Abstract
Streptococcus pneumoniae has more than 95 serotypes, each of which presumably can cause sepsis, meningitis, pneumonia, and acute otitis media. Pneumococcal conjugate vaccines (PCV) targeted against a limited number of serotypes have nonetheless revealed an impressive impact on each manifestation of pneumococcal disease. At the same time, growing evidence of significant non-vaccine type (NVT) replacement disease following implementation of infant PCV programs has raised questions about the long-term viability of PCV immunization strategies and how to optimize PCV formulations. We discuss here theoretical and practical considerations regarding serotype replacement, and provide a snapshot of the most important NVT types seen to date after implementation of the 2 higher-valent PCVs.
Abbreviations
| AOM | = | Acute otitis media |
| IPD | = | Invasive pneumococcal disease |
| NP | = | Nasopharyngeal |
| VT | = | Vaccine type |
| NVT | = | non-vaccine type |
| PCV13-CRM, Thirteen-valent pneumococcal conjugate vaccine; | = | |
| PCV7-CRM | = | Seven-valent pneumococcal conjugate vaccine |
| PHiD-CV-10 | = | Ten-valent pneumococcal conjugate vaccine |
Introduction
Pneumococcal conjugate vaccines and serotype replacement in nasopharyngeal carriage and in disease. Colonization of the nasopharynx (NP) by the pneumococcus, while perhaps occasioning local inflammation is not, in itself, a disease state. However, NP colonization is generally accepted as a precursor and prerequisite for virtually all pneumococcal disease,Citation1 as well as for the transmission of the pneumococcus to other individuals. A pneumococcal vaccine that substantially decreases colonization by vaccine serotypes (VT) could be expected to both decrease the risk of disease in the vaccinated individual and to provide herd protection to unvaccinated individuals. Conversely, vaccine-induced increases in non-vaccine serotype (NVT) colonization, referred to as "replacement" carriage, have the potential to lead to increased NVT disease in both vaccinated and unvaccinated populations.
Despite eliciting high levels of functionally-active anti-capsular antibodies, the 23-valent pneumococcal polysaccharide vaccine does not appear to show a significant impact on NP carriage.Citation1 Based on the experience with Haemophilus influenzae type b conjugate vaccines,Citation2 it was expected that the story would be different with pneumococcal conjugate vaccines (PCV).
There was thus considerable interest when, in July 1996, Stephen Obaro and colleagues at the Medical Research Council Laboratories in the Gambia published a short letter in the Lancet cautiously describing "preliminary results" from a small study in young children with an experimental 5-valent pneumococcal CRM197 PCV. Infants randomized to receive 2 or 3 doses of the PCV and then given a dose of the 23-valent polysaccharide vaccine at 18 months of ageCitation3 were shown to have statistically significantly less VT colonization compared to the control group receiving only Hib vaccine. The investigators also noted that this decrease was "countered" by a commensurate rise in NVT colonization. In other words, they ended up with similar proportions of children in each group carrying any type of pneumococcus.
Experience with PCV7-CRM
The Gambia findings emerged only 6 months after the start of the large clinical efficacy trial of a 7-valent CRM197 pneumococcal conjugate vaccine (PCV7-CRM) involving 38,000 children in Northern California. At that time, some wondered whether there would be any net impact of the PCV on overall invasive pneumococcal disease (IPD). The results of that trial (55 vs 6 IPD cases of any type in the control and vaccinated groups,Citation4 respectively) suggested that replacement disease concerns had been overblown. In addition, the observation of a net vaccine impact on acute otitis media (AOM) and pneumoniaCitation5 in the same study alleviated similar concerns about replacement in mucosal diseases. Around the same time, however, a Finnish AOM efficacy trial with PCV7-CRM that determined etiology via tympanocentesis-based diagnostic methods showed that the 56% decrease in VT AOM in vaccinees was accompanied by a 33% increase in NVT AOM that bordered on statistical significance (95% CI: -80 to 1).Citation6
Taken together, these results indicated that the virtually complete replacement of VT by NVT at the level of the nasopharynx seen in the Gambia (and subsequently in other randomized studies with higher valent CRM197 PCVs)Citation1 did not translate into complete disease replacement.
From a public health perspective, what really matters is what happened following PCV7-CRM introduction into infant immunization programs. In the US, this quickly led to a dramatic decrease in VT IPD both in the target age group as well as in older, non-vaccinated individuals, with an impressive net impact in all age groups. In children, for example, the net IPD decrease was on the order of 75%. At the same time, attention was increasingly focused on the seemingly inexorable rise of IPD in the US due to a single serotype not contained in PCV7-CRM, 19A. In absolute terms it rose 4–5-fold (from low levels) in young children in the years following introduction of PCV7-CRM, with a smaller rise in older age groups.Citation7 While there remains some debate as to the degree to which the 19A rise was driven by antibiotic selection, as opposed to vaccine alone,Citation8 there is little question that rises in disease caused by multiple NVT were consistently observed following implementation of PCV7-CRM programs and that in large part these were the consequence of serotype replacement at the level of the nasopharynx.Citation9
A recent meta-analysis systematically assessed the impact of infant immunization programs with PCV7-CRMCitation10 on IPD in different age groups in industrialized settings. It found that the statistically significant decreases in VT IPD in young children were consistently accompanied by statistically significant increases in NVT IPD, nonetheless resulting in a sustained decrease in overall IPD of approximately 50%. The "explanation" for this sustained decrease in light of complete nasopharyngeal replacement lies in the concept of "invasiveness," whereby colonizing NVT have been shown, on average, to be less likely to cause invasive disease per carriage episode than colonizing VT.Citation9 Accordingly, it has been proposed that we can quantitatively predict the net impact of a PCV on IPD in children in a given setting using only 2 parameters from the pre-PCV era: the proportion of NP colonizers with VT,Citation11 and the proportion of IPD due to VT.Citation12 Logically, it has been proposed that future PCV formulations should preferentially include highly invasive NVT.
In the meta-analysis by Feikin et al, the replacement disease story appeared more complex for older adults >50 y of age, with the increase in NVT IPD, on average, fully "countering" the decrease in VT for most of the follow-up years. More precisely, no statistically significant decrease in overall IPD in 50–64 y olds nor in >65 y olds was detected in any of the first 6 years post-introduction of PCV7-CRM; only in the 7th year was a small and significant decrease noted. As data from the 7th year analysis were derived from only a small subset of countries and statistically dominated by a single US study, it is difficult to understand the robustness and generalizability of this finding.Citation10 Curiously, the magnitude of the net effect observed in older adults after infant PCV7-CRM immunization appeared to be highly setting-specific, with the US for example reporting a 37% decrease in IPD in >65,Citation7 the UK indicating they saw about half of that (19%),Citation13 while Quebec reported no net decrease at all.Citation14
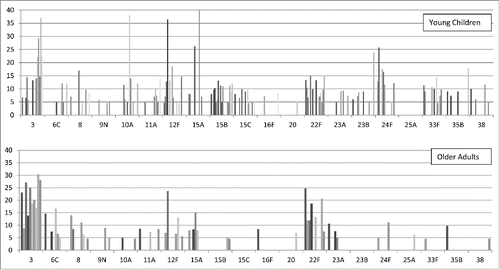
It is worth noting that a wide array of types was responsible for most NVT replacement in the older age groups, while in younger children in the same settings, the major NVT included 7F and 19A, potentially preventable by the higher-valent PCV formulations (see below).Citation7,13 This raises the question whether the broad spectrum of serotype invasiveness documented for young children is applicable to other populations, such as older adults or immunocompromised individuals. This topic is discussed more fully in Section 5.
Recent Experience with Higher-Valency Formulations
This already dynamic situation is being further influenced by the recent substitution of PCV7-CRM by 2 extended conjugate vaccines, a 13-valent CRM197 conjugate (PCV13-CRM), and a 10-valent PCV in which 8 polysaccharides are conjugated to protein D of H. influenzae, and the other 2 conjugated to tetanus or diphtheria toxoids (PHiD-CV-10). Conjugate vaccine formulations containing more serotypes are under investigation.Citation15 Currently available and future formulations will likely protect against some of the more invasive serotypes (1, 5, 7F, 19A) not found in PCV7-CRM. As these serotypes are responsible for some of the site specific variation in the PCV effect in older adults, these vaccines might be expected to mitigate some of this variability in NVT disease rates, even if further NP replacement with NVT occurs.
To date, there have been 2 randomized NP carriage studies published that each compared one of the 2 new vaccines with PCV7-CRM, and each suggested that the overall NP carriage levels did not change with vaccination.Citation16,17 These indicate, not surprisingly, that non-vaccine type replacement at the NP level will continue to occur. Conclusions as to whether more subtle differences in the density, kinetics or magnitude of the VT, NVT, or vaccine-related type carriage impact exist between the 2 higher-valent vaccines will have to await the results of head-to-head trials currently underway.
Recent reports suggest that the pattern of replacement disease observed after PCV7-CRM may be continuing following introduction of PCV13-CRM and PHiD-CV-10. In young children the general pattern seems to be for a rise in NVT IPD that is much smaller than the drop in VT IPD, resulting in substantial net benefit. This is true in PCV7-CRM-naïve countries who introduced PHiD-CV-10 (Finland, Kenya, or Chile),Citation18–20 and in countries that had previously used PCV7-CRM and then introduced PHiD-CV-10 (New Zealand, the Netherlands)Citation21,22 or PCV13-CRM (France, UK, Denmark or South Africa).Citation23–26 Whether the magnitude of those NVT increases is similar in the different groups of countries will undoubtedly remain a topic for future study.
