread of the medically important viral hemorrhagic fever viruses, after dengue virus,Citation3 and is described as an emerging virus; CCHF is distributed over much of Asia, the Middle East, Africa and expanding areas of south-eastern Europe. The continued spread of the tick vector and reservoir (Hyalomma species) through climate change and modern farming practices, has resulted in the virus becoming established in territories where it was not previously endemic; its introduction to Turkey, Greece and, more recently, Spain being testament to this.Citation4 CCHF virus is recognized as a possible agent of bioterrorism.Citation5 In Iraq, it was studied as a potential biological weapon,Citation6 and the virus has also been shown to be potentially disseminated via aerosolisation.Citation7
Recognized antiviral compounds or vaccines have not been proven to be effective against CCHF virus in controlled trials. A vaccine developed in Bulgaria and used there since 1974 is based on CCHF virus derived from suckling mouse brain and inactivation by chloroform.Citation8 The vaccine elicited both cell-mediated and humoral immunity, but multiple doses were required before neutralisation activity was observed; even then the activity was low.Citation9 To date, there are no controlled efficacy studies and the vaccine is unlicensed by the European Medicines Agency or the US Food and Drug Administration. Due to its crude preparation, it is unlikely to gain widespread international regulatory approval.
Recent vaccine approaches for CCHF include a DNA-based vaccine expressing the glycoprotein-encoding region of the virus, which induced neutralising antibodies in approximately half of vaccinated mice.Citation10 Another vaccine candidate used transgenic tobacco leaves expressing the CCHF viral glycoproteins, which were fed to mice and induced both IgG and IgA.Citation11 However, neutralisation activity was not tested and neither vaccine approach has been tested for protection against lethal disease using a challenge model, so efficacy has not been assessed. The most promising CCHF vaccine candidate published to date is a Modified Vaccinia Ankara (MVA) vector expressing the full-length glycoproteins which induced humoral and cellular immunity, along with protection in an adult small animal model of CCHF virus infection.Citation12
The genome of CCHF virus is distributed over 3 RNA segments: small (S), medium (M) and large (L) which encode the viral nucleoprotein (NP), glycoprotein and RNA polymerase, respectively. While all the vaccine reports published to date have focused on the M segment,Citation10–12 there is compelling evidence that a vaccine based on the S segment would be a feasible alternative. The NP is recognized as the predominant antigen, inducing a high immune response in most Bunyavirus infections;Citation13 it is also highly conserved between strains.Citation14 Additionally, the NP has been used as an antigenic target for vaccines that have demonstrated protective effects in a range of viral diseases (). Of particular interest is the protective effect that the NP antigen has shown against 2 other viruses of the same Bunyaviridae family of which CCHF virus is a member: HantavirusCitation15 and Rift Valley fever virus.Citation16
Table 1. Summary of the vaccines against viral diseases reported that have the viral nucleoprotein as the sole target antigen
The NP of CCHF virus consists of a large, globular domain, plus a protrusion that contains a conserved caspase-3 cleavage site.Citation17 The globular region is responsible for RNA binding,Citation18 while the role of the caspase-3 cleavage site is currently unclear. It has been shown that the nucleoprotein is cleaved in apoptotic cells at later stages of infection,Citation19 and that it may play a regulatory role as RNA polymerase is increased when cleavage is disrupted.Citation18 NP formation is essential for virus multiplication and therefore represents a potential vaccination target.Citation17
This report documents the incorporation of the CCHF virus S segment in a Modified Vaccinia virus Ankara (MVA) vector. The vaccine candidate was then tested for immunogenicity and efficacy using murine models.
Results
In vitro expression of MVA-NP constructs
In order to verify the proper expression of the inserted CCHF NP, MVA-NP3010 was used in a Western blot assay. Using an anti-V5 antibody to ascertain the molecular size of the inserted protein, a band of approximately 62.5 kDa was observed. This was consistent with the estimated size of the CCHF NP of 52 kDa,Citation29 plus the V5 tag and tPA regions.
Immunogenicity of MVA-NP
Effects of type-1 interferon receptor deficiency on vaccine induced immunity
Immunogenicity studies used A129 and 129Sv/Ev mouse strains to represent a susceptible CCHF host and the parent wild-type strain, respectively. To assess whether the type-1 interferon receptor deficiency possessed by the A129 mice affected the vaccine-induced immune responses, both strains of mice were immunised with MVA-NP3010 vaccine. As observed in , MVA-NP3010 induced similar numbers of IFN-γ secreting cells specific to peptides derived from CCHF NP in both strains of mice (P > 0 .05, Mann-Whitney statistical test). Responses to the tPA and V5 tags were similarly low in both groups, demonstrating the specificity of the response to the inserted protein. Furthermore, as shown in , Western blot analysis revealed that all (5/5) A129 mice developed an antibody response specific to NP as did all 129Sv/Ev mice (5/5). Therefore, a deficiency in the type-1 interferon receptor did not affect the induction of humoral or cell-mediated immune responses of the MVA-NP3010 vaccine in these assays.
Figure 1. IFN-γ ELISpot responses from A129 and 129Sv/Ev mice vaccinated with MVA-NP3010 (black), MVA-1974 (gray) or saline (white). Splenocytes from vaccinated mice were restimulated with peptides derived from the CCHFv nucleoprotein split into 2 pools and summed, or a single pool containing peptides from the tPA and V5 fusion partners. Mean ± SEM is plotted .
Figure 2. Antibody responses from A129 and 129Sv/Ev mice vaccinated with MVA-NP3010. A representative animal from each mouse strain is shown. Sera from vaccinated A129 and 129Sv/Ev mice were tested for reactivity with a baculovirus-expression recombinant CCHFv NP (lane 4) and CCHFv-infected (lane 3) or uninfected (lane 2) SW13 cells by Western blotting. Lane 1 shows a molecular weight marker.
