?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The evaluation of the public health impact of a vaccination program is essential in monitoring its policy relevance. Vaccine impact (VI) is usually assessed in a before-after design, in which data on disease burden without vaccination program is required from a historical reference period. It takes into account the indirect effects and therefore aims to describe the public health performance of the vaccination program in the population. Vaccine effectiveness (VE), measured in parallel settings, quantifies the benefit for an individual of being vaccinated but does not address the indirect effects of a vaccination program. The motivation of this paper is to gain insight into patterns of how VI and VE have manifested under large-scale use of a ten-valent pneumococcal conjugate vaccine in Finnish children. We construct a simple pseudo-dynamic model that mimics typical post-vaccination trends in the incidences of pneumococcal carriage and invasive disease in children when the proportion of vaccine-type carriage decreases. In the context of the model, we define the parameters of interest for VI and VE and explore how their expected values evolve over time. For comparison, we demonstrate the application of VI and VE estimation by using register data.
1. Introduction
The performance of an ongoing vaccination program can be quantified in terms of a number of different effect measures and rely on different study designs.Citation1,Citation2 Apart from assessing vaccine-induced protection at the individual level, it is essential to evaluate the population-level impact of the program to monitor its policy relevance.Citation3–5 The choices of effect measure, study design, and estimation method depend not only on the research question but also on feasibility of data collection.
Hanquet et al.Citation6 define vaccine impact as the population prevented fraction of disease incidence when exposure is the vaccination program rather than each individual’s own vaccination. The total impact for a vaccinated individual depends on both the direct and indirect protection through a vaccine-induced immune response and reduced exposure to infection, respectively. During a large-scale vaccination program, also the unvaccinated part of the population benefits from reduced transmission (indirect impact, herd protection). The overall impact of the program is a weighted average of the total impact in the vaccinated and the indirect impact in the unvaccinated (cf. Halloran et al.Citation7).
Vaccine impact is usually assessed using a before-after design, in which data on disease burden without the vaccination program is required from a historical reference period. In particular, surveillance data are essential in providing the incidence of a well-defined disease outcome, e.g. laboratory-confirmed invasive pneumococcal disease, over a long time period. Any such before-after comparison is necessarily ecological in the sense that the disease incidence is compared (ideally) within the same population but between different time periods.Citation8 Before-after designs can be considered as non-randomized analogs to community randomized trials.Citation4
In a post-licensure setting, the incidence of infection in the vaccinated and unvaccinated parts of the population are sometimes compared simultaneously.Citation9,Citation10 We call effect measures based on such parallel comparisons as vaccine effectiveness, thus making a distinction between parallel and before-after designs with differing exposure status of the control group.Citation1,Citation6 Parallel designs take the unvaccinated as the comparison group although also those are subject to the indirect effects of vaccination.
In this paper, our interest is in the pneumococcus (Streptococcus pneumoniae), a pathogen with more than 90 distinct types of capsular polysaccharides. The reduced transmission or virtual elimination of serotypes included in the pneumococcal conjugate vaccines have led to increased carriage and disease by serotypes not included in the vaccines (replacement).Citation11–16 Our motivation is to gain insight into patterns of how the vaccine impact and effectiveness of the ten-valent pneumococcal conjugate vaccine (PCV10) have manifested in young Finnish children. We construct a simple model that mimics typical post-introduction trends in the incidences of pneumococcal carriage and disease until elimination of vaccine-type disease. We formulate the effect measures of interest for vaccine effectiveness and impact and explore how their values evolve over time.
The structure of this paper is as follows. Section 2 describes the model for the dynamics of pneumococcal carriage and disease since vaccine introduction. In Section 3 we formulate the effect measures based on the model and calculate their typical evolution over time. In Section 4 we demonstrate the estimation of vaccine impact and effectiveness among vaccine-eligible children in Finland. The paper concludes with a discussion in Section 5.
2. A pseudo-dynamic model of carriage and disease
In order to relate the parameters of vaccine impact and effectiveness to the population dynamics of pneumococcal carriage and disease, we construct a simple pseudo-dynamic model for the respective incidences in cohorts of children after the introduction of a vaccination program (post-introduction period). We follow the processes until vaccine-type (VT) disease becomes eliminated and a new steady-state is reached. The model allows us to describe explicitly the expected behavior of vaccine impact and effectiveness during the post-introduction period.
Carriage. We model pneumococcal carriage based on the following assumptions:
(A1)Proportion of each vaccine-eligible birth cohort is vaccinated;
(A2)The per capita rate of carriage acquisition (force of infection) is constant over time, irrespective of the child’s age and vaccination status. In particular, this means that any reduction in the rate of VT acquisition is immediately replaced by a corresponding rate of non-vaccine-type (NVT) acquisition;Citation17,Citation18
(A3) The proportion of VT carriage acquisition declines as a function of time
because of reduced VT transmission in the population (). While this is admittedly a simplification, we thereby avoid the need for more complex dynamic modelling.