One exception may be pediatric meningitis. On the one hand, studies from the UKCitation77 and FranceCitation69 report net decreases in pneumococcal meningitis after switching from PCV7-CRM to PCV13-CRM; on the other hand a German studyCitation27 suggests replacement is quite substantial, with the authors noting a statistically significant increase in non-PCV13-CRM types in children following the switch to PCV13-CRM, and a recent US report suggests no net impact (as of yet) on this disease manifestation.Citation28
As observed with PCV7-CRM, the consequences of the rise in NVT IPD in older adults >65 vary by setting. While a few countries using PCV13-CRM (Denmark, Norway, US or UK)Citation24,25,29,30 and PHiD-CV-10 (the Netherlands)Citation22 have reported 12–25% decreases in overall disease in this age group compared to the PCV7-CRM era, others using PCV-13-CRM (South Africa, France, Sweden, Germany)Citation23,26,31,32 or PHiD-CV-10 (Finland, New Zealand; Quebec)Citation14,18,21,33 report no net IPD decrease. NVT disease in this high incidence age group in the UK is also cause for concern. An increase was already evident in the 2013/14 season, in which an estimated 1461 cases of IPD occurred in this age group in comparison with 1134 in 2008–10; this converts to an incidence rate ratio of 1.25 (95% CI 1.17–1.35).Citation24 Data on the numbers of cases of IPD due to different classes of serotypes in different age groups are made available by Public Health England and show an increasing trajectory of NVT disease across multiple age groups. At the time of writing, this is continuing for data from 2015/16.Citation34
One should exercise caution in drawing conclusions based on comparisons across settings, not only because vaccine programs, schedules, and coverage differ, and surveillance sensitivities may vary over time, but also because some studies,Citation24,30 but not others,Citation18 have relied on modeled trend analyses to quantify vaccine impact. What is clear, however, is that NVT replacement disease is widely observed in the era of the new conjugate vaccines, with especially significant consequences in the older age group. To better understand these consequences, and try to formulate predictions for the future, it is helpful to step back and consider how various factors, both at the serotype and at the population levels, could affect replacement carriage and disease.
Properties of Serotypes that Could Affect Replacement
We should start by admitting something a little embarrassing, which is that we don't have a good sense of why the pneumococcal capsule should be so antigenically diverse. Over 95 distinct serotypes are known, and it is likely that others await discovery. The reflexive answer that it is the consequence of diversifying selection by the immune system is unsatisfactory for several reasons. First, while prior disease with a given capsular type does offer protection against subsequent infection with that type, disease is an extremely rare event relative to carriage.
Secondly, while in theory carriage itself could be an immunizing (and therefore selecting) event, a study following children in daycare calculated the odds ratio for carriage of a given serogroup relative to carriage of the same serogroup in the past, with mixed results.Citation35 Clear evidence of protection was only available for 23F, while other serotypes showed non-significant trends, or no protection at all. However, considering the point estimates for the Odds Ratios, the great majority are less than one. Thus, taking these data together and considering what we know to be the plausible impact of mucosal immunity, it seems that carriage offers some protection against subsequent colonization, but it isn't clear whether this is sufficient selection to explain pneumococcal serotype diversity. The over 95 known pneumococcal serotypes should be contrasted with the relatively small amounts of capsular diversity associated with other encapsulated bacteria colonizing the nasopharynx like H. influenzae (6 serotypes, in addition to non-typeable strains), or N. meningitidis (12 serogroups).
Serotype diversity reflects differences in the structure of the underlying polysaccharide capsule, and the ability to stimulate adaptive immune responses is only one of the ways in which capsules differ. Serotypes also vary in the amount of capsule they produce, the number of carbon atoms in each repeat unit of the capsular polysaccharideCitation36 and the degree of negative charge associated with it.Citation37 Serotypes with fewer carbons per repeat unit are less energetically costly to produce, and are more heavily encapsulated and resistant to neutrophil killing. They are also typically found at higher prevalence.Citation36 The same is true of capsules with a higher negative charge, which is proposed to electrostatically repel phagocytic cells.Citation37 Furthermore, serotypes also show a varying capacity to cause invasiveCitation38,39 (or other)Citation40 disease per episode of carriage, together with variation in the severity of that disease,Citation41–43 or association with specific manifestations like empyema.Citation44 These are all potentially important properties, and while almost certainly not the only factors determining prevalence, these correlations suggest that they are important and there are plausible biological mechanisms why they should be so.
For replacement, the most important thing is the degree to which any of the above characteristics affect the competition between vaccine and non-vaccine serotypes in the absence of vaccination. If there is no competition, then the removal of the vaccine types offers no benefit for the non-vaccine types. Whereas if there is competition, then the removal of vaccine types unlocks an ecological opportunity for the non-vaccine types and they are expected to replace the vaccine types in colonization
This is an ecological question, which we can address by considering the different niches to which serotypes are adapted. Here we mean niche in the ecological sense. In classic ecological theory the niche is defined as a notional hypervolume in resource space, in which an organism can persist and grow.Citation45 This is not to be confused with the usage of the 'anatomical niche', which refers to the site of colonization and is closer to the ecological definition of 'habitat'. Organisms with overlapping ecological niches compete with each other for resources, resulting in potentially complex interactions and dynamics.Citation46 However in the simplest case of 2 organisms that share exactly the same niche, then the frequency of each will be determined by random drift.
Dimensions of the niche as experienced by different serotypes might include many factors. Among them, we can suggest that host immunity, host networks, and the presence of other co-colonizing bacteria may be important. If serotypes have exactly the same niche the predictions of replacement are simple: the vaccine serotypes will disappear, and the population will restructure itself in a fashion similar to the pre-vaccine era. What this structure looks like can be described by the rank frequency distribution of serotypes, which is to say the proportion of carriage due to the most common serotype, followed by the second and so on.Citation47 Note that the identity of the serotypes does not matter in this case. Without niche structure, the nasopharyngeal serotype carriage distribution post vaccine should be similar to that pre vaccine, except with NVT taking the place of VT. Indeed this has been empirically observed.Citation47
It was mentioned in an earlier section that if we can predict the post vaccine serotype distribution, this can be combined with data on the invasiveness of the replacing serotypes to estimate the net impact of different vaccine formulations including replacement.Citation11,48 However, such analyses rest on the very peculiar assumption that when 2 serotypes encounter each other in competition to colonize a host, the winner will be random. In other words there are no fitness differences and the population dynamics are, in a population genetic sense, neutral. In fact we can apply tests for neutrality to the rank frequency distribution observed in unvaccinated communities, and show it to be consistent with neutrality. In contrast, the flattening of this distribution in vaccinated communities, described in the previous reportCitation47 is the signature effect of selection.Citation49,50
Such competitive interactions are essential for replacement to happen. If two serotypes do not compete at all, being adapted to completely non-overlapping niches, then the removal of one will have no impact on the other. So the observation of replacement following PCV7 means we can be confident some competitive interactions exist. But it does not follow that all serotypes compete to an equal degree with one another. The amount of competition between serotypes is hence a crucial parameter, as it will determine the nature of the interactions between them, the resulting distribution and the potential for serotypes to coexist. Prior modeling work has examined this in the context of drug resistance,Citation51 but it is a concept that is also important for vaccination.Citation52–54 One obvious, almost trivial conclusion that we can draw from the observation of capsular diversity is that to maintain it there must be some niche variation, even if the nature of that variation is not obvious. What might it be and how might it impact replacement?
We know that serotypes vary in how likely they are to colonize hosts of different ages, to progress from colonization to disease, and in how common they are in different host populations. The US and Europe have been the focus of much work, but the serotypes found here can be quite different from those in carriage and disease elsewhere. This has been addressed by expanded vaccine formulations including serotypes such as 1 and 5, which are known to be especially common in many developing countries.Citation55 These are highly invasive serotypes,Citation38 and if they were not included in the vaccine we might worry that vaccination in places where they are already common would lead to a replacement-driven increase in their carriage, which will eventually lead to more disease. Indeed, a study of IPD across European countries using PCV7-CRM found serotype 1 to be among the most common.Citation56
Before ascribing these rises to PCV7-CRM, it is important to consider that some serotypes, 1, 5 and 3 among them, are ecologically distinct. In the first place serotypes 1 and 5 are very rare and transient colonizers,Citation57 even when important contributors to disease – and if they were in direct competition with the more common vaccine serotypes you would expect them to be driven to extinction by competitive exclusion. The serotype 1 ST 306 clone emerged rapidly in Sweden in the 5 years to 1997 (showing the prominence of this serotype in Europe),Citation58 but then rapidly declined,Citation59 suggesting possible epidemic dynamics. Similar observations were made in Chile.Citation60 Studies of IPD in Denmark aiming to unpack the impact of vaccine and natural cyclical fluctuations in disease prevalence found evidence for a roughly 7 year cycle in disease due to several different serotypes, notably 1, 3 and 19A.Citation25 The amplitude associated with the waves of serotype 1 was greater than the others, and that of serotype 19A was overwhelmed by the effect of vaccine. Once these fluctuations are accounted for, the apparent increase in serotype 1 can be ascribed to the natural dynamics of disease and not PCV7-CRM. (Similar fluctuations of serotype 3 have been seen in carriage in Massachusetts prior to vaccination with PCV13, although the numbers are too small to test formally; see Chang et al.Citation61 and references within.)
These observations suggest a role for the immune system in protecting against carriage. However we have not been able to directly study the impact of prior carriage on subsequent acquisition of serotypes 1, 3, or 5 because they are so rare in carriage. We don't know how many serotypes fall into this category, characterized by large fluctuations in disease incidence. However, we would not expect them to be major contributors to replacement because what limits them is not competition with other serotypes, but the resupply of susceptible hosts following waning immunity.