Immunogenicity of MVA-NP10200
To determine the efficacy of the vaccine, a challenge model developed for infection with strain IbAr10200 of CCHF virus was planned. Therefore, to develop a homologous vaccine the NP insert was changed to the equivalent region of this strain. To ensure that the new construct conferred similar immune responses in vaccinated animals, A129 mice were vaccinated with 2 doses separated by 2 weeks, and then 2 weeks after the final dose 3 animals were culled. As shown in , a NP-specific T-cell response was detected in all of the vaccinated animals. Additionally, as demonstrated in , NP-specific antibodies were detected in all of the animals tested. Therefore, MVA-NP10200 was taken forward for testing in efficacy studies.
Figure 3. IFN-γ ELISpot responses from A129 mice vaccinated with MVA-NP10200, MVA-1974 or saline. Splenocytes from vaccinated mice were restimulated with peptides derived from the CCHFv nucleoprotein split into 2 pools and summed, or a single pool containing peptides from the tPA and V5 proteins. Black, white and gray bars denote animals given saline, MVA-1974 and MVA-NP, respectively. Mean ± SEM is plotted.
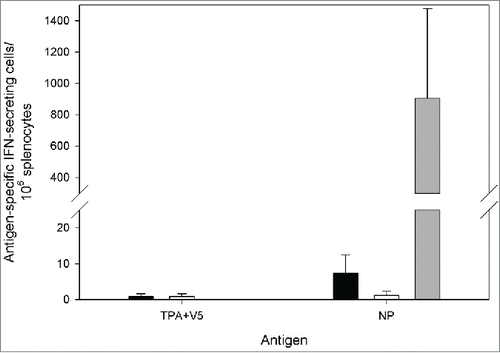
Figure 4. Antibody response from a representative A129 mouse vaccinated with MVA-NP10200. Sera from vaccinated A129 mice were tested for reactivity with a baculovirus-expression recombinant CCHFv NP (lane 4) and CCHFv-infected (lane 3) or uninfected (lane 2) SW13 cells by Western blotting. Lane 1 shows a molecular weight marker.
Efficacy of MVA-NP
Survival
A129 mice (n = 9 per group) were vaccinated with 2 doses of MVA-NP10200 spaced at 2 weeks intervals. At a time point 2 weeks after the final vaccination, they were challenged with a lethal dose of CCHF virus. As seen in , despite the induction of NP-specific immunity prior to challenge, all mice succumbed to the lethal infection between days 4 and 5 post-challenge.
Viral load
To assess whether the MVA-NP10200 vaccine gave any reduction in viral load in the target sites of CCHF viral replication, viral load analysis using RT-PCR was conducted. Blood, spleen and liver were collected 4 d post-challenge from animals challenged with MVA-1974 and MVA-NP10200. As seen in , there were no observable differences between animals challenged with the empty vector versus those that were immunised with the CCHF vaccine candidate.
Figure 6. Normalized viral load analysis of CCHFv RNA by RT-PCR. A129 mice were challenged with double the minimum lethal dose of CCHFv, 14 d after booster vaccination with MVA-NP10200 (gray bars) or MVA-1974 (black bars). Four days post-challenge, 3 randomly selected animals from each group were killed humanely and analyzed by RT-PCR for CCHFv gene expression, normalized to mouse HPRT gene expression. Data show mean ± SEM.
Histopathology
In the spleen, patchy to diffuse infiltration of parenchyma by macrophages was noted, primarily involving the red pulp, with varying degrees of effacement of white pulp (). In addition, lymphocyte loss and apoptosis were observed, with the latter characterized by the presence of tingeable body macrophages and apoptotic bodies. Using immunohistochemistry, positively staining cells, consistent with macrophages, were observed, diffusely scattered throughout the parenchyma, primarily within the red pulp. In the liver, changes comprised multifocal, hepatocyte necrosis, characterized by cytoplasmic eosinophilia and nuclear pyknosis and accompanied frequently with a mixed inflammatory cell infiltration, mainly of polymorphonuclear leukocytes (). Positively stained hepatocytes, detected by immunohistochemistry, were observed scattered throughout the parenchyma.
Table 2. Severity of spleen and liver lesions in challenged mice: distribution in treatment groups
Discussion
To elicit an immune response against the CCHF viral NP, a Modified Vaccinia virus Ankara (MVA) viral vector was used. MVA is one of the most advanced recombinant poxviral vaccine vectors used in human clinical trials,Citation30 and elicits both humoral and cellular immune responses.Citation31 This latter point is particularly pertinent as there is no defined correlate of protection against CCHF virus so the priming of both arms of the immune system may offer the best opportunity to observe protective effects. The results of these studies confirmed the induction of both antibody and cell-mediated immunity and it is noteworthy that the Bulgarian vaccine based on suckling mouse brain (inactivated by chloroform, heated at 58°C, and absorbed on aluminum hydroxide) also induced T-cell and humoral immunity,Citation9 but efficacy of this vaccine has yet to be tested in animal models. For our studies, a homologous prime-boost approach was undertaken as we have demonstrated that this induced increased numbers of antigen-specific T cells compared to a single dose (data not shown). This finding is in line with others who have used similar approaches with MVA-based vaccines against Mycobacterium tuberculosis.Citation32 and human immunodeficiency virus (HIV).Citation33 Repeat administration with MVA allows reboosting of responses, despite induction of cellular and humoral immune responses against the vector.Citation34 This has been reported in Phase I/II therapeutic cancer vaccine trial.Citation35 as well as in a Phase I HIV vaccine trial.Citation36
This use of NP is in contrast to others who have opted to use the envelope glycoprotein as the candidate antigenic target.Citation10,11 While the external location of the glycoprotein makes it a favorable target for the induction of neutralising antibody, it has been recognized that there is not a strict correlation between in vitro neutralisation and in vivo protection of CCHF virus-specific antibodies.Citation37 The NP was considered to be an appropriate vaccine antigen, due to several characteristics. The NP in Bunyavirus infection has been recognized as the predominant antigen, inducing a high immune response.Citation13 and after challenge with CCHF, it has been shown that most antibodies are directed to NP.Citation38