Figure 1. The proportion of vaccine-type (VT) carriage of all carriage acquisition over time since vaccine introduction. Functions and
show the proportions of vaccine-type acquisition in the unvaccinated and vaccinated children, respectively. The scenario shown here corresponds to the observation in Finland. The initial proportion of VT carriage of all carriage among unvaccinated children at time
is
and decreases to
in about 70 months. The vaccine efficacy is
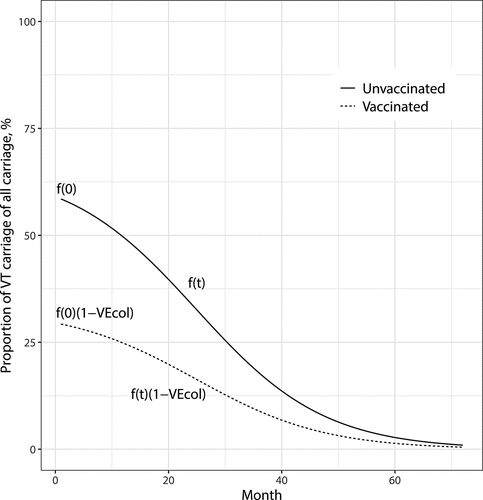
Based on assumptions,Citation1–3 summarises the per capita rates of acquisition by serotype category (VT/NVT) and vaccination status (vaccinated/unvaccinated). The forces of VT carriage acquisition at time are
and
in the unvaccinated and vaccinated children, respectively, where
is the leaky vaccine efficacy against carriage acquisition.
Table 1. Per capita rates of carriage acquisition at time after vaccine introduction per serotype category and vaccination status.
is the per capita rate of carriage acquisition,
is the proportion of VT carriage acquisition of all carriage, and
is the vaccine efficacy against carriage acquisition. Because of complete replacement in carriage, the rates of VT and NVT carriage acquisition sum up to
in all three columns of the table
Disease. summarises the per capita rates of invasive pneumococcal disease (IPD) by serotype category (VT/NVT) and vaccination status. These rates follow by multiplying the rates of carriage acquisition () with the corresponding IPD case-to-carrier ratios (i.e., ratios of IPD incidence to carriage incidence). One additional assumption is made:
Table 2. Per capita rates of IPD at time after vaccine introduction per serotype category and vaccination status.
is the rate of IPD before vaccine introduction and
is the vaccine efficacy against progression to disease
(A4) For both serotype categories, the case-to-carrier ratios are assumed to be constant and remain at their pre-vaccination values.Citation17,Citation18
Apart from the proportion of VT carriage (), the rates of IPD in the post-introduction period depend on the pre-vaccination rates of (
and
), the vaccine efficacy against acquisition (
), and the vaccine efficacy against progression from carriage to disease (invasion,
), i.e., efficacy against case-to-carrier ratio.Citation19 Note that the expressions of the IPD rates in do not depend on the absolute rate
of carriage acquisition.
3. Vaccine impact and effectiveness
3.1. Parameters of interest
Vaccine impact (VI) is here defined as the relative reduction in the rate of IPD in vaccine-eligible children in a population that experiences a vaccination program, compared to the rate in unvaccinated children in a completely unvaccinated population. presents VI parameters separately for the VT, NVT, and all IPD categories, derived from by comparing the rates in the post-introduction period to those in the pre-vaccination period. The overall impact of a vaccination program is the weighted average of the total and indirect impact parameters with the vaccinated and unvaccinated proportions of the cohort as weights, i.e., for the vaccine types. Similar expressions hold for the NVT and all IPD categories.
Table 3. The total and indirect impact of a vaccination program against VT, NVT, and all IPD in a before-after study setting
Vaccine effectiveness against VT IPD () is defined as one minus the rate of VT IPD in the vaccinated compared to the rate of VT IPD in the unvaccinated in the same child population subject to a vaccination program. It is the individual-level effectiveness against disease and depends on vaccine efficacies against VT carriage acquisition (
) and against progression of carriage to VT disease (
), i.e.,
.Citation19
presents VE parameters separately for the VT, NVT, and all IPD categories derived from by comparing the rates in the vaccinated and unvaccinated children in the post-introduction period. VE parameters are only available for vaccinated children because the unvaccinated group acts as controls.
Table 4. Vaccine effectiveness against VT, NVT, and all IPD in a parallel study setting
To conclude this section, we note that our framework allows an alternative way to correct an odds-ratio-based estimate of vaccine effectiveness in presence of replacement. The original estimate by Broome et al.Citation20 is given by the ratio of the odds of vaccination in cases of VT IPD to the odds of vaccination in cases of NVT IPD. If the odds ratio is written in terms of the rates of IPD as given in , the estimator reads as follows:
This is the same expression as in Andrews et al.Citation21 who presented an odds ratio for the indirect cohort design accounting for the effect of replacement carriage, i.e., the higher NVT carriage prevalence in the vaccinated as compared to the unvaccinated. Thus, the above hazard-ratio-based Broome-estimate is equivalent to the corrected odds-ratio-based estimate. It is clear from EquationEquation (1)(1)
(1) that
slightly overestimates VE, but the difference diminishes toward the end of follow-up as
.