Part of pneumococcal biology ignored by the simplest models of serotype dynamics is multiple carriage, when more than one serotype colonizes a single host. This is common in nature. If some serotypes are more likely to co-colonize with each other, then targeting one of them through vaccination may have a negative, rather than positive, impact on the prevalence of the other. While multiple colonization has been known for some time, it has been hard to study simply because of the practical difficulty of the number of colonies that must be serotyped in order to confidently detect a minority serotype, as well as the lack of standardized sampling and detection techniques. As a result, the possibility that some serotypes positively impact the probability of colonization with the other is speculation. Molecular methods are beginning to shed light on this, with a microarray being used to study colonizing populations in Nepal and Malawi especially promising.Citation62,63 Metagenomic studies of the nasopharyngeal microbiome will be especially valuable in extending our understanding of multiple-colonization and its importance.
Finally we have discussed differences between serotypes here as if the population of each capsule type were homogeneous, but it is well known that this is not the case. Many serotypes are made up of multiple distantly related clones. While it is often assumed that the capsule is the predominant factor determining the properties of the strain, this reflects our state of ignorance rather than settled evidence that other loci are irrelevant. One possible example is the Zinc Metalloproteinase ZmpC, recently shown to be associated with increased severity of disease in multiple serotypes and genetic backgrounds.Citation64 Strains vary in many other properties, from the obvious (antibiotic resistance) to the subtle (adaptation to specific host populations or networks). These likely interact, and the observed serotype diversity may be the product of population level interactions between capsular and non-capsular antigens.Citation65
In summary, the properties of serotypes that make them more or less likely to be prominent in replacement disease can be divided into 2 groups: those that affect replacement carriage, and those that affect disease. Success in carriage comes down to competition with other serotypes. The matrix of competitive interactions is unknown, but arises from properties of the capsule in addition to serology (such as negative charge) and other antigens. At present, serotype is considered the major factor in determining how likely a strain is to cause disease per carriage exposure, but others may await discovery.
Population Factors that Could Determine the Extent and Nature of Serotype Replacement Reported
We considered previously the differences in how individual non-vaccine serotypes might respond to vaccination, identifying the amount of competition with the vaccine serotypes as being a crucial factor. But what might produce differences between host populations in the course and consequences of serotype replacement?
The most obvious factor is the composition of the pneumococcal population before vaccination.Citation12 Making the simple assumption of high levels of competition among serotypes, then at the nasopharyngeal level we would expect the most common non-vaccine types to become more common still. This is, indeed, roughly what has been observed in multiple settings. But it falls short of offering much predictive value. The observation of considerable heterogenicity in the magnitude and even existence of a net herd effect for disease in older adults is not easily attributable to setting-specific differences in the pre-vaccine era serotype distribution, and indicates other factors are at play.
Regarding the host population, one obvious factor that could influence local dynamics is the volume of antibiotic use, which we would expect to select for any serotypes associated with resistance or diminished susceptibility. This effect will be mediated in 2 ways: both direct selection for resistant strains of non-vaccine serotype following vaccination, and the prevalence of resistance among NVTs in the population prior to vaccination. It will be hard to disentangle these effects if antibiotic use remains constant, but the consequence will be the same.
Populations may also vary in coverage, which vaccine is used, the schedule applied, and the presence or absence of a catch up program. We might expect that more immunogenic strategies would both be more associated with replacement, as a result of more effectively removing the vaccine serotypes. Countries differ in the use of 2 vs 3 primary doses of PCVs, with clear differences in immunogenicity at the end of the primary series, though the use of a booster dose may obscure any impact. For example, the US employs a 3 plus one-dose schedule (2, 4, 6 and 12–15 months), the UK uses a 2 plus one schedule (2, 4 and 12 months), but substantial VT herd effects have been documented in both, consistent with the comparable booster responses.
The most important practical feature of host populations that may affect replacement in the volume and nature of disease is the presence of vulnerable populations such as the immunocompromised, or other groups particularly susceptible to pneumococcal disease. Replacement in carriage in the pediatric population will alter the serotypes to which these populations are exposed. If the replacing serotypes retain the ability to cause disease in these vulnerable populations, and if these populations are sufficiently numerous, this will obviously lead to more replacement disease. In addition, the specific role of adults in transmission may vary a great deal among high and low burden settings, and may differ by serotype. In the US, children are considered to be responsible for the great majority of transmission, certainly of the types contained in PCV7-CRM. As noted previously, early data do suggest a VT herd benefit following PCV13 implementation in sites including the US, UK, Denmark and Israel,Citation24,25,30,66 implying children drive the majority of transmission of the additional PCV13 serotypes in these populations. Cumulative case counts of IPD, stratified by serotype and age, are made available by Public Health England and show the impressive reduction in PCV13 VT disease in all age groups.Citation67
In high burden settings, where colonization of adults is more common, it is possible children are responsible for a smaller proportion of transmission. If this is so, it is predicted to limit the benefit of the herd effect that results from vaccinating children. Nonetheless, there is cause for optimism in the observation of a herd effect on overall colonization of mothers in both HIV positive and negative from a cohort study in South Africa,Citation68and in the unvaccinated adults in Kenya.Citation69 However, we note that in both cases the prevalence of colonization in children was still higher than in adults, so they are expected to be responsible for the majority of transmission.
This returns us to one of the most important, yet little studied features of serotypes: how they might differ in their capacity to cause disease among different age groups. Almost all estimates of this have been based on data in children, so they may not be reliable guides among adults, and even less so among adults with co-morbidities or other risk factors including encroaching immunosenescence in the >65 age group. If the replacing serotypes are more invasive than VT in certain adult populations, then this could lead to an increase in IPD in this population. Studies of the Aboriginal population in Western Australia following PCV7-CRM found that while IPD due to vaccine serotypes declined precipitately following vaccination among both children and adults, this was more than offset by the increase in NVT disease among adults. The overall incidence of IPD per 100,000 persons prior to PCV7-CRM was 44.6, and following vaccination 56.4.Citation70 Another population especially vulnerable to IPD is Alaska Natives. While the impact of PCV7-CRM vaccination on IPD among children of this population was rapid, the same time period saw no benefit among adults over 45 years of age, among whom the incidence of IPD (per 100,000 people, due to all serotypes) before vaccination was 57.9, and afterwards 75.7.Citation71,72
The most recent data offer some cause for optimism in a small if far from significant reduction in overall IPD rates in Alaska Natives over 45 (90.9 – 83.0/100,000 persons per year for 2005–2008 and 2010–2013 respectively. p = 0.482).Citation73 In contrast with the 2 cases above, HIV positive children have shown a reduction in pneumococcal carriage following PCV7-CRM vaccination.Citation74 The clinical significance of this remains mysterious at the time of writing, but taken together with the above it illustrates the importance of considering the impact of replacement on particular subpopulations, and the peril of making assumptions in the absence of data. Perhaps the most interesting and perplexing results have come from a cluster-randomized trial in the Gambia. A trial of this kind is expected to provide the maximum advantage to replacing serotypes (an individually randomized trial has less population level impact on the pneumococcal population) and yet no replacement has been observed in carriage despite long follow up.Citation75,76 The reasons for this are obscure, and it should be emphasized that this is the exception rather than the rule.
These studies demonstrate the capacity for vaccine impact in some specific patient populations to diverge from that in others. The reasons for these differences are not clear, but the populations in question are known to be more vulnerable than others to pneumococcal disease.
The phenomenon of serotype switching, in which the genes conferring serotype are transferred into different genetic backgrounds by recombination,Citation77 offers another way in which different populations might impact the replacement phenomenon. For example, in settings with higher frequencies of multiple carriage (for example sub-Saharan Africa), more opportunities for recombination are expected and this is supported by statistical genetic analyses.Citation78 If vaccine types and non-vaccine types coexist in the population for a time, as might be expected in the case where vaccine coverage was relatively low for instance, this produces more opportunity for serotype switching. Prior to PCV7-CRM much concern centered around the possibility that serotype switching could produce variants that would undermine the vaccine's success. But study of multiple cases of switching between vaccine and non-vaccine types using a molecular clock to date their origins has suggested that they were generated before vaccination, not since. On reflection this may be counted unsurprising, as vaccine types removed from the carriage population are by definition unable to participate in recombination. The importance of serotype switching is instead that which has taken place prior to vaccination.
In suggesting the likely consequences of vaccination we have the great benefit of having studied the impact of PCV7-CRM, but there are reasons for caution. First and most important PCV7-CRM has been studied in a limited number of settings, with little data from developing countries, and it may not be sensible to extrapolate our experience of these wholesale. In addition, the expanded conjugate formulations may not behave identically to PCV7-CRM. It is also important to note that in order to understand transmission, which underpins all genuine replacement, we must sample carriage. Unfortunately good carriage samples are notably scarce.
Most Prominent Disease-Causing Non-Vaccine Serotypes Reported after Introduction of Higher Valent Conjugates
We were interested in examining the relative importance of serotypes for which neither higher-valent vaccine has shown convincing evidence of clinical effectiveness. Therefore, on 15 July 2015 we searched the PubMED data base using as key words "Streptococcus pneumoniae & serotype & disease." This resulted in 221 total hits. We augmented this by extracting serotype information from non-peer reviewed but publically available reports of various national and regional pneumococcal reference laboratories. We selected data sets only from countries reporting at least 2 years' experience with one or both of the higher valent vaccines; where possible, we chose only the most recent 1–2 years. When multiple age groups were available from the same country, for children we selected <2 or <5 year olds only; for adults the preference was >60 or >65 years. In each case we only included those data sets that provided individual serotype-specific information from at least 50 pneumococcal isolates of any type in the age group examined during the surveillance period.