Despite the induction of humoral responses for recent CCHF vaccine candidates,Citation10,11 neither vaccine approach was tested for efficacy, presumably due to the lack of a suitable animal model at that time. In 2010, 2 murine models susceptible to CCHF virus were published, with deletions in either STAT-1.Citation39 or the type-I interferon receptor.Citation40 The STAT-1 knockout mice exhibit signaling defects in their response to all 3 major types of interferon (type I, IFN-α and IFN-β; type II, IFN-γ; and type III, IFN-λ) that leads to a complete abolishment of the intracellular interferon response.Citation41 For the efficacy studies with the MVA-NP10200 vaccine candidate, mice deficient in the type-I interferon receptor were used since these have less immune deficiency. This was considered essential for testing vaccination approaches as IFN-γ is a major cytokine involved in the adaptive immune response.Citation42,43 For the immunogenicity of the MVA-NP vaccine, we compared the immune responses in mice with the type-I interferon receptor deficiency and the parental wild-type strain. No differences were observed in either the antibody or cell-mediated response, demonstrating that the knockout mice elicited similar responses. This was unsurprising, as others have also reported similar findings in studies with dengue virus.Citation44-46 The type-I IFN receptor knockout mice are also valuable for studying the efficacy of vaccines, as has been shown with those against Chikungunya virus,Citation47 Bluetongue virus,Citation48 Vaccinia virus.Citation49 and African Horse Sickness virus.Citation50 Therefore, our finding that the CCHF MVA-NP vaccine did not demonstrate any protective effects seems unlikely to be a consequence of the animal model utilised.
In the present studies, antibody-induced responses using Western Blot analysis using virus infected cell lysate and recombinant CCHF viral nucleoprotein as antigen were assessed. While this did not allow us to quantify antibody levels or elucidate the subclass of immunoglobulin, it did demonstrate specific antibody recognition of the protein target. Due to the NP being internally located, its main effects in viral immunity are through T lymphocytes.Citation51 However, although it recognized that antibodies against viral NP are often poor at neutralisation.Citation52 others effects include complement-mediated cytolysis,Citation53 increased T cell responses associated with enhanced dendritic cell function.Citation54 and reduced viral replication in culture.Citation55 The protective role of CCHF immunoglobulin is currently unknown, as although immune globulin therapy has been administered on several occasions its efficacy has still not been assessed in a randomized clinical trial.Citation56,57 Should the vaccine have demonstrated protective effects, further work would have been warranted in deciphering the immune response by depletion or transfer experiments.Citation58,59 Similarly, while the T-cell response looked at IFN-γ recall responses after stimulation with overlapping peptide pools, further work to identify the responding cell phenotypes, function and cytokine/chemokine secretory patterns could have been conducted had the vaccine shown any positive effect.
To our knowledge, this is the first report of a vaccine against CCHF virus based on the nucleoprotein. The MVA-NP vaccine candidate demonstrated antigen-specific immunogenicity in mice, but failed to exert any protective effects upon challenge with CCHF virus. This demonstrates that with the lack of any immune correlates of protection, vaccines against CCHF virus will need to demonstrate protection in a lethal dose model before protective efficacy can be established.
Materials and Methods
Cells
BHK-21 cells (American Type Culture Collection, USA) were cultured in modified essential eagle medium (Sigma, UK) supplemented with 10% foetal bovine serum, 2 mM L-glutamine, 100 U penicillin and 0.1 mg/ml streptomycin (Sigma). Chick Embryonic Fibroblast (CEF) cells (Institute for Animal Health, UK) were cultured in Dulbecco's modified eagle medium (Sigma) supplemented as above. SW13 and VeroE6 cells (European Collection of Cell Cultures, UK) were maintained in Leibovitz's L-15 medium containing Glutamax (Life Technologies, UK) supplemented with 10% foetal bovine serum (Sigma).
Viruses
MVA strain 1974/NIH clone 1 (kindly supplied by Prof B. Moss, NIH) was used for the vaccine construct. Virus titer was determined by plaque assay in BHK-21 cells. CCHF virus strain IbAr10200 was prepared from suckling mouse brain homogenate. Titer was determined by TCID50 in VeroE6 cells.
Animals
A129 (IFN-α/βR−/−) or 129Sv/Ev (both from B&K Universal, UK) aged 5–8 weeks were used. Animal studies were approved by the ethical review process of Public Health England, UK and the UK Home Office, via project licenses. All work involving animals was performed in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986.
Construction of plasmids
Plasmid pLW-44 (kindly provided by Prof B. Moss, NIH) encoded the green fluorescence protein (GFP) reporter gene under control of the p11 promoter and an expression cassette controlled by the artificial promoter, mH5, which allowed constitutive expression of heterologous genes.Citation60 The mH5 promoter demonstrates increased stability.Citation61 Plasmid pDEST44-TPA-V5 was derived by inserting a cassette of pLW-44. The cassette contained Gateway system attR recombination sequences (Life Technologies, UK) flanked by the human tissue plasminogen activator (tPA) leader sequence to increase secretion and neutralising antibody induction,Citation62,63 and a C-terminal V5 tag between the Xmal and SalI restriction sites for in vitro immunodetection.
The NP open reading frames from the S segments of CCHF strains 3010 (Accession number DQ099335) and IbAr10200 (Accession number NC_005302) were used in this work to generate plasmid pENTR-NP (pENTR-NP3010 and pENTR-NP10200, respectively).
Plasmid pDEST44-TPA-V5 was recombined with pENTR-NP using Gateway technology.Citation64 to generate plasmid pTP-NP. The resulting plasmids encoded the respective tPA-NP-V5 fusion proteins downstream of the poxvirus mH5 promoter.