3.2. Scenarios
Different scenarios were considered to explore the time-related behavior of VI and VE. These scenarios represent settings of moderate or high vaccination coverage , efficacy against carriage acquisition of
, and efficacy against progression to disease
. We assumed that
is a declining function that starts from
and declines to
in 6 years (). This corresponds broadly to the observations from Finland and elsewhere.Citation22 The initial proportion of NVT cases out of all IPD was set at
, similar to that in the pre-vaccination era in Finland.Citation23 The proportion of NVT in carriage was taken to be
, which means that the non-vaccine types are generally less invasive, i.e., have lower case-to-carrier ratios than the vaccine types.Citation17 Note that the impact and effectiveness parameters do not depend on the actual rates of VT or NVT IPD but on their initial (i.e., pre-vaccination) ratio.
3.3. Behavior of the impact and effectiveness over time
shows how the vaccine effectiveness and the (total and indirect) vaccine impact against VT, NVT, and all IPD evolve over time according to the expressions of . shows the overall impact against VT, NVT, and all IPD.
Figure 2. Vaccine effectiveness and total and indirect impact as function of time since vaccination onset. Parameter values: ,
,
and the initial fraction of NVT IPD out of all IPD is
. VT carriage decreases to
in about 70 months. (a) Total impact (solid line) and effectiveness (dashed line) against VT; (b) total impact (solid line) and effectiveness (dashed line) against NVT; (c) total impact (solid line) and effectiveness (dashed line) against all IPD; (d) indirect impact against VT; (e) indirect impact against NVT; (f) indirect impact against all IPD
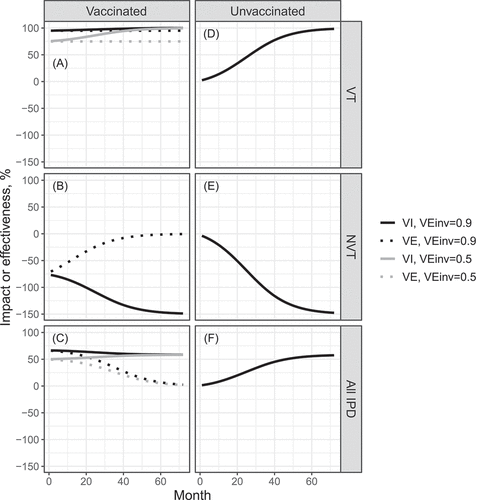
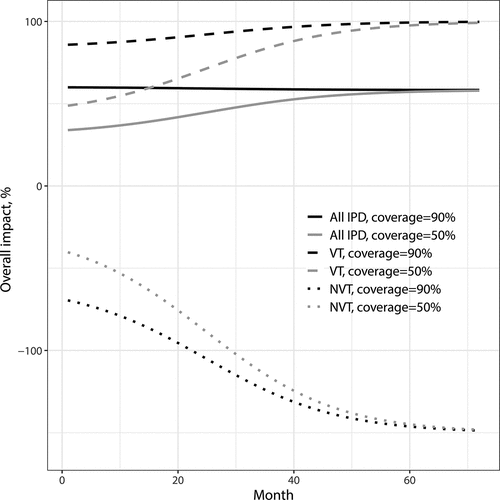
Figure 3. Overall impact against VT, NVT and all IPD as function of time since vaccination onset. Parameter values: proportion vaccinated ,
,
,
and the initial fraction of NVT IPD out of all IPD is
. VT carriage decreases to 0% in about 70 months
The total impact against VT (, )) increases over time since vaccine introduction and reaches eventually
when VT infection approaches elimination. While having the same initial value as
, VE against VT (
) remains constant. Of note, when VT IPD is eliminated,
is not estimable. Obviously, at any time after the program onset
is higher than
.
The total impact against NVT (, )) is always negative and decreases over time, capturing the replacement of VT acquisition by NVT acquisition. Eventually,
reaches the value
, which is the additive inverse of the initial VT/NVT split in carriage, i.e., the pre-vaccination odds of VT carriage.Citation17 VE against NVT (
) starts at the same negative value as the impact but increases over time and reaches zero when all VT carriage is eliminated and the non-vaccine types transmit equally in the vaccinated and unvaccinated parts of the population. This stems from using a contemporaneous cohort of unvaccinated children as the comparison group.
The total impact against all IPD (, )) depends on the initial proportion of VT carriage (
), vaccine efficacy against carriage (
) and the initial VT/NVT split in IPD before vaccine introduction. VE against all IPD (
) starts from the same level as the total impact but decreases to zero toward the end of the study period because only NVT IPD remains.
The indirect impact against VT () increases and the indirect impact against NVT (
) decreases over time, both starting from zero and describing the herd protection and replacement disease, respectively, which unvaccinated children experience under the vaccination program (). The indirect impact against all IPD (
, )) is an increasing function if vaccine coverage is high. In the event of complete replacement, the indirect impact against VT, NVT, and IPD all reach the same values as the respective total impact parameters.