Which serotypes to focus on? Since the advent of PCVs, pneumococcal serotype studies have historically categorized serotypes into 3 categories: vaccine types (serotypes actually represented in the vaccine formulation), vaccine-related types (serotypes belonging to the same serogroups as those contained in the vaccine), and non-vaccine types (belonging to non-vaccine serogroups). However, the limits of this categorization became apparent with the publication of the first PCV7-CRM efficacy and effectiveness studies, when it was recognized that the heptavalent vaccine could provide good protection against one prominent vaccine-related type (6A) but not another (19A).Citation6,79 Accordingly, many investigators began "including" 6A in their calculations of PCV7-CRM VT coverage,Citation10,66 although it was not part of PCV7-CRM. More recent evidence indicates that, although 19A is not a true "vaccine type" included in PHiD-CV-10, that vaccine provides clinically relevant cross protection against that serotype in vaccinees.Citation22,79,80 Finally, the example of serotype 3 has revealed that mere inclusion of a serotype into a vaccine formulation was no guarantee of efficacy against that serotype, even in vaccinees.Citation16,30,81,82
Thus the definitions of VT and NVT are complicated by several factors. In recognition of these complexities in nomenclature, and to use a common definition of "non-vaccine types" for both vaccines/settings, we limited our quantitative analysis to all serotypes NOT contained in PCV13-CRM, plus serotype 3, i.e., all but serotypes 1, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F, and defined these are NVT. In recognition of its prominence in many post-PCV7-CRM settings, for each data set we also show where current levels of 19A would "fit in."
As an indicator of the extent of vaccine penetration in the different settings, we calculated the percentage that NVT represented of all IPD in each age group during the surveillance period, with the assumption that the higher the number, the greater penetration with the PCV. This is admittedly an imperfect measure since the relative proportion of NVT will itself be a function of several factors, including PCV immunization coverage, time since introduction, whether catch-up programs were performed, as well as intrinsic vaccine effectiveness.
Tables 1 and 2 list those NVT representing at least 5% of the IPD or AOM in children caused by all NVT detected within each study in countries that had introduced PCV13-CRM () or PHiD-CV-10 (Table 2) in infant immunization programs. and depict the analogous information for PCV13-CRM and PHiD-CV-10, respectively, in older age groups. Although not a formal meta-analysis, several observations can be made. In most of the pediatric data sets 5–6 serogroups account for at least 50% of NVT, for IPD or for AOM. In contrast, only 2–3 serogroups had been responsible for 50% of VT in young children in the pre-PCV7-CRM era for either IPD or AOM.Citation83 In older children and adults, 4–5 serogroups generally comprise 50% of NVT for IPD, similar to the figure in the pre-PCV7-CRM era. Therefore, in all age groups a relatively broad range of NVT appears to be responsible for disease.
Table 1. Young Children: Most Prominent IPD-Causing NVT in Countries that have Introduced PCV13-CRM into their Infant Immunization Program
Table 2. Young Children: Most Prominent Non-Vaccine Types (NVT) Causing IPD in Countries that Introduced PHiD-CV-10 into the Infant Immunization Program
Table 3. Older Children and Adults: Most Prominent Non-Vaccine Types (NVT) Causing IPD in Countries that Introduced PCV13-CRM into the Infant Immunization Program
Table 4. Older Children and Adults: Most Prominent Non-Vaccine Types (NVT) Causing IPD in Countries that Introduced PHiD-CV-10 into the Infant Immunization Program
A second observation is that in most countries serotype 19A remains a prominent cause of IPD in all age groups, both in PCV13-CRM and PHiD-CV-10 countries. This is not surprising, even for PCV13-CRM countries, as we know that the effects of PCVs on VT disease are dependent both on time since introduction and vaccination coverage,Citation10 and there is some indication that vaccine effectiveness against 19A may be a bit lower than that seen with most of the other serotypes.Citation84 Although it is beyond the scope of this paper to systematically assess changes in disease rates, we note that many of the individual studies with prominent 19A reported that these levels were decreasing after introduction of the higher valent vaccines.
Looking across all pediatric data sets, no single serogroup is consistently found among the top 3 or even 5 types ( and ). Nonetheless, almost all IPD studies report 22F and/or 24F and/or serotype 15B/C among the top 3 NVT. A number of countries count serotype 3 or 33F as one of the larger NVTs, but they are absent from several other studies. This lack of consistency stands in contrast to the pre-PCV7-CRM era, when serotypes from 6, 14, 19, and often 1 were usually present among the top 5 types.Citation83
In older children and adults, serotype 3 is almost always the 1st or 2nd NVT, a finding similar to that seen in the pre-PCV-CRM7 era (Tables 3 and 4).Citation83 With only a few exceptions, 22F is almost always within the top 3 or 4 NVT, something that was not true previously.
To facilitate comparisons of the specific NVTs in the younger and older age groups, we graphically depict in the results restricted to those data sets from Tables 1 and 2 comprising only young children (<2, 3–38 months, or <5 years) or from those in Tables 3 and 4 comprising only older adults (>60 or >65 years). Based on this snapshot, on a global level some serotypes (10A, 15B, 15C, 23B, 24F, 33F and to a lesser extent 12F) appear more prominent in young children, while serotype 3 appears more important in older adults (). From visual inspection, it is not obvious that the extent of vaccine penetration (assessed by % of all types represented by NVT) in a given setting is associated with a different proportion of any individual NVT (), with one possible exception: higher serotype 3 levels in young children appear to be associated with lower vaccine penetration. This association, however, does not take into account regional or vaccine-specific variability.
Figure 1. Most prominent NVT causing IPD in young children or older adults in countries that have introduced higher-valent PCVs. Y-axis: % each serotype represented of all NVT in that study. Upper panel (young children) includes the 18 data sets from combined Tables 1 and 2 comprising age ranges <2 years, 3-38 months, or <5 years. Lower panel (older adults) includes the 10 data sets from combined Tables 3 and 4 comprising age ranges >60 years or >65 years. Within each serotype grouping, the bars from left to right are in the same order as the studies in the combined tables, i.e., the left-most bar values represents the study with the highest proportion of NVT/all types, and the right-most the lowest. To accommodate as much information from the table as possible, for graphical purposes several serotyping assumptions were made. Panel A: IsraelCitation66: 15B/C=>15B; USCitation87: 10=>10A; 11=> 11A; 12=>12F; TaiwanCitation88:15 non-B=>15A. Panel B: SpainCitation89: 23A/B=> 23A; NZCitation21: 16=>16F; SwitzerlandCitation90: 22=>22F; 9 non 9V=> 9N; 15=>15A; TaiwanCitation88: 15 non-B=>15A. For graphical reasons, the Taiwan young children 15A and older children/adults serotype 3 values are only depicted up to 40%, and it was assumed that the number of 10A isolates from Belgium,Citation91 listed as >6, was 6. Note that a missing bar doesn't necessarily mean zero, just <5% of NVT isolates. NVT was defined as all serotypes except 1,4,5,6A,6B,7F,9V,14,18C,19A,19F,23F.
PCV13-CRM and PHiD-CV-10 are not identical in immunogenicity nor impact on nasopharyngeal carriage, and these could potentially affect the replacement types seen.Citation79 provides a graphical representation of the most prominent NVT with a focus on young children data sets, extracted from Tables 1 and 2, and analyzed separated by vaccine. It is not obvious that any serotype plays a more prominent role in countries using one vaccine vs the other, with the possible exception of serotypes 3 and 11A that may comprise a greater proportion of NVT in the PHiD-CV-10 compared to the PCV13-CRM data sets. Focusing on serotype 3 and assuming this is not just a chance finding, it is unknown whether this reflects a modest vaccine effect of PCV13-CRM on serotype 3, less replacement by other NVT in the PHiD-CV-10 countries (and therefore proportionately higher serotype 3), or is independent of the specific vaccine and has more to do with the extent of vaccine penetration or previous geographical differences in serotype distribution.
Figure 2. Most prominent NVT causing IPD in young children in countries that have introduced PCV13-CRM or PHiD-CV-10. Y axis: % each serotype represented of all NVT in that study. Upper panel (PCV13-CRM) includes the 11 data sets from Table 1, and lower panel (PHiD-CV-10) includes the 7 data sets from Table 2, which comprise age ranges <2 years, 3–38 months, or <5 years, and which used only PCV13-CRM or PHiD-CV-10. Values for each serotype represent % of all NVT IPD in each data set. Same bar ordering and serotype assumptions were made as in legend to .
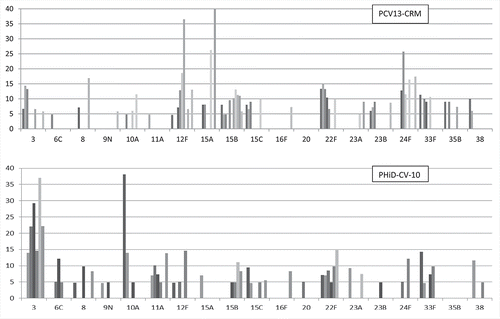
When we combine studies from both countries using either or both vaccines, retaining the focus specifically on young children, there may be some suggestion of a distinction in serotype distributions between studies from North America and Oceania on the one hand, and those from Europe on the other (Tables 1 and 2). In 4/5 of the North American/Oceania studies comprising only young children, 23B comprises >5% of all NVT, but this was not observed in any of the 6 European studies (we also included the large German IPD study encompassing the broader age pediatric age group).