Generation and characterization of recombinant MVA expressing CCHFv nucleoprotein
BHK-21 cells were infected with MVA at a multiplicity of infection of 0.05. Infected cells were transfected with pTP-NP using Lipofectamine (Life Technologies) as directed by the manufacturer. Resulting recombinant MVA-NP was serially plaque-purified 4 times in BHK-21 cells, based on GFP expression. MVA-NP was amplified on BHK-21 and CEF cells, purified by sucrose cushion centrifugation.Citation65 and titrated by plaque assay on BHK-21 cells, prior to use with in vivo studies. Plaques were visualised using GFP fluorescence or by immunostaining.Citation66 with rabbit anti-Vaccinia antibody (AbD Serotec, UK) and Vectastain Universal ABC-AP kit (Vector Laboratories, USA). Genomic DNA from infected cells was extracted using the Wizard SV genomic DNA purification system (Promega, UK) and used as a template for PCR with AccuPrime Taq DNA polymerase High Fidelity (Life Technologies, UK). Quality control testing to ensure expression of the insert proteins was confirmed in the resulting MVA-NP3010 and MVA-NP10200 by and Western Blotting and quantity of MVA evaluated using plaque assays in BHK-21 cells.
MVA-NP vaccination
Groups of 5–12 mice were injected into the caudal aspect of the proximal hindlimb musculature with 107 plaque-forming units (pfu) per animal of MVA-NP diluted in endotoxin-free PBS. A total volume of 100 μl was delivered equally across 2 sites. Animals received a booster vaccination 14 d later. Control animals received 107 pfu of non-recombinant MVA 1974 or an equivalent volume of saline. Animals were euthanised and tissues were collected 7 or 14 d after the final vaccination.
Interferon-gamma (IFN-γ) ELISpot assay
Splenocytes were assessed for antigen recall response via IFN-γ ELISpot (Mabtech, Sweden), performed as per the manufacturer's instructions. Cells were seeded in PVDF microtitre plates at 2 × 105 splenocytes per well and re-stimulated with peptide pools (Mimotopes, Australia). Overlapping peptides spanning the length of the CCHF virus nucleoprotein consisting of 20mers, offset by 8 residues, were applied at a final concentration of 25 μg/ml per peptide in pools of 28–32 peptides. Plates were developed after 18 hours at 37°C in a humidified incubator supplemented with 5% CO2. Spots were counted visually on an automated ELISpot reader (Autoimmun Diagnostika GmbH, Germany). Background values from wells containing medium but no peptides were subtracted and pools were summed across the target protein. Results were expressed as spot forming units (SFU) per 106 cells.
Antibody testing by Western Blot
SW13 cell monolayers were infected with CCHF virus strain IbAr10200 at a multiplicity of infection (MOI) of approximately 0.01, and incubated at 37°C in Leibovitz's L-15 medium containing 2% foetal bovine serum. 48 hours post-infection, the medium was removed and the cells were treated with Laemmli buffer supplemented to contain 10% sodium dodecyl sulfate (SDS) (Sigma). The resultant mixture was collected into vials and heat treated at 90°C for 10 minutes before use in Western Blot analysis. Uninfected SW13 monolayers were treated similarly for use as a negative control.
Lysates from CCHFv-infected or uninfected SW13 cells were subjected to SDS-PAGE on a 4–12% Bis-Tris gel (Life Technologies) and transferred to a PVDF membrane. After blocking in 5% milk protein, membranes were incubated with mouse serum for 2 hours, washed 6 times with PBS containing 0.05% NP40, incubated for 1 hour with HRP-conjugated rabbit anti-mouse IgG (Sigma) or goat anti-mouse IgG/A/M (AbD Serotec) and washed as before. All antibody dilutions were made in PBS containing 0.05% NP40 and 5% milk protein. Bound antibody was detected with ECL-Prime WB detection reagent (GE Life Sciences, UK) according to the manufacturer's directions and visualised on a ChemiDoc system (BioRad, UK). Molecular weights were calculated by comparison with markers of known molecular weight using QuantityOne software.
CCHF virus challenge in A129 mice
Fourteen days after the final vaccination with MVA-NP or a control substance, A129 mice received 200 TCID50 CCHF virus strain IbAr10200 intradermally in the midline of the lumbar region in a volume of 100 μl divided equally across 2 sites. 50 μl is the maximum recommended volume for intradermal inoculation of mice.Citation67 and confirmation of intradermal delivery was seen by a visible bleb formation under the skin. Post challenge, animals were weighed and body temperature measured daily by a subcutaneously located temperature chip. In addition, they were observed for clinical signs of disease twice daily (arching, ruffled fur, lethargy and immobility). Criteria for euthanasia on welfare grounds consisted of 20% weight loss or observation of 2, abnormal clinical signs. At 4 d post-challenge, randomly selected animals were euthanised and samples of blood, spleen and liver collected for viral load studies. Spleen and liver samples were also collected for histopathological examination.
Viral load analysis
Whole blood (100 μl) was collected into RNA Protect Animal Blood tubes (Qiagen) and stored at −80°C. Tubes were thawed, inverted and left for a further 2 hours at room temperature to ensure efficient cell lysis. Samples were treated with Red Blood Cell Lysis Solution (Miltenyi Biotec) before purification of total RNA using an RNeasy Mini kit (Qiagen).
For viral load analysis, spleen and liver samples were collected into RNALater (Qiagen) and stored at −80°C. Thawed tissue was transferred to RLT buffer (Qiagen), homogenized by passing through a 70 μm sieve and then treated using an RNeasy Mini kit (Qiagen) for extraction of total RNA.