Overall impact (, ) combines all divergent trends and depends on vaccine coverage. There is no concept for indirect or overall effectiveness, because the unvaccinated act as the comparison group in any of the parallel designs.
4. Vaccine impact and effectiveness based on register data in Finland
4.1. Vaccination program
PCV10 was introduced into the Finnish national vaccination program in September 2010. Prior to PCV10 vaccinations in the FinIP trialCitation24 and national vaccination program, a seven-valent conjugate vaccine was recommended for risk groups, but conjugate vaccines were not routinely used and the uptake was minimal (<2%). Since 2010, the coverage rose quickly above 90% and was estimated at 95.5% in the birth cohort of 2015.Citation25
4.2. Data sources and case definition
IPD cases, defined as isolations of Streptococcus pneumoniae from blood or cerebrospinal fluid, were identified from the National Infectious Diseases Register, a population-based electronic laboratory surveillance system maintained by the Finnish Institute for Health and Welfare (THL).Citation23,Citation26 For the analysis, the cases were categorized according to the causative serotype into three mutually exclusive groups: PCV10 serotypes (VT; 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F), PCV10-related serotypes belonging to the same serogroups as the PCV10 serotypes, and non-PCV10-related serotypes (NVT).
The study population was determined based on the Finnish Population Information System. The target cohort was defined as all vaccine-eligible children (born between 6/2010 and 6/2016, followed from 1/2011 through 12/2016, aged 6–78 months, ). Children were defined as vaccinated if at least one dose was registered at the National Vaccination Register. Individuals whose vaccinations were incompletely covered by the National Vaccination Register (altogether 9.1%, 32204 out of 354912 vaccine-eligible children) were excluded from the analysis. All register-based information was linked by using the unique national personal identity code.
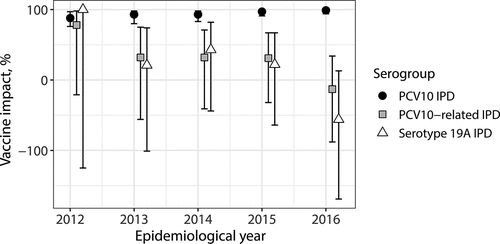
Figure 4. IPD cases and their vaccination status in the target cohort of PCV10 eligible children (years 2010–2016) and in the reference cohort (years 2004–2008) in Finland
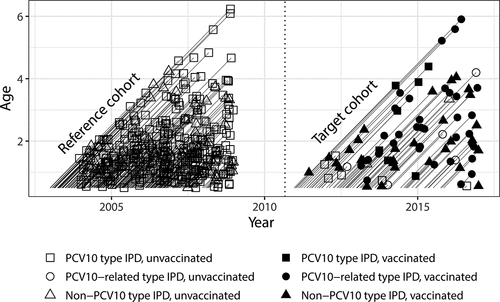
To assess the total, indirect and overall impact of the vaccination program against IPD, a season and age-matched reference cohort was chosen from the pre-vaccination period (born between 6/2002 and 6/2008, followed from 1/2003 to 12/2008, aged 6–78 months, ). IPD rates in the target and reference cohorts were compared by using Poisson regression models. VE against IPD was estimated based on the target cohort of vaccine-eligible children. Poisson regression was used to compare incidence rates in vaccinated and unvaccinated children. In all analyses, population sizes of the cohorts were used as offset.
The inferences were performed within the Bayesian framework. Uninformative prior distributions were used: normal distribution with mean 0 and variance for the effect parameters and Gamma distribution with mean 1 and intensity
for precision. Results are presented as point estimates (posterior mean) and 95% posterior probability (credible) intervals (CI). All analyses were carried out with R (version 3.4.4) and the INLA library.
It is important to note that VE was estimated as a time-average of the time-varying effectiveness measure (cf. ) over the years 2011–2016. VI was estimated both as time-average and separately for each year, comparing to the whole reference cohort (). Note also that VE and VI were estimated separately for the three serotype groups: PCV10 serotypes (VT), PCV10-related serotypes and non-PCV10-related serotypes (NVT). In addition, VI and VE were estimated for all serotypes.
4.3. Impact and effectiveness against IPD in Finland
presents the estimated vaccine impact and effectiveness against IPD in vaccine-eligible Finnish children. We compare the time trends of these estimates to patterns predicted by the theoretical model as well as to findings from previous studies in the same setting.
Table 5. Effectiveness of PCV10 vaccination in the PCV10-eligible target cohort (2010–2016), and the impact of PCV10 vaccination based on the comparison of the target cohort with a reference cohort (2002–2008), Finland
Total impact and effectiveness. Both and
were high and, consistently with the model,
was higher than
(97%; 95% CI: 95, 99% versus 92%; 95% CI: 77, 97%). Also the time trends corresponded to the model predictions, as after splitting the follow-up into yearly periods,
rose quickly over 90% and ended up at 99% (95% CI: 94, 100%) in 2016 (). In 2018, there were no cases of PCV10 IPD in the vaccine-eligible cohort, and
was thus 100%. By contrast,
in Finland has remained constant in the post-introduction period, as reported elsewhere.Citation27
For the non-PCV10-related types, was negative (−78%; 95% CI: −214, −2%), as expected due to replacement in carriage. The small number of cases prevented meaningful estimation of the time trend in VI.