Conversely, 24F constitutes >5% of NVT in 4/6 European studies and is, in fact, the most common NVT cause of IPD in Germany, France and the UK children. Yet 24F does not even feature among the top 7–9 NVT in any of the North American/Oceania studies. Interestingly, the most prominent serotype in one US study, Canada, and Australia (22F) does not feature among the NVT causing more than 5% of the NVT IPD in Germany nor in a second, hospital-based US study. In the absence of evidence of regional differences in clonal distribution for these types, we may tentatively describe replacement scenarios centered around 24F as being distinct from those centered around 23B or 22F. Whether these apparent differences hold true for other countries in the respective regions, or even persist beyond the time periods observed here, remain to be seen. We note that in contrast, we detected no obvious regional differences in NVT in older adults (Tables 3 and 4).
In a number of studies, 22F, along with 12F, 15A, and 23B, 24F were reported to be rising in recent years. In contrast, serotype 3 was reported to have statistically risen in only one study. Caution is appropriate in ascribing any of these changes to vaccine use, as periodic oscillations in disease independent of vaccine have been documented for many of these serotypes and for 12F in particular.Citation25,85 It will be important to determine whether the absolute numbers of cases of disease due to this and other serotypes remain higher than these historical oscillations.
Finally, although we didn't systematically search for antibiotic resistance data, a few studies reported such results. At least some isolates of each of the top serotypes listed in these tables were reported to have decreased susceptibility to penicillin, with the noteworthy exceptions being 2 of the most prominent types (3 and 22F), as well as serotype 8.
Conclusions
Initially following the introduction of PCV7-CRM the existence and importance of serotype replacement was controversial. Because it is difficult to securely ascribe changes in the distribution of carriage serotypes to PCV introduction, rather than 'secular trends' or other factors that would have happened in the absence of vaccine, researchers were wary of pointing to the vaccine as the cause. However, we now have experience of PCV7-CRM use in many different settings, as well as recent experience with the higher valent PCVs, and substantial serotype replacement in carriage is now undeniable (even if the specific causes of fluctuations in the frequency of individual serotypes remain difficult to assess). But replacement in carriage is acceptable if it does not lead to disease replacement to an extent that undermines the overall benefit of vaccination.
Replacement in disease depends on the serotypes involved, and their virulence in the population of interest. Fortunately, replacement disease in the pediatric population has been limited as a result of nasopharyngeal replacement with serotypes that have been, on average, less virulent than the VT they replaced in this population. However this is not necessarily the case for other age groups. Indeed, by removing vaccine serotypes that were adapted to colonization of children, we might create an opportunity for increased prevalence of other serotypes more capable of causing disease in adults. Consistent with this, invasive disease has not been consistently reduced among older adult populations following vaccination. In some cases, it has increased.
Can we predict the consequences of replacement? Only to a limited degree. It does appear that the most successful serotypes post vaccine at the NP level will be those NVTs that were most common pre vaccine, unsurprisingly. But we then run into the considerable uncertainty about the virulence of these serotypes, and how it might vary among different patient populations. The serotype snapshot provided here suggests that the diversity of the more important disease-causing types in young children is greater than that seen in the pre-conjugate era. This may reflect the progressive elimination, with the higher valent PCVs, of several of many of the most virulent serotypes. At the same time, immunocompromised populations, whether due to medical conditions, malnutrition, or immunosenescence, are likely more vulnerable to a broad diversity of types. Study-specific differences in the prominence of different serotypes, perhaps associated with different geographic regions, point to the complexity of selection pressures, the possibility that important determinants lay at the sub-serotype level, and our still limited understanding of serotype niches.
What can we learn by applying the principles we have discussed to the data shown in Tables 1 and 2? The first thing we note is the many-faceted nature of the phenomenon. In contrast with the rapid rise of 19A as a cause of pediatric IPD following PCV7-CRM use in a wide variety of settings, several different serotypes are involved here. The pneumococcus is fascinating in part because of its diversity, but that diversity makes it difficult to gain sufficient power to study this, as the numbers of each individual serotype may be quite small even in large samples.
That said, we can identify some common patterns. For example, certain NVT appear to be more prominent causes of disease in young children than in older adults, and there may be some regional differences as well. Regarding the latter, the study-specific differences in 24F and 23A/B disease are interesting, but we cannot say at this stage whether they merely reflect stochastic variation in the composition of the pre vaccine pneumococcal population in Europe in comparison with elsewhere. If some serotypes are actually fitter than others, and these NVT are among them, then the current variability will be temporary and in time surveillance will find different locations becoming more similar in terms of the serotypes causing disease. In addition, the observation that some isolates of most of the NVT noted here show diminished susceptibility to antimicrobials suggests that selection pressure from high antibiotic use will also favor the emergence of specific serotypes.
We also noted that serotype 3 is present in multiple studies and ages, especially in elderly adults, which might be in part due to relatively poor vaccine efficacy against this by PCV13-CRM.Citation84 If this is the case then any future reductions in serotype 3 disease should not be automatically ascribed to vaccine, but disaggregated into the effect of vaccine and the underlying dynamics as in Harboe et al.Citation25 Conversely, the continued prominence of serotype 19A in most of the data sets, even those with high penetration of PCV13-CRM, underscore the interim nature of this analysis—even in highly vaccinated populations, disappearance of vaccine and especially vaccine-related type disease can take several years.Citation8,79
For future efforts to control pneumococcal disease, several lessons may be drawn. First, the design of new conjugate vaccines will need to incorporate a range of serotypes—no single serotype dominates the NVT field as 19A did in the post-PCV7-CRM era in some countries. The most prominent, to date, include 3, 6C, 8, 10A, 11A, 12F, 15A/B/C, 22F, 23A/B, 24F, 33F, 35B, but these data are mostly from Europe and the Americas, and it cannot be discounted that others are more important in other regions. Evidence of the difficulty in predicting what types will be important in the future is that several of the above (6C, 15A, 23A and B, 24F, 35B) were unknown or not considered to be sufficiently epidemiologically important to be worthy of inclusion in the 23-valent polysaccharide vaccine.Citation86 In addition, the finding that we did not detect robust evidence of different NVT populations in the countries introducing PCV13-CRM vs PHiD-CV-10 cannot be taken as evidence that vaccine-specific differences in the degree of NVT replacement, or in the most prominent NVT, will not emerge with longer surveillance with each vaccine.
Secondly, while it makes sense for prevention of disease in young children, to focus next generation conjugation efforts on the more virulent types (as defined by pediatric studies of carriage and disease), early signs are that this approach will not be sufficient for the elderly or immunocompromised populations, who have long appeared more susceptible to a wider variety of types. These considerations in turn point to the potential value of common antigen approaches for the long term prevention of pneumococcal disease.
From the perspectives of public health officials, clinicians, parents, and patients, the most important attribute of a pneumococcal conjugate vaccine program is the extent to which it decreases overall pneumococcal disease, not the identity of the specific serotypes that remain. Conjugate vaccination has been an astonishing success in terms of removing the targeted serotypes from the population, and has led to a net decrease in IPD and other pneumococcal diseases in young children (and in some adult populations) that in some cases has already been sustained for more than 15 years. Precise predictions, for an organism as diverse as the pneumococcus, are hard. But we can state with some confidence that replacement will likely be complete in carriage, and that we should focus on what this means for some of the vulnerable populations discussed here.
Regarding individual serotypes, we must distinguish between the natural oscillations in disease that have been shown to complicate the inference of replacement, and actually increasing replacement disease. We also predict that we will gain an improved grasp of the invasive potential of previously rare pneumococcal serotypes as they become more common. Finally, the net impact of the 2 higher valent PCVs, and whether they differ from one another in terms of the replacement they produce, remains to be rigorously assessed in the common years. All these considerations highlight the importance of maintaining and strengthening population-based surveillance programs. After all, PCV immunization is also an ongoing ecological experiment.
Disclosure of Potential Conflicts of Interest
WP Hanage reports no conflicts of interests. At the time of manuscript submission, WPH Hausdorff was employed by the GSK group of companies, and owns GSK shares. He is co-holder of a patent for 13-valent PCV licensed to Pfizer/Wyeth, but receives no royalties as per industry practice.
Authors' Contribution
Both authors were involved in the design of the manuscript and interpretation of the data. WP Hausdorff designed and performed the serotype literature search. Both authors drafted the manuscript, revised it critically, approved it for submission and take responsibility for the content.
Acknowledgments
The authors would like to thank Dr Thomas Déplanque (XPE Pharma & Science for GSK Vaccines) for manuscript coordination, excellent data checking and editorial support. We also thank Dr. Carla Talarico (Epidemiologist, GSK Vaccines) for her incisive comments on earlier versions of the manuscript.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the development of the manuscript and its approval for submission. GlaxoSmithKline Biologicals SA also took in charge all costs associated to the development and publishing of the present manuscript.