CCHFv S segment was detected by RT-PCR on the ABi 7500 RT-PCR platform as described (Atkinson et al, 2012), with cycling conditions adjusted to those described in the QuantiFast probe assay: 50°C for 20 min, 96°C for 5 min, followed by 45 cycles of 95°C for 15 sec and 60°C for 30 sec (with quantification analysis of fluorescence performed at the end of each 60°C step), and final cooling of 40°C for 30 sec. A synthetic S segment of known concentration was used to quantify S segment copy number in blood and tissue samples. All reactions were run in triplicate.
To normalize the CCHFv expression data, the hypoxanthine guanine phosphoribosyl transferase (HPRT) housekeeping gene was used. A one-step RT-PCR with singleplex detection was performed targeting an 89 bp product in the mouse HPRT gene (NCBI Reference sequence NM_013556) using the QuantiFast probe assay (Qiagen) and the ABi 7500 RT-PCR platform. CT values for CCHFv and HPRT were each inverted by subtracting the CT value from 45 (the total number of cycles), where CT is the number of cycles to reach the fluorescence threshold value. The mean value of CCHFv was then divided by the mean value of the HPRT reference gene for each sample.
Histopathology studies
Samples of spleen and liver were placed in 10% neutral buffered formalin for 7 days, processed routinely to paraffin wax, sections cut at 3–5 μm, stained with haematoxylin and eosin (H&E) and examined microscopically. Lesions referable to infection with CCHF virus were scored subjectively using the following scale: normal, minimal, mild, moderate and marked.
For immunohistochemistry, formalin-fixed, paraffin-embedded sections of spleen and liver, cut between 3–5 μm, were mounted on positively charged X-tra Adhesive slides (Leica Biosystems, UK), deparaffinised and rehydrated. Immunohistochemical staining was achieved using a BOND-MAX Immunostainer (Leica Microsystems, UK) and a Novacastra Bond Intense R (Leica Biosystems) detection kit. A heat-induced epitope retrieval cycle with buffer ER1 (Leica Biosystems) was performed for 20 minutes. Slides were incubated with rabbit serum (4%) (Abcam, Cambridge, UK) for 20 minutes followed by an avidin/biotin blocking stage (15 minutes each) (Abcam). Polyclonal antibody raised in sheep immunised against recombinant CCHFv nucleoprotein (kindly provided by Dr John Barr, University of Leeds, UK) was incubated with the tissue for 30 minutes, followed by a biotinylated rabbit anti-sheep polyclonal antibody (Abcam) at a dilution of 1500, for 10 minutes. Haematoxylin was used as the counterstain. Positive and negative control slides were included. Immunolabelled slides were evaluated using light microscopy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank the staff of the Biological Investigations Group at PHE Porton for their technical expertise in conducting the animal experiments and Laura Hunter for processing histopathology samples. The views expressed in this publication are those of the author(s) and not necessarily those of the UK Department of Health.
References
- WHO, Crimean-Congo haemorrhagic fever. Factsheet No208, 2013; http://www.who.int/mediacentre/fact sheets/fs208/en/
- Ergonul O Crimean-Congo haemorrhagic fever. Lancet Infect Dis, 2006; 6(4): p:203-14; PMID:16554245; http://dx.doi.org/10.1016/S1473-3099(06)70435-2
- Watts DM, Kziasek TG, Linthicum KJ, Hoogstraal H.Crimean-Congo hemorrhagic fever. In: Monath TP (ed.) The Arboviruses: Epidemiology and Ecology. Vol 2. Boca Raton, FL: CRC Press; 1988; 177-260
- Maltezou HC, Andonova L, Andraghetti R, Bouloy M, Ergonul O, Jongejan F, Kalvatchev N, Nichol S, Niedrig M, Platonov A, et al. Crimean-Congo hemorrhagic fever in Europe: current situation calls for preparedness. Euro Surveill 2010; 15(10): p:19504; PMID:20403306
- Sidwell RW, Smee DF. Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res 2003; 57(1-2): p:101-11; PMID:12615306; http://dx.doi.org/10.1016/S0166-3542(02)00203-6
- Zilinskas RA. Iraq's biological weapons. The past as future? Jama 1997; 278(5): p:418-24; PMID:9244334; http://dx.doi.org/10.1001/jama.1997.03550050080037
- Bronze MS, Huycke MM, Machado LJ, Voskuhl GW, Greenfield RA. Viral agents as biological weapons and agents of bioterrorism. Am J Med Sci 2002; 323(6): p:316-25; PMID:12074486; http://dx.doi.org/10.1097/00000441-200206000-00004
- Papa A, Papadimitriou E, Christova I. The Bulgarian vaccine Crimean-Congo haemorrhagic fever virus strain. Scand J Infect Dis 2011; 43(3): p:225-9; PMID:21142621; http://dx.doi.org/10.3109/00365548.2010.540036
- Mousavi-Jazi M, Karlberg H, Papa A, Christova I, Mirazimi A. Healthy individuals' immune response to the Bulgarian Crimean-Congo hemorrhagic fever virus vaccine. Vaccine 2012; 30(44): p:6225-9; PMID:22902680; http://dx.doi.org/10.1016/j.vaccine.2012.08.003