was −17% (95% CI: −622, 65%).
and
were 79% (95% CI: 73, 84%) and 60% (95% CI: 26, 77%), respectively.
is expected to approach zero when VT disease is eliminated and vaccinated and unvaccinated children carry NVT with similar rates (). Indeed, there has been a decreasing trend in
among Finnish children.Citation27Citation28Citation29
In our data, the group of non-vaccine-types consists of PCV10-related and non-PVC10-related serotypes. The first subgroup is dominated by type 19A, which has been the main replacing serotype in Finnish children as well as in the older, unvaccinated population.Citation28,Citation29 However, in vaccine-eligible children, serotype 19A IPD seems to have occurred more often in unvaccinated children as compared to vaccinated.Citation27 This is indicated by the positive point estimate of VE against PCV10-related IPD (46%; 95% CI: −59, 77%). By contrast, the impact against PCV10-related IPD shows a decreasing trend ().
Indirect impact. was high (67%; 95% CI: 35, 86%), indicating strong herd protection in unvaccinated children.
and
were −53% (95% CI: −451, 70%) and 47% (95% CI: 13, 70%), respectively.
Overall impact. The estimates of and
(95%; 95% CI: 92, 97%, and −76%; 95% CI: −209, −3%) were almost the same as the respective estimates of
, as expected due to the small proportion of unvaccinated children.
was 77% (95% CI: 71, 82%), resulting from high
, slow replacement of NVT, and positive
against PCV10-related IPD.
5. Discussion
The vaccine impact and effectiveness against invasive pneumococcal disease (IPD) are affected by the indirect effects of vaccination through herd immunity and serotype replacement in carriage. As effect measures, the impact and effectiveness describe two different phenomena and may behave very differently over time. While vaccine impact (VI) describes the (relative) reduction in the rate of disease due to a vaccination program, vaccine effectiveness (VE) quantifies the (relative) reduction in the rate of disease that vaccination affords to an individual during ongoing vaccination. In this paper, we constructed a simple pseudo-dynamic model to describe the time-dependency of VI and VE in cohorts of children after the introduction of a pneumococcal vaccination program.
As exposure to vaccine-type (VT) infection decreases over time since vaccine introduction, the impact against vaccine-type disease increases in both vaccinated () and unvaccinated (
).
is affected by the direct and indirect effects and thus quantifies the net benefit of the vaccination program to a vaccinated individual. The impact against the non-vaccine types (
and
) is expected to decrease due to increasing exposure to NVT infection through replacement in carriage among vaccinated and unvaccinated children.
The overall impact () incorporates the divergent trends and depends additionally on vaccination coverage. If vaccination coverage is high and the majority of the population is vaccinated, the overall and total VI behave similarly. Eventually, the net impact of a vaccination program will be positive only if the replacing serotypes have a lower average case-to-carrier ratio than the vaccine types.Citation17
The vaccine effectiveness against the vaccine types (), also called field efficacy,Citation1 is a measure of the direct protection afforded by the vaccine against the vaccine serotypes. Although the incidence rates of VT IPD decrease in both vaccinated and unvaccinated children as both groups benefit from the herd effects in a similar manner, the incidence rate ratio and thus VE are expected to remain stable at any time since the program onset. This follows from the assumption that both vaccine efficacies against VT carriage acquisition and progression of carriage to disease are constant irrespective to the dynamics of the indirect effects. Once VT disease becomes eliminated,
is no longer estimable.
The fact that remains constant over time may be a simplification because it discards competition between serotypes: carriers may be additionally protected against acquiring another strain while already carrying a serotype.Citation11,Citation30,Citation31 As the vaccination program enhances NVT acquisition among the vaccinated,
could increase over time. This effect, however, should be small, especially when the VT incidence is very low.
The vaccine effectiveness against the non-vaccine types () describes the differential within-host serotype competition between the vaccinated and unvaccinated. It should approach zero in the new equilibrium when vaccine-type carriage has been replaced by non-vaccine-type carriage and exposure to NVT is equal in the vaccinated and unvaccinated parts of the population. Also
should approach zero as eventually all disease is caused by NVT.
In Finland, the assessment of vaccine impact in a before-after design is possible because serotype-specific data on IPD are available for a period starting several years prior to PCV10-introduction. Several parallel study designs are also applicable, because each child’s vaccination status is recorded in a nation-level register. In this study, we compared the estimates of VI (with a before-after design) and VE (with a parallel design) by using register data of vaccine-eligible children in Finland. Because VE is a measure of the benefit of becoming vaccinated, this comparison will be made with .
Consistent with the model, the estimated was higher than
and increased over the years reaching 100% in 2018 when no IPD cases caused by PCV10 serotypes were detected. Our conclusion about the non-vaccine serotypes, however, is not as clear. Not all serotypes in our setting fall into the two categories of VT and NVT but exhibit properties of both. In particular, this is the case with serotype 19A that constitutes the majority of PCV10-related IPD in children in the post-vaccination era. The increasing incidence of 19A IPD especially among unvaccinated children has resulted in positive and higher than expected estimate of
. By contrast, although the incidence of the serotypes not related to PCV10 serotypes (the ”true” NVT) was very low throughout the study period, the impact against them (
) was negative and
was close to the expected value of zero.