References
- Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O'Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 2012; 11:841-55; PMID:22913260; http://dx.doi.org/10.1586/erv.12.53
- Barbour ML, Mayon-White RT, Coles C, Crook DWM, Moxon ER. The Impact Of Conjugate Vaccine On Carriage Of Haemophilus Influenzae Type B. J Infect Dis 1995; 171:93-8; PMID:7798687; http://dx.doi.org/10.1093/infdis/171.1.93
- Obaro SK, Adegbola R, Banya W, Greenwood B. Carriage of pneumococci after pneumococcal vaccination. The Lancet 1996; 348:271-2; http://dx.doi.org/10.1016/S0140-6736(05)65585-7
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Elvin L, Ensor KM, Hackell J, Siber G. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J 2000; 19:187-95; PMID:10749457; http://dx.doi.org/10.1097/00006454-200003000-00003
- Black SB, Shinefield HR, Ling S, Hansen J, Fireman B, Spring D, Noyes J, Lewis E, Ray P, Lee J. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002; 21:810-5; PMID:12352800; http://dx.doi.org/10.1097/00006454-200209000-00005
- Eskola J, Kilpi T, Palmu A, Jokinen J, Eerola M, Haapakoski J, Herva E, Takala A, Käyhty H, Karma P, et al. Efficacy of a Pneumococcal Conjugate Vaccine against Acute Otitis Media. N Engl J Med 2001; 344:403-9; PMID:11172176; http://dx.doi.org/10.1056/NEJM200102083440602
- Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, et al. Sustained Reductions in Invasive Pneumococcal Disease in the Era of Conjugate Vaccine. J Infect Dis 2010; 201:32-41; PMID:19947881; http://dx.doi.org/10.1086/648593
- Van Effelterre T, Moore MR, Fierens F, Whitney CG, White L, Pelton SI, Hausdorff WP. A dynamic model of pneumococcal infection in the United States: Implications for prevention through vaccination. Vaccine 2010; 28:3650-60; PMID:20359560; http://dx.doi.org/10.1016/j.vaccine.2010.03.030
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. The Lancet 2011; 378:1962-73; http://dx.doi.org/10.1016/S0140-6736(10)62225-8
- Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, Levine OS, Whitney CG, O'Brien KL, Moore MR, et al. Serotype-Specific Changes in Invasive Pneumococcal Disease after Pneumococcal Conjugate Vaccine Introduction: A Pooled Analysis of Multiple Surveillance Sites. PLoS Med 2013; 10:e1001517; PMID:24086113; http://dx.doi.org/10.1371/journal.pmed.1001517
- Nurhonen M, Auranen K. Optimal Serotype Compositions for Pneumococcal Conjugate Vaccination under Serotype Replacement. PLoS Comput Biol 2014; 10:e1003477; PMID:24550722; http://dx.doi.org/10.1371/journal.pcbi.1003477
- Flasche S, Le Polain de Waroux O, O'Brien KL, Edmunds WJ. The serotype distribution among healthy carriers before vaccination is essential for predicting the impact of pneumococcal conjugate vaccine on invasive disease. PLoS Comput Biol 2015; 11:e1004173; PMID:25879748; http://dx.doi.org/10.1371/journal.pcbi.1004173
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11:760-8; PMID:21621466; http://dx.doi.org/10.1016/S1473-3099(11)70090-1
- De Wals P, Lefebvre B, Markowski F, Deceuninck G, Defay F, Douville-Fradet M, Landry M. Impact of 2 + 1 pneumococcal conjugate vaccine program in the province of Quebec, Canada. Vaccine 2014; 32:1501-6; PMID:24486346; http://dx.doi.org/10.1016/j.vaccine.2013.11.028
- McFetridge R, Meulen AS, Folkerth SD, Hoekstra JA, Dallas M, Hoover PA, Marchese RD, Zacholski DM, Watson WJ, Stek JE, et al. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine 2015; 33:2793-9; PMID:25913828; http://dx.doi.org/10.1016/j.vaccine.2015.04.025
- Dagan R, Juergens C, Trammel J, Patterson S, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-Valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible streptococcus pneumoniae. J Infect Dis 2015; 211:1144-53; PMID:25355940; http://dx.doi.org/10.1093/infdis/jiu576
- Bergh MR van den, Spijkerman J, Swinnen KM, François NA, Pascal TG, Borys D, Schuerman L, IJzerman EPF, Bruin JP, Ende A van der, et al. Effects of the 10-Valent pneumococcal nontypeable haemophilus influenzae protein D–conjugate vaccine on nasopharyngeal bacterial colonization in young children: A randomized controlled trial. Clin Infect Dis 2013; 56:e30-9; PMID:23118268; http://dx.doi.org/10.1093/cid/cis922
- Jokinen J, Rinta-Kokko H, Siira L, Palmu AA, Virtanen MJ, Nohynek H, Virolainen-Julkunen A, Toropainen M, Nuorti JP. Impact of Ten-Valent Pneumococcal Conjugate Vaccination on Invasive Pneumococcal Disease in Finnish Children – A Population-Based Study. PLoS One 2015; 10:e0120290; PMID:25781031; http://dx.doi.org/10.1371/journal.pone.0120290
- Scott A. The Pneumococcal Conjugate Vaccine Impact Study (PCVIS) [Internet]. Kemri Wellcome Trust 2015 [cited 2015 Jul 22]; Available from: http://www.kemri-wellcome.org/index.php/en/studies_inner/75.
- Valenzuela MT, Seoane M, Canals A, Pidal P, Hormazábal JC, Araya P, Terrazas S, Díaz J. Laboratory surveillance of Streptococcus pneumoniae from invasive disease, Chile 2007-2012. Rev Chil Infectol 2014; 31:651-8; http://dx.doi.org/10.4067/S0716-10182014000600002
- New Zealand Public Health Surveillance. IPD Quarterly Report: Oct-Dec 2014 [Internet]. 2014 [cited 2015 Jul 22]; Available from: https://surv.esr.cri.nz/surveillance/IPD.php?we_objectID=4081
- Knol M, Wagenvoort G, Sanders EAM, Elberse KEM, Vlaminckx B, de Melcker H, van der Ende A. Invasive Pneumococcal Disease 3 Years after Introduction of 10-Valent Pneumococcal Conjugate Vaccine, the Netherlands. Emerg Infect Dis 2015; 21: 2040-4; Ahead – of – Print; PMID:26488415; http://dx.doi.org/10.3201/eid2111.140780
- Lepoutre A, Varon E, Georges S, Dorléans F, Janoir C, Gutmann L, Lévy-Bruhl D. Impact of the pneumococcal conjugate vaccines on invasive pneumococcal disease in France, 2001–2012. Vaccine 2015; 33:359-66; PMID:25448105; http://dx.doi.org/10.1016/j.vaccine.2014.11.011
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MPE, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 2015; 15:535-43; PMID:25801458; http://dx.doi.org/10.1016/S1473-3099(15)70044-7
- Harboe ZB, Dalby T, Weinberger DM, Benfield T, Mølbak K, Slotved HC, Suppli CH, Konradsen HB, Valentiner-Branth P. Impact of 13-Valent Pneumococcal Conjugate Vaccination in Invasive Pneumococcal Disease Incidence and Mortality. Clin Infect Dis 2014; 59:1066-73; PMID:25034421; http://dx.doi.org/10.1093/cid/ciu524
- von Gottberg A, de Gouveia L, Tempia S, Quan V, Meiring S, von Mollendorf C, Madhi SA, Zell ER, Verani JR, O'Brien KL, et al. Effects of Vaccination on Invasive Pneumococcal Disease in South Africa. N Engl J Med 2014; 371:1889-99; PMID:25386897; http://dx.doi.org/10.1056/NEJMoa1401914
- Imöhl M, Möller J, Reinert RR, Perniciaro S, Linden M van der, Aktas O. Pneumococcal meningitis and vaccine effects in the era of conjugate vaccination: results of 20 years of nationwide surveillance in Germany. BMC Infect Dis 2015; 15:61; PMID:25885764; http://dx.doi.org/10.1186/s12879-015-0787-1
- Olarte L, Barson WJ, Barson RM, Lin PL, Romero JR, Tan TQ, Givner LB, Bradley JS, Hoffman JA, Hultén KG, et al. Impact of the 13-Valent Pneumococcal Conjugate Vaccine on Pneumococcal Meningitis in US Children. Clin Infect Dis 2015; 61(5):767-75; civ368; PMID:25972022
- Steens A, Bergsaker MAR, Aaberge IS, Rønning K, Vestrheim DF. Prompt effect of replacing the 7-valent pneumococcal conjugate vaccine with the 13-valent vaccine on the epidemiology of invasive pneumococcal disease in Norway. Vaccine 2013; 31:6232-8; PMID:24176490; http://dx.doi.org/10.1016/j.vaccine.2013.10.032
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 2015; 15:301-9; PMID:25656600; http://dx.doi.org/10.1016/S1473-3099(14)71081-3
- Darenberg J, Lindstrand A, Galanis J, Browall S, Sjöström K, Morfeldt E, Wilson J, Naucler P, Blennow M, Örtqvist Å, et al. Pneumococcal conjugate vaccination in stockholm leaves invasive disease incidence unaffected in the elderly and increases serotype diversity [Abstract ISPPD-0468]. In: pneumonia 2014; 3:214 - International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD) 9th. 2014