- Spik K, Shurtleff A, McElroy AK, Guttieri MC, Hooper JW, SchmalJohn C. Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine 2006; 24(21): p:4657-66; PMID:16174542; http://dx.doi.org/10.1016/j.vaccine.2005.08.034
- Ghiasi SM, Salmanian AH, Chinikar S, Zakeri S. Mice orally immunized with a transgenic plant expressing the glycoprotein of Crimean-Congo hemorrhagic fever virus. Clin Vaccine Immunol 2011; 18(12): p:2031-7; PMID:22012978; http://dx.doi.org/10.1128/CVI.05352-11
- Buttigieg KR, Dowall SD, Findlay-Wilson S, Miloszewska A, Rayner E, Hewson R, Carroll MW. A novel vaccine against Crimean-Congo Haemorrhagic Fever protects 100% of animals against lethal challenge in a mouse model. PLoS One 2014; 9(3): p:e91516; PMID:24621656; http://dx.doi.org/10.1371/journal.pone.0091516
- Schmaljohn CS, Nichol ST, Bunyaviridae, in Fields Virology, Knipe DM, Howley PM, Editors. Lippincott Williams & Wilkins: Philadelphia 2007. p:1741-1789
- Hewson R, Chamberlain J, Mioulet V, Lloyd G, Jamil B, Hasan R, Gmyl A, Gmyl L, Smirnova SE, Lukashev A, et al. Crimean-Congo haemorrhagic fever virus: sequence analysis of the small RNA segments from a collection of viruses world wide. Virus Res 2004; 102(2): p:185-9; PMID:15084400; http://dx.doi.org/10.1016/j.virusres.2003.12.035
- Maes P, Clement J, Van Ranst M. Recent approaches in hantavirus vaccine development. Expert Rev Vaccines 2009; 8(1): p:67-76; PMID:19093774; http://dx.doi.org/10.1586/14760584.8.1.67
- Boshra H, Lorenzo G, Rodriguez F, Brun A. A DNA vaccine encoding ubiquitinated Rift Valley fever virus nucleoprotein provides consistent immunity and protects IFNAR(−/−) mice upon lethal virus challenge. Vaccine 2011; 29(27): p:4469-75; PMID:21549790; http://dx.doi.org/10.1016/j.vaccine.2011.04.043
- Carter SD, Surtees R, Walter CT, Ariza A, Bergeron É, Nichol ST, Hiscox JA, Edwards TA, Barr JN. Structure, function, and evolution of the Crimean-Congo hemorrhagic fever virus nucleocapsid protein. J Virol 2012; 86(20): p:10914-23; PMID:22875964; http://dx.doi.org/10.1128/JVI.01555-12
- Wang Y, Dutta S, Karlberg H, Devignot S, Weber F, Hao Q, Tan YJ, Mirazimi A, Kotaka M. Structure of Crimean-Congo hemorrhagic fever virus nucleoprotein: superhelical homo-oligomers and the role of caspase-3 cleavage. J Virol 2012; 86(22): p:12294-303; PMID:22951837; http://dx.doi.org/10.1128/JVI.01627-12
- Karlberg H, Tan YJ, Mirazimi A. Induction of caspase activation and cleavage of the viral nucleocapsid protein in different cell types during Crimean-Congo hemorrhagic fever virus infection. J Biol Chem 2011; 286(5): p:3227-34; PMID:21123175; http://dx.doi.org/10.1074/jbc.M110.149369
- Wilson JA, Hart MK. Protection from Ebola virus mediated by cytotoxic T lymphocytes specific for the viral nucleoprotein. J Virol 2001; 75(6): p:2660-4; PMID:11222689; http://dx.doi.org/10.1128/JVI.75.6.2660-2664.2001
- Tsuda Y, Caposio P, Parkins CJ, Botto S, Messaoudi I, Cicin-Sain L, Feldmann H, Jarvis MA. A replicating cytomegalovirus-based vaccine encoding a single Ebola virus nucleoprotein CTL epitope confers protection against Ebola virus. PLoS Negl Trop Dis 2011; 5(8): p:e1275; PMID:21858240; http://dx.doi.org/10.1371/journal.pntd.0001275
- Xu X, Ruo SL, McCormick JB, Fisher-Hoch SP. Immunity to Hantavirus challenge in Meriones unguiculatus induced by vaccinia-vectored viral proteins. Am J Trop Med Hyg 1992; 47(4): p:397-404; PMID:1359802
- Epstein SL, Kong WP, Misplon JA, Lo CY, Tumpey TM, Xu L, Nabel GJ. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine 2005; 23(46-47): p:5404-10; PMID:16011865; http://dx.doi.org/10.1016/j.vaccine.2005.04.047
- Hashem A, Jaentschke B, Gravel C, Tocchi M, Doyle T, Rosu-Myles M, He R, Li X. Subcutaneous immunization with recombinant adenovirus expressing influenza A nucleoprotein protects mice against lethal viral challenge. Hum Vaccin Immunother 2012; 8(4): p:425-30; PMID:22370512; http://dx.doi.org/10.4161/hv.19109
- Clegg JC, Lloyd G. Vaccinia recombinant expressing Lassa-virus internal nucleocapsid protein protects guineapigs against Lassa fever. Lancet 1987; 2(8552): p. 186-8; PMID:2885642; http://dx.doi.org/10.1016/S0140-6736(87)90767-7
- Bankamp B, Brinckmann UG, Reich A, Niewiesk S, ter Meulen V, Liebert UG. Measles virus nucleocapsid protein protects rats from encephalitis. J Virol 1991; 65(4): p:1695-700; PMID:1825854
- Ozols DY, Rawls WE, Rosenthal KL, Harnish DG. The nucleoprotein of Pichinde virus expressed by a vaccinia-Pichinde virus recombinant partially protects hamsters from lethal virus challenge. Arch Virol 1994; 139(1–2): p“23-36; PMID:7826212; http://dx.doi.org/10.1007/BF01309452
- Lodmell DL, Sumner JW, Esposito JJ, Bellini WJ, Ewalt LC. Raccoon poxvirus recombinants expressing the rabies virus nucleoprotein protect mice against lethal rabies virus infection. J Virol 1991; 65(6): p:3400-5; PMID:2033678