Vaccine effectiveness is often estimated based on accumulated cases per person-time until a certain time point. This means that the effectiveness becomes a time average, although the measure itself could be time-varying. In the current setting, this is not a problem for which remains constant over time. However, as
is expected to increase from a negative level toward 0%, a time-average may overestimate the effectiveness as more follow-up time accrues toward the end of the study period.
Although prospective cohort studies are optimal in utilizing the full follow-up of the underlying cohorts,Citation10 VE is usually estimated with retrospective approaches, i.e., case-control and indirect cohort designs, in which more detailed data can be collected on the study subjects and many factors can be adjusted for.Citation20,Citation32–34 The VE estimates based on the indirect cohort design may be biased due to disproportionate NVT replacement among vaccinated and unvaccinated IPD cases.Citation21 However, the bias is small and diminishes as the proportion of VT carriage decreases. Our framework allows formulation of the bias in a similar manner as in Andrews et al.,Citation21 but in terms of rates and rate ratios rather than odds and odds ratios.
Vaccine impact is a dynamic measure affected by secular trends unrelated to vaccination, such as temporal fluctuations in the incidence of individual serotypes. Other factors such as the sensitivity of surveillance systems due to alterations in clinical awareness, reporting techniques, and blood culturing practices may also affect the analysis of data accumulating over long time periods.Citation35 A long pre-vaccination period and analysis at the serotype group level help in balancing out randomness. Time-series models have been used to adjust for pre-vaccine trends to avoid arbitrary selection of baseline incidence.Citation36–39
In our theoretical model, we defined VI and VE in terms of the per capita rates of carriage acquisition and case-to-carrier ratios. In general, VI and VE depend on vaccine efficacy against carriage and disease, the pre-vaccination split of IPD into VT and NVT, the time-varying proportion of VT carriage out of all carriage, as well as vaccination coverage. Admittedly, the model is a simplification of the true underlying processes and may thus carry many limitations. For instance, vaccine efficacy may wane in age and vaccination coverage takes time to increase to a high level. Moreover, the binary split of serotypes into VT and NVT may not be sufficient in a real setting. For example, a subgroup of vaccine serotypes might not be completely eliminated from circulation, which would show in their proportion (function f(t)) not decreasing to 0%. With regard to non-vaccine serotypes, our data indicate that due to changes in the distribution of circulating 19A clones,Citation40 the assumption about constant case-to-carrier ratios does not fully hold in Finland, leading to the need of more complicated analysis.
Another limitation of our study regarding the estimation of VE are the small case numbers that prevented estimation based on short time bands, evaluation of possible time-trends, and comparison with the model. However, the observed time trends in VI estimates corresponded to the model predictions. Nevertheless, despite the small data and the simplified structure of the model, our study should serve to exemplify how the unobservable carriage process modifies disease dynamics and how VI and VE behave in the long-term post-vaccination.
Vaccine effectiveness, measured in parallel settings, quantifies the benefit for an individual of being vaccinated but does not adjust for the indirect effects of the vaccination program. Parallel effect measures do not describe the indirect or population level overall effectiveness. Vaccine impact, by contrast, takes into account the indirect effects and therefore better describes the public health performance of the vaccination program in the whole population.
Author contributions
All authors attest they meet the ICMJE criteria for authorship. HR-K was involved in data collection and analysis. All authors were involved in study design and interpretation, and reviewed and approved submission of the final manuscript.
Disclosure of potential conflicts of interest
The Finnish Institute for Health and Welfare has received research funding from Glaxo-SmithKline Vaccines for the conduct of a nationwide effectiveness trial of the 10-valent pneumococcal conjugate vaccine. HR-K was a co-investigator in this study. The other authors have no conflicts to disclose.
References
- Haber M, Longini IM Jr., Halloran ME. Measures of effects of vaccination in a randomly mixing population. Int J Epid. 1991;20:300–10.
- Rodriques LC, Smith PG. Use of the case-control approach in vaccine evaluation: efficacy and adverse effects. Epidemiol Rev. 1999;21(1):56–72. doi:10.1093/oxfordjournals.epirev.a017988.
- Patel MM, Tate J, Cortese M, Payne DC, Armstrong G, Parashar UD, Lopman B. The impact of indirect benefits of vaccination on postlicensure vaccine effectiveness estimates: A scenario analysis. Vaccine. 2010;28(50):7987–92. doi:10.1016/j.vaccine.2010.09.044.
- Lipsitch M, Jha A, Simonsen L. Observational studies and the difficult quest for causality: lessons from vaccine effectiveness and impact studies. Int J Epid. 2016;45(6):2060–74.