- van der Linden M, Falkenhorst G, Perniciaro S, Imöhl M. Effects of Infant Pneumococcal Conjugate Vaccination on Serotype Distribution in Invasive Pneumococcal Disease among Children and Adults in Germany. PLoS ONE 2015; 10:e0131494; PMID:26132078; http://dx.doi.org/10.1371/journal.pone.0131494
- INSTITUT NATIONAL DE SANTÉ PUBLIQUE. Programme de surveillance du pneumocoque : rapport 2012 [Internet]. 2012 [cited 2015 Jul 20]; Available from: https://www.inspq.qc.ca/pdf/publications/1734_ProgSurvPneumoQc_RappAnnu2012.pdf
- Public Health England. Health protection – collection. Pneumococcal disease: guidance, data and analysis [cited 2015 Oct 26]; Available from: https://www.gov.uk/government/collections/pneumococcal-disease-guidance-data-and-analysis
- Weinberger DM, Dagan R, Givon-Lavi N, Regev-Yochay G, Malley R, Lipsitch M. Epidemiologic Evidence for Serotype-Specific Acquired Immunity to Pneumococcal Carriage. J Infect Dis 2008; 197:1511-8; PMID:18471062; http://dx.doi.org/10.1086/587941
- Weinberger DM, Trzciński K, Lu Y-J, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R, Lipsitch M. Pneumococcal Capsular Polysaccharide Structure Predicts Serotype Prevalence. PLoS Pathog 2009; 5:e1000476; PMID:19521509; http://dx.doi.org/10.1371/journal.ppat.1000476
- Li Y, Weinberger DM, Thompson CM, Trzciński K, Lipsitch M. Surface Charge of Streptococcus pneumoniae Predicts Serotype Distribution. Infect Immun 2013; 81:4519-24; PMID:24082068; http://dx.doi.org/10.1128/IAI.00724-13
- Brueggemann AB, Peto TEA, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and Geographic Stability of the Serogroup-Specific Invasive Disease Potential of Streptococcus pneumoniae in Children. J Infect Dis 2004; 190:1203-11; PMID:15346329; http://dx.doi.org/10.1086/423820
- Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal Relationships between Invasive and Carriage Streptococcus pneumoniae and Serotype- and Clone-Specific Differences in Invasive Disease Potential. J Infect Dis 2003; 187:1424-32; PMID:12717624; http://dx.doi.org/10.1086/374624
- Hanage WP, Auranen K, Syrjänen R, Herva E, Mäkelä PH, Kilpi T, Spratt BG. Ability of Pneumococcal Serotypes and Clones To Cause Acute Otitis Media: Implications for the Prevention of Otitis Media by Conjugate Vaccines. Infect Immun 2004; 72:76-81; PMID:14688083; http://dx.doi.org/10.1128/IAI.72.1.76-81.2004
- Benfield T, Skovgaard M, Schønheyder HC, Knudsen JD, Bangsborg J, Østergaard C, Slotved H-C, Konradsen HB, Thomsen RW, Lambertsen L. Serotype Distribution in Non-Bacteremic Pneumococcal Pneumonia: Association with Disease Severity and Implications for Pneumococcal Conjugate Vaccines. PLoS ONE 2013; 8:e72743; PMID:24009703; http://dx.doi.org/10.1371/journal.pone.0072743
- Burgos J, Luján M, Larrosa MN, Fontanals D, Bermudo G, Planes AM, Puig M, Rello J, Falcó V, Pahissa A. Risk factors for respiratory failure in pneumococcal pneumonia: the importance of pneumococcal serotypes. Eur Respir J 2014; 43:545-53; PMID:23845720; http://dx.doi.org/10.1183/09031936.00050413
- Alanee SRJ, McGee L, Jackson D, Chiou CC, Feldman C, Morris AJ, Ortqvist A, Rello J, Luna CM, Baddour LM, et al. Association of Serotypes of Streptococcus pneumoniae with Disease Severity and Outcome in Adults: An International Study. Clin Infect Dis 2007; 45:46-51; PMID:17554699; http://dx.doi.org/10.1086/518538
- Fletcher MA, Schmitt H-J, Syrochkina M, Sylvester G. Pneumococcal empyema and complicated pneumonias: global trends in incidence, prevalence, and serotype epidemiology. Eur J Clin Microbiol Infect Dis 2014; 33:879-910; PMID:24563274; http://dx.doi.org/10.1007/s10096-014-2062-6
- Hutchinson GE. Homage to Santa Rosalia or Why Are There So Many Kinds of Animals? Am Nat 1959; 93:145-59; http://dx.doi.org/10.1086/282070
- Chase JM, Leibold MA. Ecological Niches: Linking Classical and Contemporary Approaches. University of Chicago Press; 2003
- Hanage WP, Finkelstein JA, Huang SS, Pelton SI, Stevenson AE, Kleinman K, Hinrichsen VL, Fraser C. Evidence that pneumococcal serotype replacement in Massachusetts following conjugate vaccination is now complete. Epidemics 2010; 2:80-4; PMID:21031138
- Weinberger DM, Bruden DT, Grant LR, Lipsitch M, O'Brien KL, Pelton SI, Sanders EAM, Feikin DR. Using Pneumococcal Carriage Data to Monitor Postvaccination Changes in Invasive Disease. Am J Epidemiol 2013; 178:1488-95; PMID:24013204; http://dx.doi.org/10.1093/aje/kwt156
- Watterson GA. The Homozygosity Test of Neutrality. Genetics 1978; 88:405-17; PMID:17248803
- Ewens WJ. The sampling theory of selectively neutral alleles. Theor Popul Biol 1972; 3:87-112; PMID:4667078; http://dx.doi.org/10.1016/0040-5809(72)90035-4
- Colijn C, Cohen T, Fraser C, Hanage W, Goldstein E, Givon-Lavi N, Dagan R, Lipsitch M. What is the mechanism for persistent coexistence of drug-susceptible and drug-resistant strains of Streptococcus pneumoniae? J R Soc Interface 2010; 7:905-19; PMID:19940002; http://dx.doi.org/10.1098/rsif.2009.0400
- Lipsitch M, Colijn C, Cohen T, Hanage WP, Fraser C. No coexistence for free: Neutral null models for multistrain pathogens. Epidemics 2009; 1:2-13; PMID:21352747; http://dx.doi.org/10.1016/j.epidem.2008.07.001
- Mitchell PK, Lipsitch M, Hanage WP. Carriage burden, multiple colonization and antibiotic pressure promote emergence of resistant vaccine escape pneumococci. Philos Trans R Soc B Biol Sci 2015; 370:20140342; PMID:25918447; http://dx.doi.org/10.1098/rstb.2014.0342
- Mehtälä J, Antonio M, Kaltoft MS, O'Brien KL, Auranen K. Competition between Streptococcus pneumoniae strains: implications for vaccine-induced replacement in colonization and disease. Epidemiol Camb Mass 2013; 24:522-9; http://dx.doi.org/10.1097/EDE.0b013e318294be89
- Hausdorff WP. The roles of pneumococcal serotypes 1 and 5 in paediatric invasive disease. Vaccine 2007; 25:2406-12; PMID:17055620; http://dx.doi.org/10.1016/j.vaccine.2006.09.009
- Torné AN, Dias JG, Quinten C, Hruba F, Busana MC, Lopalco PL, Gauci AJA, Pastore-Celentano L. European enhanced surveillance of invasive pneumococcal disease in 2010: Data from 26 European countries in the post-heptavalent conjugate vaccine era. Vaccine 2014; 32:3644-50; http://dx.doi.org/10.1016/j.vaccine.2014.04.066
- Abdullahi O, Karani A, Tigoi CC, Mugo D, Kungu S, Wanjiru E, Jomo J, Musyimi R, Lipsitch M, Scott JAG. Rates of Acquisition and Clearance of Pneumococcal Serotypes in the Nasopharynges of Children in Kilifi District, Kenya. J Infect Dis 2012; 206:1020-9; PMID:22829650; http://dx.doi.org/10.1093/infdis/jis447
- Normark BH, Kalin M, Örtqvist Å, Åkerlund T, Liljequist BO, Hedlund J, Svenson SB, Zhou J, Spratt BG, Normark S, et al. Dynamics of Penicillin-Susceptible Clones in Invasive Pneumococcal Disease. J Infect Dis 2001; 184:861-9; PMID:11550126; http://dx.doi.org/10.1086/323339
- Hedlund J, Sörberg M, Normark BH, Kronvall G. Capsular Types and Antibiotic Susceptibility of Invasive Streptococcus pneumoniae Among Children in Sweden. Scand J Infect Dis 2003; 35:452-8; PMID:14514143; http://dx.doi.org/10.1080/00365540310013315
- Lagos R, Muñoz A, Martin OS, Maldonado A, Hormazabal JC, Blackwelder WC, Levine MM. Age- and Serotype-Specific Pediatric Invasive Pneumococcal Disease: Insights from Systematic Surveillance in Santiago, Chile, 1994–2007. J Infect Dis 2008; 198:1809-17; PMID:18959497; http://dx.doi.org/10.1086/593334
- Chang Q, Stevenson AE, Croucher NJ, Lee GM, Pelton SI, Lipsitch M, Finkelstein JA, Hanage WP. Stability of the pneumococcal population structure in Massachusetts as PCV13 was introduced. BMC Infect Dis 2015; 15:68; PMID:25887323; http://dx.doi.org/10.1186/s12879-015-0797-z
- Kandasamy R, Gurung M, Thapa A, Ndimah S, Adhikari N, Murdoch DR, Kelly DF, Waldron DE, Gould KA, Thorson S, et al. Multi-Serotype Pneumococcal Nasopharyngeal Carriage Prevalence in Vaccine Naïve Nepalese Children, Assessed Using Molecular Serotyping. PLoS ONE 2015; 10:e0114286; PMID:25643355; http://dx.doi.org/10.1371/journal.pone.0114286
- Kamng'ona AW, Hinds J, Bar-Zeev N, Gould KA, Chaguza C, Msefula C, Cornick JE, Kulohoma BW, Gray K, Bentley SD, et al. High multiple carriage and emergence of Streptococcus pneumoniae vaccine serotype variants in Malawian children. BMC Infect Dis 2015; 15:234; PMID:26088623; http://dx.doi.org/10.1186/s12879-015-0980-2