- Marriott AC, Nuttall PA. Comparison of the S RNA segments and nucleoprotein sequences of Crimean-Congo hemorrhagic fever, Hazara, and Dugbe viruses. Virology 1992; 189(2): p. 795-9; PMID:1641991; http://dx.doi.org/10.1016/0042-6822(92)90609-S
- Gomez CE, Nájera JL, Krupa M, Perdiguero B, Esteban M. MVA and NYVAC as vaccines against emergent infectious diseases and cancer. Curr Gene Ther 2011; 11(3): p:189-217; PMID:21453284; http://dx.doi.org/10.2174/156652311795684731
- Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AV. Viral vectors as vaccine platforms: deployment in sight. Curr Opin Immunol 2011; 23(3): p:377-82; http://dx.doi.org/10.1016/j.coi.2011.03.006
- Kolibab K, Yang A, Derrick SC, Waldmann TA, Perera LP, Morris SL. Highly persistent and effective prime/boost regimens against tuberculosis that use a multivalent modified vaccine virus Ankara-based tuberculosis vaccine with interleukin-15 as a molecular adjuvant. Clin Vaccine Immunol 2010; 17(5): p:793-801; PMID:20357059; http://dx.doi.org/10.1128/CVI.00006-10
- Mehendale S, Thakar M, Sahay S, Kumar M, Shete A, Sathyamurthi P, Verma A, Kurle S, Shrotri A, Gilmour J, et al. Safety and immunogenicity of DNA and MVA HIV-1 subtype C vaccine prime-boost regimens: a phase I randomised Trial in HIV-uninfected Indian volunteers. PLoS One 2013; 8(2): p:e55831; PMID:23418465; http://dx.doi.org/10.1371/journal.pone.0055831
- Cottingham MG, Carroll MW. Recombinant MVA vaccines: dispelling the myths. Vaccine 2013; 31(39): p:4247-51; PMID:23523407; http://dx.doi.org/10.1016/j.vaccine.2013.03.021
- Harrop R, Shingler W, Kelleher M, de Belin J, Treasure P. Cross-trial analysis of immunologic and clinical data resulting from phase I and II trials of MVA-5T4 (TroVax) in colorectal, renal, and prostate cancer patients. J Immunother 2010; 33(9): p:999-1005; PMID:20948436; http://dx.doi.org/10.1097/CJI.0b013e3181f5dac7
- Goepfert PA, Elizaga ML, Sato A, Qin L, Cardinali M, Hay CM, Hural J, DeRosa SC, DeFawe OD, Tomaras GD, et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2011; 203(5): p:610-9; PMID:21282192; http://dx.doi.org/10.1093/infdis/jiq105
- Bertolotti-Ciarlet A, Smith J, Strecker K, Paragas J, Altamura LA, McFalls JM, Frias-Stäheli N, García-Sastre A, Schmaljohn CS, Doms RW. Cellular localization and antigenic characterization of crimean-congo hemorrhagic fever virus glycoproteins. J Virol 2005; 79(10): p. 6152-61; PMID:15858000; http://dx.doi.org/10.1128/JVI.79.10.6152-6161.2005
- Blackburn NK, Besselaar TG, Shepherd AJ, Swanepoel R. Preparation and use of monoclonal antibodies for identifying Crimean-Congo hemorrhagic fever virus. Am J Trop Med Hyg 1987; 37(2): p:392-7; PMID:3116871
- Bente DA, Alimonti JB, Shieh WJ, Camus G, Ströher U, Zaki S, Jones SM. Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J Virol 2010; 84(21): p:11089-100; PMID:20739514; http://dx.doi.org/10.1128/JVI.01383-10
- Bereczky S, Lindegren G, Karlberg H, Akerström S, Klingström J, Mirazimi A. Crimean-Congo hemorrhagic fever virus infection is lethal for adult type I interferon receptor-knockout mice. J Gen Virol 2010; 91(Pt 6): p:1473-7; PMID:20164263; http://dx.doi.org/10.1099/vir.0.019034-0
- Akira S. Functional roles of STAT family proteins: lessons from knockout mice. Stem Cells 1999; 17(3): p:138-46; PMID:10342556; http://dx.doi.org/10.1002/stem.170138
- Schroder K, Hertzog PJ, RavasiT, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 2004; 75(2): p:163-89; PMID:14525967; http://dx.doi.org/10.1189/jlb.0603252
- Sen GC. Viruses and interferons. Annu Rev Microbiol 2001; 55: p:255-81; PMID:11544356; http://dx.doi.org/10.1146/annurev.micro.55.1.255
- Yauch LE, Prestwood TR, May MM, Morar MM, Zellweger RM, Peters B, Sette A, Shresta S. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J Immunol 2010; 185(9): p:5405-16; PMID:20870934; http://dx.doi.org/10.4049/jimmunol.1001709
- Yauch LE, Zellweger RM, Kotturi MF, Qutubuddin A, Sidney J, Peters B, Prestwood TR, Sette A, Shresta S. A protective role for dengue virus-specific CD8+ T cells. J Immunol 2009; 182(8): p:4865-73; PMID:19342665; http://dx.doi.org/10.4049/jimmunol.0801974
- Zompi S, Santich BH, Beatty PR, Harris E. Protection from secondary dengue virus infection in a mouse model reveals the role of serotype cross-reactive B and T cells. J Immunol 2012; 188(1): p:404-16; PMID:22131327; http://dx.doi.org/10.4049/jimmunol.1102124
- Chu H, Das SC, Fuchs JF, Suresh M, Weaver SC, Stinchcomb DT, Partidos CD, Osorio JE. Deciphering the protective role of adaptive immunity to CHIKV/IRES a novel candidate vaccine against Chikungunya in the A129 mouse model. Vaccine 2013; 31(33): p:3353-60; PMID:23727003; http://dx.doi.org/10.1016/j.vaccine.2013.05.059
- Ma G, Eschbaumer M, Said A, Hoffmann B, Beer M, Osterrieder N. An equine herpesvirus type 1 (EHV-1) expressing VP2 and VP5 of serotype 8 bluetongue virus (BTV-8) induces protection in a murine infection model. PLoS One 2012; 7(4): p:e34425; PMID:22511939; http://dx.doi.org/10.1371/journal.pone.0034425