- Wilder-Smith A, Longini I, Zuber PL, Bärnighausen T, Edmunds WJ, Dean N, Spicher VM, Benissa MR, Gessner BD. The public health value of vaccines beyond efficacy: methods, measures and outcomes. BMC Med. 2017;15(1):138. doi:10.1186/s12916-017-0911-8.
- Hanquet G, Valenciano M, Simondon F, Moren A. Vaccine effects and impact of vaccination programmes in post-licensure studies. Vaccine. 2013;31(48):5634–42. doi:10.1016/j.vaccine.2013.07.006.
- Halloran ME, Struchiner CJ, Longini IM. Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epid. 1997;146(10):789–803. doi:10.1093/oxfordjournals.aje.a009196.
- Rothman K, Greenland S, Lash T. Modern Epidemiology. 3rd ed. Philadelphia (USA): Williams & Wilkins; 2008.
- Dominguez A, Ciruela P, Hernandez S, García-García JJ, Soldevila N, Izquierdo C, Moraga-Llop F, Díaz A, F. de Sevilla M, González-Peris S. Effectiveness of the 13-valent pneumococcal conjugate vaccine in preventing invasive pneumococcal disease in children aged 7-59 months. A matched case-control study. PLoS ONE. 2017;12(8):e0183191. doi:10.1371/journal.pone.0183191.
- Gidding H, McCallum L, Fathima P, Moore HC, Snelling TL, Blyth CC, Jayasinghe S, Giele C, de Klerk N, Andrews RM, et al. Effectiveness of a 3 + 0 pneumococcal conjugate vaccine schedule against invasive pneumococcal disease among a birth cohort of 1.4 million children in Australia. Vaccine. 2018;36(19):2650–56. doi:10.1016/j.vaccine.2018.03.058.
- Lipsitch M. Vaccination against colonizing bacteria with multiple serotypes. Proc Natl Acad Sci. 1997;94(12):6571–76. doi:10.1073/pnas.94.12.6571.
- Melegaro A, Choi YH, George R, Edmunds WJ, Miller E, Gay NJ. Dynamic models of pneumococcal carriage and the impact of the heptavalent pneumococcal conjugate vaccine on invasive pneumococcal disease. BMC Infect Dis. 2010;10(1):90. doi:10.1186/1471-2334-10-90.
- Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73.
- Choi YH, Jit M, Flasche S, Gay N, Miller E. Mathematical modelling long-term effects of replacing Prevnar7 with Prevnar13 on invasive pneumococcal diseases in England and Wales. PLoS ONE. 2012;7(7):e39927. doi:10.1371/journal.pone.0039927.
- Guevara M, Barricarte A, Torroba L, Herranz M, Gil-Setas A, Gil F, Bernaola E, Ezpeleta C, Castilla J. Direct, indirect and total effects of 13-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in children in Navarra, Spain, 2001 to 2014: cohort and case–control study. Euro Surveill. 2016;21(14):pii=30186. doi:10.2807/1560-7917.ES.2016.21.14.30186.
- Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, Andrews NJ, Miller E, Ramsay ME. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51. doi:10.1016/S1473-3099(18)30052-5.
- Nurhonen M, Auranen K. Optimal serotype compositions for pneumococcal conjugate vaccination under serotype replacement. PLoS Comput Biol. 2014;10(2):e1003477. doi:10.1371/journal.pcbi.1003477.
- Flasche S, Le Polain de Waroux O, O’Brien KL, Edmunds WJ. The Serotype Distribution among Healthy Carriers before Vaccination Is Essential for Predicting the Impact of Pneumococcal Conjugate Vaccine on Invasive Disease. PLoS Comput Biol. 2015;11(4):e1004173. doi:10.1371/journal.pcbi.1004173.
- Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O’Brien KL. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines. 2012;11(7):841–55. doi:10.1586/erv.12.53.
- Broome CV, Facklam RR, Fraser DW. Pneumococcal disease after pneumococcal vaccination. An alternative method to estimate the efficacy of pneumococcal vaccine. N Eng J Med. 1980;303(10):549/52. doi:10.1056/NEJM198009043031003.
- Andrews N, Waight PA, Borrow R, Ladhani S, George RC, Slack MPE, Miller E. Using the indirect cohort design to estimate the effectiveness of the seven valent pneumococcal conjugate vaccine in England and Wales. PLoS ONE. 2011;6(12):e28435. doi:10.1371/journal.pone.0028435.
- Masala GL, Lipsitch M, Bottomley C, Flasche S. Exploring the role of competition induced by non-vaccine serotypes for herd protection following pneumococcal vaccination. J R Soc Interface. 2017;14:20170620. doi:10.1098/rsif.2017.0620.
- Jokinen J, Rinta-Kokko H, Siira L, Palmu AA, Virtanen MJ, Nohynek H, Virolainen-Julkunen A, Toropainen M, Nuorti JP. Impact of ten-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in Finnish children–a population-based study. PLoS One. 2015;10(3):e0120290. doi:10.1371/journal.pone.0120290.