- Cremers AJH, Kokmeijer I, Groh L, de Jonge MI, Ferwerda G. The role of ZmpC in the clinical manifestation of invasive pneumococcal disease. Int J Med Microbiol IJMM 2014; 304:984-9; PMID:25023076; http://dx.doi.org/10.1016/j.ijmm.2014.06.005
- Cobey S, Lipsitch M. Niche and neutral effects of acquired immunity permit coexistence of pneumococcal serotypes. Science 2012; 335:1376-80; PMID:22383809; http://dx.doi.org/10.1126/science.1215947
- Ben-Shimol S, Greenberg D, Givon-Lavi N, Schlesinger Y, Somekh E, Aviner S, Miron D, Dagan R. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children . Vaccine 2014; 32:3452-9; PMID:24690148; http://dx.doi.org/10.1016/j.vaccine.2014.03.065
- Public Health England. Pneumococcal disease: cases caused by strains covered by Prevenar 13 vaccine - GOV.UK [Internet]. GOV.UK2015 [cited 2015 Sep 29]; Available from: https://www.gov.uk/government/publications/pneumococcal-disease-cases-caused-by-strains-covered-by-prevenar-13-vaccine/pneumococcal-disease-cases-caused-by-strains-covered-by-prevenar-13-vaccine
- Nzenze SA, von Gottberg A, Shiri T, van Niekerk N, de Gouveia L, Violari A, Nunes MC, Madhi SA. Temporal Changes in Pneumococcal Colonization in HIV-infected and HIV-uninfected Mother-Child Pairs Following Transitioning From 7-valent to 13-valent Pneumococcal Conjugate Vaccine, Soweto, South Africa. J Infect Dis 2015; 212:1082-92; PMID:25784729; http://dx.doi.org/10.1093/infdis/jiv167
- Hammitt LL, Akech DO, Morpeth SC, Karani A, Kihuha N, Nyongesa S, Bwanaali T, Mumbo E, Kamau T, Sharif SK, et al. Population effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and non-typeable Haemophilus influenzae in Kilifi, Kenya: findings from cross-sectional carriage studies. Lancet Glob Health 2014; 2:e397-405; PMID:25103393; http://dx.doi.org/10.1016/S2214-109X(14)70224-4
- Lehmann D, Willis J, Moore HC, Giele C, Murphy D, Keil AD, Harrison C, Bayley K, Watson M, Richmond P, et al. The Changing Epidemiology of Invasive Pneumococcal Disease in Aboriginal and Non-Aboriginal Western Australians from 1997 through 2007 and Emergence of Nonvaccine Serotypes. Clin Infect Dis 2010; 50:1477-86; PMID:20420501; http://dx.doi.org/10.1086/652440
- Hennessy TW, Singleton RJ, Bulkow LR, Bruden DL, Hurlburt DA, Parks D, Moore M, Parkinson AJ, Schuchat A, Butler JC. Impact of heptavalent pneumococcal conjugate vaccine on invasive disease, antimicrobial resistance and colonization in Alaska Natives: progress towards elimination of a health disparity. Vaccine 2005; 23:5464-73; PMID:16188350; http://dx.doi.org/10.1016/j.vaccine.2005.08.100
- Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 2007; 297:1784-92; PMID:17456820; http://dx.doi.org/10.1001/jama.297.16.1784
- Bruce MG, Singleton R, Bulkow L, Rudolph K, Zulz T, Gounder P, Hurlburt D, Bruden D, Hennessy T. Impact of the 13-valent pneumococcal conjugate vaccine (pcv13) on invasive pneumococcal disease and carriage in Alaska. Vaccine 2015; 33:4813-9; PMID:26247901; http://dx.doi.org/10.1016/j.vaccine.2015.07.080
- Madhi SA, Izu A, Nunes MC, Violari A, Cotton MF, Jean-Philippe P, Klugman KP, von Gottberg A, van Niekerk N, Adrian PV. Longitudinal study on Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus nasopharyngeal colonization in HIV-infected and -uninfected infants vaccinated with pneumococcal conjugate vaccine. Vaccine 2015; 33:2662-9; PMID:25910923; http://dx.doi.org/10.1016/j.vaccine.2015.04.024
- Roca A, Dione MM, Bojang A, Townend J, Egere U, Darboe O, Howie SRC, Hill PC, Adegbola RA, Greenwood BM, et al. Nasopharyngeal Carriage of Pneumococci Four Years after Community-Wide Vaccination with PCV-7 in The Gambia: Long-Term Evaluation of a Cluster Randomized Trial. PLoS ONE 2013; 8:e72198; PMID:24086259; http://dx.doi.org/10.1371/journal.pone.0072198
- Roca A, Hill PC, Townend J, Egere U, Antonio M, Bojang A, Akisanya A, Litchfield T, Nsekpong DE, Oluwalana C, et al. Effects of Community-Wide Vaccination with PCV-7 on Pneumococcal Nasopharyngeal Carriage in The Gambia: A Cluster-Randomized Trial. PLoS Med 2011; 8:e1001107; PMID:22028630; http://dx.doi.org/10.1371/journal.pmed.1001107
- Wyres KL, Lambertsen LM, Croucher NJ, McGee L, Gottberg A von, Liñares J, Jacobs MR, Kristinsson KG, Beall BW, Klugman KP, et al. Pneumococcal Capsular Switching: A Historical Perspective. J Infect Dis 2013; 207:439-49; PMID:23175765; http://dx.doi.org/10.1093/infdis/jis703
- Donkor ES, Bishop CJ, Antonio M, Wren B, Hanage WP. High Levels of Recombination among Streptococcus pneumoniae Isolates from the Gambia. mBio 2011; 2:e00040-11; PMID:21693638; http://dx.doi.org/10.1128/mBio.00222-11
- Hausdorff WP, Hoet B, Adegbola RA. Predicting the impact of new pneumococcal conjugate vaccines: serotype composition is not enough. Expert Rev Vaccines 2015; 14:413-28; PMID:25266168; http://dx.doi.org/10.1586/14760584.2015.965160
- Verani JR, Domingues CMAS, Moraes JC de. Indirect cohort analysis of 10-valent pneumococcal conjugate vaccine effectiveness against vaccine-type and vaccine-related invasive pneumococcal disease. Vaccine [Internet] [cited 2015 Oct 28]; Available from: http://www.sciencedirect.com/science/article/pii/S0264410´15014188
- Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin Microbiol Rev 2015; 28:871-99; PMID:26085553; http://dx.doi.org/10.1128/CMR.00024-15
- Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels J-P, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. The Lancet 2006; 367:740-8; http://dx.doi.org/10.1016/S0140-6736(06)68304-9
- Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which Pneumococcal Serogroups Cause the Most Invasive Disease: Implications for Conjugate Vaccine Formulation and Use, Part I. Clin Infect Dis 2000; 30:100-21; PMID:10619740; http://dx.doi.org/10.1086/313608
- Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: a postlicensure indirect cohort study. Lancet Infect Dis 2014; 14:839-46; PMID:25042756; http://dx.doi.org/10.1016/S1473-3099(14)70822-9
- Robinson DA, Turner JS, Facklam RR, Parkinson AJ, Breiman RF, Gratten M, Steinhoff MC, Hollingshead SK, Briles DE, Crain MJ. Molecular Characterization of a Globally Distributed Lineage of Serotype 12F Streptococcus pneumoniae Causing Invasive Disease. J Infect Dis 1999; 179:414-22; PMID:9878026; http://dx.doi.org/10.1086/314589
- Robbins JB, Austrian R, Lee CJ, Rastogi SC, Schiffman G, Henrichsen J, Mäkelä PH, Broome CV, Facklam RR, Tiesjema RH. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis 1983; 148:1136-59; PMID:6361173; http://dx.doi.org/10.1093/infdis/148.6.1136
- Kaplan SL, Barson WJ, Lin PL, Romero JR, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Mason EO. Early Trends for Invasive Pneumococcal Infections in Children After the Introduction of the 13-valent Pneumococcal Conjugate Vaccine: Pediatr Infect Dis J 2013; 32:203-7; PMID:23558320; http://dx.doi.org/10.1097/INF.0b013e318275614b
- Taiwan CDC. Taiwan CDC report - 2014/2015 [Internet]. [cited 2015 Jul 22]; Available from: https://www.cdc.gov.tw/professional/list.aspx?treeid=098F3BC4756F62CC&nowtreeid=90960C331E13A586
- Guevara M, Ezpeleta C, Gil-Setas A, Torroba L, Beristain X, Aguinaga A, García-Irure JJ, Navascués A, García-Cenoz M, Castilla J. Reduced incidence of invasive pneumococcal disease after introduction of the 13-valent conjugate vaccine in Navarre, Spain, 2001–2013. Vaccine 2014; 32:2553-62; PMID:24674661; http://dx.doi.org/10.1016/j.vaccine.2014.03.054
- Meichtry J, Born R, Küffer M, Zwahlen M, Albrich WC, Brugger SD, Mühlemann K, Hilty M. Serotype epidemiology of invasive pneumococcal disease in Swiss adults: A nationwide population-based study. Vaccine 2014; 32:5185-91; PMID:25077419; http://dx.doi.org/10.1016/j.vaccine.2014.07.060
- Institut scientifique de Santé Publique. Maladies infectieuses pédiatriques à prévention vaccinale. Rapport annuel 2013 [Internet]. 2013 [cited 2015 Jul 20]; Available from: https://www.wiv-isp.be/SiteCollectionDocuments/Maladies%20infectieuses%20p%C3%A9diatriques%20%C3%A0%20pr%C3%A9vention%20vaccinale.%20Rapport%20annuel%202013.pdf