- Paran N, Suezer Y, Lustig S, Israely T, Schwantes A, Melamed S, Katz L, Preuss T, Hanschmann KM, Kalinke U, et al. Postexposure immunization with modified vaccinia virus Ankara or conventional Lister vaccine provides solid protection in a murine model of human smallpox. J Infect Dis 2009; 199(1): p:39-48; PMID:19012492; http://dx.doi.org/10.1086/595565
- Castillo-Olivares J, Calvo-Pinilla E, Casanova I, Bachanek-Bankowska K, Chiam R, Maan S, Nieto JM, Ortego J, Mertens PP. A modified vaccinia Ankara virus (MVA) vaccine expressing African horse sickness virus (AHSV) VP2 protects against AHSV challenge in an IFNAR -/- mouse model. PLoS One 2011; 6(1): p:e16503; PMID:21298069; http://dx.doi.org/10.1371/journal.pone.0016503
- Grant E, Wu C, Chan KF, Eckle S, Bharadwaj M, Zou QM, Kedzierska K, Chen W. Nucleoprotein of influenza A virus is a major target of immunodominant CD8+ T-cell responses. Immunol Cell Biol 2013; 91(2): p;184-94; PMID:23399741; http://dx.doi.org/10.1038/icb.2012.78
- Carragher DM, Kaminski DA, Moquin A, Hartson L, Randall TD. A novel role for non-neutralizing antibodies against nucleoprotein in facilitating resistance to influenza virus. J Immunol 2008; 181(6): p:4168-76; PMID:18768874; http://dx.doi.org/10.4049/jimmunol.181.6.4168
- Yewdell JW, Frank E, Gerhard W. Expression of influenza A virus internal antigens on the surface of infected P815 cells. J Immunol 1981; 126(5): p:1814-9; PMID:7217668
- Zheng B, Zhang Y, He H, Marinova E, Switzer K, Wansley D, Mbawuike I, Han S. Rectification of age-associated deficiency in cytotoxic T cell response to influenza A virus by immunization with immune complexes. J Immunol 2007; 179(9): p:6153-9; PMID:17947690; http://dx.doi.org/10.4049/jimmunol.179.9.6153
- Sambhara S, Kurichh A, Miranda R, Tumpey T, Rowe T, Renshaw M, Arpino R, Tamane A, Kandil A, James O, et al. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell Immunol 2001; 211(2): p:143-53; PMID:11591118; http://dx.doi.org/10.1006/cimm.2001.1835
- Vassilenko SM, Vassilev TL, Bozadjiev LG, Bineva IL, Kazarov GZ. Specific intravenous immunoglobulin for Crimean-Congo haemorrhagic fever. Lancet 1990; 335(8692): p:791-2; PMID:1969533; http://dx.doi.org/10.1016/0140-6736(90)90906-L
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res 2013; 100(1): p:159-89
- Yang ZY, Kong WP, Huang Y, Roberts A, Murphy BR, Subbarao K, Nabel GJ. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature 2004; 428(6982): p:561-4; PMID:15024391; http://dx.doi.org/10.1038/nature02463
- Zellweger RM, Miller R, EddyWE, White LJ, Johnston RE, Shresta S. Role of humoral vs. cellular responses induced by a protective dengue vaccine candidate. PLoS Pathog 2013; 9(10): p:e1003723; PMID:24204271; http://dx.doi.org/10.1371/journal.ppat.1003723
- Bisht H, Roberts A, Vogel L, Bukreyev A, Collins PL, Murphy BR, Subbarao K, Moss B. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice. Proc Natl Acad Sci U S A 2004; 101(17): p:6641-6; PMID:15096611; http://dx.doi.org/10.1073/pnas.0401939101
- Wang Z, Martinez J, Zhou W, La Rosa C, Srivastava T, Dasgupta A, Rawal R, Li Z, Britt WJ, Diamond D. Modified H5 promoter improves stability of insert genes while maintaining immunogenicity during extended passage of genetically engineered MVA vaccines. Vaccine 2010; 28(6): p:1547-57; PMID:19969118; http://dx.doi.org/10.1016/j.vaccine.2009.11.056
- Luo M, Tao P, Li J, Zhou S, Guo D, Pan Z. Immunization with plasmid DNA encoding influenza A virus nucleoprotein fused to a tissue plasminogen activator signal sequence elicits strong immune responses and protection against H5N1 challenge in mice. J Virol Methods 2008; 154(1-2): p:121-7; PMID:18789973; http://dx.doi.org/10.1016/j.jviromet.2008.08.011
- Vipond J, Vipond R, Allen-Vercoe E, Clark SO, Hatch GJ, Gooch KE, Bacon J, Hampshire T, Shuttleworth H, Minton NP, et al. Selection of novel TB vaccine candidates and their evaluation as DNA vaccines against aerosol challenge. Vaccine 2006; 24(37-39): p:6340-50; PMID:16781800; http://dx.doi.org/10.1016/j.vaccine.2006.05.025
- Katzen F. Gateway((R)) recombinational cloning: a biological operating system. Expert Opin Drug Discov 2007 2(4): p. 571-89; PMID:23484762; http://dx.doi.org/10.1517/17460441.2.4.571
- Earl PL, Moss B, Wyatt LS, Carroll MW. Generation of recombinant vaccinia viruses. Curr Protoc Protein Sci 2001 Chapter 5: p. Unit5 13; PMID:18429179; http://www.ncbi.nlm.nih.gov/pubmed/18429179
- Earl PL, Cooper N, Wyatt LS, Moss B, Carroll MW. Preparation of cell cultures and vaccinia virus stocks. Curr Protoc Protein Sci 2001 Chapter 5: p. Unit5 12; PMID:18429178; http://www.ncbi.nlm.nih.gov/pubmed/18429178
- Shimizu S. Routes of Administration, in The Laboratory Mouse. Hedrich HJ, Bullock G, Editors. Elsevier. 2004:p:527-542.