- Palmu AA, Jokinen J, Borys D, Nieminen H, Ruokokoski E, Siira L, Puumalainen T, Lommel P, Hezareh M, Moreira M, et al. Effectiveness of the ten-valent pneumococcal Haemophilus Influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: A cluster randomised trial. Lancet. 2013;381(9862):214–22. doi:10.1016/S0140-6736(12)61854-6.
- Finnish Institute for Health and Welfare (THL), Finland. The vaccination register. Helsinki, Finland: THL; [ Accessed 13 Aug 2019]. Available from: https://thl.fi/en/web/vaccination/vaccination-coverage/national-vaccination-register
- Siira L, Kaijalainen T, Lambertsen L, Nahm MH, Toropainen M, Virolainen A. From Quellung to multiplex PCR, and back when needed, in pneumococcal serotyping. J Clin Microbiol. 2012;50(8):2727–31. doi:10.1128/JCM.00689-12.
- Rinta-Kokko H, Auranen K, Toropainen M, Nuorti JP, Nohynek H, Siira L, Palmu AA. Effectiveness of 10-valent pneumococcal conjugate vaccine estimated with three parallel study designs among vaccine-eligible children in Finland. Vaccine. 2020;38(6):1559–64. doi:10.1016/j.vaccine.2019.11.049.
- Rinta-Kokko H, Palmu AA, Auranen K, Nuorti JP, Toropainen M, Siira L, Virtanen MJ, Nohynek H, Jokinen J. Long-term impact of 10-valent pneumococcal conjugate vaccination on invasive pneumococcal disease among children in Finland. Vaccine. 2018;36(15):1934–40. doi:10.1016/j.vaccine.2018.03.001.
- Nuorti P, H, Toropainen M, Siira L, Virtanen MJ, Nohynek H, Palmu AA, Jokinen J. Evidence of herd protection and serotype replacement in adults after universal 10-valent pneumococcal conjugate vaccination of infants in Finland. 10th International symposium on pneumococci and pneumococcal diseases, Glasgow, Scotland, 2016.
- Mehtälä J, Antonio M, Kaltoft MS, O’Brien KL, Auranen K. Competition between Streptococcus pneumoniae strains - Implications for vaccine-induced replacement in colonization and disease. Epidemiology. 2013;24(4):522–29. doi:10.1097/EDE.0b013e318294be89.
- Auranen K, Rinta-Kokko H, Halloran ME. Estimating strain-specific and overall efficacy of polyvalent vaccines against recurrent pathogens from a cross-sectional study. Biometrics. 2013;69(1):235–44. doi:10.1111/j.1541-0420.2012.01826.x.
- Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Nyquist A-C, Gershman KA, Vazquez M, Bennett NM. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368(9546):1495–502. doi:10.1016/S0140-6736(06)69637-2.
- Rückinger S, van der Linden M, Reinert RR, von Kries R. Efficacy of 7-valent pneumococcal conjugate vaccination in Germany: an analysis using the indirect cohort method. Vaccine. 2010;28(31):5012–16. doi:10.1016/j.vaccine.2010.05.021.
- Deceuninck G, De Serres G, Boulianne N, Lefebvre B, De Wals P. Effectiveness of three pneumococcal conjugate vaccines to prevent invasive pneumococcal disease in Quebec, Canada. Vaccine. 2015;33(23):2684–89. doi:10.1016/j.vaccine.2015.04.005.
- Flasche S, Slack M, Miller E. Long term trends introduce a potential bias when evaluating the impact of the pneumococcal conjugate vaccination programme in England and Wales. Euro Surveill. 2011;16:pii=19868.
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–09. doi:10.1016/S1473-3099(14)71081-3.
- Andrade A, Minamisava R, Policena G, Cristo EB, Domingues CMS, de Cunto Brandileone MC, Almeida SCG, Toscano CM, Bierrenbach AL. Evaluating the impact of PCV-10 on invasive pneumococcal disease in Brazil: A time-series analysis. Hum Vacc Immunother. 2016;12(2):285–92. doi:10.1080/21645515.2015.1117713.
- Bruhn CAW, Hetterich S, Schuck-Paim C, Kürüm E, Taylor RJ, Lustig R, Shapiro ED, Warren JL, Simonsen L, Weinberger DM. Estimating the population-level impact of vaccines using synthetic controls. PNAS. 2017;114(7):1524–29. doi:10.1073/pnas.1612833114.
- Richter L, Schmid D, Kanitz EE, Zwazl I, Pöllabauer E, Jasinska J, Burgmann H, Kundi M, Wiedermann U. Invasive pneumococcal diseases in children and adults before and after introduction of the 10-valent pneumococcal conjugate vaccine into the Austrian national immunization program. PLoS ONE. 2019;14(1):e0210081. doi:10.1371/journal.pone.0210081.
- Toropainen M, Nyholm O, Siira L, Jalava J, Rinta-Kokko H, Nuorti PJ, Palmu AA. Genomic epidemiology and antimicrobial resistance in invasive serotype 19A pneumococci before and after introduction of 10-valent pneumococcal conjugate vaccine (PCV10) in Finland. 11th International symposium on pneumococci and pneumococcal diseases, Melbourne, Australia, 2018.